Abstract
Many conspicuous forms of evolutionary diversity occur within species. Two prominent examples include evolutionary divergence between populations differentially adapted to their local environments (local adaptation), and divergence between females and males in response to sex differences in selection (sexual dimorphism sensu lato). These two forms of diversity have inspired vibrant research programmes, yet these fields have largely developed in isolation from one another. Nevertheless, conceptual parallels between these research traditions are striking. Opportunities for local adaptation strike a balance between local selection, which promotes divergence, and gene flow—via dispersal and interbreeding between populations—which constrains it. Sex differences are similarly constrained by fundamental features of inheritance that mimic gene flow. Offspring of each sex inherit genes from same-sex and opposite-sex parents, leading to gene flow between each differentially selected half of the population, and raising the question of how sex differences arise and are maintained. This special issue synthesizes and extends emerging research at the interface between the research traditions of local adaptation and sex differences. Each field can promote understanding of the other, and interactions between local adaptation and sex differences can generate new empirical predictions about the evolutionary consequences of selection that varies across space, time, and between the sexes.
This article is part of the theme issue ‘Linking local adaptation with the evolution of sex differences’.
Keywords: sexual dimorphism, evolutionary constraints, gene flow, sexual selection, natural selection, sexual conflict
1. Introduction
Environmental conditions vary across species' ranges, generating selection for locally adapted phenotypes. Nevertheless, gene flow—caused by dispersal and interbreeding between individuals that were born in different regions of the range—opposes genetic differentiation between populations and constrains local adaptation [1,2]. This tension between adaptation and gene flow is central to several productive research topics in modern evolutionary biology, including the genetics of adaptation and speciation [3–5], the evolutionary ecology of species' range limits [6–9], the maintenance of genetic variation [10–12], the evolution of phenotypic plasticity [13] and the empirical study of natural selection and geographical clines in the wild [14–17].
The theory of local adaptation characteristically ignores a widespread feature of biology: sexual dimorphism. Yet many classical study species for local adaptation comprise separate sexes. Such species often display pronounced sex differences in selection and demography, which can directly impact the dynamics of sex-specific adaptation and population dynamics [18–20]. Likewise, relatively few studies focusing on sex differences and/or sexual selection are conducted with spatially varying environments in mind (e.g. [21–24]).
Recent empirical and theoretical research has begun to question and extend many standard assumptions underlying theories of local adaptation with gene flow [22,25–27], and of sex-specific selection and adaptation in heterogeneous environments [23,24,28–31]. These studies reveal unexplored opportunities for research that merges the fields of spatial evolutionary ecology, sexual selection and sexual dimorphism.
There are at least two good reasons for merging studies of local adaptation with the evolution of sex differences. First, establishing conceptual connections between traditionally separated areas of study can enrich and broaden our understanding of each. For example, evolutionary predictions about the genetic basis of local adaptation with gene flow can generate novel predictions about the genetic basis of sex-specific adaptation and sexually antagonistic genetic variation [32,33] (see below). Secondly, predictions regarding single contexts of evolutionary change may break down, or change in interesting ways, when multiple contexts co-occur. Recent research shows that sex-specific selection and local adaptation can interact to drive evolutionary dynamics that are unique to the combination of processes (e.g. persistent sex asymmetries in geographical patterns of local adaptation and maladaptation across species' ranges; [30,31]).
Despite recent efforts to better integrate these two fields, several general questions remain largely unanswered, and indeed are rarely asked. How does environmental variation mediate selection on male and female traits? How do sex differences in selection impact extinction, species' range size evolution and ecological invasions? How do classical evolutionary concepts of hard and soft selection apply in species with separate sexes? How do interactions between sex and local selection shape the genetic architecture of local adaptation, and sex-specific patterns of genetic variance and covariance? In this special issue, we bring together a collection of papers that address these and related questions at the intersection between local adaptation and sex differences. The topics covered within the issue fall within four major themes, upon which we expand below:
— Parallels between sex-specific adaptation and local adaptation with gene flow. Processes that play out during the evolution of local adaptation and of sexual dimorphism bear many striking dynamical similarities with one another, with each field enriching our view of the other.
— Sex differences and the genetic basis of local adaptation. Females and males differ in both genomic architecture [34] and the intensity with which selection, migration, recombination, mutation, and genetic drift operate [35]. These fundamental sex differences shape the genetic basis of population and species divergence.
— Ecological drivers of sex-specific phenotypic selection and sexual conflict. Research over the past century has established the centrality of sexually divergent reproductive roles in the evolution of phenotypic sexual dimorphism [36,37]. The role of ecological context in promoting or constraining sex differences has only recently begun to receive the attention that it deserves.
— Environmental variation and the evolution of reproductive systems. Reproductive systems evolve in response to interactions between members each sex, their reproductive interests, and ecological conditions that affect mating and reproduction. Evolutionary diversity of reproductive systems is shaped by local environments in native and invasive species' ranges (e.g. [38–41]), providing an active arena for theoretical and empirical research.
2. Parallels between sex-specific adaptation and local adaptation with gene flow
Local adaptation hinges upon the balance between the strength of local selection, which promotes population divergence, and the magnitude of gene flow, which erodes it [2,42]. Weak gene flow allows for strong local adaptation and high genetic differentiation between populations, whereas high gene flow can severely limit such divergence. At best, local adaptation is hindered by this strong gene flow [43] (but see [44,45]). At worst, populations can be driven to extinction as a consequence of maladaptive gene flow [7,46].
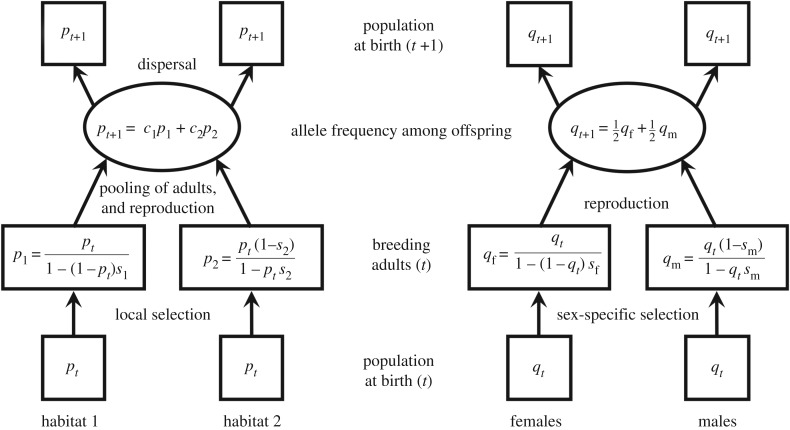
In contexts of high gene flow, evolutionary scenarios of local adaptation bear striking similarities to scenarios of selection for sex differences [33,47]. Consider an extreme example of a haploid population, where individuals disperse randomly between a pair of habitat types (‘habitat 1’ and ‘habitat 2’; figure 1, left), the direction of selection differs between habitats, and gene flow is very high (table 1). Local selection promotes genetic differentiation (p1 > pt > p2, where pt is the frequency of the focal allele before selection in generation t, and p1 and p2 are the frequencies in habitats 1 and 2 after selection; see figure 1), whereas gene flow erodes these differences, homogenizing allele frequencies between habitats following dispersal (i.e. both habitats have allele frequencies pt and pt+1 in generations t and t + 1, respectively). Sex differences in selection result in evolutionary dynamics that parallel the local adaptation scenario (figure 1, right). Selection promotes allele frequency differentiation between breeding females and males of the population (qf > qt > qm; see figure 1), whereas the equal genetic contributions of each sex to reproduction equalize allele frequencies in female and male offspring (qt+1 = (qf + qm)/2; figure 1). In fact, the evolutionary dynamics of the two scenarios of selection become mathematically indistinguishable when adults from habitats 1 and 2 contribute equally to the production of offspring of the next generation (c1 = c2 = ½, s1 = sf and s2 = sm; figure 1 and table 1).
Figure 1.
Parallels between scenarios of local adaptation with gene flow and of sex-specific adaptation. The left panel shows the life cycle of a haploid population evolving in response to local selection with high gene flow between habitats (e.g. [10,11]). Allele frequency differentiation between habitats fluctuates during the life cycle, with dispersal homogenizing the allele frequencies between habitats (pt and pt+1 are equal between habitats), and local selection favouring differentiation between habitats (p1 > pt > p2). The frequency of allele A in offspring of the next generation depends on the frequency in adults from each habitat (p1, p2) and the proportions of breeding adults from each habitat (c1, c2, where c2 = 1 − c1). The right panel shows the life cycle of a haploid population evolving in response to sexually antagonistic selection (e.g. [48,49]). Females and males make equal genetic contributions to offspring, which homogenizes the allele frequencies of female and male offspring of a given generation (qt and qt+1 are equal between sexes). Sex differences in selection elevate the frequency of the A allele in breeding females and decrease the frequency of A in breeding males (qf > qt > qm).
Table 1.
Conflicting selection between habitats and sexes.
| allele A | allele a | |
|---|---|---|
| local selection | ||
| habitat 1 fitness | 1 | 1 − s1 |
| habitat 2 fitness | 1 − s2 | 1 |
| frequency in the population | pt | 1 − pt |
| sex-specific selection | ||
| female fitness | 1 | 1 − sf |
| male fitness | 1 − sm | 1 |
| frequency in the population | qt | 1 − qt |
The dynamical similarities between scenarios of local adaptation and sex differences in selection provide an example of a wider range of parallels between the concepts. For example, over short evolutionary intervals, both scenarios can maintain stable genetic variation for fitness [10,47], stabilize linkage disequilibrium in the absence of epistasis [32,50], and select for tightly linked clusters of alleles that are exclusively beneficial within a given habitat or sex [48,51–54]. Both scenarios can also generate detectable signals of differential selection between populations or sexes (e.g. through FST analysis [55–58]). Over long evolutionary intervals, both scenarios can impact the evolution of genomic architecture, including the evolution of inversions, translocations and gene duplications [59–64], as well as the evolutionary modification of genetic dominance [65,66]. Most of the above scenarios involve simple, univariate patterns of selection (i.e. selection on single traits), providing an opportunity for future work in more complex, multivariate contexts of evolutionary change. Finally, scenarios of sex-specific selection and local adaptation can both favour the evolution of sex- or environment-dependent phenotypic plasticity, which are widely observed in nature [13,18].
3. Sex differences and the genetic basis of local adaptation
Gene flow shapes genome-wide patterns of genetic divergence, leading to empirically detectable genetic bases underlying locally adapted phenotypes. For example, whereas gene flow erodes population divergence at loci contributing weakly to local adaptation or genetic incompatibilities between species, strong selection maintains sharp genetic differentiation at loci that contribute the most to traits or genetic systems under divergent selection. These loci are identifiable as outliers with sharp genetic clines across hybrid zones (e.g. from studies of hybrid zones [67,68]), high FST between geographically diverged populations [55], or they may be enriched in genomic regions that suppress ancestral or ongoing gene flow [60,69].
There are several reasons why a view towards sex differences may be useful in research on the genetic basis of local adaptation. For example, sex differences in dispersal, genetic drift, and the strength of natural selection, can impact the chromosomal locations of loci contributing the most to local adaptation, as well as the statistical power to identify them as outliers (see [35,70]). For example, Camus et al. [71] reported strong effects of mitochondrial genetic backgrounds on local adaptation of Drosophila melanogaster to variable thermal conditions across eastern Australia. The mitochondrial genome is maternally inherited, is primarily responsive to selection in females, and contributes substantially to local adaptation in thermal tolerance, despite its small size relative to the nuclear genome. On the other hand, the lower effective population size of the mitochondrial genome should simultaneously elevate background levels of neutral divergence between populations, complicating interpretation of the evolutionary causes of geographical divergence of mitochondrial DNA. Recent theory suggests that similar considerations should also apply to X-linked genes: they exhibit female-biased transmission, are more responsive to selection than autosomal genes, are expected to disproportionately contribute to the evolution of local adaptation [70] and are more likely to fix inversions capturing locally adapted alleles [72] (this issue). However, X-linked genes also diverge more readily under genetic drift, which may mask population genetic signals (e.g. based on FST) of local adaptation involving the X chromosome (see [70,73]).
The genes and phenotypes that promote local adaptation may also differ between the sexes. Although most genes within a genome are expressed by both sexes, expression levels and phenotypic effects of mutations differ extensively between the sexes, allowing for sexual dimorphism in the genetic architecture of traits expressed by both (e.g. [74–77]). In addition, the way in which selection varies across a species' range may differ between the sexes, leading to differences in the direction or strength of local selection [23]. Although rare, studies of sex-specific genetic and phenotypic trait clines can shed light on processes of sex-specific selection and local adaptation. For example, Allen et al. [78,79] (this issue) show that male-biased genes in Drosophila exhibit more extensive clinal divergence than female-biased genes—a pattern consistent with sex differences in the intensity of local selection and/or lower pleiotropic constraints in male- relative to female-biased genes. Phenotypic body size clines show similar patterns [80], although interpretation of the body size data is complicated by potential effects of sexually dimorphic phenotypic plasticity [81] and additive genetic correlations between the sexes that may constrain geographical divergence of sexual dimorphism [82].
Combinations of sex-specific selection and sexually dimorphic genetic architecture can manifest in sexually dimorphic fitness consequences of dispersal, local adaptation and hybridization between populations or species [83,84]. In these contexts, Runemark et al. [83] (this issue) review and synthesize consequences of hybridization for the expression of sexual dimorphism, sexual conflict and the ecology of local adaptation. Svensson et al. [84] (this issue) review reciprocal transplant studies in local adaptation, and discuss the importance of recording sex-specific fitness consequences in future studies in this field. Although such studies are still rare, they can elucidate the role of sex differences in local adaptation, providing a strong impetus for further empirical attention.
4. Ecological drivers of sex-specific phenotypic selection and sexual conflict
Phenotypic sexual dimorphism can evolve in response to sexual selection or natural selection [18,19], although in practice, delineating the role of each in the evolution of sexual dimorphism is challenging (e.g. [85–87]). Selection for sexual dimorphism can potentially arise from different interactions between each sex and its environment, or from resource competition leading to ecological character displacement; both factors can drive the evolution of niche partitioning between the sexes [18,19]. Selander [88] suggested that the only reliable evidence for sexual dimorphism via niche partitioning is a sex-specific modification of trophic structures (e.g. mouthparts) beyond what would be expected from body size differences and the direction of sexual selection. However, these criteria could exclude many cases of ecologically based dimorphism, include spurious cases (e.g. dimorphic mouthparts that reflect sex-specific reproductive functions, such as digging nesting cavities or incubating eggs, rather than dimorphic diets), and have empirical difficulties in their application [85]. In some cases, sexual selection may initiate selection for sexual dimorphism, with ecological factors secondarily influencing its magnitude, for example by placing upper limits on the benefits of expressing sexually selected traits, or by favouring elaboration of initially modest sex differences. For example, the evolution of dwarf male seadevils, which parasitically feed on females, may have evolved in response to the scarcity of food and mates within deep sea environments [89]. Forsman [90] (this issue) synthesizes two decades of pygmy grasshopper research to consider how interactions between each sex and its environment shape sex differences in coloration, thermotolerance and other ecologically relevant traits.
The net outcome of natural and sexual selection is that males and females have different trait optima defined by the ecological conditions in which they evolve. The sexes also share nearly identical genomes, constraining the potential rate of evolutionary divergence between female and male traits [18,75]. Although this genetic constraint promotes adaptation when the direction of selection is the same in each sex [91,92], it becomes maladaptive when selection is misaligned between the sexes, giving rise to intralocus sexual conflict [93,94]. Recent research has shown that the manifestation of intralocus sexual conflict is sensitive to environmental conditions [29,95–97] and the degree to which each sex is adapted to its environment [31,98–100]. For example, in well-adapted populations of D. melanogaster, high-fitness males sire unfit daughters (intralocus sexual conflict is present); in maladapted populations, high-fitness males sire high-fitness offspring of both sexes (intralocus sexual conflict is absent) [98]. Using a large dataset of field-estimated selection gradients, De Lisle et al. [101] (this issue) demonstrate that environmental stressors (measured using microclimatic data) are associated with patterns of sexually concordant selection, which weakens intralocus sexual conflict in environments that are more stressful, more variable, and closer to the edge of the species' range (consistent with theory [31,99]).
Another type of sexual conflict—interlocus sexual conflict—arises from direct, antagonistic interactions between the sexes, including male sexual coercion and female resistance to mating [37,102]. The intensity and consequences of interlocus sexual conflict for sex-specific phenotypic evolution also depend on ecological context. For example, experimental populations of D. melanogaster adapted faster to a novel food resource in spatially complex environments, where interlocus sexual conflict was relatively weak, than in spatially simple environments where interlocus conflict was strong [103–104]. In this issue, Perry & Rowe [105] review the diverse ways in which ecology can affect coevolution between males and females through interlocus sexual conflict. Using water striders as a model system, they show that population-specific elaboration of sexual armaments (traits associated with male coercion and female resistance) is associated with several ecological variables, including water acidity, temperature, seasonality and winter severity (see [106]), with harsh conditions providing an advantage to females in countering male coercion.
Not only do females and males respond differently to local conditions in their environments; they can, in turn, differentially impact selection in species with which they interact, providing a context for sexual dimorphism to shape coevolutionary dynamics. Pronounced sex differences in behaviour, physiology, morphology and immune responses expose pathogens to drastically different selective environments in male and female hosts [107–109]. The adaptation of pathogens to female and male hosts may also intensify sex difference in selection on immunity [110]. In the water flea Daphnia magna, sex differences in morphology, physiology, and life-history influence potential for growth, performance, and transmission of a common pathogen, Pasteuria ramosa [111]. Hall & Mideo [111] (this issue) combine experimental data on infection and transmission of different strains of Pasteuria ramosa in hosts of each sex with an epidemiological model of pathogen virulence and transmission evolution. They show that performance (spore load), transmission and the dynamics of infection and evolution differ between pathogens infecting female versus male hosts. Pathogen evolutionary trajectories may therefore depend upon the sex with which they interact the most in nature—a scenario that is likely to differ between taxa and between different geographical populations of single species (e.g. pathogens tend to interact with females in facultatively sexual species versus both equally in obligately outcrossing taxa).
Finally, females and males differ in their spatial distributions and patterns of dispersal across their ranges [112]—an observation with implications for the manner in which each sex interfaces with environmental conditions that vary across space, as well as the evolutionary consequences of sex-biased dispersal for adaptation with gene flow (e.g. [35]). For example, sex-biased dispersal can shape the genetic architecture of local adaptation, since it mediates the effective strength of gene flow across different regions of the genome (e.g. male-biased dispersal facilitates responses of mitochondrial-encoded and X-linked genes to local selection, but dampens local adaption at Y-linked genes [70,71]). Unique responses of each sex to shared environmental conditions can also affect the expression and evolution of sex-biased dispersal and ‘dispersal syndromes’ (suites of traits that correlate with dispersal [113]). As Mishra et al. [114] show in this issue, nutrition levels shape ecologically plastic sex differences in dispersal syndromes for body size, desiccation resistance and exploratory behaviour traits. Yet these sex-specific syndromes are evolutionarily labile, and changed during the experimental evolution of dispersal (greater than 70 generations). Their results point to developmental and evolutionary mechanisms that can impact the expression of sex differences in dispersal behaviour.
5. Environmental variation and the evolution of reproductive systems
Variation in environmental conditions across native or invasive portions of a species' range can alter the economics of reproduction, including the fitness costs and benefits of different reproductive strategies and the arena in which mating competition and mate choice occurs. This provides wide scope for evolutionary divergence among subpopulations in the mode of reproduction and the nature of mating interactions between the sexes. At the most basic level, environmental variation provides an arena in which natural selection can favour the evolution and maintenance of sexual reproduction [115,116], with the details of environmental fluctuation determining the rate of sexual reproduction that evolves in a population. At a higher level, environmental variation provides an ecological context for direct and indirect selection on mating preferences, and the divergence of mating systems and species [23,24,117,118].
Individuals of many species can reproduce sexually with other individuals of the population, or individually through clonal reproduction, parthenogenesis, or self-fertilization [119–122]. The frequency with which these different reproductive tactics are employed can vary across the species' range, as evolved responses to local benefits and costs of sex and outcrossing (e.g. [38–41,123]). Geographical differences in the predictability of the environment can lead to variation across the species' range in the benefits of outcrossing, sex and recombination [124], potentially selecting for different rates of sex across habitats. Gerber & Kokko [125] (this issue) show that sex can be viewed as one of a class of bet-hedging strategies [126] for coping with environmental uncertainty. Dispersal, dormancy and sexual reproduction have bet-hedging attributes that partly complement one another, and in this context, theory predicts that the three traits (dispersal, dormancy and sex) exhibit tightly correlated coevolutionary patterns in simulated populations that evolve in spatially or temporally variable environments [125].
The role of males in selecting for or against sex can also change across a species' range. Individuals near range boundaries and those occupying recently invaded ranges may have difficulty finding suitable mates if local population densities are low, which can favour the evolution alternative modes of reproduction. The classic example is Baker's Rule (or Baker's Law), in which colonizing populations show higher capacity for selfing than their native-range populations (see [41,127]). Costs associated with males include the classic ‘twofold’ demographic cost of producing males [128], as well as indirect and direct costs to female fitness that arise from inter- and intralocus sexual conflict [105,129] (see above). Burke and Bonduriansky [130] (this issue) consider the consequences of interlocus conflict on the evolution of facultative sex; they show that conflict favours the spread of facultative sex and influences the geographical distribution of asexual reproduction and the sex ratio. Intralocus sexual conflict is also expected to promote the spread of asexual reproduction [131]. On the other hand, mate preferences can help offset costs associated with intralocus conflict, particularly when females evolve preference for males that carry genes that benefit daughters. Theory suggests that such an outcome is possible, although not inevitable, in stable environments [132,133]. Li & Holman [134] (this issue) show that metapopulation structure and spatially variable environments promote the evolution of choice for female-beneficial alleles, particularly when selection is hard (also see [30]).
Finally, the mating system of the species can impact the evolutionary response to selection on males and females (or on male and female sex-functions in hermaphrodites). While the evolutionary trajectories in outcrossing populations are equally responsive to selection in each sex (e.g. [18,47]), self-fertilization can tip this balance, leading to a stronger response to selection through female than through male sex-functions (e.g. [54,135–137]). Olito et al. [138] (this issue) merge classical theories of environmental heterogeneity and sex-specific selection (e.g. [10,47]), to reveal further complexity in the interplay between sex-specific selection, self-fertilization and hermaphrodite mating systems.
6. Where to?
Studies of local adaptation and of sexual dimorphism each have rich histories within the field of evolutionary biology. The interaction between processes that promote evolutionary differentiation between populations and processes driving intersexual divergence provides a relatively untapped area for productive research that advances our understanding of adaptation, and the many ways in which environment and sex can mediate it. Closer links between these topics also provide an ideal opportunity for new collaborations between researchers with expertise in each context of evolutionary diversification.
The papers within this special issue highlight many outstanding research questions at the interface between sex differences and the geography of adaptation, each worthy of future attention. Among them, we highlight five sets of questions that remain largely unaddressed and provide good material for future exploration:
— How much do females and males ‘agree’ in the direction or intensity of selection for local adaptation, and how do sex differences in selection shape patterns of local adaptation across species' ranges [101]?
— How do different regions of a genome—including chromosomes with symmetric versus sex-biased patterns of inheritance—contribute to local adaptation with gene flow and the maintenance of species differences [70,73,139,140]?
— How do sex differences in selection, dispersal and environmental heterogeneity interact to facilitate the maintenance of genetic variation in fitness and life-history traits, and the evolution of mating systems [130,134,138]?
— To what extent do scenarios of local adaptation and the evolution of sex differences parallel one another [32,33,47]? To what extent do these scenarios, in combination, give rise to emergent evolutionary patterns that qualitatively differ from predictions of either scenario by itself [30,31]?
— Does sexual selection tend to reinforce or conflict with natural selection promoting local adaptation and/or species divergence [24]?
Acknowledgements
The idea to edit a special issue was suggested during a summer school funded by an ESEB Special Topic Network (STN) grant to Hanna Kokko, Erik Svensson, Florence Débarre and Tim Connallon. We thank Hanna Kokko and Erik Svensson for discussion of ideas that contributed to the development of this introductory paper for the special issue, Erik Svensson and Jen Perry for providing detailed suggestions for the introductory paper, and all reviewers who took part in the evaluation and improvement of papers within this special issue.
Data accessibility
This article has no additional data.
Competing interests
We declare we have no competing interests.
Funding
F.D. received funding from Agence Nationale de la Recherche (ANR-14-ACHN-0003-01). T.C. received funding from the Australian Research Council and the School of Biological Sciences at Monash University.
References
- 1.Slatkin M. 1973. Gene flow and selection in a cline. Genetics 75, 733–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lenormand T. 2002. Gene flow and the limits to natural selection. Trends Ecol. Evol. 17, 183–189. ( 10.1016/S0169-5347(02)02497-7) [DOI] [Google Scholar]
- 3.Barton NH, Gale KS. 1993. Genetic analysis of hybrid zones. In Hybrid zones and the evolutionary process (ed. Harrison RG.), pp. 13–45. Oxford, UK: Oxford University Press. [Google Scholar]
- 4.Ralph P, Coop G. 2010. Parallel adaptation: one or many waves of advance of an advantageous allele? Genetics 186, 647–668. ( 10.1534/genetics.110.119594) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yeaman S. 2013. Genomic rearrangements and the evolution of clusters of locally adaptive loci. Proc. Natl Acad. Sci. USA 110, E1743–E1751. ( 10.1073/pnas.1219381110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haldane JBS. 1956. The relation between density regulation and natural selection. Proc. R. Soc. Lond. B 145, 306–308. ( 10.1098/rspb.1956.0039) [DOI] [PubMed] [Google Scholar]
- 7.Kirkpatrick M, Barton NH. 1997. Evolution of a spicies’ range. Am. Nat. 150, 1–23. ( 10.1086/286054) [DOI] [PubMed] [Google Scholar]
- 8.Polechová J. 2018. Is the sky the limit? On the expansion threshold of a species’ range. PLoS Biol. 16, e2005372 ( 10.1371/journal.pbio.2005372) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Connallon T, Sgrò CM. 2018. In search of a general theory of species’ range evolution. PLoS Biol. 16, e2006735 ( 10.1371/journal.pbio.2006735) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levene H. 1953. Genetic equilibrium when more than one ecological niche is available. Am. Nat. 87, 331–333. ( 10.1086/281792) [DOI] [Google Scholar]
- 11.Christiansen FB. 1975. Hard and soft selection in subdivided populations. Am. Nat. 109, 11–16. ( 10.1086/282970) [DOI] [Google Scholar]
- 12.Débarre F, Ronce O, Gandon S. 2013. Quantifying the effects of migration and mutation on adaptation and demography in spatially heterogeneous environments. J. Evol. Biol. 26, 1185–1202. ( 10.1111/jeb.12132) [DOI] [PubMed] [Google Scholar]
- 13.Via S, Lande R. 1985. Genotype-environment interaction and the evolution of phenotypic plasticity. Evolution 39, 505–522. ( 10.1111/j.1558-5646.1985.tb00391.x) [DOI] [PubMed] [Google Scholar]
- 14.Haldane JBS. 1948. The theory of a cline. J. Genet. 48, 277–284. ( 10.1007/BF02986626) [DOI] [PubMed] [Google Scholar]
- 15.Nagylaki T. 1975. Conditions for the existence of clines. Genetics 80, 595–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Endler JA. 1977. Geographic variation, speciation, and clines. Princeton, NJ: Princeton University Press. [PubMed] [Google Scholar]
- 17.Hoffmann AA, Weeks AR. 2007. Climatic selection on genes and traits after a 100 year-old invasion: a critical look at the temperate-tropical clines in Drosophila melanogaster from eastern Australia. Genetica 129, 133–147. ( 10.1007/s10709-006-9010-z) [DOI] [PubMed] [Google Scholar]
- 18.Lande R. 1980. Sexual dimorphism, sexual selection, and adaptation in polygenic characters. Evolution 34, 292–305. ( 10.1111/j.1558-5646.1980.tb04817.x) [DOI] [PubMed] [Google Scholar]
- 19.Slatkin M. 1984. Ecological causes of sexual dimorphism. Evolution 38, 622–630. ( 10.1111/j.1558-5646.1984.tb00327.x) [DOI] [PubMed] [Google Scholar]
- 20.Rankin DJ, Kokko K. 2007. Do males matter? The role of males in population dynamics. Oikos 116, 335–348. ( 10.1111/j.0030-1299.2007.15451.x) [DOI] [Google Scholar]
- 21.Day T. 2000. Sexual selection and the evolution of costly female preferences: spatial effects. Evolution 54, 715–730. ( 10.1111/j.0014-3820.2000.tb00074.x) [DOI] [PubMed] [Google Scholar]
- 22.Gosden TP, Svensson EI. 2008. Spatial and temporal dynamics in a sexual selection mosaic. Evolution 62, 845–856. ( 10.1111/j.1558-5646.2008.00323.x) [DOI] [PubMed] [Google Scholar]
- 23.Miller CW, Svensson EI. 2014. Sexual selection in complex environments. Annu. Rev. Entomol. 59, 427–445. ( 10.1146/annurev-ento-011613-162044) [DOI] [PubMed] [Google Scholar]
- 24.Servedio MR, Boughman JW. 2017. The role of sexual selection and local adaptation and speciation. Annu. Rev. Ecol. Evol. Syst. 48, 85–109. ( 10.1146/annurev-ecolsys-110316-022905) [DOI] [Google Scholar]
- 25.Gosden TP, Chenoweth SF. 2014. The evolutionary stability of cross-sex, cross-trait genetic covariances. Evolution 68, 1687–1697. ( 10.1111/evo.12398) [DOI] [PubMed] [Google Scholar]
- 26.Débarre F, Yeaman S, Guillaume S. 2015. Evolution of quantitative traits under a migration-selection balance: when does skew matter? Am. Nat. 186, S37–S47. ( 10.1086/681717) [DOI] [PubMed] [Google Scholar]
- 27.Hadfield JD. 2016. The spatial scale of local adaptation in a stochastic environment. Ecol. Lett. 19, 780–788. ( 10.1111/ele.12614) [DOI] [PubMed] [Google Scholar]
- 28.Hunt J, Hosken DJ (eds). 2014. Genotype-by-environment interactions and sexual selection. Chichester, UK: John Wiley & Sons. [Google Scholar]
- 29.Berger D, Grieshop K, Lind MI, Goenaga J, Maklakov AA, Arnqvist G. 2014. Intralocus sexual conflict and environmental stress. Evolution 68, 2184–2196. ( 10.1111/evo.12528) [DOI] [PubMed] [Google Scholar]
- 30.Harts AMF, Schwanz LE, Kokko H. 2014. Demography can favour female-advantageous alleles. Proc. R. Soc. B 281, 20140005 ( 10.1098/rspb.2014.0005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Connallon T. 2015. The geography of sex-specific selection, local adaptation, and the sexual dimorphism. Evolution 69, 2333–2344. ( 10.1111/evo.12737) [DOI] [PubMed] [Google Scholar]
- 32.Úbeda F, Haig D, Patten MM. 2011. Stable linkage disequilibrium owing to sexual antagonism. Proc. R. Soc. B 278, 855–862. ( 10.1098/rspb.2010.1201) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haig D, Úbeda F, Patten MM. 2014. Specialists and generalists: the sexual ecology of the genome. Cold Spring Harb. Perspect. Biol. 6, a017525 ( 10.1101/cshperspect.a017525) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bachtrog D, et al. 2014. Sex determination: why so many ways of doing it? PLoS Biol. 12, e1001899 ( 10.1371/journal.pbio.1001899) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hedrick PW. 2007. Sex: differences in mutation, recombination, selection, gene flow, and genetic drift. Evolution 61, 2750–2771. ( 10.1111/j.1558-5646.2007.00250.x) [DOI] [PubMed] [Google Scholar]
- 36.Andersson M. 1994. Sexual selection. Princeton, NJ: Princeton University Press. [Google Scholar]
- 37.Arnqvist G, Rowe L. 2005. Sexual conflict. Princeton, NJ: Princeton University Press. [Google Scholar]
- 38.Vandel A. 1928. La parthénogenese geographique. Contribution a l’étude biologique et cytologique de la parthénogenese naturelle. Bull. Biol. Fr. Belg. 62, 164–182. [Google Scholar]
- 39.Baker HG. 1955. Self-compatibility and establishment after ‘long-distance’ dispersal. Evolution 9, 347–348. [Google Scholar]
- 40.Hörandl E. 2006. The complex causality of geographical parthenogenesis. New Phytol. 171, 525–538. [DOI] [PubMed] [Google Scholar]
- 41.Pannell JR, et al. 2015. The scope of Baker's law. New Phytol. 208, 656–667. ( 10.1111/nph.13539) [DOI] [PubMed] [Google Scholar]
- 42.Haldane JBS. 1930. A mathematical theory of natural and artificial selection. (Part VI, Isolation). Math. Proc. Camb. Philos. Soc. 26, 220–230. ( 10.1017/S0305004100015450) [DOI] [Google Scholar]
- 43.García-Ramos G, Kirkpatrick M. 1997. Genetic models of adaptation and gene flow in peripheral populations. Evolution 51, 21–28. ( 10.1111/j.1558-5646.1997.tb02384.x) [DOI] [PubMed] [Google Scholar]
- 44.Edelaar P, Siepielski AM, Clobert J. 2008. Matching habitat choice causes directed gene flow: a neglected dimension in evolution and ecology. Evolution 62, 2462–2472. ( 10.1111/j.1558-5646.2008.00459.x) [DOI] [PubMed] [Google Scholar]
- 45.Edelaar P, Bolnick DI. 2012. Non-random gene flow: an underappreciated force in evolution and ecology. Trends Ecol. Evol. 27, 659–665. ( 10.1016/j.tree.2012.07.009) [DOI] [PubMed] [Google Scholar]
- 46.Ronce O, Kirkpatrick M. 2001. When sources become sinks: migrational meltdown in heterogeneous habitats. Evolution 55, 1520–1531. ( 10.1111/j.0014-3820.2001.tb00672.x) [DOI] [PubMed] [Google Scholar]
- 47.Kidwell JF, Clegg MT, Stewart FM, Prout T. 1977. Regions of stable equilibria for models of differential selection in the two sexes under random mating. Genetics 85, 171–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Connallon T, Jordan CY. 2016. Accumulation of deleterious mutations near sexually antagonistic genes. G3 6, 2273–2284. ( 10.1534/g3.116.031161) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Immler S, Arnqvist G, Otto SP. 2012. Ploidally antagonistic selection maintains stable genetic polymorphism. Evolution 66, 55–65. ( 10.1111/j.1558-5646.2011.01399.x) [DOI] [PubMed] [Google Scholar]
- 50.Li WH, Nei M. 1974. Stable linkage disequilibrium without epistasis in subdivided populations. Theor. Popul. Biol. 6, 173–183. ( 10.1016/0040-5809(74)90022-7) [DOI] [PubMed] [Google Scholar]
- 51.Yeaman S, Whitlock MC. 2011. The genetic architecture of adaptation under migration-selection balance. Evolution 65, 1897–1911. ( 10.1111/j.1558-5646.2011.01269.x) [DOI] [PubMed] [Google Scholar]
- 52.Patten MM, Haig D, Úbeda F. 2010. Fitness variation due to sexual antagonism and linkage disequilibrium. Evolution 64, 3638–3642. ( 10.1111/j.1558-5646.2010.01100.x) [DOI] [PubMed] [Google Scholar]
- 53.Patten MM, Úbeda F, Haig D. 2013. Sexual and parental antagonism shape genomic architecture. Proc. R. Soc. B 280, 20131795 ( 10.1098/rspb.2013.1795) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Olito C. 2017. Consequences of genetic linkage for the maintenance of sexually antagonistic polymorphism in hermaphrodites. Evolution 71, 458–464. ( 10.1111/evo.13120) [DOI] [PubMed] [Google Scholar]
- 55.Hoban S, et al. 2016. Finding the genomic basis of local adaptation: pitfalls, practical solutions, and future directions. Am. Nat. 188, 379–397. ( 10.1086/688018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cheng C, Kirkpatrick M. 2016. Sex-specific selection and sex-biased gene expression in humans and flies. PLoS Genet. 12, e1006170 ( 10.1371/journal.pgen.1006170) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kasimatis KR, Nelson TC, Phillips PC. 2017. Genomic signatures of sexual conflict. J. Hered. 108, 780–790. ( 10.1093/jhered/esx080) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Connallon T, Hall MH. 2018. Genetic constraints on adaptation: a theoretical primer for the genomics era. Ann. NY Acad. Sci. 1422, 65–87. ( 10.1111/nyas.13536) [DOI] [PubMed] [Google Scholar]
- 59.Charlesworth D, Charlesworth B. 1980. Sex differences in fitness and selection for centric fusions between the sex chromosomes. Genet. Res. 35, 205–214. ( 10.1017/S0016672300014051) [DOI] [PubMed] [Google Scholar]
- 60.Kirkpatrick M, Barton N. 2006. Chromosome inversions, local adaptation and speciation. Genetics 173, 419–434. ( 10.1534/genetics.105.047985) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pennell MW, Kirkpatrick M, Otto SP, Vamosi JC, Peichel CL, Valenzuela N, Kitano J. 2015. Y fuse? Sex chromosome fusions in fishes and reptiles. PLoS Genet. 11, e1005237 ( 10.1371/journal.pgen.1005237) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yanchukov A, Proulx S. 2012. Invasion of gene duplication through masking for maladaptive gene flow. Evolution 66, 1543–1555. ( 10.1111/j.1558-5646.2011.01551.x) [DOI] [PubMed] [Google Scholar]
- 63.Connallon T, Clark AG. 2011. The resolution of sexual antagonism by gene duplication. Genetics 187, 919–937. ( 10.1534/genetics.110.123729) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wyman MJ, Cutter AD, Rowe L. 2012. Gene duplication in the evolution of sexual dimorphism. Evolution 66, 1556–1566. ( 10.1111/j.1558-5646.2011.01525.x) [DOI] [PubMed] [Google Scholar]
- 65.Otto SP, Bourguet D. 1999. Balanced polymorphisms and the evolution of dominance. Am. Nat. 153, 561–574. ( 10.1086/303204) [DOI] [PubMed] [Google Scholar]
- 66.Spencer HG, Priest NK. 2016. The evolution of sex-specific dominance in response to sexually antagonistic selection. Am. Nat. 187, 656–666. ( 10.1086/685827) [DOI] [PubMed] [Google Scholar]
- 67.Harrison RG. 1990. Hybrid zones: windows on evolutionary process. Oxf. Surv. Evol. Biol. 7, 69–128. [Google Scholar]
- 68.Teeter KC, et al. 2008. Genome-wide patterns of gene flow across a house mouse hybrid zone. Genome Res. 18, 67–76. ( 10.1101/gr.6757907) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Navarro A, Barton NH. 2003. Chromosomal speciation and molecular divergence-accelerated evolution in rearranged chromosomes. Science 300, 321–324. ( 10.1126/science.1080600) [DOI] [PubMed] [Google Scholar]
- 70.Lasne C, Sgrò CM, Connallon T. 2017. The relative contributions of the X chromosome and autosomes to local adaptation. Genetics 205, 1285–1304. ( 10.1534/genetics.116.194670) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Camus MF, Wolff JN, Sgrò CM, Dowling DK. 2017. Experimental support that natural selection has shaped the latitudinal distribution of mitochondrial haplotypes in Australian Drosophila melanogaster. Mol. Biol. Evol. 34, 2600–2612. ( 10.1093/molbev/msx184) [DOI] [PubMed] [Google Scholar]
- 72.Connallon T, Olito C, Dutoit L, Papoli H, Ruzicka F, Yong L. 2018. Local adaptation and the evolution of inversions on sex chromosomes and autosomes. Phil. Trans. R. Soc. B 373, 20170423 ( 10.1098/rstb.2017.0423) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Presgraves DC. In press. Evaluating genomic signatures of ‘the large X-effect’ during complex speciation. Mol. Ecol. ( 10.1111/mec.14777) [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ober C, Loisel DA, Gilad Y. 2008. Sex-specific genetic architecture of human disease. Nat. Rev. Genet. 9, 911–922. ( 10.1038/nrg2415) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Poissant J, Wilson AJ, Coltman DW. 2010. Sex-specific genetic variance and the evolution of sexual dimorphism: a systematic review of cross-sex genetic correlations. Evolution 64, 97–107. ( 10.1111/j.1558-5646.2009.00793.x) [DOI] [PubMed] [Google Scholar]
- 76.Griffin RM, Dean R, Grace JL, Rydén P, Friberg U. 2013. The shared genome is a pervasive constraint on the evolution of sex-biased gene expression. Mol. Biol. Evol. 30, 2168–2176. ( 10.1093/molbev/mst121) [DOI] [PubMed] [Google Scholar]
- 77.Gilks WP, Abbott JK, Morrow EH. 2014. Sex differences in disease genetics: evidence, evolution, and detection. Trends Genet. 30, 453–463. ( 10.1016/j.tig.2014.08.006) [DOI] [PubMed] [Google Scholar]
- 78.Allen SL, Bonduriansky R, Chenoweth SF. 2018. Genetic constraints on microevolutionary divergence of sex-biased gene expression. Phil. Trans. R. Soc. B 373, 20170427 ( 10.1098/rstb.2017.0427) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Allen SL, Bonduriansky R, Sgrò CM, Chenoweth SF. 2017. Sex-biased transcriptome divergence along a latitudinal gradient. Mol. Ecol. 26, 1256–1272. ( 10.1111/mec.14015) [DOI] [PubMed] [Google Scholar]
- 80.Blanckenhorn WU, Stillwell RC, Young KA, Fox CW, Ashton KG. 2006. When Rensch meets Bergmann: does sexual size dimorphism change systematically with latitude? Evolution 60, 2004–2011. ( 10.1111/j.0014-3820.2006.tb01838.x) [DOI] [PubMed] [Google Scholar]
- 81.Stillwell RC, Blanckenhorn WU, Teder T, Davidowitz G, Fox CW. 2010. Sex differences in phenotypic plasticity affect variation in sexual size dimorphism in insects: from physiology to evolution. Annu. Rev. Entomol. 55, 227–245. ( 10.1146/annurev-ento-112408-085500) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lasne C, Hangartner SB, Connallon T, Sgrò CM. 2018. Cross-sex genetic correlations and the evolution of sex-specific local adaptation: insights from classical trait clines in Drosophila melanogaster. Evolution 72, 1317–1327. ( 10.1111/evo.13494) [DOI] [PubMed] [Google Scholar]
- 83.Runemark A, Eroukhmanoff F, Nava-Bolaños A, Hermansen JS, Meier JI. 2018. Hybridization, sex-specific genomic architecture and local adaptation. Phil. Trans. R. Soc. B 373, 20170419 ( 10.1098/rstb.2017.0419) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Svensson EI, Goedert D, Gómez-Llano MA, Spagopoulou F, Nava-Bolaños A, Booksmythe I. 2018. Sex differences in local adaptation: what can we learn from reciprocal transplant experiments? Phil. Trans. R. Soc. B 373, 20170420 ( 10.1098/rstb.2017.0420) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shine R. 1989. Ecological causes for the evolution of sexual dimorphism: a review of the evidence. Q. Rev. Biol. 64, 419–461. ( 10.1086/416458) [DOI] [PubMed] [Google Scholar]
- 86.Hedrick AV, Temeles EJ. 1989. The evolution of sexual dimorphism in animals: hypotheses and tests. Trends Ecol. Evol. 4, 136–138. ( 10.1016/0169-5347(89)90212-7) [DOI] [PubMed] [Google Scholar]
- 87.De Lisle SP, Rowe L. 2015. Ecological character displacement between the sexes. Am. Nat. 186, 693–707. ( 10.1086/683775) [DOI] [PubMed] [Google Scholar]
- 88.Selander RK. 1972. Sexual selection and dimorphism in birds. In Sexual selection and the descent of Man (ed. Campbell B.), pp. 180–230. Chicago, IL: Aldine Publ. Co. [Google Scholar]
- 89.Pietsch TW. 1975. Precocious sexual parasitism in the deep sea ceratioid anglerfish, Cryptopsaras couesi Gill. Nature 256, 38–40. ( 10.1038/256038a0) [DOI] [Google Scholar]
- 90.Forsman A. 2018. On the role of sex differences for evolution in heterogeneous and changing fitness landscapes: insights from pygmy grasshoppers. Phil. Trans. R. Soc. B 373, 20170429 ( 10.1098/rstb.2017.0429) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lorch PD, Proulx S, Rowe L, Day T. 2003. Condition-dependent sexual selection can accelerate adaptation. Evol. Ecol. Res. 5, 867–881. [Google Scholar]
- 92.Whitlock MC, Agrawal AF. 2009. Purging the genome with sexual selection: reducing mutation load through selection on males. Evolution 63, 569–582. ( 10.1111/j.1558-5646.2008.00558.x) [DOI] [PubMed] [Google Scholar]
- 93.Cox RM, Calsbeek R. 2009. Sexually antagonistic selection, sexual dimorphism and the resolution of intralocus sexual conflict. Am. Nat. 173, 176–187. ( 10.1086/595841) [DOI] [PubMed] [Google Scholar]
- 94.Pennell TM, Morrow EH. 2013. Two sexes, one genome: the evolutionary dynamics of intralocus sexual conflict. Ecol. Evol. 3, 1819–1834. ( 10.1002/ece3.540) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Delph LF, Andicoechea J, Steven JC, Herlihy CR, Scarpino SV, Bell DL. 2011. Environment-dependent intralocus sexual conflict in a dioecious plant. New Phytol. 192, 542–552. ( 10.1111/j.1469-8137.2011.03811.x) [DOI] [PubMed] [Google Scholar]
- 96.Punzalan D, Delcourt M, Rundle HD. 2014. Comparing the intersex genetic correlation for fitness across novel environments in the fruit fly, Drosophila serrata. Heredity 112, 143–148. ( 10.1038/hdy.2013.85) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Holman L, Jacomb F. 2017. The effects of stress and sex on selection, genetic covariance, and the evolutionary response. J. Evol. Biol. 30, 1898–1909. ( 10.1111/jeb.13149) [DOI] [PubMed] [Google Scholar]
- 98.Long TAF, Agrawal AF, Rowe L. 2012. The effect of sexual selection on offspring fitness depends on the nature of genetic variation. Curr. Biol. 22, 204–208. ( 10.1016/j.cub.2011.12.020) [DOI] [PubMed] [Google Scholar]
- 99.Connallon T, Hall MH. 2016. Genetic correlations and sex-specific adaptation in changing environments. Evolution 70, 2186–2198. ( 10.1111/evo.13025) [DOI] [PubMed] [Google Scholar]
- 100.Martinossi-Allibert I, Savković U, Đorđević M, Arnqvist G, Stojković B, Berger D. 2018. The consequences of sexual selection in well-adapted and maladapted populations of bean beetles. Evolution 72, 518–530. ( 10.1111/evo.13412) [DOI] [PubMed] [Google Scholar]
- 101.De Lisle SP, Goedert D, Reedy AM, Svensson EI. 2018. Climatic factors and species range position predict sexually antagonistic selection across taxa. Phil. Trans. R. Soc. B 373, 20170415 ( 10.1098/rstb.2017.0415) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chapman T, Arnqvist G, Bangham J, Rowe L. 2003. Sexual Conflict. Trends Ecol. Evol. 18, 41–47. ( 10.1016/S0169-5347(02)00004-6) [DOI] [Google Scholar]
- 103.Yun L, Chen PJ, Kwok KE, Angell CS, Rundle HD, Agrawal AF. 2018. Competition for mates and the improvement of nonsexual fitness. Proc. Natl Acad. Sci. USA 115, 6762–6767. ( 10.1073/pnas.1805435115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Arbuthnott D, Dutton EM, Agrawal AF, Rundle HD. 2014. The ecology of sexual conflict: ecologically dependent parallel evolution of male harm and female resistance in Drosophila melanogaster. Ecol. Lett. 17, 221–228. ( 10.1111/ele.12222) [DOI] [PubMed] [Google Scholar]
- 105.Perry JC, Rowe L. 2018. Sexual conflict in its ecological setting. Phil. Trans. R. Soc. B 373, 20170418 ( 10.1098/rstb.2017.0418) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Perry JC, Garroway CJ, Rowe L. 2017. The role of ecology, neutral processes and antagonistic coevolution in an apparent sexual arms race. Ecol. Lett. 20, 1107–1117. ( 10.1111/ele.12806) [DOI] [PubMed] [Google Scholar]
- 107.Rolff J. 2002. Bateman's principle and immunity. Proc. R. Soc. Lond. B 269, 867–872. ( 10.1098/rspb.2002.1959) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Duneau D, Ebert D. 2012. Host sexual dimorphism and parasite adaptation. PLoS Biol. 10, e1001271 ( 10.1371/journal.pbio.1001271) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gipson SAY, Hall MD. 2016. The evolution of sexual dimorphism land its potential impact on host-pathogen coevolution. Evolution 70, 959–968. ( 10.1111/evo.12922) [DOI] [PubMed] [Google Scholar]
- 110.Svensson EI, McAdam AG, Sinervo B. 2009. Intralocus sexual conflict over immune defense, gender load, and sex-specific signaling in a natural lizard population. Evolution 63, 3124–3135. ( 10.1111/j.1558-5646.2009.00782.x) [DOI] [PubMed] [Google Scholar]
- 111.Hall MD, Mideo N. 2018. Linking sex differences to the evolution of infectious disease life-histories. Phil. Trans. R. Soc. B 373, 20170431 ( 10.1098/rstb.2017.0431) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Trochet A, Courtois AE, Stevens VM, Baguette M, Chaine A, Schmeller DS, Clobert J. 2016. Evolution of sex-biased dispersal. Q. Rev. Biol. 91, 297–330. ( 10.1086/688097) [DOI] [PubMed] [Google Scholar]
- 113.Ronce O, Clobert J. 2012. Dispersal syndromes. In Dispersal ecology and evolution (eds Clobert J, Baguette M, Benton TG, Bullock JM), pp. 119–138. Oxford, UK: Oxford University Press. [Google Scholar]
- 114.Mishra A, Tung S, Shreenidhi PM, Aamir Sadiq M, Shree Sruti VR, Chakraborty PP, Dey S. 2018. Sex differences in dispersal syndrome are modulated by environment and evolution. Phil. Trans. R. Soc. B 373, 20170428 ( 10.1098/rstb.2017.0428) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Otto SP, Lenormand T. 2002. Evolution of sex: resolving the paradox of sex and recombination. Nat. Rev. Genet. 3, 252–261. ( 10.1038/nrg761) [DOI] [PubMed] [Google Scholar]
- 116.Hartfield M, Keightley PD. 2012. Current hypotheses for the evolution of sex and recombination. Integr. Zool. 7, 192–209. ( 10.1111/j.1749-4877.2012.00284.x) [DOI] [PubMed] [Google Scholar]
- 117.Svensson EI, Eroukhmanoff F, Karlsson K, Runemark A, Brodin A. 2010. A role for learning in population divergence of mate preferences. Evolution 64, 3101–3113. ( 10.1111/j.1558-5646.2010.01085.x) [DOI] [PubMed] [Google Scholar]
- 118.Svensson EI, Runemark A, Verzijden MN, Wellenreuther M. 2014. Sex differences in developmental plasticity and canalization shape population divergence in mate preferences. Proc. R. Soc. B 281, 20141636 ( 10.1098/rspb.2014.1636) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Harrison D. 1971. Modes of sexual reproduction. In Advanced biology notes, pp. 147–182. London, UK: Palgrave. [Google Scholar]
- 120.Bell G. 1982. The masterpiece of nature: the evolution and genetics of sexuality. Berkeley, CA: University of California Press. [Google Scholar]
- 121.Jaeckle WB. 1994. Multiple modes of asexual reproduction by tropical and subtropical sea star larvae: an unusual adaptation for genet dispersal and survival. Biol. Bull. 186, 62–71. ( 10.2307/1542036) [DOI] [PubMed] [Google Scholar]
- 122.Ram Y, Hadany L. 2016. Condition-dependent sex: who does it, when and why? Phil. Trans. R. Soc. B 371, 20150539 ( 10.1098/rstb.2015.0539) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jokela J, Dybdahl MF, Lively CM. 2009. The maintenance of sex, clonal dynamics, and host-parasite coevolution in a mixed population of sexual and asexual snails. Am. Nat. 174, S43–S53. ( 10.1086/599080) [DOI] [PubMed] [Google Scholar]
- 124.Tilquin A, Kokko H. 2016. What does the geography of parthenogenesis teach us about sex? Phil. Trans. R. Soc. B 371, 20150538 ( 10.1098/rstb.2015.0538) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gerber N, Kokko H. 2018. Abandoning the ship using sex, dispersal, and dormancy: multiple escape routes from challenging conditions. Phil. Trans. R. Soc. B 373, 20170424 ( 10.1098/rstb.2017.0424) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Li XY, Lehtonen J, Kokko H. 2017. Sexual reproduction as bet-hedging. In Advances in dynamic and mean field games (eds Apaloo J, Viscolani B), pp. 217–234. Basel, Switzerland: Springer International Publishing. [Google Scholar]
- 127.Razanajatovo M, et al. 2016. Plants capable of selfing are more likely to become naturalized. Nat. Commun. 7, 13313 ( 10.1038/ncomms13313) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Maynard Smith J. 1978. The evolution of sex. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 129.Pischedda A, Chippindale AK. 2006. Intralocus sexual conflict diminishes the benefits of sexual selection. PLoS Biol. 4, e356 ( 10.1371/journal.pbio.0040356) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Burke NW, Bonduriansky R. 2018. The geography of sex: sexual conflict, environmental gradients, and local loss of sex in facultatively parthenogenetic animals. Phil. Trans. R. Soc. B 373, 20170422 ( 10.1098/rstb.2017.0422) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Roze D, Otto SP. 2012. Differential selection between the sexes and selection for sex. Evolution 66, 558–574. ( 10.1111/j.1558-5646.2011.01459.x) [DOI] [PubMed] [Google Scholar]
- 132.Seger J, Trivers R. 1986. Asymmetry in the evolution of female mating preferences. Nature 319, 771–773. ( 10.1038/319771a0) [DOI] [Google Scholar]
- 133.Albert AY, Otto SP. 2005. Sexual selection can resolve sex-linked sexual antagonism. Science 310, 119–121. ( 10.1126/science.1115328) [DOI] [PubMed] [Google Scholar]
- 134.Li XY, Holman L. 2018. Evolution of female choice under intralocus sexual conflict and genotype-by-environment interactions. Phil. Trans. R. Soc. B 373, 20170425 ( 10.1098/rstb.2017.0425) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Charlesworth B, Charlesworth D. 1978. A model for the evolution of dioecy and gynodioecy. Am. Nat. 112, 975–997. ( 10.1086/283342) [DOI] [Google Scholar]
- 136.Charlesworth D, Charlesworth B. 1978. Population genetics of partial male-sterility and the evolution of monoecy and dioecy. Heredity 41, 137–153. ( 10.1038/hdy.1978.83) [DOI] [Google Scholar]
- 137.Jordan CY, Connallon T. 2014. Sexually antagonistic polymorphism in simultaneous hermaphrodites. Evolution 68, 3555–3569. ( 10.1111/evo.12536) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Olito C, Abbott JK, Jordan CY. 2018. The interaction between sex-specific selection and local adaptation in species without separate sexes. Phil. Trans. R. Soc. B 373, 20170426 ( 10.1098/rstb.2017.0426) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Muirhead CA, Presgraves DC. 2016. Hybrid incompatibilities, local adaptation, and the genomic distribution of natural introgression between species. Am. Nat. 187, 249–261. ( 10.1086/684583) [DOI] [PubMed] [Google Scholar]
- 140.Hoellinger I, Hermisson J. 2017. Bounds to parapatric speciation: a Dobzhansky–Muller incompatibility model involving autosomes, X chromosomes, and mitochondria. Evolution 71, 1366–1380. ( 10.1111/evo.13223) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.