Abstract
Sexual conflict can lead to rapid and continuous coevolution between females and males, without any inputs from varying ecology. Yet both the degree of conflict and selection on antagonistic traits are known to be sensitive to local ecological conditions. This leads to the longstanding question: to what extent does variation in ecological context drive sexually antagonistic coevolution? In water striders, there is much information about the impacts of ecological factors on conflict, and about patterns of antagonistic coevolution. However, the connection between the two is poorly understood. Here, we first review the multiple ways in which ecological context might affect the coevolutionary trajectory of the sexes. We then review ecological and coevolutionary patterns in water striders, and connections between them, in light of theory and new data. Our analysis suggests that ecological variation does impact observed patterns of antagonistic coevolution, but highlights significant uncertainty due to the multiple pathways by which ecological factors can influence conflict and its evolutionary outcome. To the extent that water striders are a reasonable reflection of other systems, this observation serves as both an opportunity and a warning: there is much to learn, but gaining insight may be a daunting process in many systems.
This article is part of the theme issue ‘Linking local adaptation with the evolution of sex differences'.
Keywords: arms race, Gerridae, pond skaters, sexual coevolution, sexual conflict, sexual selection
1. Introduction
Sexual selection plays out on an ecological stage, which sets the costs and benefits of sexual interactions and their outcomes. Therefore, ecological context—including variables like predation risk, food supply and population density—is expected to be key in shaping mating system variation and the strength of sexual selection [1–6]. The theory of sexual conflict similarly emphasizes the influence of ecological factors [7–10]. In this paper, we focus on sexual conflict resulting from evolutionary conflicts over mating interactions, both in general and in water striders as a case study [11–13].
We know a considerable amount about the ecology of sexual conflict in water striders [7,14,15]. Early work centred on within-population studies examining how ecological factors (such as food availability, predation risk, population density and sex ratio) affect the economics of conflict, behavioural plasticity around mating decisions and consequences for sexual selection. For example, mating becomes more costly for females in low-food environments because mating interferes with foraging. Females respond by increasing behavioural resistance to mating, which, in turn, increases sexual selection favouring male traits that overcome resistance [16]. In addition to these within-population studies, comparative studies have documented the correlated evolution of antagonistic traits across both populations and species, and demonstrated that variation in these traits relates to variation in mating behaviour among groups [15,17–22].
Given that there is ample information about the ecological forces acting on water strider mating conflicts within populations, and patterns of sexually antagonistic coevolution (SAC) among evolutionary units (populations or species), one might expect to see a straightforward link between the ecologically driven within-population processes and macroevolutionary patterns. However, there is a surprising disconnect between these levels of investigation. Studies have only recently begun to attempt to connect ecological effects on sexual conflict within populations to the coevolutionary dynamics of antagonistic traits [15]. To the question—what role does ecological context play in driving SAC?—we have only partial answers.
We believe this disconnect arises for two related reasons. First, it is challenging to connect ecological effects to coevolutionary patterns because they have been studied at different scales. Ecological effects are usually studied through manipulations within populations, but coevolution has (and must) be studied among evolutionary units. As a result, each level of study ignores the other: studies within populations have assumed a fixed and non-evolving set of sexually antagonistic traits (reviewed in [7,23]), while studies of the dynamics of SAC have frequently ignored ecological variation among evolutionary units and have always ignored behavioural plasticity within evolutionary units [15,17–19,21,22]. Second, it is challenging to discern underlying processes from coevolutionary patterns. Theory for sexual conflict predicts that coevolution of antagonistic traits can happen even in the absence of ecological variation [12,24–27]. Consequently, detecting SAC does not implicate a driving role for ecological factors. Neither of these obstacles is likely to be restricted to sexual conflict and antagonistic coevolution, given the impact of ecology on other processes of sexual selection, and given that at least some mechanisms of sexual selection (e.g. Fisherian runaway selection) can drive the coevolution of preferences and traits even in the absence of ecological variation.
Here, we address the disconnect between within-population and coevolutionary patterns, using water striders of the genus Gerris to focus the discussion. We first briefly review the extensive evidence for ecological effects on sexual conflict within populations. We then review theory and emerging evidence for ecological effects on SAC, developing a framework for how SAC should shift with the ecological setting, and present new supportive data from water striders. Finally, we highlight discrepancies between the two levels of study, and discuss means to bridge the gap between them in future research.
2. Ecological effects on sexual conflict within populations
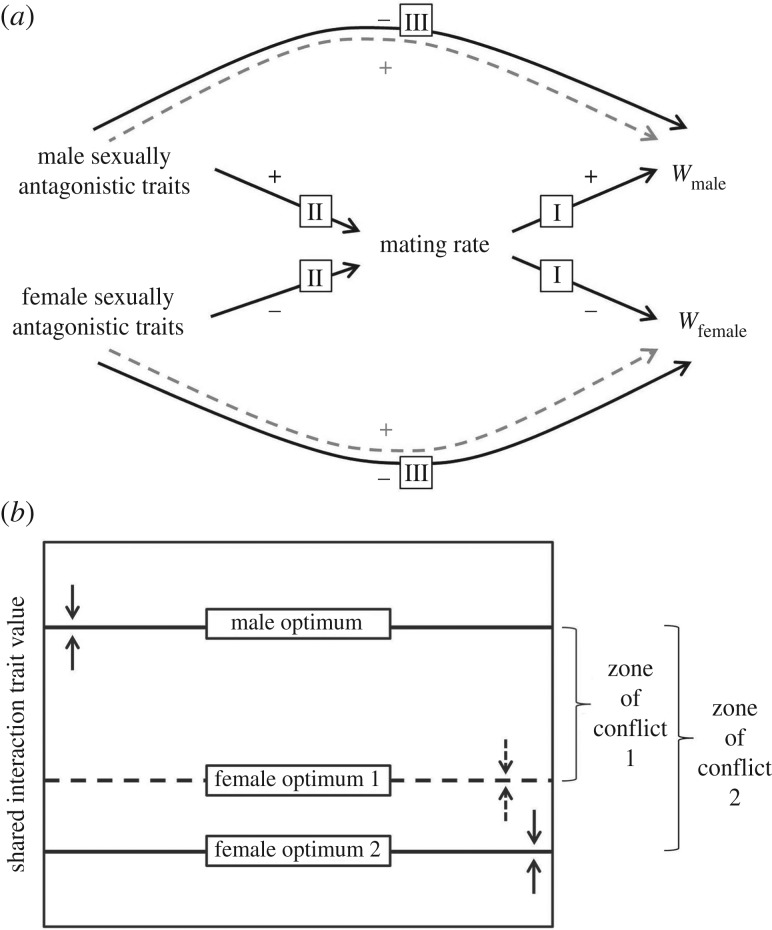
We consider ecological effects on sexually antagonistic selection and coevolution in the light of an earlier model ([28], figure 1). As in this model, males and females in many species experience conflict over mating rate, with males often favoured to mate at a higher rate than females (indicated by positive and negative relationships between mating rate and male and female fitness, respectively, in figure 1a [7,23]). There is sexually antagonistic selection favouring traits, in both sexes, that move realized mating rate in the direction favoured by the sex bearing them. Hence, male traits that increase mating rate and female traits that decrease mating rate (indicated by positive and negative relationships between antagonistic traits and mating rate, respectively; figure 1a) both experience positive indirect selection through their effects on mating rate (dashed lines in figure 1a). These sexually antagonistic traits have been characterized as ‘persistence’ traits in males—for example, behavioural harassment of females or morphological adaptations for holding on to females—and ‘resistance’ traits in females—for example, evasive and defensive behaviour and anti-grasping structures.
Figure 1.
Sexual conflict over mating rate. (a) In this example, we consider a system in which more mating increases male fitness but decreases female fitness (+ and – signs along pathways I for males and females, respectively). Sexual conflict is generated by these opposing effects of mating rate on male and female fitness (w), and results in selection favouring male and female traits that shift mating rate towards each sex's optimum (+ and – signs along pathways II). Sex-specific traits have positive indirect effects on each sex's fitness through effects on mating rate (dashed lines), along with negative direct effects borne by each sex (e.g. trait costs; solid lines). Ecological variation can be expected to shape relationships between traits, mating rate and fitness, at pathways I, II and III (see §2). Adapted with permission from Rowe and Day [29]. (b) Each sex's optimum is set by the balance of sex-specific benefits and costs; arrows indicate the direction of selection. Benefits and costs will be influenced by the local environment; for example, the sexes might experience conflict over a wider range of mating rates in one environment than another. Adapted with permission from Rowe et al. [30].
How might ecological variation influence the degree of sexual conflict and the exaggeration of antagonistic traits? Inspection of figure 1a reveals several possibilities. Ecological factors can influence the optimal mating rate for each sex (acting along pathways I, figure 1a), changing the strength of the relationship between mating rate and fitness within each sex, and hence the degree of conflict over mating (figure 1b). Alternatively, the relationship between sexual armaments and mating rate can change depending on the local environment (along pathways II, figure 1a). For example, the effectiveness of male persistence traits in increasing mating rate might vary with population density and sex ratio, as well as female behavioural resistance to mating, which itself will often be sensitive to ecological variation. Finally, the local environment can set the direct costs of sexual armaments, including costs of developing, bearing, maintaining and deploying traits (pathways III, figure 1a). In all three cases, ecological variation might affect the sexes in sex-specific or congruent ways. Hence, ecological variation can be expected to shape the relationships between mating rate, sexual armaments and fitness, and in complex ways.
Water striders within the genus Gerris share a similar mating system, characterized by multiple mating in both sexes, pre-mating struggles and (frequently) post-mating guarding [7]. Males gain the opportunity for fertilization with each mating, but most mating can be considered superfluous to females at best, beyond very infrequent matings required to replenish sperm, or costly at worst, with costs including reduced feeding efficiency during copulation and increased predation risk [31,32]. Thus, there is sexual conflict over mating (figure 1a), and encounters between the sexes often result in mating attempts by males and behavioural resistance to those attempts by females. Resistance behaviours include vigorous attempts to disengage from males and leaving the water surface [7,33]. In addition to female behavioural resistance, females of many Gerris species have sexually antagonistic morphological structures that include dorsal abdominal spines (figure 2) [34], and in G. gracilicornis, a modified pregenital segment that covers the genital opening [35]. Males have corresponding persistence traits, including frequent and vigorous harassment of females and grasping adaptations, and enlarged sexually dimorphic forelegs and elongated and curved pregenital abdominal segments, which house grasping genitalia (figure 2) [36]. Body size is also likely to evolve at least in part as a sexual armament, as larger females are better able to resist males and larger males better able to overcome resistance [21,37–42]. Furthermore, both sexes experience direct costs from their sexually antagonistic traits (figure 1a). Pre-mating struggles arising from male harassment and female resistance attract predators and can appear energetically demanding [32]. Producing morphological armaments also entails costs. For example, producing spines prolongs moulting and thereby increases risk of predation and cannibalism [43].
Figure 2.
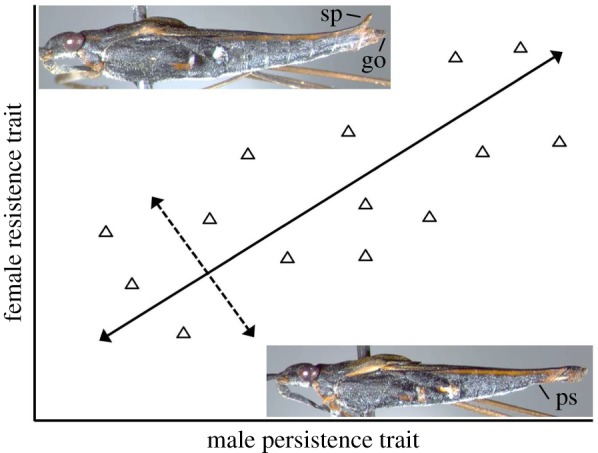
Covariation between male and female sexual armaments among populations or species (triangles, for illustration only). Groups are aligned on a coevolutionary line of equilibrium (solid line, equivalent to a first principal components axis), along which the sexes have more or less evenly matched armaments. Groups might also deviate from the line such that one sex has a relative advantage (dashed line, equivalent to a second principal components axis). Photos show female dorsal spines (sp) above the genital opening (go) and male pregenital segments (ps) of Gerris incognitus; these traits covary among populations [21]. (Online version in colour.)
Ecological effects on each of these pathways have been demonstrated in within-population studies of Gerris spp. (reviewed by [7,23]). Many studies have considered how the ecological context affects the economics of mating, and thus conflict over mating, by examining plasticity in female resistance behaviour (pathways I, figure 1a; [7,23]). In sum, as the costs of mating to females increase, females ramp up their resistance to male mating attempts. There is evidence to suggest that ecologically driven variation in the economics of mating might sometimes be congruent between the sexes (e.g. pre-mating struggles increase predation risk for both sexes) and sometimes be sex-specific (e.g. mating increases predation risk more for females than for males [32]). There is also empirical support for the hypothesis that ecological variation mediates the relationship between sexual armaments and mating rate (pathways II, figure 1a [7,13,23]). Selection favouring male grasping morphology increases when females resist mating; the strength of sexually antagonistic selection, therefore, depends on female behavioural plasticity, which depends on the costs of mating and struggling that are set, in part, by ecological context [7]. Hence, ecological variation can set the degree of sexual conflict, and sexual selection on male traits, through effects on female resistance behaviours. In total, sexual armaments in water striders should be shaped by selection that depends on the costs of mating, the costs of expressing armaments and the expression of the opposite sex's antagonistic traits (including behaviour and morphology). Ecological factors have been demonstrated to influence all of these variables.
3. Patterns of correlated evolution in sexually antagonistic traits
One of the most interesting outcomes of sexual conflict is the potential for SAC, brought about because each sex exerts selection on the other (figure 1a). In groups with strong sexual conflict (figure 1b), SAC is expected to drive rapid evolutionary divergence, and because this divergence does not typically resolve the underlying conflict, there is the potential for sexual arms races, in which armaments in both sexes are exaggerated and more or less evenly matched within groups [12,24–27]. Other coevolutionary outcomes are possible in theoretical models, including a single equilibrium, evolutionary cycling between highly and lowly armed states, and diversification within a sex into multiple phenotypic states [11,26,28,44–48].
(a). Antagonistic coevolution in Gerris
In Gerris spp., just such an arms race pattern has been documented in comparative studies among species [17–19] and among populations of a single species [15,21]. The general pattern of correlated evolution is one of matching across evolutionary units between male and female antagonistic traits [34]: populations or species with exaggerated antagonistic traits in one sex have similar exaggeration in the other sex, generating a line of covariance between male and female traits across units (figure 2). Behavioural trials support the idea that there is some balancing of these traits between the sexes during correlated evolution: in common garden trials there is little variance in mating rate along the evolutionary line of covariance [19,21]. Moreover, in groups that are displaced from the line of covariance, when females are relatively armed, mating rate is reduced, whereas when males are relatively armed, mating rate increases [19,21].
(b). Ecological effects on antagonistic coevolution
We know much less—theoretically and empirically—about the influence of ecological variation on SAC than on sexual conflict and sexually antagonistic selection within populations [10]. Here, we offer a framework to address this question in Gerris spp., and more generally.
As a starting point, we note that theory suggests that SAC can occur in the absence of any ecological variation [12,24–27]. Net selection on each sex's antagonistic traits may be dominated by selection resulting from the opposite sex's antagonistic traits. Here, evolutionary units may evolve along a coevolutionary line of equilibrium, and their position along the line should depend on initial conditions, stochastic mutation order effects and genetic drift ([12,13,24,48–50]; see also [51]). As a water strider example, suppose that a mutation invades a population that increases female investment in spines, such that (all else being equal) mating rate is reduced. This increases selection for larger male grasping traits (figure 1a), which, in turn, might increase selection for even larger female spines. This ratcheting process will move the group up and to the right on the line of equilibrium (figure 2). Because the sexes remain evenly matched, mating rate should not vary among groups along the line. We can also expect to find populations that have moved away from the line towards sexual mismatch; in the example above, females may initially gain an advantage in spines before males catch up. In any large enough set of groups there will inevitably be some groups where one sex has a temporary advantage. The mating behaviour outcome should reflect the imbalance in armaments, such that mating rates should shift towards the optimum for the sex with a relative advantage in armaments. Thus, the evolutionary dynamics of SAC alone can explain a group's position in coevolutionary space, even without ecological variation. We can use this model as a base from which to explore the potential role of ecological variation in driving units up and down an observed line of covariance.
At first pass, the pattern predicted by this non-ecological model fits the comparative data for Gerris well. For both among-species and among-population contrasts, male and female traits are positively correlated among evolutionary units, without any change in mating rates in common garden experiments, suggesting that exaggeration of male and female traits are matched. Although these results are consistent with models of ecology-free SAC, it seems unlikely to us that ecology has no role, given the many ways that ecological context can affect the economics of mating and trait expression (figure 1a).
We suggest two scenarios by which ecological variation might cause groups to move up or down such a coevolutionary trajectory. First, optimal mating rates in both sexes might vary among environments, leading to higher investment in sexual armaments in groups that experience high conflict, and lower investment in groups that experience low conflict. For example, as noted above, at high population densities, the costs of mating for females start to be outweighed by the costs of continuous male harassment when not mating. Here females tend to resist male mating attempts less vigorously, and will experience less selection favouring exaggerated spines; both factors will lead to reduced selection on persistence traits in males. The outcome will be a correlated reduction in armaments in both sexes with decreasing conflict; in other words, the same morphological pattern is generated as with ecology-free SAC.
Testing this hypothesis will require measuring the relative degree of conflict over mating across evolutionary units in their natural habitat, a challenging task given that it requires data on how variable mating rates impact fitness in both sexes in the wild. As a first pass, one can instead assess mating rates under natural conditions across units. The hypothesis above suggests that mating rates will be higher in less armed species. A weakness of the comparative datasets we have described above is that mating rate has been assessed across evolutionary units in a common garden environment. The available data on mating rate variation among populations or species, therefore, do not necessarily reflect optimal mating rates for either sex or realized mating rates in their natural habitats. These common garden experiments tell us only that when antagonistic traits are matched between the sexes across groups, mating rate will be similar when in the same environment.
A second scenario is that variation among groups along a line of covariance results from ecological effects on the costs and benefits of expressing sexually antagonistic traits, if some environments are more permissive for developing these traits in both sexes. For example, in water striders, pre-mating struggles, which reflect both male persistence and female resistance, increase predation risk for both sexes [32], and so may be similarly sensitive to variation in predation. Thus, we would expect correlated evolution across a predation gradient.
One can also ask what ecological forces might drive groups away from the line of covariance. The scenarios above might explain movement away from the line when ecological variation has sex-specific effects, rather than congruent effects on males and females. For example, if the costs of producing antagonistic traits are elevated in females alone, then we expect less investment in antagonistic traits by females, relative to males, and higher mating rates. Although the currently available data demonstrate that relatively unarmed females (from groups falling on the male side of the line of equilibrium) mate at a higher rate in a common garden environment, these data do not tell us about mating rates in the field, and cannot address the costs of armament expression.
In the end, we are left in a position where a simple ecology-free model can account for the coevolutionary pattern in sexually antagonistic traits in water striders, but there is a wealth of data suggesting a central role of ecology in conflict (§2), leaving a nagging sense that they must be connected. Moreover, although ecology can drive the comparative patterns we see, it can do so in many alternative ways. Any given ecological variable is likely to affect several aspects of sexual conflict (e.g. the pathways in figure 1a); for example, a higher predation risk increases the costs of mating in males and females, but might do so to different extents in each sex [32], while also increasing the costs of producing spines [43] and engaging in both harassment and resistance behaviours [32]. Thus, the only direct route to distinguishing the hypotheses is to measure selection acting on each pathway illustrated in figure 1a. This is more than a daunting task!
A promising and more tractable alternative is to measure ecological variables—rather than selection—across local environments, and to ask whether patterns emerge that could narrow the options. If there are no ecological correlates that explain variation in sexual armaments or mating rates among groups, then the simple non-ecological model of SAC would gain support. If one or more ecological factors emerge, then closer study of their impact on the pathways identified in figure 1a is warranted. This is the approach we have begun in analysing the correlated evolution of antagonistic traits among populations of Gerris incognitus in recently published work [15] and in new analyses below.
4. Ecological correlates of the arms race in Gerris incognitus
Our goals in a recent study were to test how candidate ecological variables are associated with population divergence in sexual armaments, and whether ecological variation could account for the positive covariation between male and female armaments without a need to invoke SAC itself [15]. We identified 12 ecological variables (table 1) all relating to aspects of growth, survival or costs of mating or pre-mating struggles, from within-population functional studies of water striders. Variables related to growth included measures of warmth, the presence of other water striders as potential competitors for food [55], and measures relating to aquatic habitat stability (water body size and precipitation), as more stable habitats are associated with larger body size [56]. Variables related to overwintering survival included measures of the severity of winter cold and the depth of snow as protection from cold [52]. Because the risk of predation is an important source of mating and resistance costs, we considered the presence of common Gerris predators, as well as ecological correlates of predator abundance (emergent vegetation, canopy cover and water acidity [57–62]). Note that the absence of a detectable relationship between morphology and ecological variables does not tell us whether there is no relationship, whether our measures are only weakly correlated with real ecological variation, or whether temporal fluctuations in the environment limit the consistency of ecological effects on morphological evolution. Many of these variables are known to affect more than one dimension of water strider life. As examples, emergent vegetation, canopy cover and water acidity affect community structure for aquatic invertebrates, including the diversity and abundance not only of predators of water striders but also of prey and heterospecific competitors [63–65]. This makes it likely that any given ecological dimension might act along several pathways related to sexual conflict simultaneously (figure 1a).
Table 1.
The strength of evidence for ecological models of mismatch in sexual armaments (scores on principal component axis 2 summarizing variation in female spines and male pregenitals), across 16 populations of G. incognitus sampled from British Columbia, Canada (see [21]). Akaike's information criterion (corrected for small sample sizes; AICc) is given along with model probability, indicating the probability of each model within the set of models given the data and evidence ratio, measuring how many times greater support is for the best supported model, compared with model i. The set of models includes a baseline (intercept-only) model for comparison and an ecological summary model (PC1 ecology, with PC1 scores for the three ecological variables with AICc values lower than the baseline model).
| model description | factors | AICci | Δi | model probability | evidence ratio | adjusted R2 |
|---|---|---|---|---|---|---|
| ecological summary model | PC1 ecology | 16.7 | 0.0 | 0.28 | 1.0 | 0.24 |
| snow depth | mean depth of snow [52] | 17.4 | 0.7 | 0.19 | 1.4 | 0.21 |
| winter severity | mean number of days annually with temperature approaching lower lethal threshold (−10°C) [52] | 18.6 | 1.9 | 0.10 | 2.6 | 0.14 |
| breeding season length | mean number of frost free days annually [53] | 18.6 | 2.0 | 0.10 | 2.7 | 0.14 |
| baseline model | intercept only | 19.1 | 2.4 | 0.08 | 3.4 | n.a. |
| elevation | 20.1 | 3.5 | 0.05 | 5.6 | 0.06 | |
| temperature | mean number of days with temperature > growth threshold for Gerris spp. (10°C) [54] | 20.4 | 3.7 | 0.04 | 6.3 | 0.04 |
| habitat stability | water body area | 21.3 | 4.6 | 0.03 | 10.0 | −0.01 |
| canopy cover | index from 0 to 5 (0, none; 1, 0–20%; 2, 21–40%; 3, 41–60%; 4, 61–80%; 5, 81–100%) | 21.5 | 4.9 | 0.02 | 11.3 | −0.03 |
| emergent vegetation | as for canopy cover | 21.9 | 5.2 | 0.02 | 13.6 | −0.05 |
| water aciditya | pH | 22.0 | 5.4 | 0.02 | 14.7 | −0.06 |
| precipitation | mean precipitation during the breeding season | 22.1 | 5.4 | 0.02 | 14.8 | −0.06 |
| predator presence | detection of corixid bugs, dytiscid or gyrinid beetles, water spiders, minnows, frogs and ducks, by net samples and visual and audial inspection | 22.1 | 5.5 | 0.02 | 15.3 | −0.07 |
| presence of other water striders | 22.1 | 5.5 | 0.02 | 15.5 | −0.07 |
aMeasured at 14 sites; imputed for two sites by the relationship between measured pH and geographical coordinates (see [15]).
From the set of ecological variables, we detected several that were associated with a population's degree of exaggeration in sexual armaments—in particular, female spines and male pregenital segments—in 16 G. incognitus populations [15]. Populations with reduced sexual armaments tend to occupy more productive local environments: warmer areas with less acidic water. Hence, a population's position along the line of covariation is associated with characteristics of its local environment. This ecological signature to variation in sexual armaments raises the question of whether ecological variation alone can explain the positive covariation between male and female armaments among groups, without a need to invoke SAC. However, we found that positive sexual covariation in armaments remained even after accounting statistically for both ecological variation and for neutral genetic variation [15]. Therefore, true coevolution is indicated—antagonistic traits in one sex appear to be driving the evolution of antagonistic traits in the other—and ecology appears to be influencing coevolution.
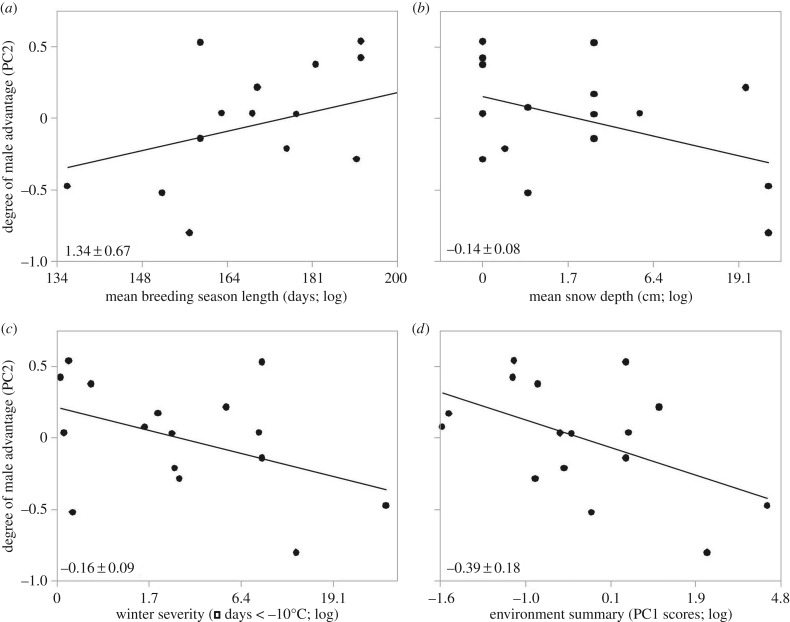
We now expand this example to ask two new questions, using data from the same G. incognitus populations (electronic supplementary material, table S1). First, can ecological variation account for which sex holds a relative advantage in sexual armaments? We test this idea by modelling the degree of mismatch between female spines and male pregenitals (measured by scores on the second principal components axis summarizing variation in female spines and male pregenitals) as a function of the ecological variables described above. We found that females had a relative advantage (here, lower PC2 scores) in populations occupying harsher abiotic environments (table 1 and figure 3)—those with shorter breeding seasons and more severe winters, with greater depth of snow and more often near the lower lethal limit for overwintering water striders. Although these two variables should have opposing effects on overwintering mortality (i.e. more snow makes winters less severe for water striders [52]), those local environments with more snow also have lower temperatures and longer winters. Hence, ecological variation is associated not only with the degree of exaggeration of sexual armaments [15], but also with who has the lead in the sexual arms race.
Figure 3.
Relationships between three ecological variables (a–c) and a summary measure of these three variables (PC1 scores; d) with the degree of male advantage in sexual morphology (scores from the second principal component summarizing variation in female spines and male pregenitals, where zero indicates balanced sexual armaments relative to this set of populations), across 16 populations of G. incognitus. Slopes with standard errors are given in the lower left of each panel. See table 1 for R2 values.
Second, is variation in mating behaviour solely an outcome of sexual morphology, or do populations diverge in mating behaviour with respect to the local environment and independent of morphology? We asked whether we could detect a relationship between ecological and behavioural variation, after accounting for behavioural variation explained by morphology. To do this, we calculated residual values for sexual behaviour from a multiple regression that included morphological variables associated with behavioural variation [21]: the relative advantage males have in sexual morphology (PC2 scores, as above), sexual size dimorphism, and their interaction. We measured behaviour in a common garden laboratory environment, as noted above, and used two measures of mating behaviour: a summary measure of sexual activity (PC1 scores summarizing variation in the frequency of male harassment, the proportion of struggles that resulted in mating, struggle duration and mating rate; see [21]) and mating rate alone.
After accounting for associations between sexual morphology and these two measures of behaviour, we did not find any detectable relationship between ecological and behavioural variation (tables 2 and 3). We, therefore, find no evidence for the independent evolution of mating behaviour in response to the local environment. Instead, variation in mating behaviour appears to arise based on relative advantage in sexual armaments or in body size.
Table 2.
The strength of evidence for ecological models of a summary measure of mating behaviour, after controlling for variation in mating behaviour associated with sexual morphology. Model variables and AICc results are described in table 1. The set of models includes a baseline (intercept-only) model for comparison.
| model description | AICci | Δi | model probability | evidence ratio | adjusted R2 |
|---|---|---|---|---|---|
| baseline | 63.6 | 0.0 | 0.21 | 1.0 | n.a. |
| breeding season length | 64.5 | 0.9 | 0.14 | 1.5 | 0.07 |
| canopy cover | 65.5 | 1.8 | 0.09 | 2.5 | 0.01 |
| snow depth | 65.7 | 2.1 | 0.08 | 2.8 | −0.01 |
| precipitation | 65.7 | 2.1 | 0.07 | 2.9 | −0.01 |
| winter severity | 66.0 | 2.3 | 0.07 | 3.2 | −0.02 |
| predator presence | 66.2 | 2.6 | 0.06 | 3.7 | −0.04 |
| presence of other water striders | 66.5 | 2.9 | 0.05 | 4.3 | −0.06 |
| emergent vegetation | 66.6 | 2.9 | 0.05 | 4.4 | −0.06 |
| temperature | 66.6 | 3.0 | 0.05 | 4.4 | −0.07 |
| habitat stability | 66.7 | 3.0 | 0.05 | 4.6 | −0.07 |
| elevation | 66.7 | 3.1 | 0.05 | 4.6 | −0.07 |
| water acidity | 66.7 | 3.1 | 0.05 | 4.6 | −0.07 |
Table 3.
The strength of evidence for ecological models of variation in mating rate, measured under laboratory conditions, after controlling for variation in mating behaviour associated with sexual morphology. Model variables and AICc results are described in table 1. The set of models includes a baseline (intercept-only) model for comparison.
| model description | AICci | Δi | model probability | evidence ratio | adjusted R2 |
|---|---|---|---|---|---|
| baseline | −9.2 | 0.0 | 0.22 | 1.0 | n.a. |
| breeding season length | −8.2 | 1.0 | 0.14 | 1.6 | 0.06 |
| canopy cover | −6.9 | 2.2 | 0.07 | 3.0 | −0.02 |
| snow depth | −6.9 | 2.2 | 0.07 | 3.0 | −0.02 |
| precipitation | −6.5 | 2.7 | 0.06 | 3.8 | −0.04 |
| winter severity | −6.5 | 2.7 | 0.06 | 3.8 | −0.04 |
| predator presence | −6.5 | 2.7 | 0.06 | 3.8 | −0.04 |
| presence of other water striders | −6.4 | 2.8 | 0.06 | 4.0 | −0.05 |
| emergent vegetation | −6.4 | 2.8 | 0.06 | 4.0 | −0.05 |
| temperature | −6.3 | 2.9 | 0.05 | 4.2 | −0.06 |
| habitat stability | −6.1 | 3.1 | 0.05 | 4.6 | −0.07 |
| elevation | −6.1 | 3.1 | 0.05 | 4.7 | −0.07 |
| water acidity | −6.1 | 3.1 | 0.05 | 4.7 | −0.07 |
5. The future
We found several ecological correlates for the outcome of SAC, suggesting that ecology may play a role in the process. The next, and more work-intensive, step is to develop an understanding of how these ecological parameters translate into those factors that we know directly impact the pathways identified in figure 1a. These include density, food supply and predator abundance. There are some behavioural metrics that should allow a short cut in narrowing down the possible role of ecology. Prime among these is an understanding of how optimal mating rates in the two sexes, and hence the degree of conflict, varies across these ecological gradients. As noted, this is a formidable task, but simply measuring mating rates across these gradients would at least indicate whether or not there was a correlated change in one or both of the sexes. We know, from the common garden laboratory studies discussed above, that in the absence of ecological change, mating rates do not vary among the populations; if they do in the wild, then ecological factors are implicated.
We also do not currently know how general the ecological correlates of SAC that we have observed are in water striders. Our findings are based on correlations across 16 populations, and it will be important to test the extent to which the correlations hold in independent population datasets of G. incognitus and congeners. Furthermore, the extent to which ecology shapes SAC at the species level is entirely unknown. On the one hand, different species are more likely to experience distinct ecological settings, which should magnify ecological effects. On the other, species-level sexual coevolution might be more strongly influenced by rapid divergence associated with speciation events [66,67], where a signature of ecology may be lost.
There are whole sets of interesting questions that remain open in this group, and in the field of sexual conflict more generally. For example, we have previously suggested that female body size and female spines might act as alternative sexual armaments in water striders [21]. Perhaps ecological variation determines the diversification of alternative phenotypes, rather than only the exaggeration of single types. Another open area is how ecological input affects the links between SAC and speciation and extinction. The relationship between sexual conflict and speciation has been controversial. Some theory predicts that conflict should enhance speciation (e.g. [26,68,69]), whereas other suggests a more complex effect [29,70,71], and yet other theoretical work predicts that it will also enhance extinction [72,73]. Empirical tests have been inconclusive, with mixed support [74,75]. To the extent that ecological factors affect the degree of sexual conflict, they can play a role in speciation and extinction.
6. Summary and outlook
We began this paper by emphasizing a surprising disconnect in water striders between our understanding of the role of local ecological factors in determining the degree of sexual conflict and selection on antagonistic traits, and the observed macroevolutionary patterns in SAC. Our initial attempts at connecting these two datasets have brought us forward. We provide evidence here and elsewhere [15] that ecological factors are correlated with movement of populations both along and off the observed line of antagonistic trait coevolution. These data implicate a role for ecology in the coevolution of sexually antagonistic traits. We also demonstrate that there is little evolution in the behaviours involved in conflict. We know these behaviours are highly plastic, and that may slow their evolution. Despite identifying some environmental correlates of SAC, we do not know how, of the many routes, they may affect optimal mating rates or trait expression, and so there is considerable distance to go. The next steps require much more attention to comparative ecological and behavioural studies, particularly local mating rates. Furthermore, the complexity of the questions is heightened by sexual coevolution: any ecologically driven change in behaviour or morphology in one sex will change selection on the other.
Although we have centred the discussion around SAC and on water striders, the complexity of the problem almost certainly extends to other species and forms of intersexual coevolution. The economics of mating interactions are known to be sensitive to local ecological conditions across a broad array of systems (e.g. [76–78]). This may be particularly true for resource-based systems where ecological factors (e.g. food resources) are part of sexual interactions. For example, in nuptial-feeding katydids, the strength of sexual selection is sensitive to food supply, and substantial changes in supply can even change which sex is exercising mate choice [79]. But even non-resource based and apparently simple systems can be highly sensitive to ecological conditions. For example, recent work on fruit flies has demonstrated that spatial structure can affect the strength of mate choice and sexual conflict [80]. Understanding the extent of the effects of ecological context on intersexual coevolution, including SAC, will require much closer study of the economy of mating, as others have noted [4,5,9]. Our experience with water striders suggests that this is a first step, and that connecting that understanding to coevolutionary pattern will be a second and perhaps longer step.
Supplementary Material
Acknowledgements
We are grateful to T. Connallon, F. Débarre and X.-Y. Li for inviting us to contribute this article, J.M. Biernaskie for discussion and two anonymous reviewers for helpful feedback that improved the manuscript.
Data accessibility
The dataset supporting this article has been uploaded as part of the electronic supplementary material.
Authors' contributions
J.C.P and L.R. contributed to the design and writing of this manuscript.
Competing interests
We have no competing interests.
Funding
J.C.P. was funded by a fellowship from the Natural Environment Research Council (NERC; NE/P017193/1).
References
- 1.Emlen ST, Oring LW. 1977. Ecology, sexual selection, and the evolution of mating systems. Science 197, 215–223. ( 10.1126/science.327542) [DOI] [PubMed] [Google Scholar]
- 2.Vehrencamp SL, Bradbury JW. 1984. Mating systems and ecology. In Behavioral ecology: an evolutionary approach (eds Krebs JR, Davies NB), pp. 251–278. Oxford, UK: Blackwell. [Google Scholar]
- 3.Davies NB. 1991. Mating systems. In Behavioral ecology: an evolutionary approach (eds Krebs JR, Davies NB), pp. 263–294. Oxford, UK: Blackwell. [Google Scholar]
- 4.Cornwallis CK, Uller T. 2010. Towards an evolutionary ecology of sexual traits. Trends Ecol. Evol . 25, 145–152. ( 10.1016/j.tree.2009.09.008) [DOI] [PubMed] [Google Scholar]
- 5.Miller CW, Svensson EI. 2014. Sexual selection in complex environments. Annu. Rev. Entomol . 59, 427–445. ( 10.1146/annurev-ento-011613-162044) [DOI] [PubMed] [Google Scholar]
- 6.Holman L, Kokko H. 2014. Local adaptation and the evolution of female choice. In Genotype-by-environment interactions and sexual selection (eds Hunt J, Hosken DJ), pp. 41–62. Chichester, UK: Wiley-Blackwell. [Google Scholar]
- 7.Rowe L, Arnqvist G, Sih A, Krupa JJ. 1994. Sexual conflict and the evolutionary ecology of mating patterns: water striders as a model system. Trends Ecol. Evol . 9, 289–293. ( 10.1016/0169-5347(94)90032-9) [DOI] [PubMed] [Google Scholar]
- 8.Chapman T, Arnqvist G, Bangham J, Rowe L. 2003. Sexual conflict. Trends Ecol. Evol . 18, 41–47. ( 10.1016/S0169-5347(02)00004-6) [DOI] [Google Scholar]
- 9.Fricke C, Perry J, Chapman T, Rowe L. 2009. The conditional economics of sexual conflict. Biol. Lett . 5, 671–674. ( 10.1098/rsbl.2009.0433) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perry JC, Rowe L. 2014. The evolution of sexually antagonistic phenotypes. In The genetics and biology of sexual conflict (eds Rice WR, Gavrilets S), pp. 83–100. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parker GA. 1979. Sexual selection and sexual conflict. In Sexual selection and reproductive competition in insects (eds Blum MS, Blum NA), pp. 123–166. New York, NY: Academic Press. [Google Scholar]
- 12.Holland B, Rice WR. 1998. Chase-away sexual selection: antagonistic seduction versus resistance. Evolution 52, 1–7. ( 10.1111/j.1558-5646.1998.tb05132.x) [DOI] [PubMed] [Google Scholar]
- 13.Arnqvist G, Rowe L. 2005. Sexual conflict. Princeton, NJ: Princeton University Press. [Google Scholar]
- 14.Sih A, Montiglio P-O, Wey TW, Fogarty S. 2017. Altered physical and social conditions produce rapidly reversible mating systems in water striders. Behav. Ecol . 28, 632–639. ( 10.1093/beheco/arx021) [DOI] [Google Scholar]
- 15.Perry JC, Garroway CJ, Rowe L. 2017. The role of ecology, neutral processes and antagonistic coevolution in an apparent sexual arms race. Ecol. Lett . 20, 1107–1117. ( 10.1111/ele.12806) [DOI] [PubMed] [Google Scholar]
- 16.Ortigosa A, Rowe L. 2002. The effect of hunger on mating behaviour and sexual selection for male body size in Gerris buenoi. Anim. Behav . 64, 369–375. ( 10.1006/anbe.2002.3065) [DOI] [Google Scholar]
- 17.Arnqvist G, Rowe L. 2002. Comparative analysis unveils antagonistic coevolution between the sexes in a group of insects. Nature 415, 787–789. ( 10.1038/415787a) [DOI] [PubMed] [Google Scholar]
- 18.Arnqvist G, Rowe L. 2002. Correlated evolution of male and female morphologies in water striders. Evolution 56, 936–947. ( 10.1111/j.0014-3820.2002.tb01406.x) [DOI] [PubMed] [Google Scholar]
- 19.Rowe L, Arnqvist G. 2002. Sexually antagonistic coevolution in a mating system: combining experimental and comparative approaches to address evolutionary processes. Evolution 56, 754–767. ( 10.1111/j.0014-3820.2002.tb01386.x) [DOI] [PubMed] [Google Scholar]
- 20.Gagnon M-C, Turgeon J. 2011. Sexual conflict in Gerris gillettei (Insecta: Hemiptera): intraspecific intersexual correlated morphology and experimental assessment of behaviour and fitness. J. Evol. Biol . 24, 1505–1516. ( 10.1111/j.1420-9101.2011.02283.x) [DOI] [PubMed] [Google Scholar]
- 21.Perry JC, Rowe L. 2012. Sexual conflict and antagonistic coevolution across water strider populations. Evolution 66, 544–557. ( 10.1111/j.1558-5646.2011.01464.x) [DOI] [PubMed] [Google Scholar]
- 22.Rowe L, Arnqvist G. 2012. Sexual selection and the evolution of genital shape and complexity in water striders. Evolution 66, 40–54. ( 10.1111/j.1558-5646.2011.01411.x) [DOI] [PubMed] [Google Scholar]
- 23.Arnqvist G. 1997. The evolution of water strider mating systems: causes and consequences of intersexual conflicts of interest. In Social competition and cooperation in insects and arachnids: evolution of mating systems (eds Choe JC, Crespi BJ), pp. 146–163. Princeton, NJ: Princeton University Press. [Google Scholar]
- 24.Gavrilets S. 2000. Rapid evolution of reproductive barriers driven by sexual conflict. Nature 403, 886–889. ( 10.1038/35002564) [DOI] [PubMed] [Google Scholar]
- 25.Gavrilets S, Arnqvist G, Friberg U. 2001. The evolution of female mate choice by sexual conflict. Proc. R. Soc. B 268, 531–539. ( 10.1098/rspb.2000.1382) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gavrilets S, Waxman D. 2002. Sympatric speciation by sexual conflict. Proc. Natl Acad. Sci. USA 99, 10 533–10 538. ( 10.1073/pnas.152011499) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gavrilets S, Hayashi TI. 2006. The dynamics of two- and three-way sexual conflicts over mating. Phil. Trans. R. Soc. B 361, 345–354. ( 10.1098/rstb.2005.1792) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rowe L, Cameron E, Day T. 2005. Escalation, retreat, and female indifference as alternative outcomes of sexually antagonistic coevolution. Am. Nat . 165, S5–S18. ( 10.1086/429395) [DOI] [PubMed] [Google Scholar]
- 29.Rowe L, Day T. 2006. Detecting sexual conflict and sexually antagonistic coevolution. Phil. Trans. R. Soc. B 361, 277–285. ( 10.1098/rstb.2005.1788) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rowe L, Cameron E, Day T. 2003. Detecting sexually antagonistic coevolution with population crosses. Proc. R. Soc. B 270, 2009–2016. ( 10.1098/rspb.2003.2453) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rowe L. 1992. Convenience polyandry in a water strider: foraging conflicts and female control of copulation frequency and guarding duration. Anim. Behav . 44, 189–202. ( 10.1016/0003-3472(92)90025-5) [DOI] [Google Scholar]
- 32.Rowe L. 1994. The cost of mating and mate choice in water striders. Anim. Behav . 48, 1049–1056. ( 10.1006/anbe.1994.1338) [DOI] [Google Scholar]
- 33.Pineaux M, Turgeon J. 2017. Behavioural consistency in female resistance to male harassment in a water strider species. Ethology 123, 83–93. ( 10.1111/eth.12575) [DOI] [Google Scholar]
- 34.Arnqvist G, Rowe L. 1995. Sexual conflict and arms races between the sexes—a morphological adaptation for control of mating in a female insect. Proc. R. Soc. B 261, 123–127. ( 10.1098/rspb.1995.0126) [DOI] [Google Scholar]
- 35.Han CS, Jablonski PG. 2009. Female genitalia concealment promotes intimate male courtship in a water strider. PLoS ONE 4, e5793 ( 10.1371/journal.pone.0005793) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Andersen NM. 1982. The semiaquatic bugs (Hemiptera, Gerromorpha): phylogeny, adaptations, biogeography and classification. Klampenborg, Denmark: Scandinavian Science Press. [Google Scholar]
- 37.Fairbairn DJ. 1988. Sexual selection for homogamy in the Gerridae: an extension of Ridley's comparative approach. Evolution 42, 1212–1222. ( 10.1111/j.1558-5646.1988.tb04181.x) [DOI] [PubMed] [Google Scholar]
- 38.Sih A, Krupa JJ. 1992. Predation risk, food deprivation and non-random mating by size in the stream water strider, Aquarius remigis. Behav. Ecol. Soc . 31, 51–56. ( 10.1007/BF00167815) [DOI] [Google Scholar]
- 39.Arnqvist G, Rowe L, Krupa JJ, Sih A. 1996. Assortative mating by size: a meta-analysis of mating patterns in water striders. Evol. Ecol . 10, 265–284. ( 10.1007/BF01237684) [DOI] [Google Scholar]
- 40.Rowe L, Arnqvist G. 1996. Analysis of the causal components of assortative mating in water striders. Behav. Ecol. Soc . 38, 279–286. ( 10.1007/s002650050243) [DOI] [Google Scholar]
- 41.Arnqvist G, Danielsson I. 1999. Postmating sexual selection: the effects of male body size and recovery period on paternity and egg production rate in a water strider. Behav. Ecol . 10, 358–365. ( 10.1093/beheco/10.4.358) [DOI] [Google Scholar]
- 42.Ortigosa A, Rowe L. 2003. The role of mating history and male size in determining mating behaviours and sexual conflict in a water strider. Anim. Behav . 65, 851–858. ( 10.1006/anbe.2003.2112) [DOI] [Google Scholar]
- 43.Arnqvist G. 1994. The cost of male secondary sexual traits: developmental constraints during ontogeny in a sexually dimorphic water strider. Am. Nat . 144, 119–132. ( 10.1086/285664) [DOI] [Google Scholar]
- 44.Kazancioğlu E, Alonzo SH. 2012. The evolution of optimal female mating rate changes the coevolutionary dynamics of female resistance and male persistence. Phil. Trans. R. Soc. B 367, 2339–2347. ( 10.1098/rstb.2012.0219) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Härdling R, Bergsten J. 2006. Nonrandom mating preserves intrasexual polymorphism and stops population differentiation in sexual conflict. Am. Nat . 167, 401–409. ( 10.1086/498946) [DOI] [PubMed] [Google Scholar]
- 46.Härdling R, Smith HG. 2005. Antagonistic coevolution under sexual conflict. Evol. Ecol . 19, 137–150. ( 10.1007/s10682-004-7917-3) [DOI] [Google Scholar]
- 47.Gavrilets S, Hayashi TI. 2005. Speciation and sexual conflict. Evol. Ecol . 19, 167–198. ( 10.1007/s10682-004-7916-4) [DOI] [Google Scholar]
- 48.Hayashi TI, Vose M, Gavrilets S. 2007. Genetic differentiation by sexual conflict. Evolution 61, 516–529. ( 10.1111/j.1558-5646.2007.00059.x) [DOI] [PubMed] [Google Scholar]
- 49.Kimura M, Ihara Y. 2009. Replicator-dynamics models of sexual conflict. J. Theor. Biol . 260, 90–97. ( 10.1016/j.jtbi.2009.06.003) [DOI] [PubMed] [Google Scholar]
- 50.Arbuthnott D. 2017. The ecology of non-ecological diversification: how non-sexual selection affects within-environment diversification via sexual conflict. Evol. Ecol. Res . 18, 503–513. [Google Scholar]
- 51.Lande R. 1981. Models of speciation by sexual selection on plolygenic traits. Proc. Natl Acad. Sci. USA 78, 3721–3725. ( 10.1073/pnas.78.6.3721) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ditrich T, Koštál V. 2011. Comparative analysis of overwintering physiology in nine species of semi-aquatic bugs (Heteroptera: Gerromorpha). Physiol. Entomol . 36, 261–270. ( 10.1111/j.1365-3032.2011.00794.x) [DOI] [Google Scholar]
- 53.Blanckenhorn WU, Fairbairn DJ. 1995. Life history adaptation along a latitudinal cline in the water strider Aquarius remigis (Heteroptera: Gerridae). J. Evol. Biol . 8, 21–41. ( 10.1046/j.1420-9101.1995.8010021.x) [DOI] [Google Scholar]
- 54.Spence JR, Spence DH, Scudder GGE. 1980. The effects of temperature on growth and development of water strider species (Heteroptera: Gerridae) of central British Columbia and implications for species packing. Can. J. Zool . 58, 1813–1820. ( 10.1139/z80-248) [DOI] [Google Scholar]
- 55.Spence JR. 1986. Relative impacts of mortality factors in field populations of the waterstrider Gerris buenoi Kirkaldy (Heteroptera: Gerridae). Oecologia 70, 68–76. ( 10.1007/BF00377112) [DOI] [PubMed] [Google Scholar]
- 56.Fairbairn DJ. 1984. Microgeographic variation in body size and development time in the waterstrider, Limnoporus notabilis. Oecologia 61, 126–133. ( 10.1007/BF00379098) [DOI] [PubMed] [Google Scholar]
- 57.Bendell BE, McNicol DK. 1987. Cyprinid assemblages, and the physical and chemical characteristics of small northern Ontario lakes. Environ. Biol. Fish . 19, 229–234. ( 10.1007/BF00005352) [DOI] [Google Scholar]
- 58.Karaouzas I, Gritzalis KC. 2006. Local and regional factors determining aquatic and semi-aquatic bug (Heteroptera) assemblages in rivers and streams of Greece. Hydrobiologia 573, 199–212. ( 10.1007/s10750-006-0274-1) [DOI] [Google Scholar]
- 59.Battle J, Golladay SW. 2001. Water quality and macroinvertebrate assemblages in three types of seasonally inundated limesink wetlands in southwest Georgia. J. Freshw. Ecol . 16, 189–207. ( 10.1080/02705060.2001.9663804) [DOI] [Google Scholar]
- 60.Spence JR. 1983. Pattern and process in co-existence of water-striders (Heteroptera: Gerridae). J. Anim. Ecol . 52, 497–511. ( 10.2307/4568) [DOI] [Google Scholar]
- 61.Binckley CA, Resetarits JWJ. 2009. Spatial and temporal dynamics of habitat selection across canopy gradients generates patterns of species richness and composition in aquatic beetles. Ecol. Entomol . 34, 457–465. ( 10.1111/j.1365-2311.2008.01069.x) [DOI] [Google Scholar]
- 62.Plenzler MA, Michaels HJ. 2015. Terrestrial habitat quality impacts macroinvertebrate diversity in temporary wetlands. Wetlands 35, 1093–1103. ( 10.1007/s13157-015-0697-4) [DOI] [Google Scholar]
- 63.Layer K, Hildrew AG, Woodward G. 2013. Grazing and detritivory in 20 stream food webs across a broad pH gradient. Oecologia 171, 459–471. ( 10.1007/s00442-012-2421-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Spence JR, Scudder GGE. 1980. Habitats, life cycles, and guild structure among water striders (Heteroptera: Gerridae) on the Fraser Plateau of British Columbia. Can. Entomol . 112, 779–792. ( 10.4039/Ent112779-8) [DOI] [Google Scholar]
- 65.Spence JR. 1981. Experimental analysis of microhabitat selection in water-striders (Heteroptera: Gerridae). Ecology 62, 1505–1514. ( 10.2307/1941507) [DOI] [Google Scholar]
- 66.McPeek MA, Shen L, Torrey JZ, Farid H. 2008. The tempo and mode of three-dimensional morphological evolution in male reproductive structures. Am. Nat . 171, E158-E178. ( 10.1086/587076) [DOI] [PubMed] [Google Scholar]
- 67.McPeek MA, Shen L, Farid H. 2009. The correlated evolution of three-dimensional reproductive structures between male and female damselflies. Evolution 63, 73–83. ( 10.1111/j.1558-5646.2008.00527.x) [DOI] [PubMed] [Google Scholar]
- 68.Parker GA, Partridge L. 1998. Sexual conflict and speciation. Phil. Trans. R. Soc. B 353, 261–274. ( 10.1098/rstb.1998.0208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bonduriansky R. 2011. Sexual selection and conflict as engines of ecological diversification. Am. Nat . 178, 729–745. ( 10.1086/662665) [DOI] [PubMed] [Google Scholar]
- 70.Härdling R, Karlsson K. 2009. The dynamics of sexually antagonistic coevolution and the complex influences of mating system and genetic correlation. J. Theor. Biol . 260, 276–282. ( 10.1016/j.jtbi.2009.05.024) [DOI] [PubMed] [Google Scholar]
- 71.Svensson EI, Abbott JK, Gosden TP, Coreau A. 2009. Female polymorphisms, sexual conflict and limits to speciation processes in animals. Evol. Ecol . 23, 93 ( 10.1007/s10682-007-9208-2) [DOI] [Google Scholar]
- 72.Kokko H, Brooks R. 2003. Sexy to die for? Sexual selection and the risk of extinction. Ann. Zool. Fennici 40, 207–219. [Google Scholar]
- 73.Kokko H, Rankin DJ. 2006. Lonely hearts or sex in the city? Density-dependent effects in mating systems. Phil. Trans. R. Soc. B 361, 319–334. ( 10.1098/rstb.2005.1784) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Martin OY, Hosken DJ. 2003. The evolution of reproductive isolation through sexual conflict. Nature 423, 979–982. ( 10.1038/nature01752) [DOI] [PubMed] [Google Scholar]
- 75.Fricke C, Andersson C, Arnqvist G. 2010. Natural selection hampers divergence of reproductive traits in a seed beetle. J. Evol. Biol . 23, 1857–1867. ( 10.1111/j.1420-9101.2010.02050.x) [DOI] [PubMed] [Google Scholar]
- 76.Magurran AE, Seghers BH. 1994. Sexual conflict as a consequence of ecology: evidence from guppy, Poecilia reticulata, populations in Trinidad. Proc. R. Soc. B 255, 31–36. ( 10.1098/rspb.1994.0005) [DOI] [Google Scholar]
- 77.Gomez-Llano MA, Bensch HM, Svensson EI. 2018. Sexual conflict and ecology: species composition and male density interact to reduce male mating harassment and increase female survival. Evolution 72, 906–915. ( 10.1111/evo.13457) [DOI] [PubMed] [Google Scholar]
- 78.Karlsson GK, Svensson EI, Bergsten J, Härdling R, Hansson B. 2014. The interplay between local ecology, divergent selection, and genetic drift in population divergence of a sexually antagonistic female trait. Evolution 68, 1934–1946. ( 10.1111/evo.12408) [DOI] [PubMed] [Google Scholar]
- 79.Gwynne DT. 1991. Sexual competition among females: what causes courtship-role reversal? Trends Ecol. Evol . 6, 118–121. ( 10.1016/0169-5347(91)90089-G) [DOI] [PubMed] [Google Scholar]
- 80.Yun L, Chen PJ, Singh A, Agrawal AF, Rundle HD. 2017. The physical environment mediates male harm and its effect on selection in females. Proc. R. Soc. B 284, 20170424 ( 10.1098/rspb.2017.0424) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset supporting this article has been uploaded as part of the electronic supplementary material.