Abstract
Local adaptation is of fundamental interest to evolutionary biologists. Traditionally, local adaptation has been studied using reciprocal transplant experiments to quantify fitness differences between residents and immigrants in pairwise transplants between study populations. Previous studies have detected local adaptation in some cases, but others have shown lack of adaptation or even maladaptation. Recently, the importance of different fitness components, such as survival and fecundity, to local adaptation have been emphasized. Here, we address another neglected aspect in studies of local adaptation: sex differences. Given the ubiquity of sexual dimorphism in life histories and phenotypic traits, this neglect is surprising, but may be partly explained by differences in research traditions and terminology in the fields of local adaptation and sexual selection. Studies that investigate differences in mating success between resident and immigrants across populations tend to be framed in terms of reproductive and behavioural isolation, rather than local adaptation. We briefly review the published literature that bridges these areas and suggest that reciprocal transplant experiments could benefit from quantifying both male and female fitness components. Such a more integrative research approach could clarify the role of sex differences in the evolution of local adaptations.
This article is part of the theme issue ‘Linking local adaptation with the evolution of sex differences'.
Keywords: female demographic dominance, gene flow, intersexual genetic correlation, local adaptation, reciprocal transplant experiments, sexual dimorphism
1. Introduction
Local adaptation is a topic of fundamental interest to researchers in ecology, evolution and conservation biology. When selection is spatially heterogeneous, populations can adapt to their local environments such that phenotypes become fitter in their native environment than elsewhere [1,2]. Patterns of local adaptation contribute to generating and maintaining diversity within and among species, influencing range shifts and species interactions as well as population persistence [3]. Empirical tests of local adaptation are of principal importance as they explore the balance between the evolutionary processes that shape populations, i.e. the strength of selection promoting local adaptation, relative to non-adaptive or even maladaptive factors such as gene flow, recombination, mutation and genetic drift, which tend to reduce local adaptation [4,5].
While natural selection is traditionally considered to enhance local adaptation, selection often differs in magnitude and/or sign between males and females. Moreover, the role of sexual selection in local adaptation is far from clear, although there has been a lot of theoretical interest in this topic [6–9]. Depending on the mechanism of selection at work (e.g. sexual selection for direct or indirect fitness benefits or Fisherian mechanisms), sexual selection could reduce or enhance local adaptation [10–13]. These effects will have broader implications for the role of sexual selection in population persistence, speciation and extinction [14–22]. Substantial empirical research has focused either on local adaptation, on sex-specific selection, or on sex differences, but only a few empirical studies [23] have combined studies of sex differences with local adaptation at the intersection between these traditionally separated fields.
Here, we discuss the missing link between these fields with the aim to stimulate future research on sex differences in local adaptation. We take a broad view of sex differences to include any traits or mechanisms that are associated with fitness variation between the sexes, either as a consequence of intrinsic genetic, developmental or physiological differences between males and females, or because of extrinsic ecological differences in natural and sexual selection. Additionally, although hermaphrodites cannot show ‘sex differences’, we occasionally consider studies of hermaphroditic systems in which selection on male function and selection on female function have been explicitly contrasted. We discuss the implications of sex differences in local adaptation, with a focus on how the classical empirical approach of reciprocal transplant experiments can be tailored to such studies. Throughout, we review the limited literature addressing sex differences in local adaptation to date. Finally, we suggest that reciprocal transplants have still much potential as a quantitative tool to assess local adaptation, particularly if used in the light of an explicit consideration of sex differences and sex-specific fitness components.
2. Sex-specific selection, sexual antagonism and local adaptation
Owing to the polygenic nature of most traits (and because the majority of the genome is shared between the sexes) a substantial amount of the genetic variation underlying even sexually dimorphic traits is due to autosomal rather than to sex-linked genes [24–26]. The resulting positive genetic correlations between the sexes can constrain the evolution of dimorphism [24,27,28]. As the two sexes often differ in their phenotypic optima, and the intersexual genetic correlation (rmf) for most phenotypic traits is high and close to unity [27], there will often be a transient period of sexual antagonism during which males and females suffer the fitness costs of intralocus sexual conflict before the full development of sexual dimorphism [29–34]. Intralocus sexual conflict has primarily been demonstrated in laboratory experiments on model organisms like Drosophila melanogaster and other insect systems [17,30,35], and more rarely in natural, free-living populations [34,36,37] (but see [38] who did not detect sexual antagonism in the population studied by Foerster et al. [36]). Moreover, intralocus sexual conflict is usually only demonstrated in a single environment, typically a benign laboratory environment to which the population has adapted over the course of many generations [30,39,40]. A few laboratory studies on insects have quantified the strength of sexual antagonism in ancestral (benign) and novel (stressful) environments [41–43]. While there is some variation in the outcomes of these ‘novel environment’ manipulations (see [41]), two of these studies [42,43] suggested that sexual antagonism was relaxed in novel environments, as selection became concordant between males and females during these stressful novel conditions when both sexes experienced selection towards the same (shared) adaptive peak (see also [23]).
It is only relatively recently that the links between sex-specific selection, sexual antagonism, sex differences and local adaptation have been incorporated in formal mathematical models [7,8,44,45]. Other models have explored spatial variation in optimal phenotypes, and how sexual selection for locally adapted phenotypes affects female preferences [46]. In parallel, the causes and consequences of sexual selection in heterogeneous, complex environments have been explored in some empirical studies [47–51]. This small but increasing literature forms the basis of an emerging research field that underscores the importance of both environmental heterogeneity and spatial structure, with one goal being to understand the geography of sex-specific selection [8]. For example, a recent population genetic model by Connallon [8] merged theory on sex-specific selection with models for range limit evolution [52]. Under the assumption that male and female phenotypic fitness optima change in different ways across a species' range and that sexual dimorphism is constrained by the intersexual genetic correlation (rmf), three different evolutionary outcomes are predicted: (i) sexually concordant selection across the species’ range; (ii) sexually antagonistic selection across the species' range; and (iii) sexual antagonism in the centre of the species’ range and sexually concordant selection at the range limits [8]. The last scenario is particularly interesting, and a meta-analysis has found that phenotypic selection estimates, when available for both sexes, provide some empirical support for its existence in nature [53]. These alternative scenarios could be experimentally evaluated using reciprocal transplants, such as between edge and core populations [54] across a species' range. Such integrative studies hold great promise to increase our knowledge about both sexual antagonism and local adaptation and the intersection between these two fields.
3. Incorporating sex differences into studies of local adaptation
(a). Reciprocal transplants: the ‘gold standard’ for detecting local adaptation
The classical empirical approach to local adaptation is that of reciprocal transplant experiments [3–5,55]. In such experiments, researchers move phenotypes between environments, so that for each phenotype some component of fitness is measured under conditions that match its environment of origin (‘resident’ phenotypes), and under conditions that match the local environment of other populations (‘immigrant’ phenotypes) [4,54]. The existence of a significant fitness-by-environment interaction is traditionally used as a criterion for the presence of local adaptation [4], because we expect each phenotype to perform best in its native environment (i.e. when ‘resident’) (figures 1d,g,h and 2f). However, an interaction alone is insufficient to conclude that populations are locally adapted, as it could also arise when one phenotype outperforms the others in all environments through a ‘general vigour’ effect [4]. A significant interaction can also arise even if local adaptation is asymmetric, with only one of the populations experiencing higher fitness in its native environment [56,58] (figure 2c). In other words, resident phenotypes and/or genotypes might have higher fitness than immigrants in only one of the environments, with no difference in the other, which is termed ‘conditional neutrality’ in the local adaptation literature [2,58–60]. Kawecki & Ebert [4] made the important point that two comparisons can be made in reciprocal transplant experiments: the ‘home versus away’ comparison and the ‘resident versus immigrant’ (or ‘local-foreign’ sensu [4]) comparison. The former comparison may indicate that further investigation into local adaptation is warranted, and might additionally be of interest for other reasons, such as for inferring ‘source’ versus ‘sink’ environments through population differences in mean absolute fitness [56,61,62]. However, if local adaptation is interpreted strictly to mean that a phenotype/genotype presents higher fitness under the local conditions of its original environment, at a cost to its potential fitness elsewhere, then only the ‘resident versus immigrant’ criterion can provide strong evidence for local adaptation [4].
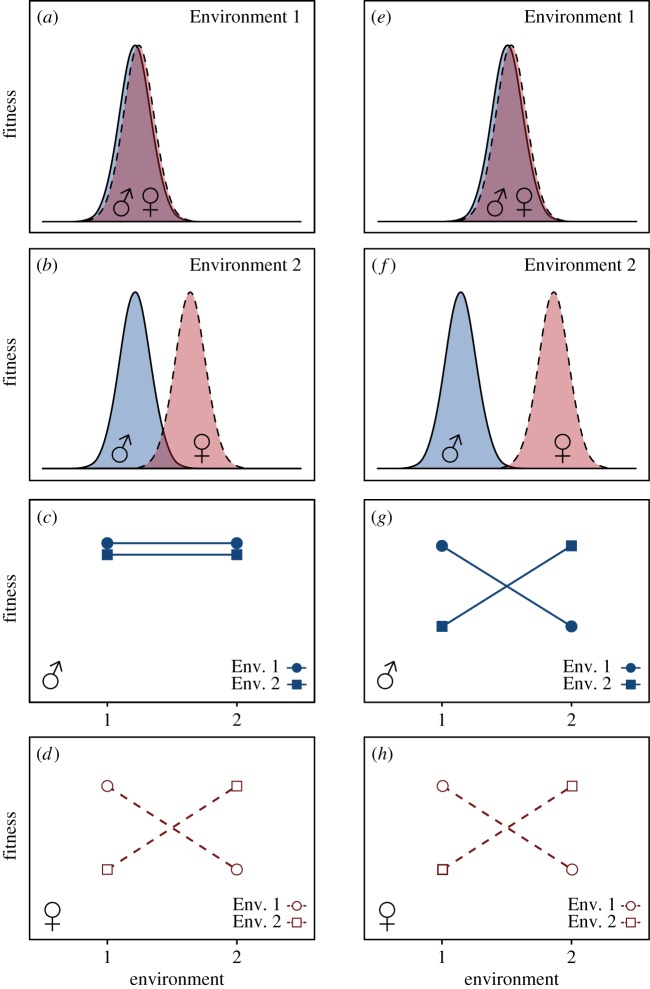
Figure 1.
Relationship between the location of sex-specific local fitness optima in two different environments (‘Environment 1’ on first row and ‘Environment 2’ on second row) and expected outcomes from reciprocal transplant experiments. Male fitness functions are shown in solid lines, blue and filled symbols, whereas female fitness functions are shown in dashed lines, red and open symbols. Left column (a–d): The location of the male fitness optimum is similar in environments 1 and 2, whereas the female optima differ between the two environments (a,b). Consequently, a reciprocal transplant experiment reveals no evidence for local adaptation or a fitness trade-off for males (c), but evidence for local adaption and a strong fitness trade-off between environments in females (d). Right column (e–h): The locations of male and female fitness optima are similar in environment 1, where selection is concordant between the two sexes (e). By contrast, in environment 2, the location of male and female optima differ, leading to sexually antagonistic selection in that environment (f). As both male and female optima differ between environments 1 and 2 (e,f), reciprocal transplant experiments reveal local adaptation and fitness trade-offs between environments in both males and females (g,h).
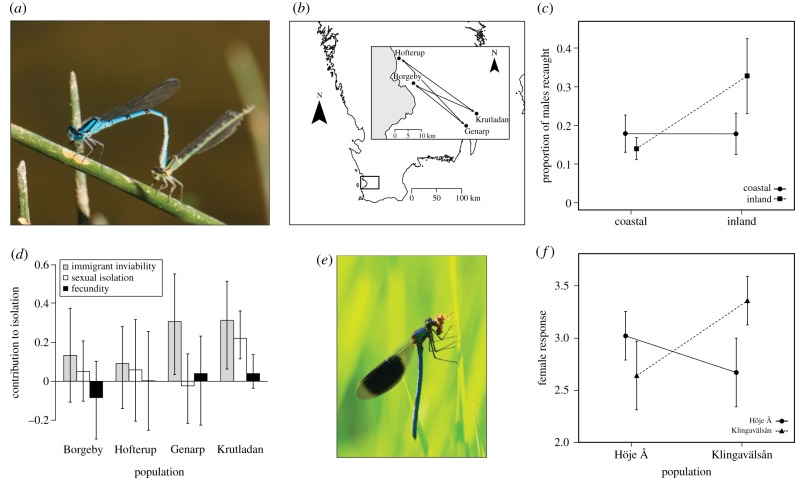
Figure 2.
Local adaptation in relation to sexual selection as revealed by reciprocal transplant experiments and assessment of male fitness components in damselflies (Odonata: Zygoptera). Reciprocal transplant experiments in two species of damselflies: Enallagma cyathigerum (a) and Calopteryx splendens (e). In E. cyathigerum, replicated reciprocal transplant experiments (two population pairs) were carried out, whereby coastal and inland males were released in native and foreign environments (b,c). A significant phenotype × environment interaction revealed local adaptation that was asymmetric in nature, with local adaptation being present in the two inland populations where residents had higher recapture probability (a measure of local survival), but not in the coastal populations, where immigrants and residents had similar survival (c). This suggests that the coastal populations form a demographic sink [56]. Closer inspection of three fitness components revealed that isolation between the environments is mainly caused by reduced survival of immigrants rather than by sexual isolation or fecundity (d). In C. splendens (e), reciprocal transplant experiments (male presentations to local females) between two populations (Klingavälsån and Höje Å) show significant evidence for a phenotype × population interaction (f), where females show strong preference for local over immigrant males [57].
(b). Sex differences may shape and limit local adaptation
The popularity of the reciprocal transplant experimental approach in studies of local adaptation is reflected in a Web of Science search using the combination of terms ‘local* adapt*’ and ‘reciprocal* trans*’, which yielded 376 records spanning from 1985 to 2017 (searched Web of Science Core Collection on 9 January 2018). Interestingly, meta-analyses of reciprocal transplant studies have revealed that although local adaptation is often detected, it is far from universal [5,55]. Hereford [5] and Leimu & Fischer [55] independently showed that local adaptation was not present in 29–55% of pairwise comparisons between residents and immigrants. In addition to the well-known constraints imposed by gene flow (migration and recombination between populations) and small population size (increasing the influence of inbreeding, mutation load and genetic drift [5,55,63,64]), conflicting selection pressures may also hinder local adaptation. There can be variation among subsets within a population, such that trait values favoured by selection in some phenotypes are selectively disadvantageous in others. Sex differences can provide a major source of such within-population variation in fitness optima: antagonistic selection between the sexes (intralocus sexual conflict) is well documented [29,31,32]. However, adding the terms ‘male*’ and ‘female*’ to the search described above reduced the list to just seven studies, of which only three actually measured fitness components separately for both translocated males and females [65–67]. It seems that, to date, local adaptation studies have largely neglected sex differences, focusing on only one sex or pooling the fitness of the two sexes with the tacit assumption that sex differences are unimportant. The vast majority of reciprocal transplant experiments either disregard the sex of individuals, or present fitness component data for only one sex [55].
We present a subset of the studies addressing local adaptation and sex differences and briefly comment upon them in the electronic supplementary material, table S1. Clearly, there is room for more empirical work in this area, and sex differences should preferably be explicitly incorporated in future experimental studies. A first step towards understanding the effect of sexual and sex-specific selection on local adaptation would be to compare the fate of male and female immigrants, as well as fitness differences between residents and immigrants, in different populations. Consider, for instance, populations that are locally adapted to one of two environments (‘Environment 1’ and ‘Environment 2’ in figure 1), which differ in their degree of sexual antagonism. If selection is concordant between males and females in an ancestral environment (figure 1a), but only the female phenotypic optimum changes when the population invades a novel environment (figure 1b), a reciprocal transplant experiment would reveal local adaptation only in females and not in males (figure 1; left column; cf. figure 1c versus 1d). By contrast, if the phenotypic fitness optimum changes for both sexes (figure 1e,f), a reciprocal transplant experiment between the two environments would reveal local adaptation for both males and females (figure 1; right column; cf. figure 1g versus 1h). Whether one or both sexes show a pattern of local adaptation can be important for understanding a population's evolutionary history, and these details can be obscured by the consideration of a single fitness measure for the population. Statistically speaking, a significant three-way interaction (fitness-by-environment-by-sex) is indicative of sex-specific local adaptation, complementing the classical two-way interaction (fitness-by-environment) that has been the traditional focus of local adaptation studies [4].
4. Reciprocal transplant experiments and sex differences
The small number of reciprocal transplant experiments and related empirical approaches that do consider sex highlight three important aspects of how sex differences can influence local adaptation: (i) patterns of sex-specific adaptation; (ii) the role of sexual compared to natural selection in local adaptation; and (iii) the role of sexual selection in regulating gene flow between populations under divergent selection. In this section, we discuss the empirical literature addressing each of these topics, and suggest a fourth key area of research into local adaptation and sex that holds promise for expansion: (iv) sex-specific development, plasticity and local adaptation.
(a). Patterns of sex-specific adaptation
Given sex-specific fitness optima in different environments, patterns of local adaptation measured in males and females may be expected to vary. In a study examining local adaptation in a range of fitness components, Li et al. [67] performed reciprocal transplants between two hermaphroditic ragweed (Ambrosia artemisiifolia) populations and measured vegetative growth traits as well as female reproductive traits. Their results suggest that the two populations may be locally adapted via different, sex-specific traits. Residents in the Beijing population outperformed immigrants from Wuhan in seed production per individual, a component of female fitness (although also dependent on fertilization by males, seed set measured per individual is a more robust proxy of fitness through female than male function, because an individual's seeds will not necessarily be fertilized by the same pollen donor, and pollen from one individual may similarly fertilize seeds across a number of recipients). By contrast, Wuhan residents had greater height at flowering, which enhances pollen dispersal and thus male fitness, compared to immigrants from Beijing. These effects were asymmetric, as for both traits there was no difference between residents and immigrants when measured in the other environment. However, several other fitness-related traits showed no pattern of local adaptation, and net fitness (or broader fitness proxies [68,69]) were not estimated in this study, leaving it unclear how these sex-specific fitness components contribute to the overall extent of local adaptation in each population. By contrast, Favre et al. [70] measured some larger fitness components both in male and in female plants from reciprocally transplanted populations of two dioecious Silene sister species. Individuals of each species had higher fitness in the habitat of conspecific populations (i.e. as residents) than in that of heterospecific populations (i.e. as immigrants). In this case, however, local adaptation was not affected by sex. Female plants performed, on average, slightly better than males across all habitats, with no evidence that males and females differed in the extent to which they were locally adapted.
(b). The role of sexual selection in local adaptation
Many observational studies and those using experimental designs other than reciprocal transplants have investigated adaptation in male and female traits separately, or asked how sexual selection in one or both sexes contributes to local adaptation. Driessens et al. [71] studied populations of an anole lizard (Anolis sagrei) inhabiting environments with either moderate (mesic) or low (xeric) humidity. They inferred local adaptation of male and female signalling traits, as well as male displays, from a correlation between these traits and the environment across populations. This study identified shared (morphological) as well as male-limited (display) traits that appear to be locally adapted. In rock dragon lizards (Ctenophorus decresii species complex), females were more cryptic against their local background than against the backgrounds of other populations, with no such correspondence for males, implying sex differences in the degree of local adaptation in cryptic coloration [72]. Using a similar approach, Morita & Tsuboi [73] related variation in sexual size dimorphism among populations of non-migratory amago salmon (Oncorhynchus masou ishikawae) to the size and complexity of their stream habitat. They found that body size in males, but not females, covaried with stream size, and suggested that different male sizes were favoured by sexual selection in different habitats. Interestingly, the threshold body size at maturation covaried with habitat size in both sexes, suggesting that males and females experienced similar natural selection pressures on this trait.
A limitation of such correlative studies is the lack of direct measures of fitness, or major fitness components, such as reproductive success and/or viability. Correlations between traits and environments are assumed to be due to adaptation of those traits to the local conditions, but in the absence of good fitness estimates, no strong inferences can be made that a trait is adaptive. Additionally, if each population is only measured in its native environment, it is difficult to exclude the possibility that phenotypic plasticity (see §4d, below) underlies the observed correlation of the focal traits with environment. These problems highlight the importance of manipulative experimental designs, such as reciprocal transplants, that allow the relative fitness of phenotypes to be compared across multiple environments. ‘Common garden’ or laboratory experiments also avoid such problems to some extent.
Sexual selection is likely to affect local adaptation in multiple ways. It can reinforce effects of natural selection or eliminate them, depending on whether there is mate preference for local phenotypes, mate preference for novel phenotypes, or mate preference for condition-dependent phenotypes. Moreover, sexual selection could be a major driver of local adaptation by selection of males with phenotypes that are favoured by local females, where females in different populations may favour alternative phenotypes [6,9,57]. This could occur through a process analogous to the adaptation of plants to the floral trait preferences of their local pollinator community (e.g. [74]). Female preferences may vary across environments due to, for example, constraints on signal detection [75–77], thereby driving local adaptation of male signalling traits in each environment. Whether sexual selection facilitates or hinders local adaptation will not only depend on the reaction norms of the traits under sexual selection, but also on the reaction norms and condition dependence of the preference itself. Condition dependence of mate choice has been demonstrated in experimental studies of diet quality in several taxa. For example, female wolf spiders (genus Schizocosa) are selective about mates when fed high-quality diets, but not when fed low-quality diets [78]. In black field crickets (Teleogryllus commodus), diet not only affects choosiness, but also the preference function of females for call frequency [79].
(c). The role of sexual selection in regulating gene flow between populations under divergent selection
The limited number of reciprocal transplant experiments addressing sex differences in local adaptation does not reflect a lack of interest in the topic (for instance, a literature search with keywords ‘local* adapt*’, ‘male’ and ‘female’ returned 289 records). There are many studies that investigate differences in mating success between residents and immigrants across populations, but these tend to be framed in the context of reproductive or behavioural isolation rather than local adaptation. The classical reciprocal transplant experimental and statistical design aiming to quantify local adaptation [4,5] is almost identical to reciprocal sexual isolation experiments in speciation research aiming to quantify the strength of reproductive isolation [80]. Such speciation research includes studies of asymmetric sexual isolation [81–83], which is conceptually and methodologically very similar to studies of asymmetric local adaptation [56].
Tobler et al. [84] used reciprocal transplants between populations of mollies (Poecilia mexicana) inhabiting sulphidic streams inside a cave system and outside of it (‘cave’ versus ‘surface’ habitats), as well as between populations from sulphidic and non-sulphidic surface stream habitats, to test whether immigrants had reduced viability compared to residents. They also tested whether females showed mating preferences for local over immigrant males using two-choice trials. There were no mortality differences between residents and immigrants in the cave and surface stream environments. However, local adaptation through viability selection was apparent between the sulphidic and non-sulphidic surface environments, with residents having higher survival than immigrants in both habitats. Additionally, females preferred resident males to immigrants in three of the four environment-population combinations, consistent with sexual selection reinforcing natural selection against immigrant males.
A similar design allowed Svensson et al. [57] to evaluate the contribution of naturally selected and sexually selected components of fitness to local adaptation in a damselfly (Calopteryx splendens). Although they found no difference in survival of local and immigrant male damselflies that were experimentally translocated between two Swedish populations, there was a clear female preference for local males over immigrants in two-choice female mate preference tests (figure 2e,f), suggesting that local adaptation might be reinforced by sexual selection in these populations. In a study of another damselfly species (Enallagma cyathigerum; figure 2a–d), Gosden et al. [56] transplanted males between two replicate populations from coastal and inland environments (figure 2b). They found evidence for a pattern of asymmetric local adaptation in both pairs of replicates, where immigrants had lower fitness in the inland but not in the coastal environment (figure 2c). However, while this pattern was found for male survival (estimated as recapture probability), other fitness components, namely male mating success and fecundity (measured through the females with whom they mated), did not differ between residents and immigrants (figure 2d). Sexual selection thus did not work in synergy with the viability selection preventing gene flow across these two different environments.
When females’ preferences are tested in their own habitat (females are ‘residents’), the pattern of female preference for local over immigrant males can be explained in at least two distinct ways (see [9]). First, because many sexually selected traits show substantial condition dependence, locally adapted males may have enhanced attractiveness and greater mating success than non-adapted immigrants, if being adapted imparts better general condition [11,85,86]. In this case, variation in a trait is expressed under certain environmental conditions, but not in others, affecting the opportunity for sexual selection. For example, cactus bugs (Narnia femorata, Hemiptera: Coreidae) varied in the degree of sexual dimorphism of sexually selected traits when reared on a novel host plant [87]. The novel environment affected not only the degree of sexual dimorphism in body size, with an increase in dimorphism resulting from smaller body size in males, but also in the size of the hind femur (a weapon), with no sexual dimorphism when bugs were reared on the novel host. Alternatively, preferences for local over immigrant males could result if females prefer mates from their own population (assortative mating) due to diverging male-female (trait-preference) coevolution. Studies that test preferences of immigrant as well as resident females have the potential to disentangle these two mechanisms. Klappert & Reinhold [65] translocated juvenile male and female grasshoppers (Chorthippus biguttulus) between two populations differing in environmental conditions, and tested whether females preferred local males in mate choice trials. Females, whether resident or immigrant, displayed no preference for local over immigrant males (rejecting the condition-dependence mechanism for local male mating advantage) and also showed no preference for males from their population of origin (rejecting the assortative mating mechanism). While no mating advantage to resident males due to either mechanism was detected in this study, the experimental design allowed each to be ruled out separately. Plath et al. [88] similarly tested the preferences of females in their local or in foreign environmental conditions (i.e. as residents or as immigrants) in two sister species of poeciliid fishes: Poecilia sulphuraria inhabit sulphidic streams, while P. mexicana inhabit non-sulphidic streams. Females of both species were given the choice between a conspecific and a heterospecific male in both sulphidic and non-sulphidic test environments. In non-sulphidic conditions, females of both species preferred the resident, P. mexicana males, ruling out assortative mating as an explanation and suggesting that P. mexicana could show enhanced attractiveness to females due to local adaptation to the non-sulphidic environment. However, in the sulphidic test conditions, females of both species showed no preference for either species' males. Local adaptation to sulphidic conditions thus does not appear to correlate with mating success of resident P. sulphuraria males.
Apart from field transplant experiments, laboratory experiments have also been used in studies addressing whether locally adapted males have greater mating success than non-adapted males [89–91]. In these studies, male Drosophila melanogaster from laboratory populations that had been experimentally evolved under different environmental conditions (different temperatures [89,90] or different larval densities [91]) were competed against each other in mating trials. In each of these studies, trials were carried out in both environments, allowing males from both environments to play the ‘adapted’ and ‘non-adapted’ role. Males adapted to their local thermal environment had, on average, greater mating success than their non-adapted competitors in one study [89]. This supports the hypothesis that males in better condition are better at attracting mates, perhaps due to the better match between their phenotype and the environment [85,86]. However, in the other two studies, there was no difference in mating success between adapted and non-adapted males [90,91].
(d). Sex differences in development, plasticity and local adaptation
Another interesting but relatively unexplored evolutionary outcome of sex-specific selection in heterogeneous environments is the evolution of sex-specific phenotypic plasticity [92]. For instance, males and females could differ in their degree of plasticity or developmental canalization in traits like mate preferences, depending on the presence or absence of heterospecific competitors, i.e. in sympatry versus allopatry [48] or host plant choice that could influence the realized degree of sexual dimorphism [87]. Moreover, variation in the preference function and choosiness for different traits can respond differently to earlier versus later developmental environments. In the swordtail fish (Xiphophorus multilineatus), the embryonic environment experienced by females influenced female preference for male colour pattern symmetry, while the post embryonic juvenile environment influenced female preference for male body size [93]. Juvenile and adult environmental conditions can also affect choosiness and preference functions differently, as well as affecting the sexes differently. Although female cactus bugs (N. femorata) always choose larger males, females were choosier when their adult environments were of low quality, with the shape of selection for male body size depending on female rearing environment [94]. Males, on the other hand, only exhibited a preference for larger females if both their juvenile and adult environments were of high quality [94].
The timing of when condition is determined should be an important concern in future studies integrating local adaptation and sex differences: phenotypes developing in the environment that they are adapted to are expected to be in better condition than phenotypes that have developed in a foreign environment. Moving to a novel and stressful environment as an adult might, however, lead to smaller effects on an individual's life-history and condition-dependent traits. In this case, while preference and choosiness of resident and immigrant individuals may not be affected by translocation experiments involving adults, the translocation of juveniles would highlight such early environmental effects, which have been documented in several taxa [95–97]. Schultzhaus et al. [98] demonstrated through diet manipulations in both sexes that variation in the quality of the rearing environment can lead to assortative mating in D. melanogaster. Specifically, males reared on low-quality diets courted females of the control and the low-quality treatments equally, but more often mated with females reared on low-quality diets. By contrast, males in the control treatment courted control females almost exclusively, and mated with these females more often than with females reared on low-quality diets. Although researchers did not test variation in female receptivity or choosiness in this work, either or both could explain the results. These studies highlight that studies of sex differences in local adaptation can become greatly enriched by translocation experiments not only in the adult stage (as common in animal studies), but also during earlier stages of development (as typical of plant translocation studies, i.e. those transplanting seeds). Translocations at different life stages in the same study system can help to disentangle whether the extent of mismatch between developmental and test environments contributes to observed differences in fitness between residents and immigrants.
5. Outlook and summary: new methods and directions in the study of local adaptation
Apart from the topics we have highlighted in this review, there are several other interesting extensions that hold great promise if studied within the emerging new framework of local adaptation and the evolution of sex differences. Adopting an ontogenetic and developmental perspective on sex differences will increase our understanding of the role of genetic causes of sexual dimorphism between populations and the role of sex-specific phenotypic plasticity and environment-dependent growth rate differences between males and females [87,92,99,100]. It is common to find differences in the degree of sexual dimorphism between different habitats and environments, and sometimes such differences can be related to differences in sexual selection regimes and/or intensity of sexual conflict [47,73,87]. It would be interesting to know how, why and when those differences emerge and the role of sex-specific maternal effects [34,101], sex differences in growth rates [99] and early environmental effects [87].
Nowadays reciprocal transplant experiments are often combined with genomic approaches, creating new, powerful and integrative research opportunities for the study of local adaptation [1,102–105]. Rapid methodological developments in molecular biology and genomics, such as Next Generation Sequencing of genome-wide variation of thousands or tens of thousands of loci or single nucleotide polymorphisms, are now rapidly transforming the research fields of local adaptation and the evolution of sex differences [102,103,105,106]. While reciprocal transplant experiments can provide more mechanistic insights into the ecological causes of selection [107–109], complementary genomic approaches present many methodological and statistical challenges, particularly on how to disentangle demographic and neutral processes from local adaptation driven by environment-dependent selection [102]. Additionally, genomic approaches will largely reflect local adaptation due to the combined effects of selection across the organism's whole life cycle, averaged across the two sexes. While for some applications this integrated measure is an advantage, it offers limited mechanistic insights into the ecological causes of selection [110–113] that drive adaptive population genetic differentiation. For these reasons, genomic approaches are probably most powerful when combined with traditional empirical tools like ‘common garden’ and reciprocal transplant experiments [102]. One genomic approach with great potential to study sex differences in local adaptation is the use of massive DNA- and RNA-sequencing of juveniles and adults of both sexes, sampled across many different populations. One could then investigate, for example, whether sexual antagonism results in a signature of increasing genetic differentiation (i.e. increasing FST-values) between males and females during the course of ontogeny, as was recently demonstrated in large-scale genomic studies of humans and fruit flies (Drosophila) [106]. This promising approach is not without its own challenges: simulations show that the power to detect antagonistic selection over the threshold of FST for neutral variation in male and female allele frequencies is low [114]. However, this problem may be mitigated by the analysis of genome-wide patterns to detect subclasses of (thousands of) genes exhibiting enriched FST relative to the rest of the genome [106,114]. Additionally, it has recently been suggested [115] that this method may be most informative when combined with other population genomic measures, such as estimates of non-neutral genetic diversity using Tajima's D.
Some conceptual and statistical issues with the incorporation of sex differences into reciprocal transplant experiments involve how to compare male and female fitness. Ideally, male and female fitness should be estimated in the same currency, to be comparable and to be able to estimate a three-way, fitness-by-environment-by-sex interaction. In practice, although males and females can be similar for some fitness components (e.g. survival), male and female reproductive traits are often measured on different scales, such as the number of eggs produced by females and the number of mates obtained by males. In these situations, the issue of how to standardize and relativize fitness becomes important for ensuring unbiased comparisons of fitness between the sexes [116]. It could be argued that fitness should be standardized separately within each sex—equivalent to assuming soft selection—because in genetic terms both sexes contribute equally to the next generation of offspring (i.e. males and females have the same total fitness) [116,117]. A more pragmatic approach avoids the problem of directly comparing non-equivalent male and female fitness measures by instead comparing the difference in fitness of male residents and immigrants separately from the difference in female resident and immigrant fitness. Alternatively, where experiments can be monitored over several generations post-translocation, measuring the change in genotype frequencies in populations where either males or females have been introduced could be an informative approach (see [118]). Finally, based on demographic and life-history arguments, some ecologists might argue that male fitness can be safely ignored in local adaptation studies. Owing to female demographic dominance (offspring production depends heavily on female abundance, because female reproductive output is rather insensitive to male abundance while male reproduction is strongly determined by female abundance [7]), female fitness contributes disproportionately to population growth and establishment in novel environments. However, male fitness can have a strong indirect effect on population growth and establishment success via the male contribution to gene flow, constraining local adaptation (e.g. pollen dispersal [44,45]).
Our overview of experimental local adaptation studies and their intersection with studies on the evolution of sex differences has identified several promising avenues for future work aiming to integrate these two fields. Recently published theoretical models have identified interesting questions that should be addressed in experimental studies. These questions include the role of female demographic dominance in local adaptation and in source and sink habitats [7], geographical variation in sexual antagonism and differences between range limits and central populations [8], and the relative importance of male and female gene flow and their effects on local adaptation and niche evolution [44,45]. Here, we have suggested that these models can be tested and evaluated using the classical tool of reciprocal transplant experiments. The reciprocal transplant design could also be productively applied to additional open questions by incorporating sex differences in life history: for example, how natural differences in patterns of dispersal affect and are affected by local adaptation. What are the consequences when dispersal is constrained to one sex or life stage (e.g. when one sex/stage is immobile)? Does the optimal offspring sex ratio differ for a resident or immigrant female? Sex- and stage-limited dispersal may be easily incorporated into a reciprocal transplant experimental design (see [119]). By explicitly considering sex differences in reciprocal transplant studies and by measuring fitness components in males and females separately, informed experiments could provide rich insights into the presence and consequences of sex differences in local adaptation. Theoretical and empirical studies of both local adaptation [120] and the evolution of phenotypic plasticity [121,122] typically ignore the effects of sex differences, yet as we have argued in this article incorporating such information is likely to yield many new insights [7,8,45,48,92].
Supplementary Material
Acknowledgements
This paper grew out of a Special Topic Network (STN) group funded by the European Society for Evolutionary Biology entitled ‘Linking local adaptation with the evolution of sex differences' (http://eseb.org/prizes-funding/special-topic-networks/linking-local-adaptation-with-the-evolution-of-sex-differences/) awarded to H. Kokko, E.I. Svensson, F. Débarre and T. Connallon. The ideas and outline for this paper were put together during our first workshop in Lund (Sweden) 14–17 August 2017. D.G. is a fellow of the CAPES Science without Borders programme (SwB, 13442/13-9).
Data accessibility
This article has no additional data.
Competing interests
We declare we have no competing interests.
Funding
Funding for this study was provided by a research grant from the Swedish Research Council (VR: grant no. 2016-03356 to E.I.S.), the Australian Research Council (DE170101193, to I.B.) and Stiftelsen for Zoologisk Forskning (to F.S.).
References
- 1.Whitlock MC. 2015. Modern approaches to local adaptation. Am. Nat . 186, S1–S4. ( 10.1086/682933) [DOI] [PubMed] [Google Scholar]
- 2.Delph LF. 2017. The study of local adaptation: a thriving field of research. J. Hered . 109, 1–2. ( 10.1093/jhered/esx099) [DOI] [PubMed] [Google Scholar]
- 3.Blanquart F. 2013. A practical guide to measuring local adaptation. Ecol. Lett . 16, 1195–1205. ( 10.1111/ele.12150) [DOI] [PubMed] [Google Scholar]
- 4.Kawecki TJ, Ebert D. 2004. Conceptual issues in local adaptation. Ecol. Lett . 7, 1225–1241. ( 10.1111/j.1461-0248.2004.00684.x) [DOI] [Google Scholar]
- 5.Hereford J. 2009. A quantitative survey of local adaptation and fitness trade-offs. Am. Nat . 173, 579–588. ( 10.1086/597611) [DOI] [PubMed] [Google Scholar]
- 6.Servedio MR. 2004. The evolution of premating isolation: local adaptation and natural and sexual selection against hybrids. Evolution 58, 913–924. ( 10.1111/j.0014-3820.2004.tb00425.x) [DOI] [PubMed] [Google Scholar]
- 7.Harts AMF, Schwanz LE, Kokko H.. 2014. Demography can favour female-advantageous alleles. Proc. R. Soc. B 281, 20140005 ( 10.1098/rspb.2014.0005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Connallon T. 2015. The geography of sex-specific selection, local adaptation, and sexual dimorphism. Evolution 69, 2333–2344. ( 10.1111/evo.12737) [DOI] [PubMed] [Google Scholar]
- 9.Servedio MR, Boughman JW. 2017. The role of sexual selection in local adaptation and speciation. Annu. Rev. Ecol. Evol. Syst . 48, 85–109. ( 10.1146/annurev-ecolsys-110316-022905) [DOI] [Google Scholar]
- 10.Doherty PF, Sorci G, Royle JA, Hines JE, Nichols JD, Boulinier T. 2003. Sexual selection affects local extinction and turnover in bird communities. Proc. Natl Acad. Sci. USA 100, 5858–5862. ( 10.1073/pnas.0836953100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lorch PD, et al. 2003. Condition-dependent sexual selection can accelerate adaptation. Evol. Ecol. Res . 5, 867–881. [Google Scholar]
- 12.Svensson EI, Gosden TP. 2007. Contemporary evolution of secondary sexual traits in the wild. Funct. Ecol . 16, 422–433. ( 10.1111/j.1365-2435.2007.01265.x) [DOI] [Google Scholar]
- 13.Doorn GS, Edelaar P, Weissing FJ. 2009. On the origin of species by natural and sexual selection. Science 326, 1704–1707. ( 10.1126/science.1181661) [DOI] [PubMed] [Google Scholar]
- 14.Ritchie MG. 2007. Sexual selection and speciation. Annu. Rev. Ecol. Evol. Syst . 38, 79–102. ( 10.1146/annurev.ecolsys.38.091206.095733) [DOI] [Google Scholar]
- 15.Maan ME, Seehausen O. 2011. Ecology, sexual selection and speciation. Ecol. Lett . 14, 591–602. ( 10.1111/j.1461-0248.2011.01606.x) [DOI] [PubMed] [Google Scholar]
- 16.Kraaijeveld K, Kraaijeveld-Smit FJL, Maan ME. 2011. Sexual selection and speciation: the comparative evidence revisited. Biol. Rev . 86, 367–377. ( 10.1111/j.1469-185X.2010.00150.x) [DOI] [PubMed] [Google Scholar]
- 17.Berger D, Martinossi-Allibert I, Grieshop K, Lind MI, Maklakov AA, Arnqvist G. 2016. Intralocus sexual conflict and the tragedy of the commons in seed beetles. Am. Nat . 188, E98–E112. ( 10.1086/687963) [DOI] [PubMed] [Google Scholar]
- 18.Rankin DJ, Dieckmann U, Kokko H. 2011. Sexual conflict and the tragedy of the commons. Am. Nat . 177, 780–791. ( 10.1086/659947) [DOI] [PubMed] [Google Scholar]
- 19.Svensson EI, Waller JT. 2013. Ecology and sexual selection: evolution of wing pigmentation in calopterygid damselflies in relation to latitude, sexual dimorphism and speciation. Am. Nat . 182, E174–E195. ( 10.1086/673206) [DOI] [PubMed] [Google Scholar]
- 20.Martins MJF, Puckett TM, Lockwood R, Swaddle JP, Hunt G. 2018. High male sexual investment as a driver of extinction in fossil ostracods. Nature 566, 366–369. ( 10.1038/s41586-018-0020-7) [DOI] [PubMed] [Google Scholar]
- 21.Parrett JM, Knell RJ. 2018. The effect of sexual selection on adaptation and extinction under increasing temperatures. Proc. R Soc. B 285, 20180303 ( 10.1098/rspb.2018.0303) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lumley AJ, et al. 2015. Sexual selection protects against extinction. Nature 522, 470–473. ( 10.1038/nature14419) [DOI] [PubMed] [Google Scholar]
- 23.Lasne C, Hangartner SB, Connallon T. 2018. Cross-sex genetic correlations and the evolution of sex-specific local adaptation: insights from classical trait clines in Drosophila melanogaster. Evol. Int. J. Org. Evol . 72, 1317–1327. ( 10.1111/evo.13494) [DOI] [PubMed] [Google Scholar]
- 24.Chenoweth S, Rundle H, Blows M. 2008. Genetic constraints and the evolution of display trait sexual dimorphism by natural and sexual selection. Am. Nat . 171, 22–34. ( 10.1086/523946) [DOI] [PubMed] [Google Scholar]
- 25.Roulin A, Altwegg R, Jensen H, Steinsland I, Schaub M. 2010. Sex-dependent selection on an autosomal melanic female ornament promotes the evolution of sex ratio bias. Ecol. Lett . 13, 616–626. ( 10.1111/j.1461-0248.2010.01459.x) [DOI] [PubMed] [Google Scholar]
- 26.Mank JE, Nam K, Brunstrom B, Ellegren H. 2010. Ontogenetic complexity of sexual dimorphism and sex-specific selection. Mol. Biol. Evol . 27, 1570–1578. ( 10.1093/molbev/msq042) [DOI] [PubMed] [Google Scholar]
- 27.Lande R. 1980. Sexual dimorphism, sexual selection, and adaptation in polygenic characters. Evolution 34, 292–305. ( 10.1111/j.1558-5646.1980.tb04817.x) [DOI] [PubMed] [Google Scholar]
- 28.Rhen T. 2000. Sex-limited mutations and the evolution of sexual dimorphism. Evolution 54, 37–43. ( 10.1111/j.0014-3820.2000.tb00005.x) [DOI] [PubMed] [Google Scholar]
- 29.Rice WR, Chippindale AK. 2001. Intersexual ontogenetic conflict. J. Evol. Biol . 14, 685–693. ( 10.1046/j.1420-9101.2001.00319.x) [DOI] [Google Scholar]
- 30.Chippindale AK, Gibson JR, Rice WR. 2001. Negative genetic correlation for adult fitness between sexes reveals ontogenetic conflict in Drosophila. Proc. Natl Acad. Sci. USA 98, 1671–1675. ( 10.1073/pnas.98.4.1671) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bonduriansky R, Chenoweth SF. 2009. Intralocus sexual conflict. Trends Ecol. Evol . 24, 280–288. ( 10.1016/j.tree.2008.12.005) [DOI] [PubMed] [Google Scholar]
- 32.Cox RM, Calsbeek R. 2009. Sexually antagonistic selection, sexual dimorphism, and the resolution of intralocus sexual conflict. Am. Nat . 173, 176–187. ( 10.1086/595841) [DOI] [PubMed] [Google Scholar]
- 33.Barker BS, Phillips PC, Arnold SJ. 2010. A test of the conjecture that G-matrices are more stable than B-matrices. Evolution 64, 2601–2613. ( 10.1111/j.1558-5646.2010.01023.x) [DOI] [PubMed] [Google Scholar]
- 34.Svensson EI, McAdam AG, Sinervo B. 2009. Intralocus sexual conflict over immune defense, gender load, and sex-specific signaling in a natural lizard population. Evolution 63, 3124–3135. ( 10.1111/j.1558-5646.2009.00782.x) [DOI] [PubMed] [Google Scholar]
- 35.Fedorka KM, Mousseau TA. 2004. Female mating bias results in conflicting sex-specific offspring fitness. Nature 429, 65–67. ( 10.1038/nature02492) [DOI] [PubMed] [Google Scholar]
- 36.Foerster K, Coulson T, Sheldon BC, Pemberton JM, Clutton-Brock TH, Kruuk LEB. 2007. Sexually antagonistic genetic variation for fitness in red deer. Nature 447, 1107–1109. ( 10.1038/nature05912) [DOI] [PubMed] [Google Scholar]
- 37.Cox RM, Calsbeek R. 2010. Cryptic sex-ratio bias provides indirect genetic benefits despite sexual conflict. Science 328, 92–94. ( 10.1126/science.1185550) [DOI] [PubMed] [Google Scholar]
- 38.Walling CA, Morrissey MB, Foerster K, Clutton-Brock TH, Pemberton JM, Kruuk LEB. 2014. A multivariate analysis of genetic constraints to life history evolution in a wild population of red deer. Genetics 198, 1735 ( 10.1534/genetics.114.164319) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pischedda A, Chippindale AK. 2006. Intralocus sexual conflict diminishes the benefits of sexual selection. PLoS Biol. 4, 2099–2103. ( 10.1371/journal.pbio.0040356) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prasad NG, Bedhomme S, Day T, Chippindale AK. 2007. An evolutionary cost of separate genders revealed by male-limited evolution. Am. Nat . 169, 29–37. ( 10.1086/509941) [DOI] [PubMed] [Google Scholar]
- 41.Delcourt M, Blows MW, Rundle HD. 2009. Sexually antagonistic genetic variance for fitness in an ancestral and a novel environment. Proc. R. Soc. Lond. B 276, 2009–2014. ( 10.1098/rspb.2008.1459) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Punzalan D, Delcourt M, Rundle HD. 2014. Comparing the intersex genetic correlation for fitness across novel environments in the fruit fly, Drosophila serrata. Heredity 112, 143–148. ( 10.1038/hdy.2013.85) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Berger D, Berg EC, Widegren W, Arnqvist G, Maklakov AA. 2014. Intralocus sexual conflict and environmental stress. Evolution 68, 2184–2196. ( 10.1111/evo.12528) [DOI] [PubMed] [Google Scholar]
- 44.Aguilé R, Shaw FH, Rousset F, Shaw RG, Ronce O. 2013. How does pollen versus seed dispersal affect niche evolution? Evolution 67, 792–805. ( 10.1111/j.1558-5646.2012.01816.x) [DOI] [PubMed] [Google Scholar]
- 45.Aguile R, Raoul G, Rousset F, Ronce O. 2016. Pollen dispersal slows geographical range shift and accelerates ecological niche shift under climate change. Proc. Natl Acad. Sci . 113, E5741–E5748. ( 10.1073/pnas.1607612113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Day T. 2000. Sexual selection and the evolution of costly female preferences: spatial effects. Evolution 54, 715–730. ( 10.1111/j.0014-3820.2000.tb00074.x) [DOI] [PubMed] [Google Scholar]
- 47.Gosden TP, Svensson EI. 2008. Spatial and temporal dynamics in a sexual selection mosaic. Evolution 62, 845–856. ( 10.1111/j.1558-5646.2008.00323.x) [DOI] [PubMed] [Google Scholar]
- 48.Svensson EI, Runemark A, Verzijden MN, Wellenreuther M. 2014. Sex differences in developmental plasticity and canalization shape population divergence in mate preferences. Proc. R. Soc. B 281, 20141636 ( 10.1098/rspb.2014.1636) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miller CW, Svensson EI. 2014. Sexual selection in complex environments. Annu. Rev. Entomol . 59, 427–445. ( 10.1146/annurev-ento-011613-162044) [DOI] [PubMed] [Google Scholar]
- 50.Verzijden MN, Svensson EI. 2016. Interspecific interactions and learning variability jointly drive geographic differences in mate preferences. Evolution 70, 1896–1903. ( 10.1111/evo.12982) [DOI] [PubMed] [Google Scholar]
- 51.Yun L, Chen PJ, Singh A, Agrawal AF, Rundle HD. 2017. The physical environment mediates male harm and its effect on selection in females. Proc. R Soc. B 284, 20170424. ( 10.1098/rspb.2017.0424) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kirkpatrick M, Barton NH. 1997. Evolution of a species range. Am. Nat . 150, 1–23. ( 10.1086/286054) [DOI] [PubMed] [Google Scholar]
- 53.De Lisle S, Goedert D, Reedy A, Svensson E. 2018. Climatic factors and species range position predict sexually antagonistic selection across taxa. Phil. Trans. R. Soc. B 373, 20170415 ( 10.1098/rstb.2017.0415) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee-Yaw JA, Kharouba HM, Bontrager M, Mahony C, Csergo AM, Noreen AME, Li Q, Schuster R, Angert AL.. 2016. A synthesis of transplant experiments and ecological niche models suggests that range limits are often niche limits. Ecol. Lett . 19, 710–722. ( 10.1111/ele.12604) [DOI] [PubMed] [Google Scholar]
- 55.Leimu R, Fischer M. 2008. A meta-analysis of local adaptation in plants. PLoS ONE 3, e4010 ( 10.1371/journal.pone.0004010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gosden TP, Waller JT, Svensson EI. 2015. Asymmetric isolating barriers between different microclimatic environments caused by low immigrant survival. Proc. R. Soc. B 282, 20142459 ( 10.1098/rspb.2014.2459) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Svensson EI, Eroukhmanoff F, Friberg M. 2006. Effects of natural and sexual selection on adaptive population divergence and premating isolation in a damselfly. Evolution 60, 1242–1253. ( 10.1111/j.0014-3820.2006.tb01202.x) [DOI] [PubMed] [Google Scholar]
- 58.Anderson JT, Willis JH, Mitchell-Olds T. 2011. Evolutionary genetics of plant adaptation. Trends Genet. 27, 258–266. ( 10.1016/j.tig.2011.04.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fry JD. 1996. The evolution of host specialization: are trade-offs overrated? Am. Nat . 148, S84–S107. ( 10.1086/285904) [DOI] [Google Scholar]
- 60.Kawecki TJ. 1997. Sympatric speciation via habitat specialization driven by deleterious mutations. Evolution 51, 1751–1763. ( 10.1111/j.1558-5646.1997.tb05099.x) [DOI] [PubMed] [Google Scholar]
- 61.Gomulkiewicz R, Holt RD. 1995. When does evolution by natural selection prevent extinction? Evolution 49, 201–207. ( 10.1111/j.1558-5646.1995.tb05971.x) [DOI] [PubMed] [Google Scholar]
- 62.Holt RD, Gomulkiewicz R. 1997. How does immigration influence local adaptation? A reexamination of a familiar paradigm. Am. Nat . 149, 563–572. ( 10.1086/286005) [DOI] [Google Scholar]
- 63.Lenormand T. 2002. Gene flow and the limits to natural selection. Trends Ecol. Evol . 17, 183–189. ( 10.1016/S0169-5347(02)02497-7) [DOI] [Google Scholar]
- 64.Lohr JN, Haag CR. 2015. Genetic load, inbreeding depression, and hybrid vigor covary with population size: an empirical evaluation of theoretical predictions. Evolution 69, 3109–3122. ( 10.1111/evo.12802) [DOI] [PubMed] [Google Scholar]
- 65.Klappert K, Reinhold K. 2005. Local adaptation and sexual selection: a reciprocal transfer experiment with the grasshopper Chorthippus biguttulus. Behav. Ecol. Sociobiol . 58, 36–43. ( 10.1007/s00265-004-0902-6) [DOI] [Google Scholar]
- 66.Hedderson TA, Longton RE. 2008. Local adaptation in moss life histories: population-level variation and a reciprocal transplant experiment. J. Bryol . 30, 1–11. ( 10.1179/174328208X282175) [DOI] [Google Scholar]
- 67.Li X-M, She D-Y, Zhang D-Y, Liao W-J. 2015. Life history trait differentiation and local adaptation in invasive populations of Ambrosia artemisiifolia in China. Oecologia 177, 669–677. ( 10.1007/s00442-014-3127-z) [DOI] [PubMed] [Google Scholar]
- 68.Barker JSF. 2009. Defining fitness in natural and domesticated populations. In Adaptation and fitness in animal populations (eds van der Werf J, Graser HU, Frankham R, Gondro C), pp. 3–14. Dordrecht, The Netherlands: Springer. [Google Scholar]
- 69.Christiansen FB, Prout T. 2000. Aspects of fitness. In Evolutionary genetics: from molecules to morphology, 8 vols (eds RS Singh, CB Krimbas), pp. 146–156. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 70.Favre A, Widmer A, Karrenberg S. 2017. Differential adaptation drives ecological speciation in campions (Silene): evidence from a multi-site transplant experiment. New Phytol. 213, 1487–1499. ( 10.1111/nph.14202) [DOI] [PubMed] [Google Scholar]
- 71.Driessens T, Baeckens S, Balzarolo M, Vanhooydonck B, Huyghe K, Van Damme R. 2017. Climate-related environmental variation in a visual signalling device: the male and female dewlap in Anolis sagrei lizards. J. Evol. Biol . 30, 1846–1861. ( 10.1111/jeb.13144) [DOI] [PubMed] [Google Scholar]
- 72.Stuart-Fox DM, Moussalli A, Johnston GR, Owens IPF. 2004. Evolution of color variation in dragon lizards: quantitative tests of the role of crypsis and local adaptation. Evolution 58, 1549–1559. ( 10.1111/j.0014-3820.2004.tb01735.x) [DOI] [PubMed] [Google Scholar]
- 73.Morita K, Tsuboi J. 2017. Sexual size dimorphism in a landlocked Pacific salmon in relation to breeding habitat features. Evol. Ecol . 31, 653–661. ( 10.1007/s10682-017-9902-7) [DOI] [Google Scholar]
- 74.Kalske A, Muola A, Laukkanen L, Mutikainen P, Leimu R. 2012. Variation and constraints of local adaptation of a long-lived plant, its pollinators and specialist herbivores. J. Ecol . 100, 1359–1372. ( 10.1111/j.1365-2745.2012.02008.x) [DOI] [Google Scholar]
- 75.Endler J. 1992. Signals, signal conditions, and the direction of evolution. Am. Nat . 139, S125–S153. ( 10.1086/285308) [DOI] [Google Scholar]
- 76.Schluter D, Price T. 1993. Honesty, perception and population divergence in sexually selected traits. Proc. R. Soc. Lond. B 253, 117–122. ( 10.1098/rspb.1993.0089) [DOI] [PubMed] [Google Scholar]
- 77.Jennions MD, Petrie M. 1997. Variation in mate choice and mating preferences: a review of causes and consequences. Biol. Rev . 72, 283–327. ( 10.1017/S0006323196005014) [DOI] [PubMed] [Google Scholar]
- 78.Hebets EA, Wesson J, Shamble PS. 2008. Diet influences mate choice selectivity in adult female wolf spiders. Anim. Behav . 76, 355–363. ( 10.1016/j.anbehav.2007.12.021) [DOI] [Google Scholar]
- 79.Hunt J, Brooks R, Jennions MÂD. 2005. Female mate choice as a condition-dependent life-history trait. Am. Nat . 166, 79–92. ( 10.1086/430672) [DOI] [PubMed] [Google Scholar]
- 80.Svensson EI, Karlsson K, Friberg M, Eroukmanhoff F. 2007. Gender differences in species recognition and the evolution of asymmetric sexual isolation. Curr. Biol. 22, 1943–1947. ( 10.1016/j.cub.2007.09.038) [DOI] [PubMed] [Google Scholar]
- 81.Coyne JA, Orr HA. 2004. Speciation. Sunderland, MA: Sinauer Associates Inc. [Google Scholar]
- 82.Shine R, Reed RN, Shetty S, Lemaster M, Mason RT. 2002. Reproductive isolating mechanisms between two sympatric sibling species of sea snakes. Evolution 56, 1655–1662. ( 10.1111/j.0014-3820.2002.tb01477.x) [DOI] [PubMed] [Google Scholar]
- 83.Svensson EI, Waller JT, Runemark A. 2016. Linking intra- and interspecific assortative mating: consequences for asymmetric sexual isolation. Evolution 70, 1165–1179. ( 10.1111/evo.12939) [DOI] [PubMed] [Google Scholar]
- 84.Tobler M, Riesch R, Tobler CM, Schulz-Mirbach T, Plath M. 2009. Natural and sexual selection against immigrants maintains differentiation among micro-allopatric populations. J. Evol. Biol . 22, 2298–2304. ( 10.1111/j.1420-9101.2009.01844.x) [DOI] [PubMed] [Google Scholar]
- 85.Proulx SR. 1999. Matings systems and the evolution of niche breadth. Am. Nat . 154, 89–98. ( 10.1086/303218) [DOI] [PubMed] [Google Scholar]
- 86.Proulx SR. 2001. Female choice via indicator traits easily evolves in the face of recombination and migration. Evolution 55, 2401–2411. ( 10.1111/j.0014-3820.2001.tb00755.x) [DOI] [PubMed] [Google Scholar]
- 87.Allen PE, Miller CW. 2017. Novel host plant leads to the loss of sexual dimorphism in a sexually selected male weapon. Proc. R Soc. B 284, 20171269. ( 10.1098/rspb.2017.1269) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Plath M, et al. 2013. Genetic differentiation and selection against migrants in evolutionarily replicated extreme environments. Evolution 67, 2647–2661. ( 10.1111/evo.12133) [DOI] [PubMed] [Google Scholar]
- 89.Dolgin ES, Whitlock MC, Agrawal AF. 2006. Male Drosophila melanogaster have higher mating success when adapted to their thermal environment. J. Evol. Biol . 19, 1894–1900. ( 10.1111/j.1420-9101.2006.01168.x) [DOI] [PubMed] [Google Scholar]
- 90.Correia L, Yeaman S, Whitlock MC. 2010. Local adaptation does not always predict high mating success. J. Evol. Biol . 23, 875–878. ( 10.1111/j.1420-9101.2010.01957.x) [DOI] [PubMed] [Google Scholar]
- 91.Shenoi VN, Prasad NG. 2016. Local adaptation to developmental density does not lead to higher mating success in Drosophila melanogaster. J. Evol. Biol . 29, 2036–2042. ( 10.1111/jeb.12927) [DOI] [PubMed] [Google Scholar]
- 92.Stillwell RC, Blanckenhorn WU, Teder T, Davidowitz G, Fox CW. 2010. Sex differences in phenotypic plasticity affect variation in sexual size dimorphism in insects: from physiology to evolution. Annu. Rev. Entomol . 55, 227–245. ( 10.1146/annurev-ento-112408-085500) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lyons SM, Goedert D, Morris MR. 2014. Male-trait-specific variation in female mate preferences. Anim. Behav . 87, 39–44. ( 10.1016/j.anbehav.2013.10.001) [DOI] [Google Scholar]
- 94.Gillespie SR, Scarlett Tudor M, Moore AJ, Miller CW. 2014. Sexual selection is influenced by both developmental and adult environments. Evolution 68, 3421–3432. ( 10.1111/evo.12526) [DOI] [PubMed] [Google Scholar]
- 95.Taborsky B. 2006. The influence of juvenile and adult environments on life-history trajectories. Proc. R. Soc. B 273, 741–750. ( 10.1098/rspb.2005.3347) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nussey DH, Kruuk LEB, Morris A, Clutton-Brock TH. 2007. Environmental conditions in early life influence ageing rates in a wild population of red deer. Curr. Biol. 17, R1000–R1001. ( 10.1016/j.cub.2007.10.005) [DOI] [PubMed] [Google Scholar]
- 97.Hooper AK, Spagopoulou F, Wylde Z, Maklakov AA, Bonduriansky R. 2017. Ontogenetic timing as a condition-dependent life history trait: high-condition males develop quickly, peak early, and age fast. Evolution 71, 671–685. ( 10.1111/evo.13172) [DOI] [PubMed] [Google Scholar]
- 98.Schultzhaus JN, Nixon JJ, Duran JA, Carney GE. 2017. Diet alters Drosophila melanogaster mate preference and attractiveness. Anim. Behav . 123, 317–327. ( 10.1016/j.anbehav.2016.11.012) [DOI] [Google Scholar]
- 99.Badyaev AV. 2002. Growing apart: an ontogenetic perspective on the evolution of sexual size dimorphism. Trends Ecol. Evol . 17, 369–378. ( 10.1016/S0169-5347(02)02569-7) [DOI] [Google Scholar]
- 100.Abbott JK, Svensson EI. 2007. Ontogeny of sexual dimorphism and phenotypic integration in heritable morphs. Evol. Ecol . 22, 103–121. ( 10.1007/s10682-007-9161-0) [DOI] [Google Scholar]
- 101.Badyaev AV, Hill GE, Beck ML, Dervan AA, Duckworth RA, McGraw KJ, Nolan PM, Whittingham LA. 2002. Sex-biased hatching order and adaptive population divergence in a passerine bird. Science 295, 316–318. ( 10.1126/science.1066651) [DOI] [PubMed] [Google Scholar]
- 102.Hoban S, et al. 2016. Finding the genomic basis of local adaptation: pitfalls, practical solutions, and future directions. Am. Nat . 188, 379–397. ( 10.1086/688018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bay RA, Rose N, Barrett R, Bernatchez L, Ghalambor CK, Lasky JR, Brem RB, Palumbi SR, Ralph P. 2017. Predicting responses to contemporary environmental change using evolutionary response architectures. Am. Nat . 189, 463–473. ( 10.1086/691233) [DOI] [PubMed] [Google Scholar]
- 104.Lancaster LT, Dudaniec RY, Chauhan P, Wellenreuther M, Svensson EI, Hansson B. 2016. Gene expression under thermal stress varies across a geographic range expansion front. Mol. Ecol . 25, 1141–1156. ( 10.1111/mec.13548) [DOI] [PubMed] [Google Scholar]
- 105.Dudaniec RY, Yong CJ, Lancaster LT, Svensson EI, Hansson B. 2018. Signatures of local adaptation along environmental gradients in a range-expanding damselfly (Ischnura elegans). Mol. Ecol. 27, 2576–2593. ( 10.1111/mec.14709) [DOI] [PubMed] [Google Scholar]
- 106.Cheng C, Kirkpatrick M. 2016. Sex-specific selection and sex-biased gene expression in humans and flies. PLoS Genet. 12, e1006170 ( 10.1371/journal.pgen.1006170) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kaufmann J, Lenz TL, Kalbe M, Milinski M, Eizaguirre C. 2017. A field reciprocal transplant experiment reveals asymmetric costs of migration between lake and river ecotypes of three-spined sticklebacks (Gasterosteus aculeatus). J. Evol. Biol . 30, 938–950. ( 10.1111/jeb.13057) [DOI] [PubMed] [Google Scholar]
- 108.Macel M, et al. 2007. Climate vs. soil factors in local adaptation of two common plant species. Ecology 88, 424–433. ( 10.1890/0012-9658(2007)88%5B424:CVSFIL%5D2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 109.Hall MC, Willis JH. 2006. Divergent selection on flowering time contributes to local adaptation in Mimulus guttatus populations. Evolution 60, 2466–2477. ( 10.1111/j.0014-3820.2006.tb01882.x) [DOI] [PubMed] [Google Scholar]
- 110.Wade MJ, Kalisz SM. 1990. The causes of natural selection. Evolution 44, 1947–1955. ( 10.1111/j.1558-5646.1990.tb04301.x) [DOI] [PubMed] [Google Scholar]
- 111.Svensson E, Sinervo B. 2000. Experimental excursions on adaptive landscapes: density-dependent selection on egg size. Evolution 54, 1396–1403. ( 10.1111/j.0014-3820.2000.tb00571.x) [DOI] [PubMed] [Google Scholar]
- 112.Maccoll AD. 2011. The ecological causes of evolution. Trends Ecol. Evol . 26, 514–522. ( 10.1016/j.tree.2011.06.009) [DOI] [PubMed] [Google Scholar]
- 113.Siepielski AM, et al. 2017. Precipitation drives global variation in natural selection. Science 355, 959–962. ( 10.1126/science.aag2773) [DOI] [PubMed] [Google Scholar]
- 114.Connallon T, Hall MD. 2018. Genetic constraints on adaptation: a theoretical primer for the genomics era. Ann. N. Y. Acad. Sci . 1422, 65–87. ( 10.1111/nyas.13536) [DOI] [PubMed] [Google Scholar]
- 115.Mank JE. 2017. Population genetics of sexual conflict in the genomic era. Nat. Rev. Genet . 18, 721–730. ( 10.1038/nrg.2017.83) [DOI] [PubMed] [Google Scholar]
- 116.De Lisle SP, Svensson EI. 2017. On the standardization of fitness and traits in comparative studies of phenotypic selection. Evolution 71, 2313–2326. ( 10.1111/evo.13325) [DOI] [PubMed] [Google Scholar]
- 117.Fisher RA. 1930. The genetical theory of natural selection. Oxford, UK: Clarendon Press. [Google Scholar]
- 118.Boynton PJ, Stelkens R, Kowallik V, Greig D. 2017. Measuring microbial fitness in a field reciprocal transplant experiment. Mol. Ecol. Resour . 17, 370–380. ( 10.1111/1755-0998.12562) [DOI] [PubMed] [Google Scholar]
- 119.Raabova J, Fischer M. 2007. Ecological rather than geographic or genetic distance affects local adaptation of the rare perennial herb, Aster amellus. Biol. Conserv . 139, 348–357. ( 10.1016/j.biocon.2007.07.007) [DOI] [Google Scholar]
- 120.Siepielski AM, Nemirov A, Cattivera M, Nickerson A, 2016. Experimental evidence for an eco-evolutionary coupling between local adaptation and intraspecific competition. Am. Nat . 187, 447–456. ( 10.1086/685295) [DOI] [PubMed] [Google Scholar]
- 121.Chevin LM, Lande R. 2011. Adaptation to marginal habitats by evolution of increased phenotypic plasticity. J. Evol. Biol . 24, 1462–1476. ( 10.1111/j.1420-9101.2011.02279.x) [DOI] [PubMed] [Google Scholar]
- 122.Chevin LM, Lande R, Mace GM. 2010. Adaptation, plasticity, and extinction in a changing environment: towards a predictive theory. PLoS Biol. 8, e1000357 ( 10.1371/journal.pbio.1000357) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This article has no additional data.