Abstract
In multiple endocrine neoplasia type 1 (MEN1), nonfunctional pancreatic neuroendocrine tumors (NF-pNETs) are the most frequently diagnosed NETs and a leading cause of MEN1-related death. The high prevalence and malignant potential of NF-pNETs outline the need for an evidence-based screening program, as early diagnosis and timely intervention could reduce morbidity and mortality. Controversies exist regarding the value of several diagnostic tests. This systematic review aims to evaluate current literature and amplify an up-to-date evidence-based approach to NF-pNET diagnosis in MEN1. Three databases were systematically searched on the diagnostic value of biomarkers and imaging modalities. Twenty-seven studies were included and critically appraised (modified Quality Assessment of Diagnostic Accuracy Studies). Another 12 studies, providing data on age-related penetrance and tumor growth, were included to assess the optimal frequency and timing of screening. Based on current literature, biomarkers should no longer play a role in the diagnostic process for NF-pNETs, as accuracies are too low. Studies evaluating the diagnostic value of imaging modalities are heterogeneous with varying risks of bias. For the detection of NF-pNETs, endoscopic ultrasound (EUS) has the highest sensitivity. A combined strategy of EUS and MRI seems to be the most useful. Gallium 68 octreotate-DOTA positron emission tomography-CT could be added if NF-pNETs are diagnosed to identify metastasis. Reported growth rates were generally low, and two distinct phenotypes were observed. Surveillance programs should focus on and be adapted to the presence of substantial growth in NF-pNETs. The optimal age to start screening must yet be determined, as insufficient evidence for an evidence-based recommendation was available.
Keywords: imaging modalities, multiple endocrine neoplasia type 1, pancreatic neuroendocrine tumors, screening, tumor markers
Current literature on various diagnostic modalities, tumor growth, and tumor penetrance was systematically reviewed to provide an up-to-date evidence-based approach to nonfunctional pNETs in MEN1.
Multiple endocrine neoplasia type 1 (MEN1) is a rare familial tumor syndrome, primarily caused by germline mutations in the MEN1 gene, encoding the tumor-suppressor protein menin [1]. Glandular hyperplasia and neoplastic endocrine tumors of the pituitary, parathyroid glands, duodenum, and pancreas form the major manifestations of the syndrome. Other manifestations of MEN1 are neuroendocrine tumors (NETs) of gastric, bronchial, or thymic origin; breast cancer; adrenal adenomas; and cutaneous manifestations, such as lipomas, collagenomas, and facial angiofibromas [2, 3].
NETs are manifest in MEN1, and particularly, thymic carcinoid and duodenopancreatic NETs (dpNETs) cause a decreased life expectancy in MEN1 [4–6]. dpNETs are the most prevalent NETs and can be divided in functional, e.g., hormone producing, and nonfunctional. Nowadays, nonfunctional pancreatic NETs (NF-pNETs) are the most frequently diagnosed NETs in MEN1 and a leading cause of MEN1-related death [7, 8]. NF-pNETs cause symptomatic disease in only up to 13% of patients, despite their multicentric appearance [9]. The high prevalence and malignant potential outline the need for an evidence-based screening program to diagnose NF-pNETs at an early stage to enable meticulous follow-up and timely intervention to prevent metastasized disease.
Studies focusing on sporadically occurring NF-pNETs are difficult to extrapolate to MEN1-related NF-pNETs, which are characterized by their multifocal occurrence and a more indolent course of disease in contrast to their sporadic counterparts. Moreover, the onset in MEN1 is at a younger age, and NF-pNETs are diagnosed in an earlier stage because of the screening programs [9, 10]. This illustrates the importance to substantiate guidelines providing recommendations for MEN1 patients, based on evidence derived from MEN1 populations.
Current guidelines advise MEN1 mutation analysis already at the age of five and subsequent presymptomatic screening for MEN1 manifestations [3]. Because of their “silent” behavior and the correlation between metastases and tumor size [7], identification of NF-pNETs depends on sensitive biochemical biomarkers and imaging modalities [5, 7].
The use of biochemical markers for the diagnosis of NF-pNETs is currently under debate, as the most recent studies on biomarkers reported low diagnostic accuracies for pNETs in MEN1 [11, 12]. In addition to biochemical testing, clinical practice guidelines recommend diagnosis and surveillance of NF-pNETs by anatomical imaging modalities, such as CT scan, MRI, or endoscopic ultrasound (EUS) [3]. Functional imaging, such as somatostatin receptor scintigraphy (SRS) and [gallium 68 octreotate (68Ga)]-labeled somatostatin analogs positron emission tomography (PET; 68Ga-dodecanetetraacetic acid (DOTA) PET-CT), is emerging, and therefore, the best approach to NF-pNETs needs to be re-evaluated. In addition, recent studies showed insights in the very low growth rate of small NF-pNETs, fueling the discussion on timing and frequency of surveillance [13, 14]. The diagnosis of small NF-pNETs with a possible indolent course of disease creates a high risk for unnecessary and expensive screening and consequently, a high burden for the patients.
Whereas consensus on the indications for surgery could not be established in 2012 [3], current cohort studies give substantial evidence that a conservative approach for tumors up to 2 cm fits within treatment goals to reduce morbidity and mortality associated with metastatic disease [14, 15]. This frames the screening dilemma in MEN1: imaging modalities should reliably detect tumors below the cutoff of 2 cm, but the indolent behavior of a large proportion of NF-pNETs could lead to overdiagnosis.
The current guideline dates from 2012 and gives rise to the need of an evidence-based approach for diagnosis and follow-up in MEN1 [3]. Recently, controversies in the diagnostic approach in MEN1 were outlined, but a systematic overview of up-to-date literature on NF-pNETs is lacking [16–18]. We systematically reviewed and critically appraised the present literature on the diagnostic value of biochemical biomarkers and various imaging modalities to diagnose NF-pNETs in patients with MEN1. In addition, we evaluated the optimal timing of follow-up by reviewing current literature on the age-related penetrance and tumor growth of NF-pNETs in MEN1.
1. Methods
A. Search Strategies
The electronic bibliographic databases Medline/Pubmed, Embase, and Web of Science were searched December 2017 to review systematically current literature on the diagnostic value of biomarkers and imaging modalities for NF-pNET in MEN1 patients. Keywords are reported in Table 1, and the complete search string is documented in Supplemental Material 1. To gain insight into the penetrance and behavior of NF-pNETs in MEN1 and subsequently answer the question on the optimal timing and frequency of follow-up, a third search was operated, also including our study domain (MEN1 patients). The literature searches were reviewed by an experienced librarian. Database subject terms, such as Mesh terms (Medline) and Emtree terms (Embase), were used as appropriate. Selection of articles was restricted to English, Dutch, German, and French, and for original research, there was no restriction for the year of publication of the studies.
Table 1.
Keywords
| Tumor markers for diagnosis NF-pNETs in MEN1 | ||||
| Biomarker OR CgA OR PP OR glucagon | AND | NET OR endocrine tumor OR NET OR nonfunctioning tumor | AND | Pancreas OR dpNET OR gastroenteropancreatic OR pNET |
| Imaging for diagnosis NF-pNETs in MEN1 | ||||
| OR CT OR MRI OR EUS OR ultrasonography OR scintigraphy OR PET | AND | NET OR endocrine tumor OR NET OR nonfunctioning tumor | AND | Pancreas OR dpNET OR gastroenteropancreatic OR pNET |
| Growth Rate and Penetrance of NF-pNETS in MEN1 | ||||
| MEN 1 OR MEN1 OR Werner syndrome OR hereditary | AND | NET OR endocrine tumor OR NET OR nonfunctioning tumor | AND | Pancreas OR dpNET OR gastroenteropancreatic OR pNET |
Searches were conducted in December 2017.
Abbreviations: CgA, chromogranin A; PP, pancreatic polypeptide.
B. Study Selection
Original studies assessing the diagnostic value of biomarkers or imaging modalities for the diagnosis (NF-)pNETs in patients with MEN1 were eligible for inclusion. In addition, articles were selected if tumor growth and/or penetrance were studied. Studies that included both sporadic NF-pNETs and MEN1-related NF-pNETs were eligible if it was possible to extract data for MEN1-related (NF-)pNETs separately. We excluded reviews, case reports, and studies including only functional dpNETs. Functional dpNETs were defined as tumors with biologically active hormone secretion and consequently, distinct clinical syndromes or symptoms, e.g., gastrinomas, insulinomas, and glucagonomas. NF-pNETs were NETs without a distinct clinical syndrome as a result of excessive hormone production. pNETs immunoreactive to other gastrointestinal hormones without a clinical syndrome are regarded as NF-pNETs. To minimize selection bias, studies with five or less MEN1 NF-pNET patients were excluded.
C. Data Extraction
All identified articles were entered in Covidence® and after the removal of duplicates, independently screened on title and abstract by two authors (M.J.C.v.T. and D.-J.v.B.). Thereafter, independent full text review of potentially relevant studies was performed, and studies were selected if eligibility criteria were fulfilled (M.J.C.v.T. and D.-J.v.B.). Authors resolved any disagreements by consensus and when unsuccessful, with the help of a third reviewer (G.D.V.). Reasons for exclusion at full text screening were recorded. All included articles were cross referenced for additional relevant articles.
Diagnostic accuracy measures, sensitivity, specificity, positive predictive value (PPV), negative predictive value, and area under the curve (AUC) were obtained from the included studies. In case these measures were not provided, data were obtained, and 2 × 2 contingency tables were calculated. Thereafter, sensitivity and specificity were calculated using the standard formulas: sensitivity (%) = true positives/(true positive + false negative); specificity (%) = true negative/(true negative + false positive). In addition, NF-pNET growth rates and the age-related penetrance of NF-pNETs were obtained.
With the consideration of the rarity of the MEN1 syndrome and the expected heterogeneity between studies, as a result of long inclusion periods, differences in patient care, and patient characteristics, narrative data analysis was preferred over meta-analysis.
D. Risk of Bias Assessment
Study and patient characteristics were retrieved from the included articles. Included articles on biomarkers and imaging modalities were critically appraised using a modified Quality Assessment of Diagnostic Accuracy Studies tool by two reviewers independently (M.J.C.v.T. and D.-J.v.B.) [19]. Quality Assessment of Diagnostic Accuracy Studies addresses four important domains: patient selection, index test, reference standard, and flow and timing (Supplemental Material 2). We developed a risk of bias tool to appraise critically studies assessing the growth rate in NF-pNETs (Supplemental Material 3). Based on the Quality In Prognosis Studies tool [20] for prognostic studies, four important domains and subsequent criteria were formulated: study participation and attrition, identification of NF-pNETs, outcome measurement, analysis, and reporting.
To grade the strength of recommendations and quality of evidence, we used the Grading of Recommendations, Assessment, Development, and Evaluation system [21, 22].
2. Results
A. Biochemical Tumor Markers
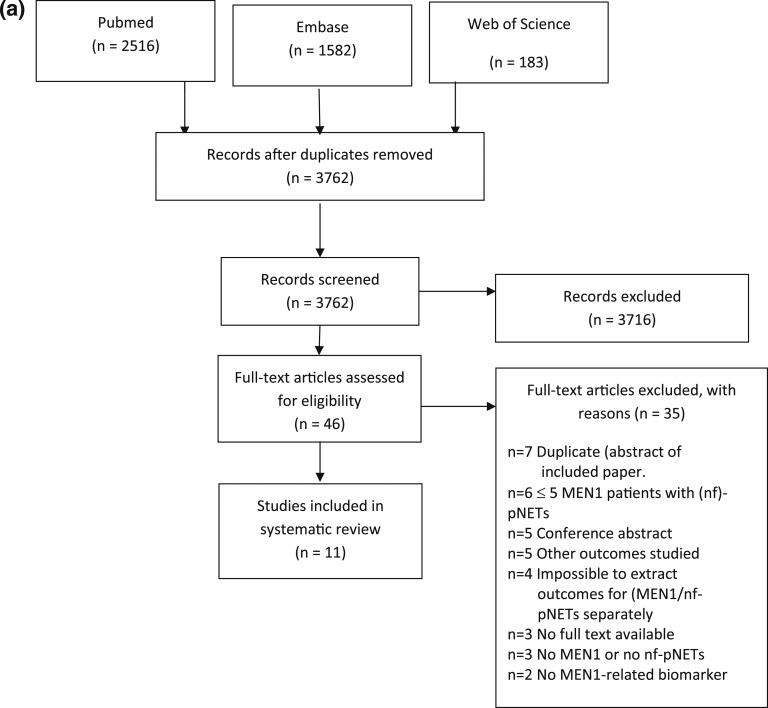
A total of 4281 studies were identified in the databases, of which 519 were duplicates (Fig. 1a) [23]. After removal of duplicates, 3762 studies were screened on title/abstract, and subsequently, full texts were retrieved for 46 potentially relevant studies. Eventually, 11 studies were included for risk of bias assessment.
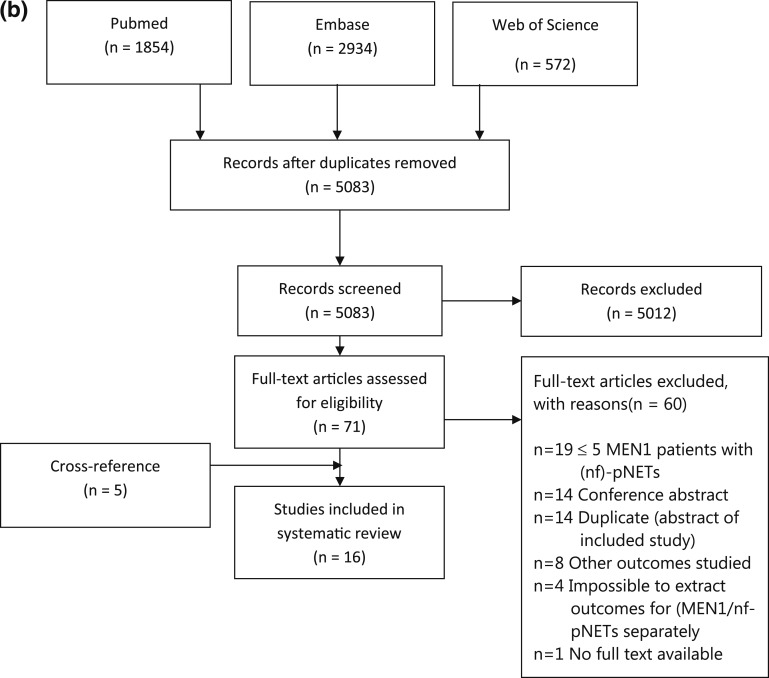
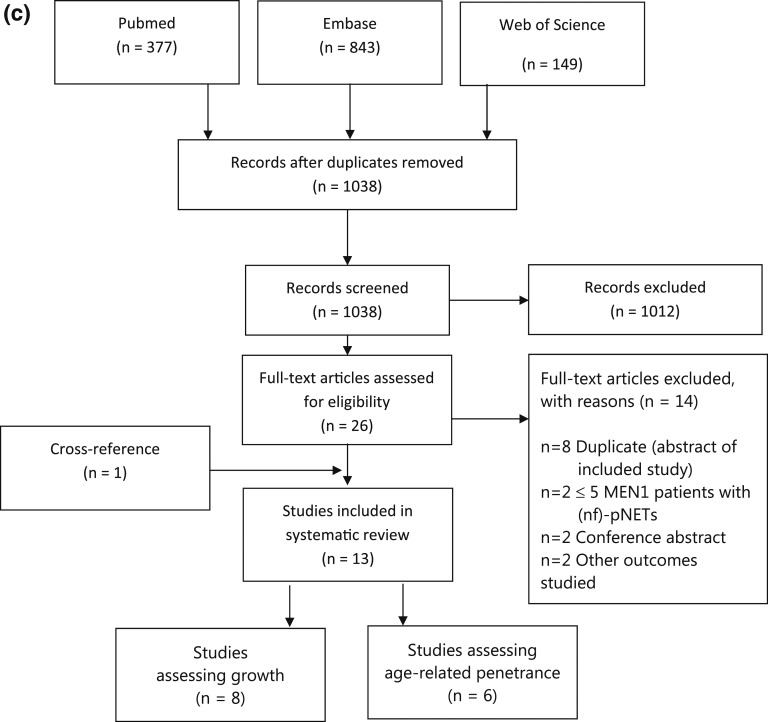
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram for identified (a) biomarker studies, (b) imaging studies, and (c) studies on growth and penetrance [23].
Characteristics of the included studies are reported in Table 2. Overall, considerable heterogeneity was observed in study designs and in study populations. The majority of studies was derived from single center patient populations, whereas only one study assessed the biomarker accuracy in a multicenter population-based cohort [12]. Several studies used a case-control design to observe possible differences in tumor markers among MEN1 (NF-)pNET patients, MEN1 patients, sporadic pNET patients, and/or healthy controls. Most studies focused on a single tumor marker.
Table 2.
Study Characteristics of Included Biomarker Studies
| Authors, Year, Ref. | Country | Single/Multicenter | Population | No. MEN1 Patients | MEN1 Tumor Markers | MEN1 (NF-)pNET | Index Test(s) | Reference Test |
|---|---|---|---|---|---|---|---|---|
| de Laat et al., 2013 [12] | The Netherlands | Multicenter | Population-based cohort | 274 | 159 | 159 | CgA n = 81, PP n = 73, glucagon n = 94 | Pathology. If not available CT/MRI/EUS |
| Granberg et al., 1999 [24] | Sweden | Single center | Case control | 36 | 36 | 27 | CgA | CT/US |
| Langer et al., 2001 [30] | Germany | Single center | Case control | 23 | 12 | 12, 6 NF-pNET | PP (stimulated) | Pathology, CT/SRS/EUS, biochemistry |
| Lewis et al., 2012 [28] | USA | Single center | Cohort | 52 | 52 | 52 | CgA n = 4, PP n = 30, glucagon n = 29 | Pathology |
| Mutch et al., 1997 [29] | USA | Single center | Cohort | 459 | 202 | 20 | PP | CT/MRI/SRS/selective angiography |
| Nehar et al., 2004 [25] | France | Single center | Case control | 34 | 34 | 22, 11 NF-pNET | CgA | CT/EUS |
| Perrachi et al., 2003 [26] | Italy | Single center | Case control | 25 | 25 | 16, 6 NF-pNET | CgA | ? |
| Stridsberg et al., 1995 [27] | Sweden | Single center | Case control | 11 | 11 | ? | CgA | Pathology |
| Qui et al., 2016 [11] | USA | Single center | Cohort | 293 | 113 | 55 pNET, 58 non-pNET | CgA n = 79, PP n = 63, glucagon n = 24 | Pathology. If not available CT/MRI/EUS/SRS |
Abbreviations: n, total number; US, ultrasonography.
The methodological quality of the studies and their risk of bias varied among the studies (Table 3). In all studies, except for de Laat et al. [12], patient selection could have introduced bias. Exclusion criteria [e.g., proton-pump inhibitor (PPI) use or chronic kidney failure], selection of certain subgroups (e.g., preoperative estimation of tumor markers), or a case-control design could have overestimated biomarker accuracy. In almost all included studies, the reference standard or patient flow could have introduced bias, or insufficient data were available to score these risks. Most studies scored a high risk of bias for the reference test, because of variation in reference standards within the study (including reference standards with low accuracy), lack of blinding, or the latency between index and reference test. This is the result of retrospective research on a rare syndrome where data usually were collected in the course of patient care without standardization.
Table 3.
Risk of Bias for Included Studies Assessing the Diagnostic Value of Biomarkers for pNETs in MEN1
|
|
|
Risk of Bias |
Applicability |
|||||
|---|---|---|---|---|---|---|---|---|
| Authors, Year | Biomarker | Patient Selection | Index Test | Reference Standard | Flow and Timing | Patient Selection | Index Test | Reference Standard |
| de Laat et al., 2013 [12] | CgA, PP, glucagon | + | + | ? | − | + | + | + |
| Qui et al., 2016 [11] | CgA, PP, glucagon, gastrin | − | + | ? | − | + | + | + |
| Granberg et al., 1999 [24] | CgA | − | + | − | − | + | + | − |
| Mutch et al., 1997 [29] | PP | − | + | − | − | − | + | + |
| Nehar et al., 2004 [25] | CgA | − | − | + | − | − | + | + |
| Perrachi et al., 2003 [26] | CgA | − | − | ? | ? | − | + | ? |
| Langer et al., 2001 [30] | Meal stimulation test | − | + | − | − | − | + | − |
| Lewis et al., 2012 [28] | PP, gastrin, glucagon | − | + | + | ? | − | − | + |
| Stridsberg et al., 1995 [27] | CgA | − | − | + | ? | ? | + | + |
Abbreviations: +, low risk/low applicability concerns; −, high risk/high applicability concerns; ?, unclear.
A-1. Chromogranin A
Six studies evaluated chromogranin A (CgA) as a diagnostic tumor marker in MEN 1 patients [11, 12, 24–27]. Two studies had maximum applicability for this review [11, 12]. de Laat et al. [12] had the lowest overall risk of bias and estimated the accuracy of CgA in 81 consecutive Dutch MEN1 patients. AUC for CgA was 0.48 with a reported sensitivity of 33% [12]. Subgroup analysis showed only a slight improvement of accuracy in patients without PPI use compared with those with PPI: AUC 0.56 vs AUC 0.47, respectively. The accuracy of CgA (AUC 0.66) for metastatic disease was evaluated, as well with a sensitivity of 53%. Qiu et al. [11] evaluated CgA in 79 patients. Reported AUC was 0.60, but patients on PPI were excluded from the analysis. No correlation was observed between CgA and tumor size, tumor load, or tumor stage nor an association with overall survival.
The three remaining studies at higher risk of bias, especially because of the selection of patients and size of the study (Table 3), concluded insufficient accuracy for CgA as a screening biomarker to identify (early) pancreatic involvement in MEN1, and none of the included studies advised CgA as a screening tool for diagnosis and staging. Sensitivity in these studies ranged from 27% up to 70%, and a low specificity was reported. No data were presented on the accuracy of CgA as a marker for progressive disease.
Based on two studies with maximum applicability, low risks of bias, and equivalent reported outcomes, we conclude an inadequate diagnostic value for CgA. Therefore, CgA should not be routinely used in MEN1 NF-pNET screening programs.
A-2. Pancreatic polypeptide
Four studies evaluated pancreatic polypeptide (PP) [11, 12, 28, 29], of which two studies met applicability criteria for this review (Table 3). de Laat et al. [12] reported a sensitivity of 36%, specificity 74%, and AUC 0.64 for the diagnosis of a pNET. Qiu et al. [11] reported the same AUC (0.64) for PP. For metastatic disease, a sensitivity of 50%, specificity of 74%, and AUC of 0.73 were reported [12]. No correlation was found between PP and tumor size, number of tumors, or tumor stage, but PP levels correlated with age and functional status of pNET. No association was found with survival [11]. Both studies concluded an unsatisfactory diagnostic value. Lewis et al. [28] reported a decline in PP after surgery in 81% of the population. This assumes a correlation with tumor load, but no quantitative data were shown. Therefore, it remains unclear whether the postoperative decrease in PP had any clinical relevance. Mutch et al. [29] published PP outcomes in 202 patients with MEN1 and reported specificity of 88% and sensitivity of 95%. However, metrics were likely to be overestimated as a result of verification bias, as only patients with elevated PP levels and eight patients with normal fasting plasma PP, but with clinical suggestive symptoms, received radiographical evaluation (reference standard; n = 28). One study evaluated the serum PP after a standard meal stimulation test in patients with MEN1 [30] and concluded that a meal stimulation test was not reliable and added no extra information on the presence of pNETs.
In conclusion, current literature does not substantiate the use of PP as a diagnostic for NF-pNETs in MEN1.
A-3. Glucagon
Three studies reported outcomes on glucagon in MEN1-related pNETs (Table 2). Reported AUCs were 0.77 and 0.58 [11, 12]. One study reported a sensitivity and specificity of 43% and 73% [12] in 94 patients. No significant correlation was found among tumor size, number of tumors, tumor stage, or location in 24 patients [11], but another study reported a moderate but significant correlation between 68Ga-dodecanetetraacetic acid tyrosine-3-octreotate (DOTATATE)-avid tumor volume of pNETs to plasma glucagon levels in 25 patients with MEN1 (r = 0.5) [31]. This study was not included in the risk of bias evaluation, as the diagnostic value for diagnosis of a pNET was not assessed, but the correlation between tumor markers and maximum standardized uptake value was assessed. Nevertheless, a large proportion of glucagon levels was within reference range, whereas only patients with proven NETs were included, confirming the low diagnostic sensitivity [31]. In 29 of the 56 cases studied by Lewis et al. [28], elevated glucagon levels were observed in 24 (83%).
Based on low accuracies and lack of correlation with disease status in the first two studies, we conclude that glucagon cannot play a vital role in MEN1 screening programs for NF-pNETs.
A-4. Other biomarkers
Gastrin was evaluated by some included studies, but gastrinomas are beyond the scope of this review.
Two studies estimated the diagnostic value of a combination of biomarkers. Qiu et al. [11] found an AUC of 0.60 for the combination of CgA, PP, and gastrin. This result is consistent with de Laat et al. [12], who reported an AUC of 0.59 for CgA, PP, and glucagon. Therefore, we conclude that the combined use of biomarkers is not of added value to the use of the individual biomarkers for the diagnosis of NF-pNETs in MEN1 patients.
No studies on the diagnostic value of circulating tumor cells or molecular markers, such as micro RNA and mRNA of cell-free DNA in MEN1-related pNETs, were encountered in this study using our search strategy.
B. Imaging
The search strategy yielded 5360 results (Fig. 1b). After the removal of duplicates, 5083 were screened on title and abstract, of which 71 potentially relevant articles were selected for full-text screening. Sixteen studies were included for risk of bias assessment.
Except for one study [32], all included articles were single-center studies. Seven studies collected the data prospectively [32–38].
Most studies reported results on EUS, followed by CT, SRS, 68Ga-DOTA PET-CT, and MRI (Table 4). Seven studies had no concerns regarding applicability for this review (Table 5). There was a high concern on applicability for patient selection in most studies because of the high proportion of included-functioning dpNETs or because a surgical cohort was analyzed. Most studies scored a high risk of bias on flow and timing because of different reference standards within the study population and the lack of standardization of index and reference test in the majority of studies.
Table 4.
Study Characteristics of Included Imaging Studies
| Authors, Year, Ref. | Country | Single/Multicenter | Study Design and Data Collection | No. MEN1 Patients | MEN1/pNET | MEN1 NF-pNET | Index Test | Reference Test |
|---|---|---|---|---|---|---|---|---|
| Albers et al., 2017 [33] | Germany | Single center | Cross-sectional, prospective data collection | 33 | 33 | 31 | 68Ga-DOTATOC PET-CT | MRI/EUS |
| Barbe et al., 2012 [32] | France | Multicenter | Cross-sectional, prospective inclusion/data collection | 90 | 90 | 90 | MRI/EUS | MRI/EUS |
| Camera et al., 2011 [40] | Italy | Single center | Cross-sectional | 14 | 9 | ? | CT | Pathology (n = 4), EUS |
| Gauger et al., 2003 [43] | USA | Single center | Cross-sectional, retrospective data collection | 66 | 15 | 13 | EUS | Pathology |
| Goroshi et al., 2016 [39] | India | Single center | Retrospective data collection | 18 | 13 | 6 | 68Ga-DOTANOC PET/CT | CT/pathology |
| Hellman et al., 2005 [44] | Sweden | Single center | Cross-sectional, retrospective data collection | 25 | 25 | 23 | EUS 5-HTP PET (selectively) | CT, US (n = 3), pathology (n = 8). Rest biochemical |
| Kornaczewski Jackson 2017 [46] | Australia (Tasmania) | Single center | Retrospective data collection | 49 | 25 | 12 | 18F-FDG PET/CT | Pathology, CT, ultrasound, EUS, MRI |
| Langer et al., 2004 [34] | Germany | Single center | Prospective data collection | 36 | 22 | 13 | EUS, CT, 111In SRS | Pathology or clinical FU |
| Lastoria et al., 2016 [45] | Italy | Single center | Cross-sectional | 18 | 11 | ? | 68Ga-DOTATATE PET/CT | Pathology or clinical FU |
| Lewis et al., 2012 [28] | USA | Single center | Cross-sectional, retrospective data collection | 52 | 52 | ? | 111In SRS, CT, MRI, EUS | Pathology |
| Morgat et al., 2016 [35] | France | Single center | Cross-sectional, prospective data collection | 19 | 19 | ? | 68Ga-DOTA-TOC PET/CT, 111In SRS, CT | Pathology, CT/MRI/EUS/18F-FDG PET/CT |
| Skogseid et al., 1998 [41] | Sweden | Single center | Cross-sectional, retrospective data collection | 25 | 25 | 13 | CT, US, angiography, SRS | Pathology |
| van Asselt et al., 2015 [36] | Netherlands | Single center | Cross-sectional study, prospective data collection | 41 | 35 | ? | EUS, 11C-5-HTP PET | Pathology, CT/MRI |
| Waldmann et al., 2009 [37] | Germany | Single center | Prospective data collection | 35 | 24 | 18 | CT, SRS, EUS | Pathology |
| Wamsteker et al., 2003 [42] | USA | Single center | Cross-sectional study, retrospective data collection | 65 | 13 | 11 | EUS | Pathology |
| Yim et al., 1998 [38] | USA | Single center | Prospective data collection | 29 | ? | ? | 111In SRS | Pathology, CT/MRI/arteriogram |
Abbreviations: 11C-5-HTP, 11C-5-hydroxytryptophan; 18F-FDG, 18F-fluorodeoxyglucose.
Table 5.
Risk of Bias for Included Studies Assessing the Diagnostic Value of Imaging Modalities for pNETs in MEN1
|
|
|
Risk of Bias |
Applicability |
|||||
|---|---|---|---|---|---|---|---|---|
| Authors, Year | Imaging | Patient Selection | Index Test | Reference Standard | Flow and Timing | Patient Selection | Index Test | Reference Standard |
| Albers et al., 2017 [33] | EUS/MRI/68Ga-PET/CT | + | − | − | + | + | + | + |
| Barbe et al., 2012 [32] | EUS/MRI | + | + | − | + | + | + | + |
| Lastoria et al., 2016 [45] | 68Ga-PET/CT | + | ? | − | − | + | + | + |
| van Asselt et al., 2015 [36] | MRI, CT, EUS, SRS, 11C-5-HTP PET | − | + | − | − | + | + | + |
| Morgat et al., 2016 [35] | 68Ga-PET/CT, CE-CT, SRS | + | + | − | − | + | + | + |
| Gauger et al., 2003 [43] | EUS | + | + | − | − | + | + | + |
| Hellman et al., 2005 [44] | EUS | + | ? | − | − | + | + | − |
| Goroshi et al., 2016 [39] | 68Ga-PET/CT, CT | + | − | + | − | − | + | + |
| Wamsteker et al., 2003 [42] | EUS | − | + | + | − | + | + | + |
| Kornaczewski Jackson 2017 [46] | 18F-FDG | + | ? | − | − | − | + | + |
| Langer et al., 2004 [34] | EUS, CT, SRS | + | + | − | − | − | + | + |
| Lewis et al., 2012 [27] | EUS, CT, MRI, SRS | − | + | + | ? | − | + | + |
| Camera et al., 2011 [40] | CT | + | − | − | − | − | + | + |
| Waldmann et al., 2009 [37] | EUS, SRS, CT | + | − | − | − | − | + | + |
| Skogseid et al., 1998 [41] | CT, SRS | − | + | + | ? | − | + | + |
| Yim et al., 1998 [38] | SRS | − | ? | + | − | − | − | + |
Abbreviation: CE, contrast-enhanced.
B-1. Conventional imaging
CT
Sensitivity varied between 54% and 81%, with a specificity of 50% (Table 6) [28, 35, 37, 39–41]. Langer et al. [34] reported 54% sensitivity in a group of patients with dpNET with surgery as a reference standard. All patients with a false-negative CT had small duodenal or pancreatic gastrinomas (largest 14 mm). Lewis et al. [28] reported the highest sensitivity (81%) with a PPV of 96% on preoperative CT. Eight of the 43 CTs were negative, with the largest missed pNET measuring 4 cm. In a prospective series of 19 consecutive patients with MEN1 suspected for dpNETs undergoing 68Ga-dodecanetetraacetic acid–tyrosine-3-octreotide (DOTA-TOC) and contrast-enhanced CT, the reported sensitivity and specificity of CT were 60% and 50%, respectively. However, on a per-lesion basis, solely of the pancreas, CT revealed 37 of the 46 lesions (80%) identified by 68Ga-DOTA-TOC PET scanning [35].
Table 6.
Accuracy of Imaging Modalities
| Authors, Year | EUS | MRI | CT | SRS | 68Ga-PET/CT |
|---|---|---|---|---|---|
| Albers et al., 2017 [33] | |||||
| n | 27 | 27 | 27 | ||
| Sensitivity, % | 100 | 74 | 78 | ||
| Barbe et al., 2012a [32] | |||||
| n | 75 | 67 | |||
| Sensitivity, % | 83 | 74 | |||
| Lastoria et al., 2016 [45] | |||||
| n | 11 | ||||
| Sensitivity, % | 100 | ||||
| van Asselt et al., 2015a,b,c [36] | |||||
| n | 35 | 35 | |||
| Sensitivity, % | 97 | 51 | |||
| Morgat et al., 2016c [35] | |||||
| n | 76 | 76 | 76 | ||
| Sensitivity, % | 60 | 20 | 76 | ||
| Specificity, % | 50 | 50 | 100 | ||
| Gauger et al., 2003 [43] | |||||
| n | 13 | ||||
| Sensitivity, % | 92 | ||||
| Hellman et al., 2005d [44] | |||||
| n | 22/8 | ||||
| Sensitivity, % | 64/50 | ||||
| Goroshi et al., 2016c [39] | |||||
| n | 13 | 13 | |||
| Sensitivity, % | 63 | 100 | |||
| Wamsteker et al., 2003c [42] | |||||
| n | 10 | ||||
| Sensitivity, % | 82 | ||||
| Langer et al., 2004 [34] | |||||
| n | 16 | 13 | 17 | ||
| Sensitivity, % | 75 | 54 | 71 | ||
| Lewis et al., 2012 [28] | |||||
| n | 35 | 8 | 43 | 32 | |
| Sensitivity, % | 100 | 88 | 81 | 84 | – |
| Camera et al., 2011c [40] | |||||
| n | 11 | ||||
| Sensitivity, % | 78 | ||||
| Skogseid et al., 1998e [41] | |||||
| n | 15/10 | 15/10 | |||
| Sensitivity, % | 57/20 | 75/0 | |||
| Waldmann et al., 2009 [37] | |||||
| n | 20 | 24 | 24 | ||
| Sensitivity, % | 100 | 62 | 54 | ||
| Yim et al., 1998c [38] | |||||
| n | 16 | ||||
| Sensitivity, % | 58 |
Abbreviation: n, number of included patients in the study.
Results from analysis for pNETs > 1 cm.
Not every patient received an MRI or CT (either MRI or CT), so sensitivity could not be extracted.
Sensitivity based on per-lesion analysis in n patients.
No reference standard was described for the index test. Results in table are distracted from the article with biochemical signs (n = 22)/histopathology (n = 8) as reference standard.
Population and sensitivity for major disease/limited disease.
All included studies had high risks of bias, but most results were uniform. Studies reported inferior diagnostic values of CT compared with 68Ga-DOTA PET-CT [35, 39] and EUS [28, 34, 37]. The reported differences in accuracy between CT and SRS were varying (Table 6), and no direct comparison was made between MRI and CT. Studies describing the characteristics of missed lesions on CT reported small sizes, mostly below 17 mm, but a few exceptions were recorded [28, 34, 35].
MRI
Three studies evaluated the diagnostic value of MRI with reported sensitivities varying between 74% and 88% [28, 32, 33] and a PPV of 100% [28].
Barbe et al. [32] compared the diagnostic value of MRI (1.5 T) with EUS in 90 consecutive patients with MEN1 to study the concordance of both modalities for the detection of pNETs ≥ 10 mm. MRI and EUS (reference standard was the combination of both) identified 57 (63%) patients with tumors between 10 and 20 mm. Overall, sensitivities for EUS and MRI were 84% and 81%, but for tumors >20 mm, sensitivities were 65% for EUS and 85% for MRI, respectively. Barbe et al. [32] concluded that for the detection of ≥10 mm pNETs, EUS is superior to MRI, but the latter performed homogeneous throughout the entire the pancreas, whereas 94% of all tumors missed by EUS were in the body and tail. Therefore, they advised that both should be performed at the initial evaluation [32]. It is important to note that the diagnostic value may be underestimated, as imaging outcomes were classified as negative, whereas a tumor <10 mm was identified on one of the modalities (∼25% of patients). Albers et al. [33] compared EUS, 68Ga-DOTA PET-CT, and MRI (1.5 T) and reported MRI sensitivity of 74% on a patient-based level. The authors also performed a per-lesion subgroup analysis based on the size of pNETs. Sensitivities for 0 to 5, 5 to 10, 10 to 20, and >20 mm were 17%, 22%, 35%, and 83%, respectively, concluding a reliable MRI detection for larger pNETs. Lewis et al. [28] preoperatively evaluated MRI in eight patients; with seven out of eight patients positive, sensitivity was 88%, and PPV was 100%.
All three studies had a high risk of bias in one of the four domains. No study directly compared MRI with CT as diagnostic for NF-pNETs in MEN1, so no conclusions can be made on the preferred noninvasive conventional imaging modality. Based on the reported sensitivities, quality of the included studies, and the risks associated with cumulative exposure to ionizing radiation, one could suggest MRI should be preferred above CT.
EUS
EUS sensitivity ranged from 75% to 100% [28, 32, 34, 36, 37, 42–44] in most studies. One study reported a sensitivity of 50%, but this study has a high risk of bias, as no reference standard for pNETs was described (selection of patients was based on elevated biochemical markers), and histopathology was only available in a small subgroup [44]. In general, histopathology, as reference standard, could also lead to lower sensitivities of imaging modalities if very small pNETs are included in per-lesion analysis as well.
van Asselt et al. [36] compared four imaging modalities [CT or MRI + SRS + 11C-5-hydroxytryptophan (11C-5-HTP) PET + EUS] in 41 patients. In 35 patients, 107 pNETs were identified by combining all modalities. EUS identified 97% of the patients and 94% of the pNETs, which was significantly better than compared with the other modalities. In the subgroup analysis of pNETs > 1 cm, EUS remained superior, as 97% of the pNETs were identified. Albers et al. [33] showed similar superiority for EUS over 68Ga-DOTA PET-CT and MRI, but this difference was only statistically significant for pNETs < 1 cm.
Lewis et al. [28] evaluated preoperative imaging in 52 individuals who underwent 56 pancreatic surgeries. EUS was performed preoperatively in 63% and had the highest sensitivity (100%) on a patient basis. Two series derived from Ann Arbor, Michigan, compared preoperative EUS with histopathology. In 13 asymptomatic patients who underwent surgery, Gauger et al. [43] identified pNETs on preoperative EUS in 12 (sensitivity 92%). Wamsteker et al. [42] found 23/28 pNETs (sensitivity 82%) on preoperative EUS in 10 asymptomatic patients.
All studies that reviewed EUS concluded that EUS is the most sensitive procedure. Therefore, EUS seems to have the highest diagnostic accuracy for the detection of small NF-pNETs [32, 33]. However, some clinically relevant NF-pNETs are missed, especially in the pancreatic tail; EUS is an invasive procedure and is operator dependent. To overcome this issue, a multimodal strategy could be initiated, preferably with MRI. Concordance between MRI and EUS in tumors ≥10 mm was moderate (Kappa coefficient = 0.55) [32].
B-2. Functional Imaging
111In pentetreotide scan (SRS)
SRS was evaluated in seven studies [28, 34–38, 41]. Sensitivity varied between 20% and 84%. Morgat et al. [35] prospectively compared SRS with CT and 68Ga-DOTA PET-CT for the detection of dpNETs in 31 patients and had the lowest risk of bias and the highest applicability. SRS showed a sensitivity of 20% and a specificity of 50%. Eleven pNETs were identified on SRS, whereas 68Ga-DOTA-TOC PET/CT and CT identified 46 and 37 pNETs, respectively. All lesions depicted by SRS were positive in 68Ga-DOTA PET-CT as well. Mean pNET size of those identified by SRS was 15 mm. Furthermore, 68Ga-DOTA PET-CT depicted smaller lesions than SRS, leading to overall superior diagnostic performance [35]. The highest sensitivity (84%) was reported by Lewis et al. [28] and is possibly overestimated, as preoperative SRS was reviewed. It is possible that the average tumor size in this study was larger compared with asymptomatic MEN1 patients not undergoing surgery, but the average tumor size was not reported. The overall inferiority of SRS compared with 68Ga-DOTA PET-CT and the insufficient sensitivity reported in studies with the lowest risk of bias assume no further indication for SRS in the screening of NF-pNETs in MEN1.
68Ga-DOTA PET-CT
68Ga-DOTA PET-CT was evaluated in three prospective studies [33, 35, 45] and one study reported the diagnostic value in a retrospective case series [39]. Albers et al. [33] compared the diagnostic value of combined conventional imaging (EUS/MRI) with 68Ga-DOTATOC-PET-CT for the diagnosis of dpNETs in routine follow-up in 33 MEN1 patients. Subgroup analysis for pNETs revealed a sensitivity of 78% for 68Ga-DOTA PET-CT. Sensitivities depended on pNET size; for pNETs, <5, 5 to 10, 10 to 19, and ≥20 mm sensitivities were 0%, 29%, 81%, and 100%, respectively. In addition, the authors concluded that the routine use for 68Ga-dodecanetetraacetic acid 1-NaI3-octreotide (DOTANOC)-PET-CT is limited for the detection of metastasis [33].
A similar sensitivity (76%) was reported by Morgat et al. [35], who compared 68Ga-DOTA PET-CT with SRS and CT in a per-lesion analysis (75 dpNETs in 19 individuals). 68Ga-DOTA PET-CT outperformed both in this study, and the reported specificity was 100%, but this was possibly overestimated, as a combination of imaging modalities, instead of histopathology, was used as a reference standard. The smallest reported lesion on 68Ga-DOTA-TOC PET-CT measured 2 mm [35].
The two remaining studies on 68Ga-DOTA PET-CT had small sample sizes and reported sensitivities of 100% [39, 45]. Lastoria et al. [45] prospectively compared 68Ga-DOTA PET-CT with conventional imaging for four MEN1-related tumor sites. The diagnostic value for pNETs was compared with EUS/CT or histology as reference standard [45]. Goroshi et al. [39] described a retrospective case series of 11 patients with 16 histopathologically proven dpNETs. Despite the maximum sensitivity in these two studies, 68Ga-DOTA PET-CT should not be recommended as a first-choice imaging modality to screen MEN1 patients based on the reported results from Albers et al. [33] and Morgat et al. [35]. Both prospective studies had lower risks of bias and larger sample sizes and reported sensitivities of almost 80%. In addition, clinical management did not change after 68Ga-DOTA PET-CT in 97% of all patients who also underwent conventional techniques for the complete screening of MEN1 [33]. As the diagnostic value of 68Ga-DOTA PET-CT depends on tumor size, and the detecting of metastases is a major advantage, 68Ga-DOTA PET-CT could be applied in patients with prevalent tumors >10 mm and not as a screening modality for detection of incident NF-pNETs.
B-3. Other imaging techniques
van Asselt et al. [36] reviewed 11C-5-HTP PET and although superior to SRS, was of no additional value compared with standard screening (EUS), as only 54% of the patients and 32% of the pNETs were diagnosed. Kornaczewski Jackson et al. [46] evaluated the use of 18F-fluorodeoxyglucose (18F-FDG) PET in 49 patients with MEN1 . Twenty-five patients had evidence of a pNET on conventional imaging, but 18F-FDG PET was positive in only five (20%). 18F-FDG PET avidity was positively associated with the Ki-67 index and pNET aggressiveness [46]. For risk stratification of aggressive disease, 18F-FDG PET revealed a sensitivity of 86% and specificity of 95%. However, the value of 18F-FDG PET for risk stratification over EUS and fine needle aspiration to determine Ki-67 needs to be determined in prospective studies. Current evidence withholds its applicability for routine screening in MEN1-related NF-pNETs.
C. Growth and Penetrance
The systematic search identified 1369 articles (Fig. 1c). After duplicates were removed, 1038 articles were screened on title/abstract. Twenty-six studies were full-text reviewed for eligibility, of which 13 were included.
Eight studies assessed the growth of NF-pNETs [13, 37, 47–52] and six, the age-related penetrance of (NF-)-pNETs in patients with MEN1 [7, 51, 53–56]. Of the studies assessing NF-pNET growth rate, two were derived from population-based cohorts [13, 50], five assessed growth by consecutive EUS [37, 47–49, 51], and four collected data prospectively [37, 48, 50, 51]. Studies evaluating growth rates were critically appraised (Table 7). One study had a low risk in all four domains [13]. Almost all studies have selection bias, as data were collected during patient follow-up. Patients with progressive tumors demanding surgery on short term are excluded in most studies, as two consecutive evaluations were not available. Therefore, reported growth rates are possibly underestimated, and the proportion patients with progressive tumors could be larger. Most studies did not address or report possible effect modifiers for tumor growth. Many studies did not assess current systemic treatment (e.g., somatostatin analogs), possibly influencing growth rates. Two studies on age-related penetrance were derived from a population-based cohort [7, 54], whereas three other studies were multicenter studies [51, 55, 56].
Table 7.
Risk of Bias for Included Studies Assessing Growth Rate in MEN1-Related NF-pNETs
| Authors, Year | Risk of Bias |
|||
|---|---|---|---|---|
| Patient Selection | Diagnosis | Outcome Measurement | (Statistical) Analysis | |
| D’souza et al., 2014 [47] | − | + | + | − |
| Kann et al., 2006 [48] | ? | + | + | − |
| Kappelle et al., 2017 [49] | − | + | + | − |
| Pieterman et al., 2017 [13] | + | + | + | + |
| Sakurai et al., 2007 [52] | − | − | + | ? |
| Triponez et al., 2017 [50] | + | − | − | ? |
| Waldmann et al., 2009 [37] | + | − | ? | − |
C-1. Growth
The annual growth rate and incidence of new lesions are summarized in Table 8. Growth rates varied between 0.1 and 1.32 mm per year. Reported factors associated with increased growth rate are scarce: MEN1 genotype [13], age [37], and number of pNETs visualized [49]. EUS and conventional imaging (CT/MRI) were used for growth assessment.
Table 8.
Reported Growth in NF-pNETs From Included Studies
| Authors, Year | n | No pNETs | NF-pNET Size, mm | Design | Modality Used for Assessment | FU in Months | Size at First Detection (in mm Median) | Annual Growth (All Lesions; mm/y) | Incidence New Lesions (per pt/y) | Growth New Lesions (mm/y) |
|---|---|---|---|---|---|---|---|---|---|---|
| D’souza et al., 2014 [47] | 11 | 18 | NA | R | EUS | 79 (18–134) | 10.3 (5–24) | 1.32 | 0.17 | 3.0 |
| Kann et al., 2006 [48] | 20 | 84 | <15 | P | EUS | 20 ± 12 | 5.9 (1.5–14.5) | 1.3% ± 3.2%/mm = 0.9 ± 2.3 | 0.62 | |
| Kappelle et al., 2017a [49] | 38 | 226 | <20 | R | EUS | 38.4 (1.1–5.6)b | 5.0 | 0.10 | 0.79 | No growth |
| Pieterman et al., 2017 [13] | 99 | 115 | <20 | R | CT/MRI | 156 (84–276)b | 10 ± 4 | 0.4 Stable: no growth; progressive: 1.6 | 1.04 | |
| Triponez et al., 2017 [50] | 46 | 96 | <20 | P | CT/MRI/EUS | 128 ± 50,4 | 9.3 ± 5 | Stable: <0.1; progressive: 0.54 | ||
| Waldmann et al., 2009a [37] | 29 | 88 | NA | P | EUS | 72 (24–108) | 9.0 | 11.7 ± 24.1% = 1.1 ± 2.17 mm | 0.52 | 1.28 |
| Sakurai et al., 2007 [52] | 14 | 26 | NA | R | CT | 78 ± 36 | 20 ± 18 (5–78) | Not reported | Not reported | Not reported |
FU: median (range) or means ± SD. Size: median (range) or means ± SD.
Abbreviations: NA, not applicable; P, prospective study; R, retrospective study.
Population with NF-pNETs and (possibly) functional pNETs.
Interquartile range.
Conventional imaging
A population-based study evaluated the growth rate of 115 NF-pNETs <2 cm in 99 MEN1 patients and assessed the incidence of new NF-pNETs after a median follow-up of 13 years per patient. The growth rate was 0.4 mm/y and the incidence of new tumors was 1.04 per year [13]. An association between growth rate and tumor numbers could not be confirmed in this study. Noteworthy, subgroup analysis identified 35 tumors in 34 patients as progressive (growth rate of 1.6 mm/year), whereas the majority of pNETs (n = 80, 70%) was stable (no growth). Genotype was an important effect modifier for growth velocity, as patients with missense mutations had a significantly higher growth rate than nonsense/frameshift mutations [13]. The finding of a large proportion patients with stable disease without substantial growth is in line with Triponez et al. [14]. Differences in growth between those with progressive disease (increase in tumor size, number or development of a hypersecretion syndrome) and stable disease in NF-pNETs were estimated. Sixty-one percent had stable disease and showed no substantial growth [14]. Sakurai et al. [52] described 14 patients with MEN1 with 26 NF-pNETs and prospectively followed 13 NF-pNETs by CT. No substantial growth (increase in tumor size of >20%) was observed in 12/13 (92%), all of them smaller than 20 mm.
EUS
The fastest growth was reported by D’souza et al. [47] in a retrospective study, including 11 patients with a mean EUS surveillance of 79 months. Sixty-one percent of all lesions were stable during follow-up. Importantly, new lesions had a significantly faster growth rate compared with the index lesions. The authors suggested a variation in phenotypic expression of the disease [47]. The lowest growth rate was estimated in a larger EUS-based surveillance study in 226 patients [49]. Annual tumor growth was 0.10 mm in pNETs ≤2 cm, but if split for prevalent pNETs (0.21 mm/year) and incident pNETs (no growth), a significant difference was found [49]. Interestingly, the absence of growth in new lesions was in contrast with the findings of D’souza et al. [47]. Thomas-Marques et al. [51] reported outcomes of systematic follow-up with EUS in 51 MEN1 patients of whom 55% had NF-pNETs. Sixty-three percent of the patients with initial NF-pNETs had stable disease (e.g., no growth nor new lesions), whereas 25% developed new NF-pNETs, and 13% showed tumor growth only.
C-2. Penetrance
The results on age-related penetrance are described in Table 9.
Table 9.
Data on Age-Related Penetrance (NF-pNETs) From Included Studies
| Study | No. of MEN1 Patients (pNETs) | Design | Age, y | Modality Used | Penetrance | Youngest Patient, y |
|---|---|---|---|---|---|---|
| Gonçalves et al., 2014 [53] | 19 (8) | R | 12–20 | EUS (74%) CT/MRI | 42% NF-pNETs by age 20 y | 16 |
| Goudet et al., 2015 [54] | 160 | R | 1–21 | CT/MRI/EUS | 9% NF-pNETs by age 21 y | 13 |
| Manoharan et al., 2017 [56] | 166 (8) | P | 8–18 | MRI/EUS | 1.8% NF-pNETs by age 19 y | 15 |
| Machens et al., 2007 [55] | 258 (126) | Cross | 43 (mean) | CT/MRI/EUS | Age-related penetrance dpNETs (NF-pNETs) | NA |
| Mean age 14: 4% (0%) | ||||||
| Mean age 33: 45% (18%) | ||||||
| Mean age 48: 57% (14%) | ||||||
| Mean age 64: 60% (13%) | ||||||
| Triponez et al., 2006 [7] | 579 (108) | P | CT/MRI/EUS | Penetrance dpNET (NF-pNET) | NA | |
| Age 20: 9% (3%) | ||||||
| Age 50: 53% (34%) | ||||||
| Age 80: 84% (53%) | ||||||
| Thomas-Marques et al., 2006 [51] | 51 | P | 39 (16–71) | EUS | Frequency: 54.9% in cohort | 16 |
Abbreviation: Cross, cross-sectional.
The largest study on penetrance in MEN1 is derived from the French Groupe d’Etude des Tumeurs Endocrines population-based registry. Triponez et al. [7] reported isolated NF-pNETs penetrances of 3%, 34%, and 53% at 20, 50, and 80 years, respectively. A large multicenter study from Germany reported lower age-related penetrances for NF-pNETs [55]. Machens et al. [55] also evaluated differences between penetrance and type of mutation (e.g., out-of-frame or truncating vs in-frame mutations), but no disparities were found.
Gonçalves et al. [53] systematically screened 19 MEN1 mutation carriers in their second decade of life with EUS and/or MRI/CT. A much higher penetrance of NF-pNETs (42%) was observed in 19 patients. This difference is probably contributable to the imaging modality used for screening (mostly EUS). Fifty percent had multicentric NF-pNETs, 21% harbored a NF-pNET >2 cm, and the largest pNET measured 40 mm [53]. Case reports were not included in this review, but Gonçalves et al. [53] also reviewed case reports of both functional and NF-pNETs in young MEN1 mutation carriers. The youngest patient with a NF-pNET >2 cm was 12 years old [57]. Goudet et al. [54] evaluated the penetrance and natural history of NF-pNETs in 160 young MEN1 patients from the Groupe d’Etude des Tumeurs Endocrines. By the age of 21, 23% harbored a pNET, of which NF-pNETs were present in 9%. The mean size of the largest NF-pNET was 18 mm. Five patients demanded surgical resection of the NF-pNET, on whom four were operated at the age of 13 to 15 years. In the operated patients, 43% of the NF-pNETs measured 2 cm or more, the largest being 4 cm [54]. Another German study investigated the age-related penetrance in 166 MEN1 patients <19 years derived from two centers. Twenty patients had MEN1 manifestations, of whom three had NF-pNETs at the ages of 15, 17, and 18, respectively (penetrance 1.8%) [56]. Two patients underwent pancreatic surgery for NF-pNETs <15 mm.
The reported penetrance below 10% by the age of 20 in large prospective cohorts, the sporadic need for surgical treatment below the age of 16, and the psychological burden of the screening program for young asymptomatic MEN1 children might be arguments to defend the start of screening for NF-pNETs at the age of 16. However, given the paucity of evidence, the refrainment from screening before the age of 16 cannot be advised. Results on age-related penetrance for young MEN1 patients are diverge and depend on the imaging modality used. Therefore, future studies are needed to establish the optimum timing for screening.
3. Discussion
We conducted a thorough systematic literature search to identify studies assessing our research questions, as well as a critical appraisal to assess methodological quality and applicability of these studies. Two extensive search strings were generated, not specifically focusing on patients with MEN1, to discover studies on sporadic pNETs, also including patients with MEN1. As MEN1 is a very rare disease with heterogeneous disease manifestations and studies covering long time spans, modified risk of bias tools was composed to account for these issues as much as possible. A complete overview of currently available literature was generated, and subsequent conclusions for clinical care were drawn on current best-available evidence. In addition, important topics to assess in future research are generated.
Inherent to the rarity of the disease, only a few studies of sufficient methodological quality were included. The majority of the studies was retrospective by design, and data were collected from routine patient care, often without standardization. In line, blinding of observers (radiologists) was not done in most studies, and different reference standards were used. Most studies were conducted on populations derived from single centers, leading to a case mix of patients with MEN1. In addition, selected cohorts, such as surgically treated cohorts, were included. Sample size of most studies was limited, leading to insufficient power to detect statistically significant results and subsequent imprecise estimates. Lastly, because of the rarity of MEN1, studies included patients over a long time period. Changes in patient care, improved knowledge on MEN1, the intensive MEN1 screening program, and increased quality of imaging modalities are hard to account for in the study design and statistical analysis.
The inferior sensitivity of CT compared with EUS and 68Ga-DOTA PET-CT and the cumulative exposure to ionizing radiation, already exceeding levels deemed safe during 8 years of follow-up [58], make CT less useful as a radiologic screening modality in a life-long disease. CT seems to have advances in the preoperative assessment, but this is beyond the scope of this review. Based on the available literature, MRI turned out to be a more sensitive and convenient screening modality, as there is no radiation exposure, but reservations should be made, as currently, no direct comparison between CT and MRI has been undertaken in patients with MEN1. MRI studies evaluated 1.5 T MRI, but higher tesla imaging is currently common standard in expert centers. The use of better MRI might increase sensitivity, so the question remains as to whether these older MRI studies reflect current clinical practice. Although this is the case, the choice for the optimal screening modality to detect NF-pNETs remains ambiguous. We concluded EUS being the most sensitive imaging modality, detecting up to 2 mm. Serial assessments of tumor size to evaluate tumor growth are reliable with EUS [59], fine needle aspiration can be added, and adrenals glands can be visualized. On the other hand, EUS is limited by the operator dependence, has a decreased sensitivity in the pancreatic tail, and is an invasive procedure. Furthermore, small NF-pNETs, without therapeutic consequences, are detected but with the necessity of follow-up. This could theoretically lead to a higher psychological burden of disease. MRI has the advantage of homogenous performance throughout the pancreas, but a significant proportion of NF-pNETs >2 cm is missed. The latter also applies for EUS. To ensure maximum sensitivity, both modalities can be used alternately to detect lesions as early as possible and reduce the burden of invasive EUS (Table 10).
Table 10.
Summary of Recommendations
| Recommendations | Evidence (According to GRADE [21, 22]) |
|---|---|
| The annual use of CgA, PP, and glucagon as a tumor marker for the diagnosis of NF-pNETs is not recommended. | (1|⊕⊕⊕○) |
| Radiological screening for NF-pNET should include MRI or endoscopic ultrasonography. | (2|⊕⊕○○) |
| 68Ga-DOTA PET/CT should be preferred over 111In single photon emission CT/CT for the diagnosis of NF-pNETs. | (1|⊕⊕○○) |
| 68Ga-DOTA PET/CT should not be routinely used for the diagnosis of NF-pNETs. | (2|⊕○○○) |
| Based on the growth rate and NF-pNET size, pancreatic visualization can be extended to once per 1 to 2 y. | (2|⊕⊕○○) |
| Screening for NF-pNETs in asymptomatic MEN1 patients should not be extended until the age of 16. | (2|⊕○○○) |
Abbreviation: GRADE, Grading of Recommendations, Assessment, Development, and Evaluation.
Regarding the high prevalence of somatostatin receptors on NETs, these tumors seem specifically interesting for somatostatin-labeled radionuclides. The recent advances 68Ga-DOTA PET-CT in sporadic NETs have not been unnoticed in MEN1 research. Unfortunately, not all MEN1 studies on 68Ga-DOTA PET-CT were included, as we could not extract data on (NF-)pNETs [60, 61]. Contrary to Albers et al. [33], two studies report more promising results regarding changes in patient management based on 68Ga-DOTA PET-CT. Both studies evaluated the impact of 68Ga-DOTATOC PET-CT on diagnosis of MEN-associated lesions and its influence on therapeutic management [60, 61]. They illustrated the advantage of “full body imaging” with 68Ga-DOTA PET-CT in a disease with multiple organs involved and the risk of locoregional and distant metastases. As 68Ga-DOTA PET-CT was introduced in recent years, studies on the diagnostic ability are scarce. From the current available evidence, we can conclude that although not superior in the detection of incident NF-pNETs, 68Ga-DOTA PET-CT could be integrated in the follow-up program for NF-pNETs >1 cm to detect metastases in an early stage. The optimal timing and frequency of screening remain to be established. No biomarker reflects tumor behavior or predicts the course of disease over time, so imaging modalities remain the cornerstone within the surveillance program. Studies on molecular markers in MEN1 were not encountered, although the search string did not specifically focus on these new biomarkers. Data on tumor growth can frame the clinical relevance of small NF-pNETs, as the malignant course of NF-pNETs seems to be correlated to tumor size [7]. We evaluated the current available data on growth to outline the average growth rate of NF-pNETs to distinguish those with more aggressive disease. Two large population-based cohorts reported very slow growth [13, 14]. In addition, two recognizable phenotypes can be distinguished in patients with NF-pNETs. Most patients (60% to 70%) had stable disease with no growth at all, whereas a subgroup demonstrated tumor growth [13, 14, 51, 52]. Pieterman et al. [13] speculated a multistep process of MEN1 pNET development; tumor initiation and tumor growth are two distinct steps with additional events needed for tumor growth and disease progression rather than germline mutation subtype alone. The effect of MEN1 germline mutation on menin protein may drive tumor initiation but could also be inversely correlated with tumor growth. Genetic or epigenetic events, such as mutations in DAXX or ATRX genes, might play important roles in growth-driving events [13]. This could explain the heterogeneity in incidence and annual growth among included studies. To tailor a follow-up regimen, surveillance programs should focus on identifying the course of disease in patients with NF-pNETs. Frequency of screening should be adapted for the growth rate in an individual, starting with repeated measurements every year after detection of NF-pNETs by EUS or MRI. After confirmation of stability of the tumor, surveillance could be extended to every 1 to 2 years over the course of time. In growing tumors, imaging should be repeated at least every year, and 68Ga-DOTA PET-CT could be added in routine surveillance when tumors >10 mm are present to identify metastasis timely. Future studies should investigate the role of molecular biomarkers, such as the NETest® [62] for MEN1-related NF-pNETs, as current screening tools lack insight in the dynamics of individual tumor behavior.
The starting age of screening young mutation carriers remains controversial. The age-related penetrance for NF-pNETs is low under 20 years (1.8% to 9%) in the larger studies, but cases of large NF-pNETs requiring surgical intervention are reported. Furthermore, penetrance is underestimated in some studies because of the imaging modality used to screen included patients. One study systematically used EUS in young patients, revealing a much higher penetrance of almost 50% [53]. Included studies recommended to start screening between 10 and 16 years [54, 56]. Because of the diversity in studies and outcomes, future research is needed to estimate the optimal age to start screening. In patients who elect not to have genetic testing but are at risk for MEN1, screening for pNETs should start at the same age as mutation carriers, as all manifestation can occur as first manifestation [54, 63].
This study has some limitations. The inclusion criterion of more than five MEN1 patients with (NF-)pNETs in individual studies led to the exclusion of studies reporting on MEN1 patients with NF-pNETs. Regarding the high chance of selection bias for these small studies (less than six NF-pNETs) and subsequent imprecise estimations of diagnostic accuracy measures, exclusion of these studies seems reasonable regarding achievement of unbiased and precise results. Another limitation is the language restriction implemented in our search string. Based on our current understanding of MEN1, most of the available literature is published in the used languages. We assume that no studies were missed using this strategy. Some studies were not available for full text eligibility or did not report outcomes for (NF)-pNETs. No attempts were made to obtain individual patients records from studies not reporting separate outcomes. Lastly, no critical appraisal of studies assessing age-related penetrance was performed.
This systematic review collected additional evidence to substantiate and update the evidence-based approach for NF-pNET screening. Current clinical practice guidelines recommend annual surveillance, whereas recent data illustrate that less aggressive surveillance is reasonable in a substantial number of MEN1 patients. Studies on growth reported very slow rates and two distinct phenotypes in the course of disease with long-term stable disease in a large subgroup. Therefore, we promote a more individualized approach based on the observed growth tendency. Our review of recent literature offers recommendations on the use of biomarkers and imaging modalities (Table 10). Biomarkers should not play a role in the diagnostic process, as accuracies are too low. Studies evaluating the diagnostic value of imaging modalities are heterogeneous with varying risks of bias, and reported outcomes diverge for most modalities. For the detection of NF-pNETs, EUS has the highest sensitivity but also has disadvantages. A combined strategy of EUS and MRI seems to be the most useful with important advantages of MRI over CT. To estimate the growth rate of NF-pNETs, we would advise use of the same imaging modality, as concordance between EUS and MRI is moderate [32]. 68Ga-DOTA PET-CT could be added if NF-pNETs are diagnosed in a patient to identify metastasized disease. The superior diagnostic performance of 68Ga-DOTA PET-CT over SRS makes it the preferred functional imaging modality when available [35]. The optimal age to start screening must yet be determined, as study methods and reported age-related penetrance are varying.
Supplementary Material
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- 11C-5-HTP
11C-5-hydroxytryptophan
- 18F-FDG
18F-fluorodeoxyglucose
- 68Ga
gallium 68 octreotate
- AUC
area under the curve
- CgA
chromogranin A
- DOTA
dodecanetetraacetic acid
- DOTANOC
dodecanetetraacetic acid 1-NaI3-octreotide
- DOTATATE
dodecanetetraacetic acid tyrosine-3-octreotate
- DOTA-TOC
dodecanetetraacetic acid tyrosine-3-octreotide
- dpNET
duodenopancreatic neuroendocrine tumor
- EUS
endoscopic ultrasound
- MEN1
multiple endocrine neoplasia type 1
- NET
neuroendocrine tumor
- NF-pNET
nonfunctional pancreatic neuroendocrine tumor
- PET
positron emission tomography
- PP
pancreatic polypeptide
- PPI
proton-pump inhibitor
- PPV
positive predictive value
- SRS
somatostatin receptor scintigraphy
References and Notes
- 1. Chandrasekharappa SC, Guru SC, Manickam P, Olufemi SE, Collins FS, Emmert-Buck MR, Debelenko LV, Zhuang Z, Lubensky IA, Liotta LA, Crabtree JS, Wang Y, Roe BA, Weisemann J, Boguski MS, Agarwal SK, Kester MB, Kim YS, Heppner C, Dong Q, Spiegel AM, Burns AL, Marx SJ. Positional cloning of the gene for multiple endocrine neoplasia-type 1. Science. 1997;276(5311):404–407. [DOI] [PubMed] [Google Scholar]
- 2. Dreijerink KMA, Goudet P, Burgess JR, Valk GD; International Breast Cancer in MEN1 Study Group . Breast-cancer predisposition in multiple endocrine neoplasia type 1. N Engl J Med. 2014;371(6):583–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Thakker RV, Newey PJ, Walls GV, Bilezikian J, Dralle H, Ebeling PR, Melmed S, Sakurai A, Tonelli F, Brandi ML; Endocrine Society . Clinical practice guidelines for multiple endocrine neoplasia type 1 (MEN1). J Clin Endocrinol Metab. 2012;97(9):2990–3011. [DOI] [PubMed] [Google Scholar]
- 4. Goudet P, Murat A, Binquet C, Cardot-Bauters C, Costa A, Ruszniewski P, Niccoli P, Ménégaux F, Chabrier G, Borson-Chazot F, Tabarin A, Bouchard P, Delemer B, Beckers A, Bonithon-Kopp C. Risk factors and causes of death in MEN1 disease. A GTE (Groupe d’Etude des Tumeurs Endocrines) cohort study among 758 patients. World J Surg. 2010;34(2):249–255. [DOI] [PubMed] [Google Scholar]
- 5. de Laat JM, van der Luijt RB, Pieterman CRC, Oostveen MP, Hermus AR, Dekkers OM, de Herder WW, van der Horst-Schrivers AN, Drent ML, Bisschop PH, Havekes B, Vriens MR, Valk GD. MEN1 redefined, a clinical comparison of mutation-positive and mutation-negative patients. BMC Med. 2016;14(1):182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brandi ML, Gagel RF, Angeli A, Bilezikian JP, Beck-Peccoz P, Bordi C, Conte-Devolx B, Falchetti A, Gheri RG, Libroia A, Lips CJ, Lombardi G, Mannelli M, Pacini F, Ponder BA, Raue F, Skogseid B, Tamburrano G, Thakker RV, Thompson NW, Tomassetti P, Tonelli F, Wells SA Jr, Marx SJ. Guidelines for diagnosis and therapy of MEN type 1 and type 2. J Clin Endocrinol Metab. 2001;86(12):5658–5671. [DOI] [PubMed] [Google Scholar]
- 7. Triponez F, Dosseh D, Goudet P, Cougard P, Bauters C, Murat A, Cadiot G, Niccoli-Sire P, Chayvialle J-A, Calender A, Proye CAG. Epidemiology data on 108 MEN 1 patients from the GTE with isolated nonfunctioning tumors of the pancreas. Ann Surg. 2006;243(2):265–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ito T, Igarashi H, Uehara H, Berna MJ, Jensen RT. Causes of death and prognostic factors in multiple endocrine neoplasia type 1: a prospective study: comparison of 106 MEN1/Zollinger-Ellison syndrome patients with 1613 literature MEN1 patients with or without pancreatic endocrine tumors. Medicine (Baltimore). 2013;92(3):135–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jensen RT, Berna MJ, Bingham DB, Norton JA. Inherited pancreatic endocrine tumor syndromes: advances in molecular pathogenesis, diagnosis, management, and controversies. Cancer. 2008;113(Suppl 7):1807–1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pieterman CRC, Vriens MR, Dreijerink KMA, van der Luijt RB, Valk GD. Care for patients with multiple endocrine neoplasia type 1: the current evidence base. Fam Cancer. 2011;10(1):157–171. [DOI] [PubMed] [Google Scholar]
- 11. Qiu W, Christakis I, Silva A, Bassett RL Jr, Cao L, Meng QH, Gardner Grubbs E, Zhao H, Yao JC, Lee JE, Perrier ND. Utility of chromogranin A, pancreatic polypeptide, glucagon and gastrin in the diagnosis and follow-up of pancreatic neuroendocrine tumours in multiple endocrine neoplasia type 1 patients. Clin Endocrinol (Oxf). 2016;85(3):400–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. de Laat JM, Pieterman CRC, Weijmans M, Hermus AR, Dekkers OM, de Herder WW, van der Horst-Schrivers ANA, Drent ML, Bisschop PH, Havekes B, Vriens MR, Valk GD. Low accuracy of tumor markers for diagnosing pancreatic neuroendocrine tumors in multiple endocrine neoplasia type 1 patients. J Clin Endocrinol Metab. 2013;98(10):4143–4151. [DOI] [PubMed] [Google Scholar]
- 13. Pieterman CRC, de Laat JM, Twisk JWR, van Leeuwaarde RS, de Herder WW, Dreijerink KMA, Hermus ARMM, Dekkers OM, van der Horst-Schrivers ANA, Drent ML, Bisschop PH, Havekes B, Borel Rinkes IHM, Vriens MR, Valk GD. Long-Term Natural Course of Small Nonfunctional Pancreatic Neuroendocrine Tumors in MEN1-Results From the Dutch MEN1 Study Group. J Clin Endocrinol Metab. 2017;102(10):3795–3805. [DOI] [PubMed] [Google Scholar]
- 14. Triponez F, Sadowski SM, Pattou F, Cardot-Bauters C, Mirallié E, Le Bras M, Sebag F, Niccoli P, Deguelte S, Cadiot G, Poncet G, Lifante JC, Borson-Chazot F, Chaffanjon P, Chabre O, Menegaux F, Baudin E, Ruszniewski P, du Boullay H, Goudet P. Long-term Follow-up of MEN1 Patients Who Do Not Have Initial Surgery for Small ≤2 cm Nonfunctioning Pancreatic Neuroendocrine Tumors, an AFCE and GTE study: Association Francophone de Chirurgie Endocrinienne & Groupe d’Etude des Tumeurs Endocrines. Ann Surg. 2018;268(1):158–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Conemans EB, Nell S, Pieterman CRC, de Herder WW, Dekkers OM, Hermus AR, van der Horst-Schrivers AN, Bisschop PH, Havekes B, Drent ML, Vriens MR, Valk GD. Prognostic factors for survival of MEN1 patients with duodenopancreatic tumors metastatic to the liver: results from the DMSG Study Group. Endocr Pract. 2017;23(6):641–648. [DOI] [PubMed] [Google Scholar]
- 16. Manoharan J, Albers MB, Bartsch DK. The future: diagnostic and imaging advances in MEN1 therapeutic approaches and management strategies. Endocr Relat Cancer. 2017;24(10):T209–T225. [DOI] [PubMed] [Google Scholar]
- 17. Ito T, Jensen RT. Imaging in multiple endocrine neoplasia type 1: recent studies show enhanced sensitivities but increased controversies. Int J Endocr Oncol. 2016;3(1):53–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yates CJ, Newey PJ, Thakker RV. Challenges and controversies in management of pancreatic neuroendocrine tumours in patients with MEN1. Lancet Diabetes Endocrinol. 2015;3(11):895–905. [DOI] [PubMed] [Google Scholar]
- 19. Whiting PF, Rutjes AWS, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, Leeflang MMG, Sterne JAC, Bossuyt PMM; QUADAS-2 Group . QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529–536. [DOI] [PubMed] [Google Scholar]
- 20. Hayden JA, van der Windt DA, Cartwright JL, Côté P, Bombardie C. Research and Reporting Methods: annals of internal medicine assessing bias in studies of prognostic factors. Ann Intern Med. 2013;158:280–286. [DOI] [PubMed] [Google Scholar]
- 21. Swiglo BA, Murad MH, Schünemann HJ, Kunz R, Vigersky RA, Guyatt GH, Montori VM. A case for clarity, consistency, and helpfulness: state-of-the-art clinical practice guidelines in endocrinology using the grading of recommendations, assessment, development, and evaluation system. J Clin Endocrinol Metab. 2008;93(3):666–673. [DOI] [PubMed] [Google Scholar]
- 22. Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, Guyatt GH, Harbour RT, Haugh MC, Henry D, Hill S, Jaeschke R, Leng G, Liberati A, Magrini N, Mason J, Middleton P, Mrukowicz J, O’Connell D, Oxman AD, Phillips B, Schünemann HJ, Edejer T, Varonen H, Vist GE, Williams JW Jr, Zaza S; GRADE Working Group . Grading quality of evidence and strength of recommendations. BMJ. 2004;328(7454):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269, W64. [DOI] [PubMed] [Google Scholar]
- 24. Granberg D, Stridsberg M, Seensalu R, Eriksson B, Lundqvist G, Oberg K, Skogseid B. Plasma chromogranin A in patients with multiple endocrine neoplasia type 1. J Clin Endocrinol Metab. 1999;84(8):2712–2717. [DOI] [PubMed] [Google Scholar]
- 25. Nehar D, Lombard-Bohas C, Olivieri S, Claustrat B, Chayvialle JA, Penes MC, Sassolas G, Borson-Chazot F. Interest of chromogranin A for diagnosis and follow-up of endocrine tumours. Clin Endocrinol (Oxf). 2004;60(5):644–652. [DOI] [PubMed] [Google Scholar]
- 26. Peracchi M, Conte D, Gebbia C, Penati C, Pizzinelli S, Arosio M, Corbetta S, Spada A. Plasma chromogranin A in patients with sporadic gastro-entero-pancreatic neuroendocrine tumors or multiple endocrine neoplasia type 1. Eur J Endocrinol. 2003;148(1):39–43. [DOI] [PubMed] [Google Scholar]
- 27. Stridsberg M, Oberg K, Li Q, Engström U, Lundqvist G. Measurements of chromogranin A, chromogranin B (secretogranin I), chromogranin C (secretogranin II) and pancreastatin in plasma and urine from patients with carcinoid tumours and endocrine pancreatic tumours. J Endocrinol. 1995;144(1):49–59. [DOI] [PubMed] [Google Scholar]
- 28. Lewis MA, Thompson GB, Young WF Jr. Preoperative assessment of the pancreas in multiple endocrine neoplasia type 1. World J Surg. 2012;36(6):1375–1381. [DOI] [PubMed] [Google Scholar]
- 29. Mutch MG, Frisella MM, DeBenedetti MK, Doherty GM, Norton JA, Wells SA Jr, Lairmore TC. Pancreatic polypeptide is a useful plasma marker for radiographically evident pancreatic islet cell tumors in patients with multiple endocrine neoplasia type 1. Surgery. 1997;122(6):1012–1019, discussion 1019–1020. [DOI] [PubMed] [Google Scholar]
- 30. Langer P, Wild A, Celik I, Kopp I, Bergenfelz A, Bartsch DK. Prospective controlled trial of a standardized meal stimulation test in the detection of pancreaticoduodenal endocrine tumours in patients with multiple endocrine neoplasia type 1. Br J Surg. 2001;88(10):1403–1407. [DOI] [PubMed] [Google Scholar]
- 31. Tirosh A, Papadakis GZ, Millo C, Sadowski SM, Herscovitch P, Pacak K, Marx SJ, Yang L, Nockel P, Shell J, Green P, Keutgen XM, Patel D, Nilubol N, Kebebew E. Association between neuroendocrine tumors biomarkers and primary tumor site and disease type based on total 68Ga-DOTATATE-Avid tumor volume measurements. Eur J Endocrinol. 2017;176(5):575–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Barbe C, Murat A, Dupas B, Ruszniewski P, Tabarin A, Vullierme M-P, Penfornis A, Rohmer V, Baudin E, Le Rhun M, Gaye D, Marcus C, Cadiot G; Groupe d’étude des Tumeurs Endocrines (GTE) . Magnetic resonance imaging versus endoscopic ultrasonography for the detection of pancreatic tumours in multiple endocrine neoplasia type 1. Dig Liver Dis. 2012;44(3):228–234. [DOI] [PubMed] [Google Scholar]
- 33. Albers MB, Librizzi D, Lopez CL, Manoharan J, Apitzsch JC, Slater EP, Bollmann C, Kann PH, Bartsch DK. Limited value of Ga-68-DOTATOC-PET-CT in routine screening of patients with multiple endocrine neoplasia type 1. World J Surg. 2017;41(6):1521–1527. [DOI] [PubMed] [Google Scholar]
- 34. Langer P, Kann PH, Fendrich V, Richter G, Diehl S, Rothmund M, Bartsch DK. Prospective evaluation of imaging procedures for the detection of pancreaticoduodenal endocrine tumors in patients with multiple endocrine neoplasia type 1. World J Surg. 2004;28(12):1317–1322. [DOI] [PubMed] [Google Scholar]
- 35. Morgat C, Vélayoudom-Céphise F-L, Schwartz P, Guyot M, Gaye D, Vimont D, Schulz J, Mazère J, Nunes M-L, Smith D, Hindié E, Fernandez P, Tabarin A. Evaluation of (68)Ga-DOTA-TOC PET/CT for the detection of duodenopancreatic neuroendocrine tumors in patients with MEN1. Eur J Nucl Med Mol Imaging. 2016;43(7):1258–1266. [DOI] [PubMed] [Google Scholar]
- 36. van Asselt SJ, Brouwers AH, van Dullemen HM, van der Jagt EJ, Bongaerts AHH, Kema IP, Koopmans KP, Valk GD, Timmers HJ, de Herder WW, Feelders RA, Fockens P, Sluiter WJ, de Vries EGE, Links TP. EUS is superior for detection of pancreatic lesions compared with standard imaging in patients with multiple endocrine neoplasia type 1. Gastrointest Endosc. 2015;81(1):159–167.e2. [DOI] [PubMed] [Google Scholar]
- 37. Waldmann J, Fendrich V, Habbe N, Bartsch DK, Slater EP, Kann PH, Rothmund M, Langer P. Screening of patients with multiple endocrine neoplasia type 1 (MEN-1): a critical analysis of its value. World J Surg. 2009;33(6):1208–1218. [DOI] [PubMed] [Google Scholar]
- 38. Yim JH, Siegel BA, DeBenedetti MK, Norton JA, Lairmore TC, Doherty GM. Prospective study of the utility of somatostatin-receptor scintigraphy in the evaluation of patients with multiple endocrine neoplasia type 1. Surgery. 1998;124(6):1037–1042. [DOI] [PubMed] [Google Scholar]
- 39. Goroshi M, Bandgar T, Lila AR, Jadhav SS, Khare S, Shrikhande SV, Uchino S, Dalvi AN, Shah NS. Multiple endocrine neoplasia type 1 syndrome: single centre experience from western India. Fam Cancer. 2016;15(4):617–624. [DOI] [PubMed] [Google Scholar]
- 40. Camera L, Paoletta S, Mollica C, Milone F, Napolitano V, De Luca L, Faggiano A, Colao A, Salvatore M. Screening of pancreaticoduodenal endocrine tumours in patients with MEN 1: multidetector-row computed tomography vs. endoscopic ultrasound. Radiol Med (Torino). 2011;116(4):595–606. [DOI] [PubMed] [Google Scholar]
- 41. Skogseid B, Oberg K, Akerström G, Eriksson B, Westlin JE, Janson ET, Eklöf H, Elvin A, Juhlin C, Rastad J. Limited tumor involvement found at multiple endocrine neoplasia type I pancreatic exploration: can it be predicted by preoperative tumor localization? World J Surg. 1998;22(7):673–677, discussion 667–668. [DOI] [PubMed] [Google Scholar]
- 42. Wamsteker EJ, Gauger PG, Thompson NW, Scheiman JM. EUS detection of pancreatic endocrine tumors in asymptomatic patients with type 1 multiple endocrine neoplasia. Gastrointest Endosc. 2003;58(4):531–535. [DOI] [PubMed] [Google Scholar]
- 43. Gauger PG, Scheiman JM, Wamsteker E-J, Richards ML, Doherty GM, Thompson NW. Role of endoscopic ultrasonography in screening and treatment of pancreatic endocrine tumours in asymptomatic patients with multiple endocrine neoplasia type 1. Br J Surg. 2003;90(6):748–754. [DOI] [PubMed] [Google Scholar]
- 44. Hellman P, Hennings J, Akerström G, Skogseid B. Endoscopic ultrasonography for evaluation of pancreatic tumours in multiple endocrine neoplasia type 1. Br J Surg. 2005;92(12):1508–1512. [DOI] [PubMed] [Google Scholar]
- 45. Lastoria S, Marciello F, Faggiano A, Aloj L, Caracò C, Aurilio M, D’Ambrosio L, Di Gennaro F, Ramundo V, Camera L, De Luca L, Fonti R, Napolitano V, Colao A. Role of (68)Ga-DOTATATE PET/CT in patients with multiple endocrine neoplasia type 1 (MEN1). Endocrine. 2016;52(3):488–494. [DOI] [PubMed] [Google Scholar]
- 46. Kornaczewski Jackson ER, Pointon OP, Bohmer R, Burgess JR. Utility of FDG-PET imaging for risk stratification of pancreatic neuroendocrine tumors in MEN1. J Clin Endocrinol Metab. 2017;102(6):1926–1933. [DOI] [PubMed] [Google Scholar]
- 47. Dʼsouza SL, Elmunzer BJ, Scheiman JM. Long-term follow-up of asymptomatic pancreatic neuroendocrine tumors in multiple endocrine neoplasia type I syndrome. J Clin Gastroenterol. 2014;48(5):458–461. [DOI] [PubMed] [Google Scholar]
- 48. Kann PH, Balakina E, Ivan D, Bartsch DK, Meyer S, Klose K-J, Behr T, Langer P. Natural course of small, asymptomatic neuroendocrine pancreatic tumours in multiple endocrine neoplasia type 1: an endoscopic ultrasound imaging study. Endocr Relat Cancer. 2006;13(4):1195–1202. [DOI] [PubMed] [Google Scholar]
- 49. Kappelle WF, Valk GD, Leenders M, Moons LMG, Bogte A, Siersema PD, Vleggaar FP. Growth rate of small pancreatic neuroendocrine tumors in multiple endocrine neoplasia type 1: results from an endoscopic ultrasound based cohort study. Endoscopy. 2017;49(1):27–34. [DOI] [PubMed] [Google Scholar]
- 50. Triponez F, Sadowski SM, Pattou F, Cardot-Bauters C, Mirallié E, Le Bras M, Sebag F, Niccoli P, Deguelte S, Cadiot G, Poncet G, Lifante J-C, Borson-Chazot F, Chaffanjon P, Chabre O, Menegaux F, Baudin E, Ruszniewski P, Du Boullay H, Goudet P. Long-term Follow-up of MEN1 Patients Who Do Not Have Initial Surgery for Small ≤2 cm Nonfunctioning Pancreatic Neuroendocrine Tumors, an AFCE and GTE Study. Ann Surg. 2018;268(1):158–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Thomas-Marques L, Murat A, Delemer B, Penfornis A, Cardot-Bauters C, Baudin E, Niccoli-Sire P, Levoir D, Choplin H du B, Chabre O, Jovenin N, Cadiot G; Groupe des Tumeurs Endocrines (GTE) . Prospective endoscopic ultrasonographic evaluation of the frequency of nonfunctioning pancreaticoduodenal endocrine tumors in patients with multiple endocrine neoplasia type 1. Am J Gastroenterol. 2006;101(2):266–273. [DOI] [PubMed] [Google Scholar]
- 52. Sakurai A, Katai M, Yamashita K, Mori J, Fukushima Y, Hashizume K. Long-term follow-up of patients with multiple endocrine neoplasia type 1. Endocr J. 2007;54(2):295–302. [DOI] [PubMed] [Google Scholar]
- 53. Gonçalves TD, Toledo RA, Sekiya T, Matuguma SE, Maluf Filho F, Rocha MS, Siqueira SAC, Glezer A, Bronstein MD, Pereira MAA, Jureidini R, Bacchella T, Machado MCC, Toledo SPA, Lourenço DM Jr. Penetrance of functioning and nonfunctioning pancreatic neuroendocrine tumors in multiple endocrine neoplasia type 1 in the second decade of life. J Clin Endocrinol Metab. 2014;99(1):E89–E96. [DOI] [PubMed] [Google Scholar]
- 54. Goudet P, Dalac A, Le Bras M, Cardot-Bauters C, Niccoli P, Lévy-Bohbot N, du Boullay H, Bertagna X, Ruszniewski P, Borson-Chazot F, Vergès B, Sadoul JL, Ménégaux F, Tabarin A, Kühn JM, d’Anella P, Chabre O, Christin-Maitre S, Cadiot G, Binquet C, Delemer B. MEN1 disease occurring before 21 years old: a 160-patient cohort study from the Groupe d’étude des Tumeurs Endocrines. J Clin Endocrinol Metab. 2015;100(4):1568–1577. [DOI] [PubMed] [Google Scholar]
- 55. Machens A, Schaaf L, Karges W, Frank-Raue K, Bartsch DK, Rothmund M, Schneyer U, Goretzki P, Raue F, Dralle H. Age-related penetrance of endocrine tumours in multiple endocrine neoplasia type 1 (MEN1): a multicentre study of 258 gene carriers. Clin Endocrinol (Oxf). 2007;67(4):613–622. [DOI] [PubMed] [Google Scholar]
- 56. Manoharan J, Raue F, Lopez CL, Albers MB, Bollmann C, Fendrich V, Slater EP, Bartsch DK. Is routine screening of young asymptomatic MEN1 patients necessary? World J Surg. 2017;41(8):2026–2032. [DOI] [PubMed] [Google Scholar]
- 57. Newey PJ, Jeyabalan J, Walls GV, Christie PT, Gleeson FV, Gould S, Johnson PRV, Phillips RR, Ryan FJ, Shine B, Bowl MR, Thakker RV. Asymptomatic children with multiple endocrine neoplasia type 1 mutations may harbor nonfunctioning pancreatic neuroendocrine tumors. J Clin Endocrinol Metab. 2009;94(10):3640–3646. [DOI] [PubMed] [Google Scholar]
- 58. Casey RT, Saunders D, Challis BG, Pitfield D, Cheow H, Shaw A, Simpson HL. Radiological surveillance in multiple endocrine neoplasia type 1: a double-edged sword? Endocr Connect. 2017;6(3):151–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kann PH, Kann B, Fassbender WJ, Forst T, Bartsch DK, Langer P. Small neuroendocrine pancreatic tumors in multiple endocrine neoplasia type 1 (MEN1): least significant change of tumor diameter as determined by endoscopic ultrasound (EUS) imaging. Exp Clin Endocrinol Diabetes. 2006;114(7):361–365. [DOI] [PubMed] [Google Scholar]
- 60. Froeling V, Röttgen R, Collettini F, Rothe J, Hamm B, Brenner W, Schreiter N. Detection of pancreatic neuroendocrine tumors (PNET) using semi-quantitative [68Ga]DOTATOC PET in combination with multiphase contrast-enhanced CT. Q J Nucl Med Mol Imaging. 2014;58(3):310–318. [PubMed] [Google Scholar]
- 61. Sadowski SM, Millo C, Cottle-Delisle C, Merkel R, Yang LA, Herscovitch P, Pacak K, Simonds WF, Marx SJ, Kebebew E. Results of (68)gallium-DOTATATE PET/CT scanning in patients with multiple endocrine neoplasia type 1. J Am Coll Surg. 2015;221(2):509–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Modlin IM, Drozdov I, Kidd M. The identification of gut neuroendocrine tumor disease by multiple synchronous transcript analysis in blood. PLoS One 2013;8(5):e63364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Pieterman CR, Schreinemakers JM, Koppeschaar HP, Vriens MR, Rinkes IH, Zonnenberg BA, van der Luijt RB, Valk GD. Multiple endocrine neoplasia type 1 (MEN1): its manifestations and effect of genetic screening on clinical outcome. Clin. Endocrinol (Oxf). 2009;70(4):575–581. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.