Abstract
The standard treatment for locally advanced unresectable (UR-LA) pancreatic ductal adenocarcinoma (PDAC) is chemo-radiotherapy. Surgery following chemo-radiotherapy (conversion surgery), has been considered a useful strategy and has been used for UR-LA PDAC. The current study presents the case of a 43-year-old woman who complained of back pain. A radiological examination revealed a pancreatic tumor in contact with >270 degrees of the superior mesenteric artery (SMA) perimeter, with invasion extending from the superior mesenteric vein (SMV) to the portal vein (PV). An endoscopic ultrasonography-guided fine needle aspiration biopsy revealed adenocarcinoma as the pathological diagnosis and the patient was diagnosed with UR-LA PDAC. Following 12 courses of combined gemcitabine plus nab-paclitaxel (GnP) for 9 months, the extent of tumor invasion to the SMA and SMV was improved and the level of cancer antigen (CA) 19-9 decreased. A pancreatoduodenectomy with PV resection and reconstruction using a left renal vein graft were performed. Pathological examination revealed that the operative outcome was R0 (no residual tumor) resection and the patient was alive 19 months after the initial treatment (9 months post surgery), however, there was local tumor recurrence. Between March 2015 and February 2016 a total of 10 cases of UR-LA PDAC were encountered at the Department of General Surgery, Chiba University Hospital (Chiba, Japan), in which GnP therapy was performed. Including the present case, 6 of the 11 cases (55%) underwent conversion surgery with curative resection. Kaplan-Meier analysis revealed that patients treated with conversion surgery presented significantly longer overall survival (OS) than those treated with no conversion surgery (median OS, 22.5 vs. 11 months; P=0.047, Wilcoxon test). The minimum reduction of CA19-9 was 67%. In conclusion, conversion surgery following GnP therapy is a desirable option for UR-LA PDAC. A significant reduction in the CA19-9 levels may be useful in determining the timing of changeover from medicine to surgery in patients with UR-LA PDAC in whom conversion surgery is being considered.
Keywords: conversion surgery, gemcitabine plus nab-paclitaxel, locally advanced unresectable pancreatic cancer, cancer antigen 19-9
Introduction
More than 50% of all cases of pancreatic ductal adenocarcinoma (PDAC) are initially considered unresectable (UR) (1), and the standard treatment for locally advanced unresectable (UR-LA) PDAC is chemo-radiotherapy (2). Even if an effective regimen, such as combination therapy with gemcitabine and nab-paclitaxel (GnP), is administered, the median overall survival (OS) is only 8.5 months (3). Recent case reports and retrospective studies of chemo-radiotherapy prior to surgery, i.e., conversion surgery, for UR PDAC have been published (4,5), and the significance of conversion surgery is now being evaluated. This report describes a case of successful conversion surgery after GnP therapy for UR-LA PDAC.
Case report
A 43-year-old woman was referred to the Department of General Surgery, Chiba University Hospital (Chiba, Japan) from a local hospital with the complaint of back pain. Initial laboratory findings showed a high level of cancer antigen 19-9 (CA 19-9), at 205.7 U/ml. Abdominal computed tomography (CT) revealed a hypovascular tumor measuring 24 mm in the head of the pancreas. The tumor was in contact with more than 270 degrees of the superior mesenteric artery (SMA) perimeter, with invasion extending from the superior mesenteric vein (SMV) to the portal vein (PV) (the longitudinal axis was 30 mm) (Fig. 1A and B). Endoscopic ultrasonography (EUS) indicated that the tumor was in contact with >180 degrees of the SMA perimeter, and the histological finding of fine needle aspiration biopsy was adenocarcinoma. Both positron emission tomography (PET) and ethoxybenzyl-magnetic resonance imaging (MRI) showed no evidence of distant metastasis.
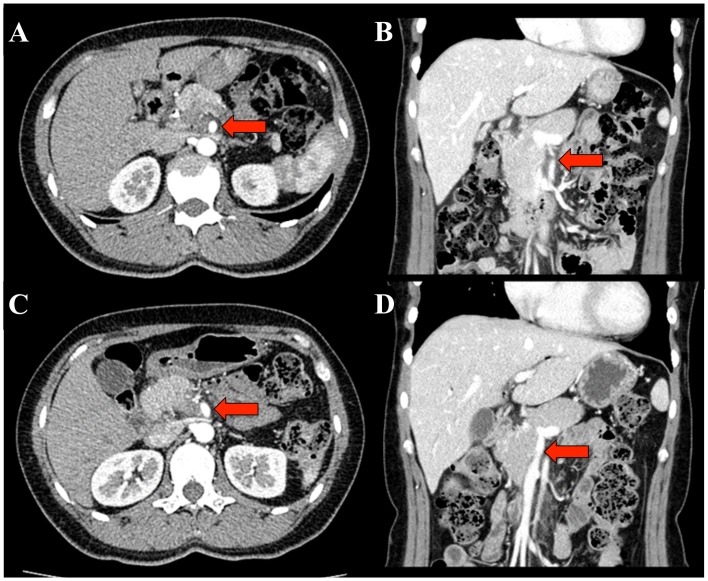
Figure 1.
CT prior to and post GnP therapy. (A) A CT on the patient's initial visit revealed a hypovascular tumor measuring 24 mm in the head of the pancreas. The tumor was in contact with >270 degrees of the SMA perimeter (red arrow). (B) The tumor invaded from the SMV to the PV. The longitudinal axis was 30 mm (red arrow). Following GnP therapy, (C) the tumor size decreased to 20 mm and the contact with the SMA decreased to 90 degrees (red arrow) and (D) the longitudinal tumor axis invading from the SMV to the PV decreased to 15 mm (red arrow). CT, computed tomography; SMV, superior mesenteric vein; PV, portal vein; SMA, superior mesenteric artery; GnP, gemcitabine plus nab-paclitaxel.
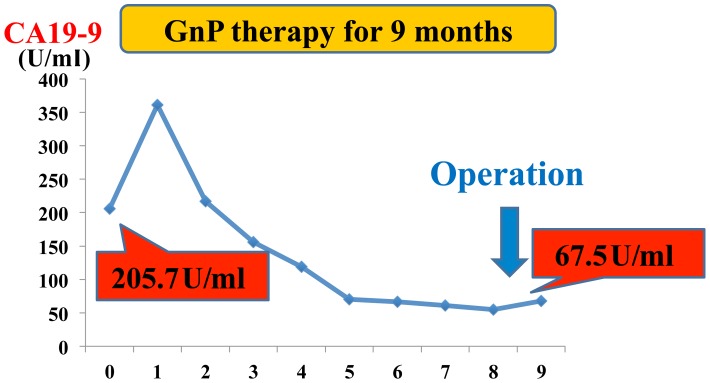
On the basis of these clinical findings, the patient was diagnosed with UR-LA PDAC, and subsequently treated with a combined chemotherapy regimen of gemcitabine (GEM, 1,000 mg/m2) and nab-paclitaxel (125 mg/m2), aimed at conversion surgery. This combination chemotherapy was intravenously administered on days 1 and 8 and repeated every 3 weeks. After 12 courses of combination chemotherapy for 9 months, CT and EUS imaging demonstrated an effective response to chemotherapy. The tumor size decreased to 20 mm and the contact with the SMA was reduced to 90 degrees (Fig. 1C). The length of tumor invasion to the SMV decreased from 30 to 15 mm in the longitudinal axis (Fig. 1D). EUS examination also showed that the extent of tumor invasion to the SMA and SMV had decreased. Preoperative stage was T4N2M0 stage III according to the 8th edition of the UICC (International Union Against Cancer)-TNM classification (6). Furthermore, the level of CA 19-9 decreased from 205.7 to 67.5 U/ml (Fig. 2). The radiological efficacy of chemotherapy was stable disease (SD) on the Response Evaluation Criteria in Solid Tumors (RECIST) (7). After discussion with the patient and her family, conversion surgery was planned.
Figure 2.
The transition of serum CA 19-9 level during GnP therapy. GnP, gemcitabine plus nab-paclitaxel; CA, cancer antigen.
Pancreaticoduodenectomy with portal vein resection and reconstruction using a left renal vein graft were performed (8) (Fig. 3A and B). The margins of the bile duct and stump of the pancreas were negative for cancer on intraoperative pathological diagnosis of a frozen section. Microscopic pathological examination showed R0 (no residual tumor) resection, and 10–50% of the tumor cells were replaced with fibrosis (Evans' criteria IIa) (9). Based on the pathological findings (moderately differentiated tubular adenocarcinoma, pT2 (24 mm), pN2 (4/32), pM0), the tumor was defined as stage III (Fig. 3C). After surgery, the patient showed bleeding from the ligated inferior pancreatic duodenal artery due to a pancreatic fistula (grade C) (10). Embolization with coiling and reoperation (remnant pancreatectomy) were performed to stop bleeding. The patient made a satisfactory recovery and was discharged on postoperative day 53. The patient is alive at 19 months after initial treatment (9 months after surgery), but with local tumor recurrence.
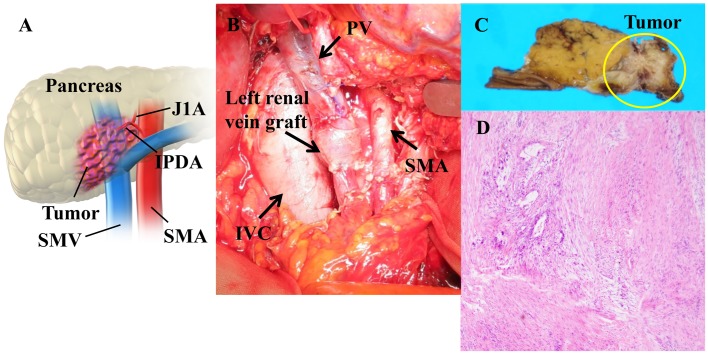
Figure 3.
Pancreatoduodenectomy with portal vein resection and reconstruction using a left renal vein graft. (A) The schema of operative findings. (B) Intraoperative image. Pathological findings. (C) Macroscopic findings. (D) Microscopic findings of the surgical specimen with hematoxylin/eosin staining revealed a change to 10–50% fibrous tissue with grade IIa per Evans' criteria following chemotherapy. Magnification, ×40. PV, portal vein; SMA, superior mesenteric artery; SMV, superior mesenteric vein; IVC, IVC; inferior vena cava; IPDA, inferior pancreatoduodenal artery; J1A, first jejunal artery.
Discussion
Chemotherapy for PDAC has advanced since gemcitabine was introduced (11). The MPACT trial demonstrated the effectiveness of GnP therapy for UR PDAC (3). Ueno et al reported that GnP therapy (response rate: 69.2%) shows better efficacy compared to gemcitabine or gemcitabine plus S-1 therapy (response rate: 30%) for patients with UR PDAC (12,13). To utilize these chemotherapies before surgery, it is possible to exclude the cases showing aggressive growth or having distant metastasis afterward. The selection of patients with a good response to chemotherapy is important for successful conversion surgery in UR PDAC. Satoi et al demonstrated that the median OS of patients with UR PDAC treated with conversion surgery after gemcitabine or S-1 therapy was significantly improved compared to that with chemotherapy alone (39.7 vs. 20.8 months, respectively; P<0.001) (14). Furthermore, Ielpo et al reported that OS of patients treated with conversion surgery with GnP therapy for resectable or borderline resectable (BR) PDAC was significantly improved compared to that with chemotherapy alone (21 vs. 12.5 months, respectively; P<0.0002) (15). Taken together, these results suggest that GnP therapy is one of the most useful options for the treatment of UR-LA PDAC and is expected to improve prognosis when followed by conversion surgery.
Recent cases of conversion surgery with GnP therapy for UR-LA PDAC have been reported. Saito et al demonstrated that the median OS of patients with UR-LA PDAC treated with conversion surgery after GnP therapy was 13.3 months in a retrospective study (5). FOLFIRINOX (5-fluorouracil/leucovorin combined with irinotecan and oxaliplatin) is another effective chemotherapeutic regimen to UR PDAC (16). Suker et al demonstrated that the median OS of patients with UR-LA PDAC treated with conversion surgery after FOLFIRINOX therapy was 24.2 months in a systematic review (17). Based on the efficacy of chemotherapy, the use of conversion surgery for UR-LA PDAC will likely increase. However, it is important to determine whether the soft tissue around major vessels such as the SMA is truly involved with tumor invasion. Therefore, it is difficult to make a decision to convert treatment from chemotherapy to surgery solely on the basis of radiological examination. A retrospective cohort study reported that a >50% decrease in pretreatment CA 19-9 levels after chemotherapy resulted in improved OS, compared to that with a ≤50% decrease (28.0 vs. 11.1 months; P<0.0001) (18). Thus, the CA 19-9 level should be taken into account in the evaluation of chemotherapy efficacy.
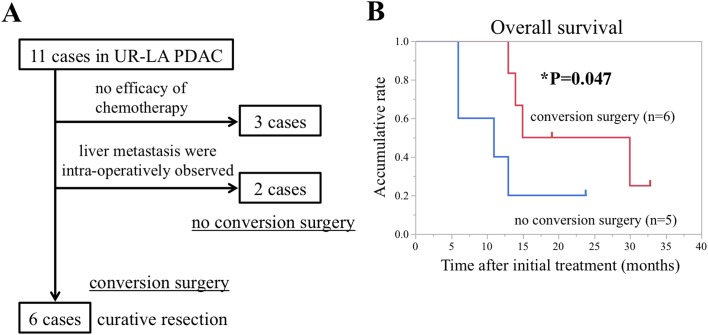
We performed GnP therapy in 10 consecutive cases of UR-LA PDAC between March 2015 and February 2016 in the Department of General Surgery, Chiba University Hospital. Although there is still no definite surgical indication for conversion surgery during multidisciplinary treatment in patients with initially UR PDAC, the following points were considered; i) tumor shrinkage from UR to resectable or BR PDAC on radiological examinations, ii) serum CA 19-9 level is clearly decreased, and iii) good performance status. Adding the present case to these 10 cases, 3 of the 11 cases (27%) were not converted to resection because chemotherapy was ineffective, while 6 of 11 cases (55%) excluding 2 cases which intra-operatively showed liver metastases in successfully underwent conversion surgery with curative resection (Fig. 4A). In this retrospective cohort study, the Kaplan-Meier analysis showed that patients treated with conversion surgery presented significantly longer overall survival (OS) than those treated with no conversion surgery (the median OS: 22.5 vs. 11 months, P=0.047, Wilcoxon test; Fig. 4B). The average duration of chemotherapy before conversion surgery was 4.3 months and the minimum reduction rate of CA 19-9 was 67% among 6 curative resection cases (Table I). The rate of R0 resection was 83% (Table II). Based on these clinical data, a significant decrease in CA 19-9 levels might be useful in determining the time of changeover from medicine to surgery in patients with UR-LA PDAC in whom conversion surgery is being considered.
Figure 4.
(A) Flow chart of patient selection for conversion surgery following gemcitabine plus nab-paclitaxel therapy in UR-LA PDAC. (B) The Kaplan-Meier survival curve revealed a favorable prognosis in conversion surgery group compared with the no conversion surgery group. *P=0.047, Wilcoxon test. UR-LA PDAC, locally advanced unresectable pancreatic ductal adenocarcinoma.
Table I.
Preoperative characteristics of locally advanced unresectable pancreatic ductal adenocarcinoma patients preparing for conversion surgery with GnP therapy.
| No. | Age/gender | Location | Factors determining unresectability | Period of Tx (months) | CA19-9 (before Tx) (U/ml) | CA19-9 (after Tx) (U/ml) | Reduction rate of CA19-9 (%) | RECIST |
|---|---|---|---|---|---|---|---|---|
| 1 | 61/M | Head | Contact with SMA 360° | 5 | 0.8 | 0.1 | 87.5 | PR |
| 2 | 56/F | Head | Contact with CHA with extension to hepatic artery bifurcation | 3.9 | 150.4 | 35.2 | 76.6 | PR |
| 3 | 45/M | Body | Contact with CEA | 2.4 | 2909 | 884 | 69.6 | SD |
| 4 | 71/M | Body | Contact with SMA and CEA | 1.4 | 165.7 | 35.4 | 78.0 | SD |
| 5 | 77/M | Head | Contact with SMA >270° | 3.8 | 726.0 | 51.3 | 92.8 | PR |
| Present case | 43/M | Head | Contact with SMA >270° | 9 | 205.7 | 67.5 | 67.1 | SD |
CEA, celiac artery; CHA, common hepatic artery; F, female; M, male; PR, partial response; RECIST, Response Evaluation Criteria in Solid Tumor; SD, stable disease; Tx, chemotherapy.
Table II.
Clinical characteristics and outcomes of locally advanced unresectable pancreatic ductal adenocarcinoma in patients who underwent conversion surgery following gemcitabine plus nab-paclitaxel therapy.
| No. | Operation method | Curability | Evans' criteria | OS from initial treatment (months) | Survival |
|---|---|---|---|---|---|
| 1 | PD | R0 | IIa | 14 | No |
| 2 | PD-CAR, PVR | R0 | I | 33 | Yes |
| 3 | DP-CAR, PVR | R0 | IIa | 30 | No |
| 4 | DP-CAR | R0 | IIa | 15 | No |
| 5 | PD, PVR | R1 | IIa | 16 | No |
| Present case | PD, PVR | R0 | IIa | 19 | Yes |
DP-CAR, distal pancreatectomy with en block celiac axis resection; OS, overall survival; PD, pancreaticoduodenectomy; PVR, portal vein resection; PD-CAR, pancreaticoduodenectomy with en block common hepatic artery resection.
In conclusions, we described a case of successful conversion surgery with gemcitabine plus nab-paclitaxel for UR-LA PDAC. GnP therapy decreased the level of CA 19-9, enabling surgical resection. Conversion surgery after GnP therapy is a useful treatment option for UR-LA PDAC. Further evidence and prospective cohort studies are required to establish the optimal strategy for treatment of UR-LA PDAC.
Acknowledgements
The authors would like to thank Dr Sugiura (Department of Pathology, Chiba University Hospital) for their assistance and the pathological information provided regarding the chemotherapeutic effects.
Glossary
Abbreviations
- BR
borderline resectable
- CA19-9
cancer antigen 19-9
- CT
computed tomography
- EUS
endoscopic ultrasonography
- GnP
gemcitabine plus nab-paclitaxel
- MST
median survival time
- OS
overall survival
- PET
positron emission tomography
- PDAC
pancreatic ductal adenocarcinoma
- PV
portal vein
- SMA
superior mesenteric artery
- SMV
superior mesenteric vein
- UR-LA
locally advanced unresectable
Funding
This study was supported by the Grant-in-Aid for Scientific Research, the Challenge Exploratory Research (grant no. 16K15607) and KIBAN B (grant no. 17H04287).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
RO gathered the patient's data and wrote the manuscript. ST treated the patient with preoperative and adjuvant chemotherapy. ST, TY, and MM performed the surgery. TM and MO were responsible for the pathological diagnosis of the case. ST, TY, HY, KF, TT, SaK, DS, NS, ShK, HN, MM and MO discussed and analyzed the data with RO and assisted in writing the manuscript. All authors approved the final manuscript.
Ethics approval and consent to participate
The Ethics Committees of Chiba University approved the content of this manuscript (approval no. 2732) and written informed consent was obtained from each patient prior to surgery.
Patient consent for publication
Written informed consent was obtained from the patient for the publication of this case report and any accompanying images.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Tempero MA, Malafa MP, Al-Hawary M, Asbun H, Bain A, Behrman SW, Betnson AB, III, Binder E, Cardin DB, Cha C, et al. Pancreatic adenocarcinoma, version 2. 2017. NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2017;15:1028–1061. doi: 10.6004/jnccn.2017.0131. [DOI] [PubMed] [Google Scholar]
- 3.Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hiyoshi M, Nanashima A, Wada T, Tsuchimochi Y, Hamada T, Yano K, Imamura N, Fujii Y. A successful case of locally advanced pancreatic cancer undergoing curative distal pancreatectomy with en bloc celiac axis resection after combination chemotherapy of nab-paclitaxel with gemcitabine. Clin J Gastroenterol. 2017;10:551–557. doi: 10.1007/s12328-017-0793-5. [DOI] [PubMed] [Google Scholar]
- 5.Saito T, Ishido K, Kudo N, Kimura N, Wakiya T, Nakayama Y, Hakamada K. Combination therapy with gemcitabine and nab-paclitaxel for locally advanced unresectable pancreatic cancer. Mol Clin Oncol. 2017;6:963–967. doi: 10.3892/mco.2017.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brierley JD, Gospodarowicz MK, Wittekind C, editors. TNM Classification of Malignant Tumors (8th edition) Wiley-Blackwell; New York: 2017. [Google Scholar]
- 7.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancy J, Arbuck S, Gwyther S, Mooney M, et al. New response evaluation criteria in solid tumors: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 8.Miyazaki M, Itoh H, Kaiho T, Ambiru S, Togawa A, Sasada K, Shiobara M, Shimizu Y, Yoshioka S, Yoshitome H, et al. Portal vein reconstruction at the hepatic hilus using a left renal vein graft. J Am Coll Surg. 1995;180:497–498. [PubMed] [Google Scholar]
- 9.Evans DB, Rich TA, Byrd DR, Cleary KR, Connelly JH, Levin B, Charnsangavej C, Fenoglio CJ, Ames FC. Preoperative chemoradiation and pancreaticoduodenectomy for adenocarcinoma of the pancreas. Arch Surg. 1992;127:1335–1339. doi: 10.1001/archsurg.1992.01420110083017. [DOI] [PubMed] [Google Scholar]
- 10.Bassi C, Marchegiani G, Dervenis C, Sarr M, Hilal Abu M, Adham M, Allen P, Andersson R, Asbun HJ, Besselink MG, et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surgery. 2017;161:584–591. doi: 10.1016/j.surg.2016.11.014. [DOI] [PubMed] [Google Scholar]
- 11.Burris HA, III, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: A randomized trial. J Clin Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 12.Ueno H, Ikeda M, Ueno M, Mizuno N, Ioka T, Nakajima TE, Furuse J. Phase I/II study of nab-paclitaxel plus gemcitabine for chemotherapy-naive Japanese patients with metastatic pancreatic cancer. Cancer Chemother Pharmacol. 2016;77:595–603. doi: 10.1007/s00280-016-2972-3. [DOI] [PubMed] [Google Scholar]
- 13.Ueno H, Ioka T, Ikeda M, Ohkawa S, Yanagimoto H, Boku N, Fukutomi A, Sugimori K, Baba H, Yamao K, et al. Randomized phase III study of gemcitabine plus S-1, S-1 alone, or gemcitabine alone in patients with locally advanced and metastatic pancreatic cancer in Japan and Taiwan: GEST study. J Clin Oncol. 2013;31:1640–1648. doi: 10.1200/JCO.2012.43.3680. [DOI] [PubMed] [Google Scholar]
- 14.Satoi S, Yamaue H, Kato K, Takahashi S, Hirono S, Takeda S, Eguchi H, Sho M, Wada K, Shinchi H, et al. Role of adjuvant surgery for patients with initially unresectable pancreatic cancer with a long-term favorable response to non-surgical anti-cancer treatments: Results of a project study for pancreatic surgery by the Japanese Society of Hepato-Biliary-Pancreatic Surgery. J Hepatobiliary Pancreat Sci. 2013;20:590–600. doi: 10.1007/s00534-013-0616-0. [DOI] [PubMed] [Google Scholar]
- 15.Ielpo B, Duran H, Diaz E, Fabra I, Caruso R, Ferri V, Malavé L, Hidalgo M, Alvarez R, Plaza C, et al. Preoperative treatment with gemcitabine plus nab-paclitaxel is a safe and effective chemotherapy for pancreatic adenocarcinoma. Eur J Surg Oncol. 2016;42:1394–1400. doi: 10.1016/j.ejso.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 16.Conroy T, Desseifne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardière C, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 17.Suker M, Beumer BR, Sadot E, Marhey L, Faris JE, Mellon EA, EI-Rayes BF, Wang-Gillam A, Lacy J, Hosein PJ, et al. FOLFIRINOX for locally advanced pancreatic caner: A systematic review and patient-lebel meta-analysis. Lancet Oncol. 2016;17:801–810. doi: 10.1016/S1470-2045(16)00172-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boone BA, Steve J, Zenati MS, Hogg ME, Singhi AD, Barlett DL, Zureikat AH, Bahary N, Zeh HJ., III Serum CA 19-9 response to neoadjuvant therapy is associated with outcome in pancreatic adenocarcinoma. Ann Surg Oncol. 2014;21:4351–4358. doi: 10.1245/s10434-014-3842-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.