Abstract
Purpose
Oral lichen planus (OLP) is a chronic oral mucosal disease that has been recognized as an immune condition. The purpose of this study was to evaluate factors affecting the clinical outcomes of topical corticosteroid application on OLP lesions using dexamethasone gargle and ointment.
Methods
The charts of patients who were clinically diagnosed with OLP and treated with dexamethasone from July 2003 to August 2017 at the Section of Dentistry of Seoul National University Bundang Hospital were thoroughly evaluated to identify subjects who were suitable for this retrospective study. For each patient, age at the index date, gender, medical history, and dental records related to OLP lesions and dexamethasone treatment were reviewed.
Results
In total, 113 of the 225 patients were included in the present study. Among them, 79 patients were female (69.9%) and 34 were male (30.1%), with a mean age of 57.6 years. The average duration of dexamethasone treatment was 4.7 months and the mean follow-up period was 2.24 years. Improvements were observed within 1 year after dexamethasone treatment in most cases, and 17.7% of patients had a new OLP lesion after treatment. New OLP lesions were more frequently gingival than mucosal, although mucosal OLP lesions were more common than gingival OLP lesions in all age groups. In age- and gender-adjusted multivariate logistic regression, a history of malignant disease was found to be a significant factor affecting the formation of new lesions. Gingival OLP lesions and intermittent use of dexamethasone showed near-significant associations. In Kaplan-Meier failure analysis, history of malignancy, menopausal status, age, and the site of the OLP lesion were significant factors affecting clinical outcomes.
Conclusions
The treatment outcomes of OLP were significantly influenced by age, history of malignancy, menopausal status, and the site of the OLP lesion, but not by factors related to dexamethasone treatment.
Keywords: Corticosteroid methanetriol mixture, Logistic models, Oral lichen planus, Retrospective studies, Survival analysis
Graphical Abstract
INTRODUCTION
Oral lichen planus (OLP) is a chronic oral mucosal disease that has been recognized as an immune condition. OLP primarily affects middle-aged or elderly patients, and occurs 2–3 times more frequently in women than in men [1]. Many epidemiologic studies of OLP have been performed. The prevalence of OLP has been estimated to range from 0.55% to 2%, and there might be some ethnic differences [1,2].
Although the etiology of OLP has not been fully elucidated, it has been theorized that genetic factors might play a role, so that some endogenous factors may be related to the onset and progress of the cell-mediated immune response. CD4 cells, CD8 cells, T cells, natural killer cells, and mast cells are well recognized as associated with OLP [3].
OLP shows a wide range of clinical features, from painless white lesions to painful erosive or ulcerative lesions [4,5]. It can present bilaterally or unilaterally and as a white or red lesion. The shape of the lesion can vary widely, with possibilities including reticular lesions, papillary lesions, erythema, erosion, and ulceration with or without symptoms. The most common site is the oral mucosa, especially the buccal mucosa, but the gingiva, tongue, lip, and palate can also be involved.
It is generally accepted that corticosteroids are the main treatment option for OLP lesions. Additionally, retinoids, vitamin A analogues, dapsone, griseofulvin, azathioprine, levamisole, and photochemotherapy can be recommended for OLP treatment [6,7,8]. While only regular observation is needed for asymptomatic OLP, patients with symptomatic OLP need interventions to relieve pain and to reduce the extent of ulcerative or erythematous lesions. Topical corticosteroids, such as dexamethasone, are the most commonly used therapeutic agent for symptomatic cases, since topical treatments have fewer adverse effects than systemic drugs. Nonetheless, despite the wide use of topical corticosteroids by dentists, the efficacy of this treatment modality remains poorly understood and controversial.
The purpose of the present study was to evaluate the clinical outcomes of topical corticosteroid application on OLP lesions by using dexamethasone gargle (0.05% or 0.1%) and/or ointment. In addition, factors affecting the clinical outcomes of OLP were also analyzed by multivariate logistic regression analysis and Kaplan-Meier failure analysis.
MATERIALS AND METHODS
Subjects
The charts of patients who were clinically diagnosed with OLP and treated with dexamethasone from July 2003 to August 2017 at the Section of Dentistry of Seoul National University Bundang Hospital were evaluated to identify subjects who were suitable for this retrospective study. This study was approved by the Institutional Review Board of the Seoul National University Dental Hospital (B-1804-463-102).
Those who had white, reticular-patterned, atrophic lesions with or without erosion in the oral cavity and showed hypersensitivity to spicy or hot food were diagnosed with OLP. Biopsies were only performed in patients with erosive or ulcerative lesions as part of the differential diagnosis to exclude malignancy or other autoimmune lesions, including pemphigus vulgaris.
A mouth rinse with 10 mL of 0.05% dexamethasone gargle 2 times a day was used as the treatment. Recall checks were done every 2–3 weeks and the treatment was changed to 0.1% dexamethasone gargle in those who had not shown any improvements. For patients with difficulty gargling or in whom only small lesions remained, the treatment was changed to an application of 0.1% dexamethasone ointment (Peridex, Green Cross Corp., Yongin, Korea). Once symptoms or lesions disappeared, treatment was discontinued and regular observations were made. Annual cortisol and adrenocorticotropic hormone (ACTH) tests were performed in patients who received long-term treatment (over 6 months) to check their hormone levels. Patients with adverse effects, such as increased appetite and weight gain, also received cortisol and ACTH tests.
Variables
For each patient, age at the index date, gender, medical history (hypertension, diabetes mellitus, hypothyroidism, cardiovascular disease, dyslipidemia, Sjögren syndrome, Behçet syndrome, asthma, menopause, malignancy), symptoms of the OLP lesion (pain, hypersensitivity), site of the OLP lesion (buccal mucosa, vestibule, keratinized gingiva, palate, or tongue), subtype of OLP (red or whitish lesion, erosive lesion, mixed type), mode of dexamethasone treatment (gargle, ointment), pattern of dexamethasone use (continuous, intermittent), duration of dexamethasone treatment, treatment response (improved, new lesion or expansion, no change), adverse effects (nausea, facial swelling, dry mouth), and follow-up duration (from index date to final visit within the study period) were recorded for the analysis.
Statistical analysis
Prior to the statistical analysis, a power analysis was performed. At the given sample size of subjects (n=113) and with an odds ratio of 2, a 0.3 prevalence of new keratinized gingival lesions, and a significance level of 0.05, the power analysis for the logistic regression analysis was calculated as 88% using G*Power 3.1.5 (Franz Faul, Universität Kiel, Kiel, Germany) The main outcome of interest was new lesion formation after treatment.
Statistical analysis was performed to evaluate the distribution of the patients by the location of the OLP lesion using the Pearson's χ2 test and 1-way analysis of variance with the Bonferroni post hoc test, to analyze the factors that affected clinical outcomes using univariate and multivariate logistic regression, and to investigate the factors affecting new lesion formation using the log-rank test with Kaplan-Meier failure analysis (failure was defined as a new lesion or expansion, and the time variable was defined as days from index date to the date of a new lesion or expansion). For the multivariate logistic regression, variables were analyzed using univariate logistic regression analysis, and the final model was constructed using age, gender, and variables with P values <0.1 in the univariate analysis. The statistical analyses were performed using STATA/SE14 (StataCorp, College Station, TX, USA).
RESULTS
In total, the charts of 225 patients were reviewed, of whom 112 were excluded due to being diagnosed with other diseases, missing records, and lack of follow-up after treatment. In one of the excluded cases, a white lesion showed a malignant transformation. This patient was a 65-year-old man who had a white mucosal lesion in the buccal vestibule measuring less than 2 cm and suffered from pain while eating. The white lesion was clinically diagnosed as an OLP lesion at the initial exam, but a verrucous change occurred after 1 month. An excisional biopsy was done and the lesion was diagnosed as squamous cell carcinoma (stage 1) without metastasis to the lymph nodes or other organs. There was no recurrence during 5 years of follow-up.
The remaining 113 patients who received dexamethasone treatment for OLP lesions were included in this study. Among the OLP patients, 79 were female (69.9%) and 34 were male (30.1%), reflecting a female-to-male ratio greater than 2:1. The patients ranged in age from 31 to 91 years, with a mean age of 49.52 years. Thirty-four patients (30.1%) had circulatory problems; 9 (8.0%) had endocrine problems, including diabetes and thyroid disease; and 9 (8.0%) had a history of malignancy. Thirty-two patients were post-menopausal women (28.3% of all patients, 40.5% of the female patients).
Biopsies were performed in 4 patients. One biopsy sample was analyzed using immunofluorescence and histopathology, while the other 3 were only analyzed using histopathology. The immunofluorescence analysis was negative for immunoglobulin (Ig) G, IgM, IgA, and C3. In histopathology, 3 of the lesions showed chronic inflammation and 1 was diagnosed as lichen planus.
Dexamethasone was mostly prescribed as a gargle, and 29 patients received adjunctive dexamethasone ointment. The average duration of dexamethasone treatment was 4.7 months and the mean follow-up period was 2.24 years. Most cases showed improvements with treatment, including patients who received a dexamethasone gargle (75%) and those who received a combination of a dexamethasone gargle and ointment (60%). However, new lesions occurred in 20 patients. Most patients experienced a reduction of symptoms in the short term, but 22 patients received treatment for over 6 months. Some of the patients who received long-term treatment used it intermittently depending on the severity of discomfort. Eight patients who used corticosteroids for over 6 months requested a blood test of cortisol and ACTH levels and the results were in the normal range. Five patients discontinued dexamethasone treatment due to adverse effects, such as dry mouth, facial swelling, nausea, and weight gain.
Distribution of OLP lesions
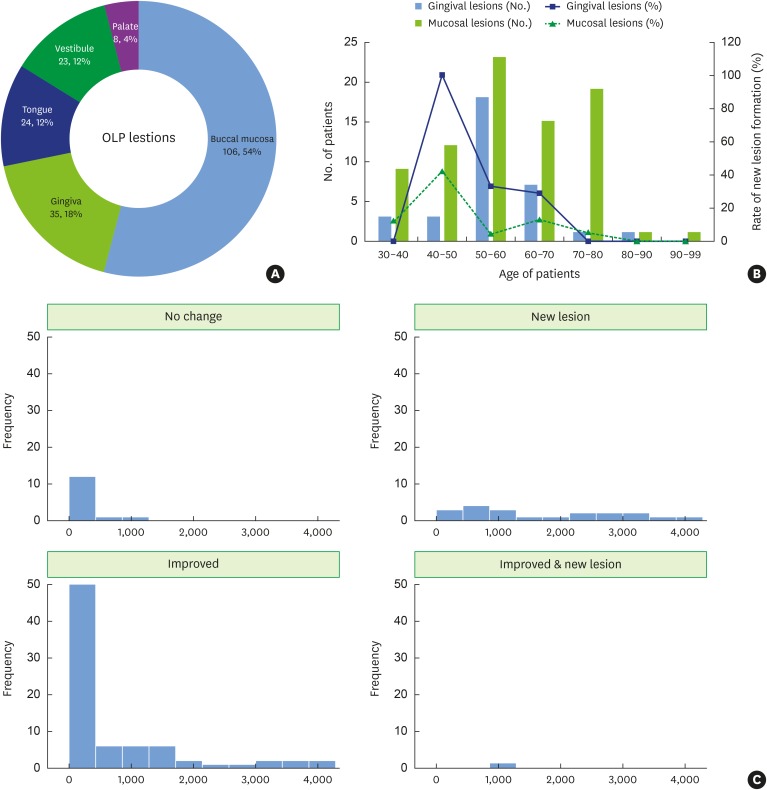
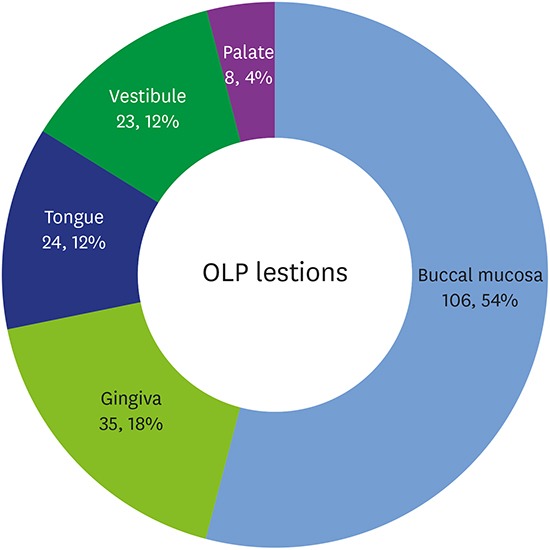
More than half of the OLP lesions were observed in the buccal mucosa, followed by the gingiva, tongue, vestibule, and palate (Figure 1A). The distribution of OLP lesions according to age is shown in Figure 1B. OLP lesions most frequently occurred in patients from 50 to 70 years of age, and mucosal lesions (buccal mucosa, vestibule, and tongue) were more frequent than gingival lesions (gingiva and palate) in all age groups. New gingival lesions were more likely to form after dexamethasone treatment than mucosal lesions. Regarding the distribution according to clinical outcomes (Figure 1C), approximately 50% of patients improved within 1 year after treatment. No change was seen in roughly 10% of patients. New lesion formation occurred throughout the follow-up period.
Figure 1. Distribution of OLP lesions in this study. (A) Distribution of OLP lesions according to site (number of patients, percentile to total), (B) distribution of OLP lesions according to patients' age (number of patients) and rate of new lesion formation in gingival and mucosal lesions (percentile), (C) histogram of clinical outcomes according to follow-up duration (days).
OLP: oral lichen planus.
Factors affecting the clinical outcome of OLP lesions
To examine the effects of the site of OLP lesions on the clinical outcomes, subgroup analysis was performed (Table 1). Age and gender were not significantly different between subgroups. However, digestive problems were only observed among patients with gingival lesions, and the OLP lesions in menopausal women were mucosal or mixed lesions. These trends were statistically significant (P<0.001 and P=0.018, respectively). Pain in gingival OLP lesions was more frequently observed than in mucosal and mixed lesions. The subtype of OLP also significantly differed by lesion site. Most mucosal lesions were white or red lesions, while gingival lesions tended to be erosive and mixed-type (P=0.007). Variables related to dexamethasone treatment were not significantly different by lesion site. However, improvement after treatment was more frequently observed in mucosal lesions, and the ratio of new lesion formation was greater in gingival and mixed lesions. The follow-up period was longer in mixed lesions, but did not significantly differ between subgroups.
Table 1. General characteristics of the patients according to the location of the OLP lesion.
| Characteristics | OLP lesion | Total (n=113) | P value | |||
|---|---|---|---|---|---|---|
| Mucosal lesion (n=78) | Gingival lesion (n=10) | Mixed lesion (n=25) | ||||
| Age (yr) | 58.8±13.4 | 60.1±10.2 | 53±8.8 | 57.6±12.4 | 0.157 | |
| Female | 56 (71.8) | 6 (60) | 17 (68) | 79 (69.9) | 0.725 | |
| Medical history | ||||||
| Circulatory problems | 23 (29.5) | 4 (40) | 7 (28) | 34 (30.1) | 0.766 | |
| Endocrine problems | 5 (6.4) | 1 (10) | 3 (12) | 9 (8.0) | 0.648 | |
| Digestive problems | 0 (0) | 2 (20) | 0 (0) | 2 (1.8) | <0.001a) | |
| Rheumatic problems | 3 (3.9) | 0 (0) | 0 (0) | 3 (2.7) | 0.501 | |
| Menopause | 28 (35.9) | 0 (0) | 4 (16) | 32 (28.3) | 0.018a) | |
| Malignancy | 5 (6.4) | 0 (0) | 4 (16) | 9 (8) | 0.190 | |
| Symptoms of OLP | ||||||
| Pain | 11 (14.1) | 4 (40) | 1 (4) | 16 (14.2) | 0.022a) | |
| Hypersensitivity | 47 (60.3) | 5 (50) | 13 (52) | 65 (57.5) | 0.676 | |
| Subtype of OLP | 0.007a) | |||||
| White or red lesion | 71 (91) | 6 (60) | 23 (92) | 92 (87.6) | ||
| Erosive lesion | 3 (3.8) | 3 (30) | 2 (8) | 8 (7.6) | ||
| Mixed | 4 (5.1) | 1 (10) | 0 (0) | 5 (4.8) | ||
| Mode of dexamethasone treatment | 0.840 | |||||
| Ointment alone | 2 (2.8) | 0 (0) | 1 (4.2) | 3 (2.8) | ||
| Gargle alone | 52 (72.2) | 6 (60) | 16 (66.7) | 74 (69.8) | ||
| Gargle + ointment | 18 (25) | 4 (40) | 7 (29.2) | 29 (27.4) | ||
| Pattern of dexamethasone use | 0.166 | |||||
| Continuous | 72 (92.3) | 8 (80) | 20 (80) | 100 (88.5) | ||
| Intermittent | 6 (7.7) | 2 (20) | 5 (20) | 13 (11.5) | ||
| Duration of dexamethasone use (day) | 131.1±190.1 | 204.4±331.3 | 186.5±189.1 | 149.8±205.3 | 0.344 | |
| Adverse effects | 3 (3.9) | 0 (0) | 2 (8) | 5 (4.4) | 0.527 | |
| Response | 0.078 | |||||
| No change | 11 (14.1) | 2 (20) | 1 (4) | 14 (12.4) | ||
| Improved | 58 (74.4) | 5 (50) | 16 (64) | 79 (69.9) | ||
| New lesion | 9 (11.5) | 3 (30) | 8 (32) | 20 (17.7) | ||
| Follow-up (day) | 702.2±1,021 | 710.6±1,073.6 | 1,229.1±1,297.9 | 819.5±1,103.8 | 0.109 | |
Values are presented as mean ± standard deviation or number (%). Circulatory problems included hypertension, cardiovascular disease, and dyslipidemia. Endocrine problems included hypothyroidism and diabetic mellitus. Rheumatic problems included asthma, Sjögren syndrome, and Behçet disease. Digestive problems included gastrointestinal trouble and hepatitis C. Adverse effects included dry mouth, facial swelling, nausea, and weight gain.
OLP: oral lichen planus.
a)Statistically significant difference compared to the baseline.
To investigate the factors affecting new lesion formation, logistic regression analysis was performed (Table 2). In the univariate analysis, history of malignancy, gingival lesions, intermittent use of dexamethasone, and the duration of dexamethasone use were found to be significant factors. In the age- and gender-adjusted multivariate analysis, history of malignancy was a significant factor (odds ratio , 11.48; 95% confidence interval, 1.96–67.31; P=0.007). Gingival lesions and intermittent use of dexamethasone showed near-significant associations.
Table 2. Logistic regression of factors related to new lesion formation.
| Variables | Unadjusted model | Multivariable-adjusted model | |||
|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | ||
| Age (yr) | 0.96 (0.93–1) | 0.077 | 0.94 (0.89–0.99) | 0.023a) | |
| Female | 1.47 (0.49–4.41) | 0.489 | 1.92 (0.53–6.93) | 0.318 | |
| Medical history | |||||
| Circulatory problems | 0.68 (0.23–2.03) | 0.489 | |||
| Endocrine problems | N/A | - | |||
| Rheumatic problems | N/A | - | |||
| Menopause | 0.36 (0.1–1.33) | 0.125 | |||
| Malignancy | 6.88 (1.66–28.4) | 0.008 | 11.48 (1.96–67.31) | 0.007a) | |
| Location of lichen planus | |||||
| Mucosa | Reference | - | |||
| Gingiva | 3.5 (1.31–9.34) | 0.012a) | 3.03 (0.98–9.34) | 0.054 | |
| Subtype | |||||
| White or red | Reference | - | |||
| Erosion | N/A | - | |||
| Mixed | 0.9 (0.1–8.51) | 0.927 | |||
| Mode of dexamethasone treatment | |||||
| Ointment alone | Reference | - | |||
| Gargle alone | 0.24 (0.02–2.98) | 0.269 | |||
| Gargle and ointment | 1.22 (0.1–15.11) | 0.876 | |||
| Pattern of dexamethasone use | |||||
| Continuous use | Reference | - | Reference | - | |
| Intermittent use | 7.17 (2.1–24.47) | 0.002a) | 7.3 (0.86–61.84) | 0.068 | |
| Duration of dexamethasone use | 1 (1–1) | 0.017a) | 1 (1–1) | 0.910 | |
| Adverse effects | 3.12 (0.49–19.99) | 0.229 | |||
OR: odds ratio, CI: confidence interval, N/A: not applicable.
a)Statistically significant difference compared to the baseline.
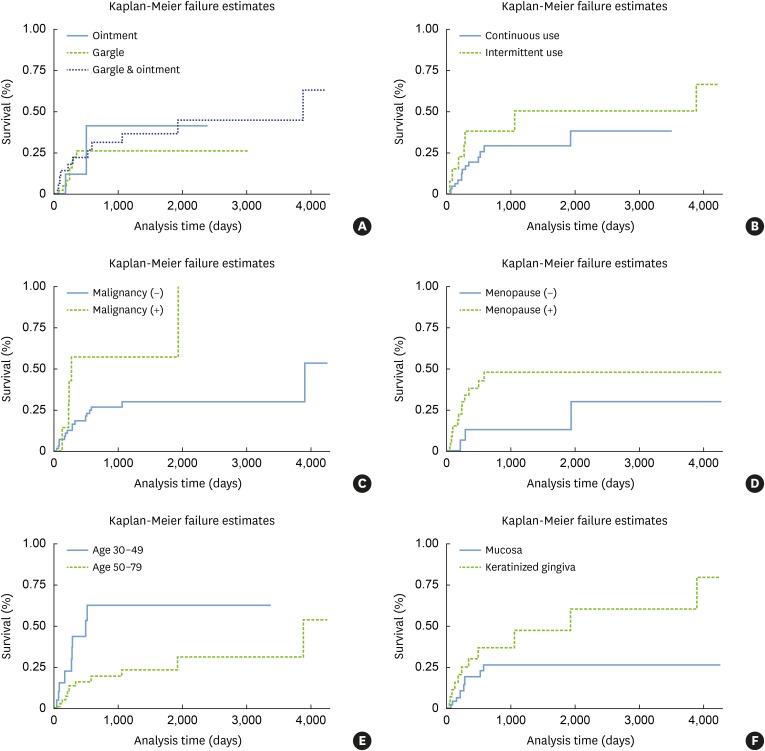
In Kaplan-Meier failure analysis examining the interval until new lesion formation, the mode of dexamethasone administration and use pattern did not significantly influence new lesion formation (Figure 2). However, new lesion formation was significantly affected by history of malignancy and menopausal status in females, relatively young age, and gingival lesions (P=0.008 for history of malignancy, P=0.039 for menopausal status, P=0.013 for age from 30 to 49, and P=0.049 for gingival lesions).
Figure 2. Kaplan-Meier survival curves showing failure estimates. The time to new lesion formation or expansion of OLP was calculated from the index date to the date when a new lesion was observed. The pattern of dexamethasone use and mode of dexamethasone treatment did not significantly influence the time to failure (P=0.234 and P=0.789, respectively). However, a history of malignancy significantly affected the time to failure (P=0.008). Menopause showed a near-significant relationship (P=0.039). Younger patients had a higher failure rate than elderly patients (P=0.013). The location of the OLP lesion was a significant factor affecting new lesion formation or lesion expansion (P=0.049).
OLP: oral lichen planus.
DISCUSSION
In this study, we retrospectively investigated the distribution of OLP lesions and the clinical outcomes of topical dexamethasone used to treat OLP lesions.
Treatments for OLP lesions include local corticosteroid therapy in general and systemic therapy for severe cases. Topical applications have the benefits of minimal systemic absorption and fewer adverse effects [9]. In this study, a mouth rinse was used, and the concentration of dexamethasone was changed from 0.05% to 0.1% depending on the severity of symptoms. In some patients, an ointment (Peridex) was also applied. Most patients in this study showed improvement within 6 months. Corticosteroids such as dexamethasone are effective for OLP due to their anti-inflammatory effect and suppression of T cells [10]. A gargle is recommended over an ointment considering the difficulty of applying the ointment, especially to the vestibular area. Since a mouth rinse can reach throughout the oral cavity, it is generally superior to an ointment, except for localized small lesions of the gingiva or palatal areas. However, for patients who experience difficulties using a mouth rinse due to the bitterness of the taste and challenges in carrying the liquid, an ointment can be an alternative choice. Both the dexamethasone gargle and ointment led to improvements in over 60% of patients in this study, meaning that those therapies can be used together or separately as needed. Fewer than 20% of patients experienced new lesion formation after treatment. In this study, the factors affecting treatment outcomes were analyzed, and age, history of malignancy, menopausal status in women, and the location of the OLP lesion were significant predictors.
OLP lesions in this study were most prevalent in patients in the 50–60 age group, but new lesion formation was mostly observed in patients younger than 50 years. The rate of new lesion formation was 30% in patients younger than 50 years, while it was 12% in patients over 50 years. The pathophysiology of OLP is controversial, and has been proposed to involve impaired immunity and inflammatory reactions to various stimuli such as infectious agents, trauma, and stress through the following possible mechanisms: 1) antigen-specific cell-mediated immune response via activation of cytotoxic T cells and apoptosis of basal keratinocytes, 2) non-specific mechanisms triggered by pre-existing inflammation, 3) an anti-keratinocyte autoimmune response, and 4) humoral immunity including autoantibodies against desmogleins 1 and 3 [11]. In younger patients, the immune reaction tends to be more aggressive than in the elderly, which may help explain the higher rate of new lesion formation around OLP lesions in younger patients.
In patients with a history of malignancy, oral mucositis is one of the most common side effects of radiotherapy and/or chemotherapy as a result of the cytotoxic effects of the chemotherapy drugs used [12]. During chemotherapy and/or radiation therapy, a non-specific inhibitory effect on mitosis in the basal epithelium occurs in the oral cavity, followed by reduction of the renewal rate of the epithelium and atrophic changes [13]. However, the inhibition appears to remain after the end of chemotherapy and worsens the treatment outcome after OLP lesion formation. Therefore, OLP patients with a history of malignancy should be carefully managed to improve their quality of life and to prevent the malignant transformation of OLP lesions.
In another study, medication for diabetes or hypertension also affected OLP lesion formation [14]. Dry mouth induced by medication could weaken the oral tissue, resulting in severe damage by mechanical irritants. Others have suggested that a relationship may exist between thyroid disease and OLP based on the finding of a significant correlation between OLP and thyroid disease, but this proposal remains controversial [15,16]. In this study, the number of patients with diabetes, thyroid disease, and hypertension were insufficient for analysis and did not yield meaningful results.
OLP lesions were mostly observed in the oral mucosa. About 30%–40% of OLP lesions showed gingival involvement together with mucosal lesions, while fewer than 10% of lesions were confined to the gingiva. The distribution of OLP lesions was similar to what has been reported in previous studies [17,18]. However, the treatment outcomes were unfavorable when gingival lesions were involved. The accumulation of plaque and calculus also contributes to the exacerbation of OLP lesions. In this vicious cycle, OLP lesions interfere with the correct performance of daily oral hygiene, leading to increased bacterial deposits and food remnants, which cause inflammatory and immune reactions and worsen the lesions [19]. This vicious cycle may be a reason for the unfavorable prognosis of gingival OLP lesions. However, it has been reported that plaque control consisting of supragingival and/or subgingival scaling in conjunction with topical corticosteroids and oral hygiene instructions was effective for improving gingival OLP lesions [20,21,22].
However, most patients with gingival OLP lesions in this study did not receive periodontal treatment, except for 1 patient who was followed up at the Department of Periodontology after the initial appointment. Most of the patients were referred from a local clinic for a differential diagnosis to exclude malignancy. After treatment of the lesions, patients feared the possibility of malignant transformation, and therefore scheduled follow-up appointments with oral surgeons. Even though oral surgeons referred patients to periodontology for supportive periodontal treatment (SPT), the patients generally did not visit the periodontal clinic. They did not understand why periodontal treatment is helpful for promoting remission of the lesions. In addition, traumatic pain after periodontal treatment was another reason for non-compliance. It has recently been reported that lidocaine gel application during scaling and root planning or SPT was effective in pain control and increased patient compliance [23].
Even though the frequency of malignant transformation is low (0.2%–0.5% annually [24]), the risk of malignancy is non-zero. Some studies reported that erosive, ulcerative, and atrophic lesions could develop into malignancies in response to mucosal damage from carcinogenic agents, but this proposal still requires more evidence [25]. In addition, malignant transformation is more common in women, in the sixth decade of life, and in lesions in the tongue area [26]. Although it is not recommended to perform biopsies in all patients suspected to have OLP in terms of costs and the invasiveness of the procedure, OLP in the lateral tongue is particularly difficult to diagnose since the lesions may not be obviously distinct from oral candidiasis and leukoplakia [27]. In previous studies, malignant transformation occurred from several months to more than 20 years after OLP diagnosis, although the maximum risk was observed between 3 and 6 years after diagnosis [28]. Candida albicans has been suggested to be a risk factor of malignancy as a consequence of N-nitrosobenzylmethylamine production; even though this has still not been fully proven, special care of fungal infections is recommended in patients with OLP [25,28].
In conclusion, dexamethasone gargle or ointment could be considered as the treatment of choice for OLP. Gargle, ointment, or the combination of both modes of treatment can be used effectively depending on a patient's situation and needs. The treatment outcomes of OLP were significantly influenced by age, history of malignancy, menopause, and the site of the OLP lesion, but not by factors related to dexamethasone treatment. In order to improve the prognosis of gingival OLP lesions, periodontal treatment with dexamethasone is suggested. In addition, regular monitoring is recommended to detect malignant transformation and adverse effects of corticosteroids, although the malignant transformation rate is low.
Footnotes
Author Contributions: Conceptualization: Pil-Young Yun; Formal analysis: Shin-Young Park; Investigation: So-Hyun Kim, Sung-Beom Kim; Methodology: Shin-Young Park; Project administration: Shin-Young Park, Pil-Young Yun; Writing - original draft: So-Hyun Kim, Sung-Beom Kim, Shin-Young Park; Writing - review & editing: Hyo-Jung Lee, Yong-Hoon Choi.
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Alrashdan MS, Cirillo N, McCullough M. Oral lichen planus: a literature review and update. Arch Dermatol Res. 2016;308:539–551. doi: 10.1007/s00403-016-1667-2. [DOI] [PubMed] [Google Scholar]
- 2.McCartan BE, Healy CM. The reported prevalence of oral lichen planus: a review and critique. J Oral Pathol Med. 2008;37:447–453. doi: 10.1111/j.1600-0714.2008.00662.x. [DOI] [PubMed] [Google Scholar]
- 3.Kurago ZB. Etiology and pathogenesis of oral lichen planus: an overview. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;122:72–80. doi: 10.1016/j.oooo.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 4.Dissemond J. Oral lichen planus: an overview. J Dermatolog Treat. 2004;15:136–140. doi: 10.1080/09546630410030720. [DOI] [PubMed] [Google Scholar]
- 5.Naylor GD. Treating erosive lichen planus with griseofulvin: a report of four cases. Quintessence Int. 1990;21:943–947. [PubMed] [Google Scholar]
- 6.Carrozzo M, Gandolfo S. The management of oral lichen planus. Oral Dis. 1999;5:196–205. doi: 10.1111/j.1601-0825.1999.tb00301.x. [DOI] [PubMed] [Google Scholar]
- 7.McCreary CE, McCartan BE. Clinical management of oral lichen planus. Br J Oral Maxillofac Surg. 1999;37:338–343. doi: 10.1054/bjom.1999.0131. [DOI] [PubMed] [Google Scholar]
- 8.Scully C, Beyli M, Ferreiro MC, Ficarra G, Gill Y, Griffiths M, et al. Update on oral lichen planus: etiopathogenesis and management. Crit Rev Oral Biol Med. 1998;9:86–122. doi: 10.1177/10454411980090010501. [DOI] [PubMed] [Google Scholar]
- 9.Hambly JL, Haywood A, Hattingh L, Nair RG. Comparison between self-formulation and compounded-formulation dexamethasone mouth rinse for oral lichen planus: a pilot, randomized, cross-over trial. J Investig Clin Dent. 2017;8:e12225. doi: 10.1111/jicd.12225. [DOI] [PubMed] [Google Scholar]
- 10.Pedersen A, Klausen B. Glucocorticosteroids and oral medicine. J Oral Pathol. 1984;13:1–15. doi: 10.1111/j.1600-0714.1984.tb01395.x. [DOI] [PubMed] [Google Scholar]
- 11.Roopashree MR, Gondhalekar RV, Shashikanth MC, George J, Thippeswamy SH, Shukla A. Pathogenesis of oral lichen planus--a review. J Oral Pathol Med. 2010;39:729–734. doi: 10.1111/j.1600-0714.2010.00946.x. [DOI] [PubMed] [Google Scholar]
- 12.Singh N, Scully C, Joyston-Bechal S. Oral complications of cancer therapies: prevention and management. Clin Oncol (R Coll Radiol) 1996;8:15–24. doi: 10.1016/s0936-6555(05)80034-2. [DOI] [PubMed] [Google Scholar]
- 13.Lockhart PB, Sonis ST. Alterations in the oral mucosa caused by chemotherapeutic agents. A histologic study. J Dermatol Surg Oncol. 1981;7:1019–1025. doi: 10.1111/j.1524-4725.1981.tb00208.x. [DOI] [PubMed] [Google Scholar]
- 14.Lamey PJ, Gibson J, Barclay SC, Miller S. Grinspan's syndrome: a drug-induced phenomenon? Oral Surg Oral Med Oral Pathol. 1990;70:184–185. doi: 10.1016/0030-4220(90)90116-a. [DOI] [PubMed] [Google Scholar]
- 15.Lavaee F, Majd M. Evaluation of the association between oral lichen planus and hypothyroidism: a retrospective comparative study. J Dent (Shiraz) 2016;17:38–42. [PMC free article] [PubMed] [Google Scholar]
- 16.Li D, Li J, Li C, Chen Q, Hua H. The association of thyroid disease and oral lichen planus: a literature review and meta-analysis. Front Endocrinol (Lausanne) 2017;8:310. doi: 10.3389/fendo.2017.00310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eisen D. The clinical features, malignant potential, and systemic associations of oral lichen planus: a study of 723 patients. J Am Acad Dermatol. 2002;46:207–214. doi: 10.1067/mjd.2002.120452. [DOI] [PubMed] [Google Scholar]
- 18.Scully C, el-Kom M. Lichen planus: review and update on pathogenesis. J Oral Pathol. 1985;14:431–458. doi: 10.1111/j.1600-0714.1985.tb00516.x. [DOI] [PubMed] [Google Scholar]
- 19.Mignogna MD, Lo Russo L, Fedele S. Gingival involvement of oral lichen planus in a series of 700 patients. J Clin Periodontol. 2005;32:1029–1033. doi: 10.1111/j.1600-051X.2004.00761.x. [DOI] [PubMed] [Google Scholar]
- 20.Holmstrup P, Schiøtz AW, Westergaard J. Effect of dental plaque control on gingival lichen planus. Oral Surg Oral Med Oral Pathol. 1990;69:585–590. doi: 10.1016/0030-4220(90)90241-j. [DOI] [PubMed] [Google Scholar]
- 21.Guiglia R, Di Liberto C, Pizzo G, Picone L, Lo Muzio L, Gallo PD, et al. A combined treatment regimen for desquamative gingivitis in patients with oral lichen planus. J Oral Pathol Med. 2007;36:110–116. doi: 10.1111/j.1600-0714.2007.00478.x. [DOI] [PubMed] [Google Scholar]
- 22.López-Jornet P, Camacho-Alonso F. Application of a motivation-behavioral skills protocol in gingival lichen planus: a short-term study. J Periodontol. 2010;81:1449–1454. doi: 10.1902/jop.2010.100245. [DOI] [PubMed] [Google Scholar]
- 23.Petersilka GJ, Arweiler NB, Otto J, Wittig T. Non-interventional study to collect data for the application of lidocaine gel 2% during scaling and root planing and professional mechanical plaque removal. Clin Oral Investig. 2018 doi: 10.1007/s00784-018-2468-0. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Al-Hashimi I, Schifter M, Lockhart PB, Wray D, Brennan M, Migliorati CA, et al. Oral lichen planus and oral lichenoid lesions: diagnostic and therapeutic considerations. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103 Suppl:S25.e1–S25.e12. doi: 10.1016/j.tripleo.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 25.van der Meij EH, Schepman KP, van der Waal I. The possible premalignant character of oral lichen planus and oral lichenoid lesions: a prospective study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;96:164–171. doi: 10.1016/s1079-2104(03)00305-6. [DOI] [PubMed] [Google Scholar]
- 26.Fitzpatrick SG, Hirsch SA, Gordon SC. The malignant transformation of oral lichen planus and oral lichenoid lesions: a systematic review. J Am Dent Assoc. 2014;145:45–56. doi: 10.14219/jada.2013.10. [DOI] [PubMed] [Google Scholar]
- 27.Cheng YS, Gould A, Kurago Z, Fantasia J, Muller S. Diagnosis of oral lichen planus: a position paper of the American Academy of Oral and Maxillofacial Pathology. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;122:332–354. doi: 10.1016/j.oooo.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 28.Gonzalez-Moles MA, Scully C, Gil-Montoya JA. Oral lichen planus: controversies surrounding malignant transformation. Oral Dis. 2008;14:229–243. doi: 10.1111/j.1601-0825.2008.01441.x. [DOI] [PubMed] [Google Scholar]