Abstract
Background:
Current periodontal disease taxonomies have limited utility for predicting disease progression and tooth loss; in fact, tooth loss itself can undermine precise person-level periodontal disease classifications. To overcome this limitation, the current group recently introduced a novel patient stratification system using latent class analyses of clinical parameters, including patterns of missing teeth. This investigation sought to determine the clinical utility of the Periodontal Profile Classes and Tooth Profile Classes (PPC/TPC) taxonomy for risk assessment, specifically for predicting periodontal disease progression and incident tooth loss.
Methods:
The analytic sample comprised 4,682 adult participants of two prospective cohort studies (Dental Atherosclerosis Risk in Communities Study and Piedmont Dental Study) with information on periodontal disease progression and incident tooth loss. The PPC/TPC taxonomy includes seven distinct PPCs (person-level disease pattern and severity) and seven TPCs (tooth-level disease). Logistic regression modeling was used to estimate relative risks (RR) and 95% confidence intervals (CI) for the association of these latent classes with disease progression and incident tooth loss, adjusting for examination center, race, sex, age, diabetes, and smoking. To obtain personalized outcome propensities, risk estimates associated with each participant’s PPC and TPC were combined into person-level composite risk scores (Index of Periodontal Risk [IPR]).
Results:
Individuals in two PPCs (PPC-G: Severe Disease and PPC-D: Tooth Loss) had the highest tooth loss risk (RR = 3.6; 95% CI = 2.6 to 5.0 and RR = 3.8; 95% CI = 2.9 to 5.1, respectively). PPC-G also had the highest risk for periodontitis progression (RR = 5.7; 95% CI = 2.2 to 14.7). Personalized IPR scores were positively associated with both periodontitis progression and tooth loss.
Conclusions:
These findings, upon additional validation, suggest that the periodontal/tooth profile classes and the derived personalized propensity scores provide clinical periodontal definitions that reflect disease patterns in the population and offer a useful system for patient stratification that is predictive for disease progression and tooth loss.
Keywords: Diagnosis, epidemiology, oral medicine, periodontal medicine, periodontitis, prognosis
The recently introduced concept of precision medicine offers a new vision for the prevention and treatment of disease, as well as for biomedical research.1 Along the lines of the “personalized medicine” paradigm, precision medicine entails prevention and treatment strategies that take individual variability into account.1 A complete account of environmental and innate influences of disease susceptibility is a daunting task—nevertheless, recent advances in the biomedical sciences have made possible the comprehensive characterization of individuals’ genomes, transcriptomes, proteomes, and metabolomes, and the superimposition of this “panomics” information with detailed health and disease endpoints.2 There is promise that “precision dentistry” will emerge from this new wave of systems biology and big data-driven science and practice and will bring about meaningful improvements in the health of individuals and populations.3,4
Recent efforts in periodontal medicine have built upon the principles of precision medicine to refine periodontal health and disease classifications and to dissect the biologic basis of disease susceptibility, with the ultimate goal of tailoring or targeting prevention and treatment strategies.5 Along these lines, the development of a precise stratification system that reflects distinct periodontal disease patterns can serve as the basis for precise risk assessment (at the population level) and estimation of individual susceptibilities (at the person level), with disease progression and tooth loss being the main endpoints of interest. However, current periodontal disease taxonomies have limited utility for predicting disease progression and tooth loss; in fact, tooth loss itself can undermine precise person-level periodontal disease classifications.
To date, periodontal disease risk assessment tools have used clinical measurements and known risk factors to predict tooth loss or periodontal disease progression with the goal of establishing more specific prognoses and to optimize treatment choices.6–11 Although some prediction models incorporate well-established risk factors, such as smoking history, diabetes, age, race, and sex, there are currently no validated risk assessment tools utilizing clinical parameters including tooth-specific patterns. Most models utilize subject-level summary variables of clinical parameters, such as mean or extent scores for various signs of disease including plaque scores, gingival indices, probing depths, and clinical attachment levels, that reflect subject-level disease and are not always linked to tooth type or tooth loss patterns. Most prediction models do not explicitly classify missing teeth, which can be lost for a variety of reasons, and are not informed by existing tooth loss patterns when considering risk of natural disease progression, which has important prognostic value.12 Individual tooth-specific measures of crown-to-root ratios, mobility, tooth position, and other factors can be used to improve estimates of individual tooth-level prognoses, but irrespective of the model, the final estimates of risk are qualitative in nature based upon clinical impressions. In sum, no predictive models exist that provide quantitative tooth-based risk estimates and account for the informative heterogeneity in clinical presentation by patterns of tooth loss. These elements are critical in the realm of precision dentistry, and they are necessary for informing personalized periodontal, restorative, and prosthodontics plans of care.
To overcome these limitations, our group recently developed a novel periodontitis stratification system, University of North Carolina-Periodontal Profile Class (UNC-PPC), based on latent class analyses of clinical parameters, including patterns of missing teeth. In a previous publication, we demonstrated how multiple clinical characteristics can be used to identify and stratify clinically distinct periodontal and tooth profile classes that incorporate these tooth loss patterns.13 The aim of the present investigation was to determine the clinical utility of the UNC-PPC taxonomy for risk assessment and “precision periodontal medicine,” specifically, for predicting periodontal disease progression and incident tooth loss.
1 |. MATERIALS AND METHODS
1.1 |. Study populations
The analytic sample comprised 4,682 adult participants of two prospective cohort studies (Dental Atherosclerosis Risk in Communities Study [DARIC] and Piedmont Dental Study [PDS]) with information on periodontal disease progression and incident tooth loss. All participants provided written informed consent to a protocol that was reviewed and approved by the Institutional Review Board on research involving human subjects at the University of North Carolina and/or at each study performance site.
The DARIC sample was recruited from the ARIC population study and included dentate participants who did not have contraindications for periodontal probing.14 The entire DARIC study comprised 6,793 dentate individuals living in four United States communities who received a baseline dental and periodontal examination between 1996 and 1998. In 2012 to 2013, DARIC participants were asked via follow-up calls to assess their tooth loss in the previous 10 years (“Have you lost any teeth in the past ten years?”). Their answers were categorized as none, one or two, three or more, and don’t know.15
The PDS was based on a stratified, random, clustered sample of all people aged >65 years in the five adjacent counties in the Piedmont area of North Carolina.16 The longitudinal study began in 1988 with a random subsample of 697 dentate individuals. Additional study design and population characteristics are described in detail in previous publications.17,18
1.2 |. Calculation of index of periodontal classes (IPC) as risk score
We used the DARIC 10-year tooth loss data to compute the risk of tooth loss for each tooth profile class (TPC) within each periodontal profile class (PPC) assignment. A composite risk score for each individual was then calculated based on tooth loss risks; this continuous score (ranging theoretically between 0 and 100) is referred to as the Index of Periodontal Risk (IPR). The analytic approach used to calculate IPR was based on a 7 × 7 table (PPC × TPC) of predicted probabilities for 10-year tooth loss. First, each participant was assigned to one of the seven PPCs; then, each tooth was classified to one of the seven TPCs. The IPR was calculated as the mean predicted probability for 10-year tooth loss across all teeth present for each individual. The development of the IPR included traditional risk factors for periodontal disease, such as age, sex, race, diabetes, and smoking status. We used information from the DARIC dataset to develop an IPR adjustment score for each of these traditional risk factors. Later, we validated IPR using the longitudinal PDS dataset.
1.3 |. Statistical analyses
Logistic regression models adjusting for examination center, race, sex, age, diabetes, and smoking were used to quantify the association of the seven PPCs and the seven TPCs with tooth loss (DARIC and PDS datasets) and periodontitis progression (PDS dataset) by obtaining corresponding relative risk (RR) estimates and 95% confidence intervals (CI). Generalized lineal model was used to compute RR. Similar models were developed using the Center for Disease Control/American Academy of Periodontology (CDC/AAP) definition of periodontal disease,19 to allow the contrast of tooth loss and periodontitis estimates of association with the ones obtained from PPC/TPC-based analyses. The PDS estimates for tooth loss and attachment loss data have been weighted to represent the population in the five counties and account for the design effect of the sample. Also, P values were obtained through methods that account for such design effects.20
Predicted probabilities and 95% CI were computed to estimate the risk for tooth loss across the observed range of IPR scores using the DARIC dataset. Effect estimates (beta coefficients) for race, age, sex, diabetes, and smoking status were also computed in the DARIC dataset. This IPR-developed model was subsequently applied to the PDS longitudinal dataset for validation. Predicted probabilities were calculated to estimate the risk for tooth loss, periodontitis progression, and incidence of edentulism in the PDS. Periodontitis progression was defined as >10% of sites exhibiting >3 mm attachment loss in a 3-year period. To evaluate the sensitivity and specificity of the predicted probability model, a receiver operator curve (ROC)21 and C-statistic22 were calculated for each predicted model. IPR thresholds for defining risk categories as IPC low, moderate, and high were identified using the Bayesian Information Criterion (BIC)23 and confirmed with Classification and Regression Trees (CART).24
2 |. RESULTS
The demographic and clinical characteristics of the study participants as well the seven PPCs were reported in an earlier publication.13 There were significant differences between the PPC groups with regard to race, sex, age, diabetes, smoking (history and pack/years), obesity, access to dental care, socioeconomic status, and educational level.
2.1 |. Risk models for tooth loss and periodontal disease progression by periodontal profile class
The DARIC dataset was originally used to derive the PPC classification,13 and in this study it was used to estimate associations with incident tooth loss and periodontal disease progression (i.e., clinical attachment loss). The PDS dataset was used as an independent validation dataset. Table 1 presents the RR and 95% CI for a person losing >three teeth during a 10-year period for the DARIC sample, stratified by PPC assignment and CDC/AAP disease definition. Estimates from both crude and fully adjusted (for race, sex, age, diabetes, and smoking status) models are presented. Individuals assigned to PPC-D and PPC-G had the highest tooth loss risk: RR = 3.8 (95% CI = 2.9 to 5.1) and 3.6 (2.6 to 5.0), respectively. These estimates, derived from the UNC-PPC system demonstrated a stronger association with tooth loss compared with the CDC/AAP severe disease classification (RR = 2.8; 95% CI = 2.0 to 4.0).
TABLE 1.
Relative risk and 95% confidence intervals for incident loss of ≥three teeth during 10-year period (n = 3,985) among DARIC participants stratified by PPC and CDC/AAP classification
| Classification | Description | Crude Model | Adjusted Modela |
|---|---|---|---|
| PPC-A | Health | Ref | Ref |
| PPC-B | Mild Disease | 2.10(1.53–2.87) | 1.84 (1.34–2.53) |
| PPC-C | High GI Scores | 3.84 (2.82–5.23) | 2.15 (1.51–3.05) |
| PPC-D | Tooth Loss | 4.91 (3.71–6.51) | 3.83 (2.87–5.12) |
| PPC-E | Posterior Disease | 2.89 (2.15–3.90) | 2.72 (2.01–3.69) |
| PPC-F | Severe Tooth Loss | 3.32 (2.45–4.50) | 2.29 (1.66–3.15) |
| PPC-G | Severe Disease | 5.91 (4.39–7.96) | 3.62 (2.61–5.01) |
| CDC/AAP | Health | Ref | Ref |
| CDC/AAP | Mild | 1.24 (0.88–1.75) | 1.13 (0.78–1.61) |
| CDC/AAP | Moderate | 1.90 (1.37–2.62) | 1.72 (1.22–2.42) |
| CDC/AAP | Severe | 3.65 (2.63–5.05) | 2.82 (1.97–4.04) |
DARIC, Dental Atherosclerosis in Communities Study; PPC, periodontal profile class; CDC/AAP, Center for Disease Control/American Academy ofPeriodontology; GI, gingival inflammation.
Adjusted for examination center, race/center, sex, age, diabetes, smoking (five levels.)
Five-year tooth loss risk estimates (>two and >three teeth) are presented in Table 2. Similarly, Table 2 presents periodontal disease progression (3-year attachment loss of >3 mm in >10% of sites in the PDS) risk estimates according to the UNC-PPC system and the CDC/AAP definition. Individuals assigned to PPC-D, -E, and -G classes showed the highest risk of losing >two teeth, with corresponding estimates of RR = 3.4 (95% CI = 1.8 to 6.6), 3.7 (1.4 to 9.7), and 3.6 (1.7 to 7.4), respectively. As expected, risk estimates for attachment loss were the highest among PPCs associated with disease. For example, for attachment loss, PPC-G had the highest RR at 5.7 (95% CI = 2.2 to 14.7) followed by PPC-D with RR = 4.2 (95% CI = 1.7 to 10.1) and PPC-F with RR = 3.0 (95% CI = 1.1 to 8.0). In contrast, the severe disease CDC/AAP group had RR = 3.8 (95% CI = 1.9 to 7.8). These results highlight the higher risks of attachment loss associated with PPC-D and PPC-G assignment compared with the CDC/AAP severe category, even after adjustments for race,sex, age, diabetes, and smoking status. These findings support the utility of the PPC method to identify subjectswith elevated disease progression risk in an independent sample (PDS).
TABLE 2.
Adjusted* relative risk and 95% confidence interval for losing ≥two and ≥three teeth during 5-year period and 10% of sites with 3+mm attachment loss increase (n = 363) during 3-year period for PDS population stratified by PPC and CDC/AAP classification
| Class | Description | Tooth loss ≥2 Teeth during 5 years |
Tooth loss ≥ Three teeth during 5 years |
Attachment loss 10% of sites with ≥3 mm AL increase |
|---|---|---|---|---|
| PPC | ||||
| PPC-A | Health | Ref | Ref | Ref |
| PPC-B | Mild Disease | 2.92(1.34–6.38) | 3.21 (1.06–9.77) | 1.13 (0.20–6.39) |
| PPC-C | High GI Scores | 2.68 (1.38–5.19) | 3.50 (1.40–8.74) | 2.17 (0.86–5.49) |
| PPC-D | Tooth Loss | 3.45 (1.80–6.63) | 4.45 (1.79–11.1) | 4.22(1.76–10.1) |
| PPC-E | Posterior Disease | 3.74(1.43–9.79) | 3.68 (0.84–16.2) | 2.56 (0.23–28.3) |
| PPC-F | Severe Tooth Loss | 3.23 (1.66–6.28) | 4.20 (1.68–10.5) | 3.06 (1.17–8.01) |
| PPC-G | Severe Disease | 3.63 (1.78–7.41) | 5.51 (2.11–14.4) | 5.71 (2.22–14.7) |
| CDC/AAP | ||||
| CDC/AAP | Health | Ref | Ref | Ref |
| CDC/AAP | Mild | 0.74 (0.45–1.20) | 0.66 (0.31–1.39) | 0.71 (0.27–1.91) |
| CDC/AAP | Moderate | 1.09 (0.76–1.56) | 1.05 (0.60–1.82) | 1.41 (0.69–2.87) |
| CDC/AAP | Severe | 1.72(1.20–2.47) | 2.30 (1.34–3.96) | 3.88 (1.93–7.84) |
The estimates were weighted for study design.
PDS, Piedmont Dental Study; PPC, periodontal profile class; CDC/AAP, Center for Disease Control/American Academy of Periodontology; GI, gingival inflammation.
Adjusted for race, sex, age, diabetes, and smoking.
2.2 |. Risk models for tooth loss and periodontal disease progression by tooth profile class
Tooth loss and disease progression risk estimates in the PDS sample according to TPC are presented in Table 3. Evidently, teeth classified under the TPC associated with periodontal disease showed higher risk for loss. TPC-G had the highest tooth loss risk RR = 3.9 (95% CI = 3.4 to 4.7), followed by TPCF: RR = 2.5 (95% CI = 2.1 to 2.9) and TPC-E: RR = 2.3 (95% CI = 1.9 to 3.0). Attachment loss estimates were significantly higher for TPC-B (recession), TPC-E (interproximal periodontal disease), and TPC-G (severe periodontal disease) compared with TPC-A (health).
TABLE 3.
Tooth-level adjusted* relative risk and 95% confidence interval stratified by TPC for observed 5-year tooth loss and attachment loss increase (≥3 mm increase) for PDS dataset
| Tooth profile class | Description | Tooth loss | Attachment loss increase (≥3 mm) |
|---|---|---|---|
| TPC-A | Health | Ref | Ref |
| TPC-B | Recession | 1.24(1.00–1.55) | 1.56 (1.40–1.77) |
| TPC-C | Crown | 0.85 (0.62–1.14) | 0.49 (0.38–0.65) |
| TPC-D | Gingival Inflammation | 2.02(1.69–2.41) | 1.36 (1.19–1.56) |
| TPC-E | Interproximal Disease | 2.34(1.89–3.02) | 1.77(1.49–2.10) |
| TPC-F | Diminished Periodontium | 2.50 (2.14–2.92) | 1.44(1.28–1.63) |
| TPC-G | Severe Disease | 3.95 (3.35–4.66) | 1.73 (1.46–2.04) |
TPC, tooth profile class; PDS, Piedmont Dental Study.
Adjusted for race, age, sex, diabetes and smoking
2.3 |. Predicted probability of 10-year tooth loss stratified by PPC and TPC
The tooth-level risk scores, which are computed probabilities for 10-year tooth loss (≥three teeth) using the DARIC dataset are shown in Figure 1 as a 7 × 7 table (PPC/TPC). For example, periodontally healthy teeth in a periodontallyhealthy person (TPC-A/PPC-A) had a pooled 4.5% probability of belonging to a personwho lost ≥three teeth during 10 years. As expected, healthy teeth (TPC-A) had lower risk than diseased teeth (TPC-G) to be in a person with periodontal disease (PPC-G). Periodontally healthy teeth in a participant with severe disease (TPC-A/PPC-G) had an increased probability to be lost (20.2%). In contrast, a severely diseased tooth in a person with severe disease (TPC-G/PPC-G) had 45.8% probability of being lost (Figure 1). As mentioned earlier, these pooled-risk tooth-level scores stratified by PPC were averaged across teeth. For example, if a PPC-A (health) person had all teeth classified as TPC-A (health), the IPR would be 4.5. Similarly, a person classified as PPC-G (severe disease) with all teeth classified as TPC-G (severe disease) would have an IPR score of 45.8. A clinical example of how to calculate the IPR for a hypothetic PPC-E person having teeth representing multiple TPC classes is presented in supplementary Figure 1 in the online Journal of Periodontology. The person-level IPR score is a composite risk score based on the DARIC dataset that is unadjusted for traditional person-based risk factors. Supplementary Table 1 in the online Journal of Periodontology provides a numeric adjustment of the IPR score that accounts for the effect of age, race, sex, diabetes, and smoking. Thus, a male 65-year-old subject who is a current smoker will have an IPR score that is increased by eight points, based upon these risk factors (3 + 5 + 0) using estimates described under Methods.
FIGURE 1.
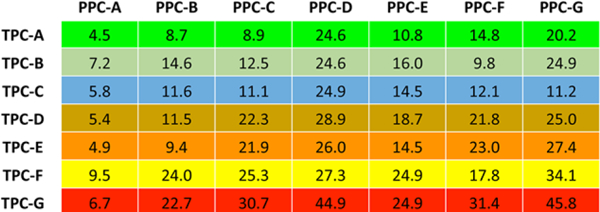
Computed probabilities presented as percentage for 10-year tooth loss (≥three teeth) using the DARIC dataset stratified by Periodontal Profile Classes (PPC) and Tooth Profile Classes (TPC). DARIC, Dental Atherosclerosis in Communities Study
The association of the IPR score and 10-year tooth loss (≥three teeth) in theDARIC dataset is illustrated in Figure 2A. The validation of IPR as a predictor of tooth loss was done using PDS 5-year tooth loss (≥three teeth) data and is shown in Figure 2C. For example, the predicted probability of tooth loss with an IPR of 20, based on the DARIC dataset, is 19%. The observed probability in the PDS dataset for an IPC of 20 was 23%. The predicted probability of disease progression in 3 years and incident edentulism using the PDS dataset appear in Figures 2E and 2G, respectively. Similar to the risk for tooth loss, the IPR score is positively associated with periodontitis progression and edentulism. The C-statistics for all predicted estimates are shown in Figure 2. The C-statistics for predicted tooth loss for both DARIC and PDS was 0.72 (Figure 2B and 2D). The C-statistic for attachment loss for the PDS dataset was 0.75 (Figure 2F). A detailed presentation of predicted probabilities for tooth loss according to IPR scores can be found in supplementary Table S2 in the online Journal of Periodontology.
FIGURE 2.
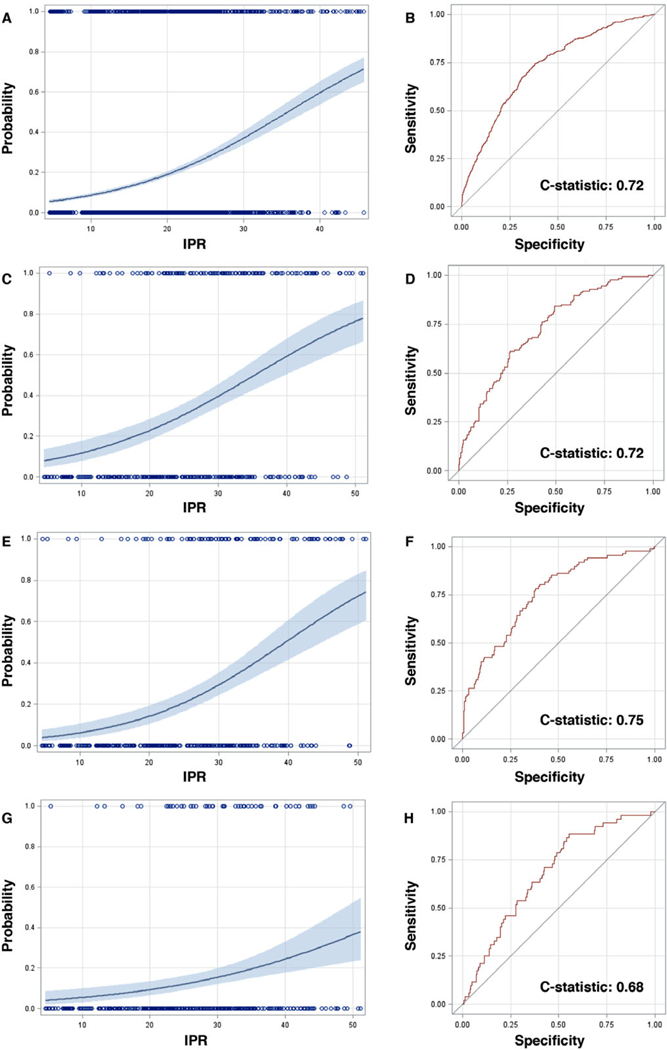
Predicted probability for the Index of Periodontal Risk (IPR) score associated with tooth loss, attachment loss, and edentulism. A) Association of the IPR score and 10-year tooth loss (≥three teeth) in the DARIC dataset. B) Receiver Operator Curve (ROC) and C-statistic for 10-year tooth loss (≥three teeth) in the DARIC population. C) Association of the IPR score and 5-year tooth loss (≥three teeth) in the Piedmont Dental Study (PDS) dataset. D) ROC and C-statistic for 5-year tooth loss (≥three teeth) in the PDS dataset. E) Association of the IPR score and 3-year attachment loss in the PDS dataset. F) ROC and C-statistic for 3-year attachment loss in the PDS dataset. G) Association of the IPR score and edentulism in the PDS dataset. H) ROC and C-statistic for edentulism in the PDS dataset. DARIC, Dental Atherosclerosis in Communities Study
2.4 |. Risk models for tooth loss and periodontal disease progression by IPC
Tooth loss and disease progression risk estimates based on classes developed from specific IPR cut-points are presented in Table 4. Rather than defining these from percentiles (e.g., quartiles) of IPR values, which are distribution-based, we used CART methods24 to select optimal cut-points to establish three levels or classes of risk for tooth loss: IPC-low (0 to 10), IPC-moderate (11 to 20), and IPC-high (>20). The DARIC dataset demonstrated a significantly higher RR (CI) for tooth loss for both moderate (RR = 2.4, 95% CI = 1.8 to 3.1) and high (RR = 5.6, 95% CI = 3.5 to 5.9) relative to low. Similar estimates were found in the PDS dataset. Using IPC-low as a reference, IPC-moderate showed a 200% increased risk (RR = 3.2, 95% CI = 0.8 to 12.1) for tooth loss, while IPC-high had RR = 5.8 (95% CI = 1.6 to 20.7). Estimates of attachment loss of >3 mm risk in the PDS dataset were low for IPC-moderate: RR = 0.6 (95% CI = 0.2 to 2.2) and substantially higher for IPC-high: RR = 2.5 (95% CI = 0.9 to 6.5). Attachment loss estimates were not calculated in DARIC because there are no available longitudinal data for attachment loss. Of note, exploratory adjustment for dental caries did not influence the risk estimates obtained for either tooth loss or periodontal disease progression (i.e., attachment loss).
TABLE 4.
Relative risk and 95% confidence interval for losing >three teeth during 5-year period and 10% of sites with ≥3 mm attachment loss increase (n = 363) during 3-year period for the PDS dataset and for 10-year tooth loss (≥three teeth) for DARIC dataset stratified by IPC
| PDS Dataset | Tooth Loss | Attachment Loss |
|---|---|---|
| IPC Low (0–10) | Ref | Ref |
| IPC Moderate (11–20) | 3.21 (0.85–12.1) | 0.69 (0.21–2.28) |
| IPC High (> 20) | 5.83 (1.64–20.7) | 2.51 (0.96–6.58) |
| DARIC Dataset |
Tooth Loss (≥three teeth) |
|
| IPC Low (0–10) | Ref | |
| IPC Moderate (11–20) | 2.36 (1.81–3.07) | |
| IPC High (> 20) | 5.55 (3.50–5.92) |
The PDS estimates were weighted for study design.
PDS, Piedmont Dental Study; DARIC, Dental Atherosclerosis in Communities Study; IPC, index of profile classes.
3 |. DISCUSSION
The development and application of the IPR, as a means to inform precise periodontal disease risk assessment, prevention, and therapy, is presented. The IPR is based upon the novel patient stratification system for periodontal disease classification, UNC-PPC/TPC, to provide summary estimates for tooth loss risk and periodontitis progression. This study used two independent population-based cohort samples with more than 4,500 participants and demonstrated that the IPR and its derived classes (IPC-low, -moderate, and -severe) offer substantial gains in precise classification and accurate estimation of prospectively assessed disease endpoints, such as tooth loss and disease progression, compared with existing taxonomies of disease.
There are several strategic advantages of the proposed UNC-PPC/TPC classification that were previously described by our group.13 The IPR was generated based on specific PCC/TPC classifications using the DARIC 10-year tooth loss data. For example, Figure 1 shows that a severely disease tooth (TPC-G) has a probability of 6.7% for tooth loss when found in a PPC-A (health) individual. This probability increases to 45.8% in a PPC-G (severe disease) individual. The IPR simply computes a composite probability based upon the individual’s PPC category and TPC values determined by the specific clinical status of the teeth present. In this study, we demonstrated the application of the IPR score in a manner that offers quantitative assessments for attachment and tooth-loss risk rather than broadly defined and overlapping categories of prognostic terms, such as good, fair, questionable, unfavorable, poor, or hopeless. A strength of this study is that this risk tool was developed using the DARIC cohort of 3,985 individuals and validated using the PDS longitudinal study (n = 697), representing a total of 4,682 individuals. The validation in the PDS dataset was important because it had a different composition of age, sex, and race. The IPR score also accommodates the well-established risk factors, i.e., smoking, diabetes, age, sex, and race, into the risk models with effect sizes that are comparable to previous publications.6,8,25 Additionally, this method can be extended to make specific risk inferences at the tooth-type level.
In this study, we demonstrate that the UNC-PPC system classification enables a more detailed and precise stratification for risk assessment and clinical outcome prediction than the CDC/AAP classification. Admittedly, the CDC/AAP classification was developed for epidemiologic surveys rather than risk assessment, but it has been widely used for this purpose. The World Workshop classification is based on presence of attachment/bone loss that reflects history of disease,26 which is relatively insensitive to changes in person-level factors, tooth loss, or disease activity, but these factors are widely used in healthcare settings. The PPC classification system is similarly insensitive to change with time, either in response to treatment or in the natural history of disease. However, treatment or disease progression does influence the TPC values and therefore can modify the IPR score. This characteristic makes the IPR score useful for clinicians as a means for monitoring and illustrating individual patients’ disease and specific outcome (e.g., tooth loss) propensity. Once treatment is provided, the IPR score can change as it relates directly to risk for attachment and tooth loss. In our opinion, this is a more valid outcome measure and of greater utility than, for example, changes in mean probing depths and bleeding scores. The utility of the IPR score as a measure of clinical outcomes in response to therapy will be explored and presented in future publications. The IPR score incorporates a large set of tooth-specific periodontal clinical indicators, but it also includes information on tooth-specific coronal and root caries that appear to result in excess risk for tooth loss. Nevertheless, exclusion of caries scores did not change the risk estimates obtained for attachment loss.
In a previous publication, the current authors demonstrated that the Latent Class Analysis (LCA) model grouped individuals into separate clinical phenotypes that would be collapsed (or hidden) under the CDC/AAP classification.13 For example, 46% of individuals in PPC-D (tooth loss) were classified with moderate periodontal disease (CDC/AAP). The PPC-D class has sites with periodontal disease, but these individuals have also lost about half of their dentition (mean 16.8 teeth present). Nonetheless, here it is demonstrated (Table 1) that the adjusted relative risk for losing >three teeth among individuals in that group was 3.8, more than double compared with the CDC/AAP moderate level of disease category (RR = 1.7). This is an emphatic demonstration of the gains in precise risk assessment that can be achieved by defining periodontal disease classes that accommodate missing teeth.
The PDS included tooth status assessment over time that enabled us to measure rates of tooth loss and periodontitis progression as shown in Tables 2 and 3. We defined periodontitis progression as a minimum of 10% of sites with >3 mm of attachment loss within 3 years. This is a very stringent definition of disease progression. We found that PPC-G (severe disease) had a very high relative risk (RR = 5.7) to experience periodontitis progression compared with periodontally healthy individuals. Also, PPC-D demonstrated a significantly higher adjusted RR for periodontitis progression. Considering that the majority of PPC-D (tooth loss) individuals were classified into the mild/moderate CDC/AAP disease category,13 a large proportion of individuals would be unable to receive appropriate preventive care due to the underestimation of risk attributed to the CDC/AAP classification. Interestingly, it was found that TPC-C (crown) assignment was protective against attachment loss and had a similar, yet non-significant tooth loss prevention effect (Table 3). This is likely due to the TPC clustering of these teeth within individuals who had disposable income and were willing to spend it for restorative dental care.
The predicted values for tooth loss based upon the composite IPR score by individuals in the DARIC dataset is closely reproduced in the PDS dataset. The predicted probabilities of attachment loss for the PDS dataset also demonstrate a similar pattern. For attachment and tooth loss the ROC reflected in the C-statistic were > 70%, which is considered strong for a single clinical score.22 The key to the utility of IPC is based on the incorporation of the longitudinal data in the 7 × 7 table (Figure 1). The overall precision of the model and the granularity at a tooth level of establishing prognosis could be improved with the addition of more prospective clinical data that could refine the scoring. This method fulfills the requirement of generating a “learning algorithm” that could improve prediction with more data. For example, with more individuals in the dataset, the risk for tooth and attachment loss for a second maxillary molar in a specific PPC class individual could be quantified.
Potential limitations in our study include the mean age of the DARIC and the PDS populations, 62 and 73 years, respectively; thus, the model was developed among older adults. Nevertheless, in a previous publication, it appears that the model performs well among younger populations, as in the two NHANES samples (NHANES 2009–2010: mean age 51 years [range 30 to 80 years]; NHANES 2011–2012: mean age 52 years [range 30 to 80 years]).13 A second limitation of the LCA method lies in its “analytic sophistication,” in that it requires the application of a statistical algorithm for class assignment rather than simple rules associated with specific periodontal measures. To overcome this shortcoming, the algorithm could be easily and efficiently made available via a web-based application, and then made widely available for analyses and patient class assignment. Regarding individual misclassification, the model demonstrates a relative consistency on correctly assigning individuals into classes even with some clinical parameters completely missing.13 More importantly, the model assigns individuals to a single, mutually exclusively periodontal profile class. It is important to clarify that the model was based on self-reported tooth loss data from the DARIC dataset. However, the longitudinal PDS dataset was used for model validation and demonstrated consistency in results.
4 |. CONCLUSIONS
This study demonstrates the clinical application and utility of the UNC-PPC/TPC system, as well as its derived risk score and risk classes (IPR/IPC), for patient stratification, risk assessment, and personalized outcome propensity estimation. To develop precision dentistry models we need to create large datasets that harmonize clinical, biomarker, and genetic data. This method has clear application for using clinical data to assign an individual to a diagnostic category and assign a person-level risk score. It will also enable the creation of harmonized datasets that have clinical information for precision dentistry model development. This newly developed system, upon additional validation, can inform and improve patient care decisions and outcomes, consistent with the vision of precision periodontal medicine.
Supplementary Material
ACKNOWLEDGMENTS
The authors declare that there are no conflicts of interest in this study. This study was funded by the National Institute of Dental and Craniofacial Research R01-DE021418. Thiago Morelli was funded by the National Institute of Dental and Craniofacial Research K23-DE025093. Kimon Divaris was funded by the National Institute of Dental and Craniofacial Research U01-DE025046.
Funding information
National Institute ofDental and Craniofacial Research, Grant/Award Numbers: R01-DE021418, K23-DE025093, U01-DE025046
REFERENCES
- 1.Collins FS, Varmus H. A new initiative on precision medicine. N Engl JMed. 2015;372:793–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Voros S, Maurovich-Horvat P, Marvasty IB, et al. Precision phe-notyping, panomics, and system-level bioinformatics to delineate complex biologies of atherosclerosis: rationale and design of the “genetic loci and the burden of atherosclerotic lesions” study. J Car-diovasc Comput Tomogr. 2014;8:442–451. [DOI] [PubMed] [Google Scholar]
- 3.Sankar PL, Parker LS. The precision medicine initiative’s all of us research program: an agenda for research on its ethical, legal, and social issues. Genet Med. 2017;19:743–750. [DOI] [PubMed] [Google Scholar]
- 4.Kusiak JW, Somerman M. Data science at the national institute of dental and craniofacial research: changing dental practice. J Am Dent Assoc. 2016;147:597–599. [DOI] [PubMed] [Google Scholar]
- 5.Offenbacher S, Divaris K, Barros SP, et al. Genome-wide association study of biologically informed periodontal complex traits offers novel insights into the genetic basis of periodontal disease. Hum Mol Genet. 2016;25:2113–2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lang NP, Tonetti MS. Periodontal risk assessment (pra) for patients in supportive periodontal therapy (spt). Oral Health Prev Dent. 2003;1:7–16. [PubMed] [Google Scholar]
- 7.Chandra RV. Evaluation of a novel periodontal risk assessment model in patients presenting for dental care. Oral Health Prev Dent. 2007;5:39–48. [PubMed] [Google Scholar]
- 8.Page RC, Krall EA, Martin J, Mancl L, Garcia RI. Validity and accuracy of a risk calculator in predicting periodontal disease. J Am Dent Assoc. 2002;133:569–576. [DOI] [PubMed] [Google Scholar]
- 9.Trombelli L, Farina R, Ferrari S, Pasetti P, Calura G. Comparison between two methods for periodontal risk assessment. Minerva Stomatol. 2009;58:277–287. [PubMed] [Google Scholar]
- 10.Busby M, Chapple L, Matthews R, Burke FJ, Chapple I. Continuing development of an oral health score for clinical audit. Br Dent J. 2014;216:E20. [DOI] [PubMed] [Google Scholar]
- 11.Lindskog S, Blomlof J, Persson I, et al. Validation of an algorithm for chronic periodontitis risk assessment and prognostication: analysis of an inflammatory reactivity test and selected risk predictors. JPeriodontol. 2010;81:837–847. [DOI] [PubMed] [Google Scholar]
- 12.Lang NP, Suvan JE, Tonetti MS. Risk factor assessment tools for the prevention of periodontitis progression a systematic review. J Clin Periodontol. 2015;42(Suppl.6):S59–S70. [DOI] [PubMed] [Google Scholar]
- 13.Morelli T, Moss KL, Beck J, et al. Derivation and validation of the periodontal and tooth profile classification system for patient stratification. J Periodontol. 2017;88:153–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beck JD, Elter JR, Heiss G, Couper D, Mauriello SM, Offenbacher S. Relationship of periodontal disease to carotid artery intima-media wall thickness: the atherosclerosis risk in communities (aric) study. Arterioscler Thromb Vasc Biol. 2001;21:1816–1822. [DOI] [PubMed] [Google Scholar]
- 15.Naorungroj S, Slade GD, Divaris K, Heiss G, Offenbacher S, Beck JD. Racial differences in periodontal disease and 10-year self-reported tooth loss among late middle-aged and older adults: the dental aric study. J Public Health Dent. 2017;77(4):372–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beck JD, Sharp T, Koch GG, Offenbacher S. A study of attachment loss patterns in survivor teeth at 18 months, 36 months and 5 years in community-dwelling older adults. J Periodontal Res. 1997;32:497–505. [DOI] [PubMed] [Google Scholar]
- 17.Beck JD, Koch GG, Rozier RG, Tudor GE. Prevalence and risk indicators for periodontal attachment loss in a population of older community-dwelling blacks and whites. J Periodontol. 1990;61:521–528. [DOI] [PubMed] [Google Scholar]
- 18.Beck JD, Koch GG, Offenbacher S. Attachment loss trends over 3 years in community-dwelling older adults. J Periodontol. 1994;65:737–743. [DOI] [PubMed] [Google Scholar]
- 19.Eke PI, Page RC, Wei L, Thornton-Evans G, Genco RJ. Update of the case definitions for population-based surveillance of periodontitis. J Periodontol. 2012;83:1449–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fuller WA KW, Schnell D, Sullivan G, Park HJ. Statistical Laboratory. Ames: Iowa State University; 1986. [Google Scholar]
- 21.Griner PF, Mayewski RJ, Mushlin AI, Greenland P. Selection and interpretation of diagnostic tests and procedures. Principles and applications. Ann Intern Med. 1981;94:557–592. [PubMed] [Google Scholar]
- 22.Hosmer DW, Lemeshow S. Applied Logistic Regression. New York: John Wiley & Sons; 2000. [Google Scholar]
- 23.Schwarz G, Estimating the dimension of a model. Annals of Statistics. 1978;6:461–464. [Google Scholar]
- 24.Breiman L, Friedman JH, Olshen RA, Stone CJ. Classification andRegression Trees. Monterey, CA: Wadsworth & Brooks/Cole Advanced Books & Software; 1984. [Google Scholar]
- 25.Giannobile WV, Braun TM, Caplis AK, Doucette-Stamm L, Duff GW, Kornman KS. Patient stratification for preventive care in dentistry. J Dent Res. 2013;92:694–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999;4:1–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


