Abstract
While gene flow can reduce the potential for local adaptation, hybridization may conversely provide genetic variation that increases the potential for local adaptation. Hybridization may also affect adaptation through altering sexual dimorphism and sexual conflict, but this remains largely unstudied. Here, we discuss how hybridization may affect sexual dimorphism and conflict due to differential effects of hybridization on males and females, and then how this, in turn, may affect local adaptation. First, in species with heterochromatic sexes, the lower viability of the heterogametic sex in hybrids could shift the balance in sexual conflict. Second, sex-specific inheritance of the mitochondrial genome in hybrids may lead to cytonuclear mismatches, for example, in the form of ‘mother's curse’, with potential consequences for sex ratio and sex-specific expression. Third, sex-biased introgression and recombination may lead to sex-specific consequences of hybridization. Fourth, transgressive segregation of sexually antagonistic alleles could increase sexual dimorphism in hybrid populations. Sexual dimorphism can reduce sexual conflict and enhance intersexual niche partitioning, increasing the fitness of hybrids. Adaptive introgression of alleles reducing sexual conflict or enhancing intersexual niche partitioning may facilitate local adaptation, and could favour the colonization of novel habitats. We review these consequences of hybridization on sex differences and local adaptation, and discuss how their prevalence and importance could be tested empirically.
This article is part of the theme issue ‘Linking local adaptation with the evolution of sex differences'.
Keywords: hybridization, sex-specific recombination, asymmetric introgression, intersexual correlations, sex-specific inheritance, local adaptation
1. Introduction
Here we outline how hybridization, through its effects on sex-specific viability, sexual conflict and sexual dimorphism, can contribute to sex-specific local adaptation. Recent research has highlighted the importance of understanding sex-specific local adaptation [1]. Sometimes, sexual dimorphism evolves in the same way and for the same reasons as sympatric ecological divergence and speciation, namely to reduce competition for resources [2]. Ecological divergence and sexual dimorphism may evolve at once [3] to maximize niche packing (see Glossary) [2–4]. In addition to classical examples such as the extreme sexual dimorphism in the beaks of the Huia [5], evidence from a wide range of taxa (e.g. birds [6], reptiles [7] and fish [8]) suggests that sexual dimorphism and niche partitioning may be important mechanisms to decrease competition for food resources between males and females. Moreover, different reproductive roles may lead to different requirements on body size, habitat use or diet. While such niche division can be advantageous, the genetic correlation between the sexes may constrain the evolution of sexual dimorphism [9]. Unless resolved, selection towards different optima may result in both sexes residing away from their fitness peaks and hence sexual conflict [9].
Despite a long-standing research tradition investigating sex-specific viability and fitness effects of hybridization [10], and an increasing appreciation of the importance of mitonuclear co-adaptation for hybridizing taxa [11], the effects of these phenomena on the potential for local adaptation following hybridization remain largely unexplored. Sex-specific inheritance and recombination mechanisms could affect sexual dimorphism, interlocus sexual conflict (Glossary), sex-specific expression patterns or sex ratios in hybrids (figure 1), but this has never been the main focus of hybridization studies. Moreover, hybridization may reshuffle sexually antagonistic alleles leading to transgressive segregation [12], which may enhance sexual dimorphism in niche use. This could dampen intersexual competition and have important consequences for ecological niche breadth.
Figure 1.
How hybridization may alter sex-specific local adaptation through its effects on sexual dimorphism, sex ratio and sexual conflict. This schematic illustrates the outline of the manuscript. In §2, we discuss how patterns resulting from hybridization may result in sexual dimorphism, sex-ratio distortion and affect sexual conflict. In §3, we address how resulting changes in sexual dimorphism, sex ratio and sexual conflict may affect local adaptation. (Online version in colour.)
It is increasingly recognized that under certain conditions, hybridization may have a positive impact on local adaptation [13]. Traditionally, plant ecologists viewed hybridization as potentially beneficial to adaptive evolution [14,15], while zoologists viewed it mostly as a cause of maladaptive breakdown of isolating mechanisms [16]. Recent studies suggest that the tree of life is rather a net of life with frequent introgression events [13,17–19]. Currently, a plethora of examples of evolutionary consequences of hybridization, ranging from local extinction to speciation, are described [13]. While adaptation to novel niches by hybrid species with trait values that differ from those of both parent species is documented (e.g. in Helianthus sunflowers where hybrid species inhabit more extreme habitats compared with the parent species [20,21]), other consequences of hybridization for local adaptation are less understood [22]. In particular, we argue that there is a gap between the multitude of studies documenting sex-specific viability, sex-specific expression and sex-biased introgression in hybrid species and introgressed taxa, and the lack of studies of how these factors affect sexual dimorphism in ecological niche and local adaptation in these taxa. Here, we review how hybridization interacts with sex-specific inheritance and recombination mechanisms, their effects on hybrid fitness, sex-specific fitness, sex ratio and how this can lead to sexual dimorphism and/or alter the prospects for local adaptation (figure 1). Following the structure outlined in figure 1, we first present how sex-specific effects of hybridization may affect sexual dimorphism, sex ratios and sexual conflict in §2, then we outline how altered sexual dimorphism, sex ratios and sexual conflict may affect local adaptation in §3, and finally, we discuss ideas for how to test our novel predictions in §4. We identify exciting areas for future research and suggest analyses to elucidate effects of hybridization on the prospects of local adaptation.
2. How hybridization can affect sexual conflict, sex ratio and sexual dimorphism
(a). Interactions with sex chromosomes
Patterns of sex-specific inheritance related to differentiated sex chromosomes are long known. However, little is known of how these patterns may affect sexual conflict, sex ratio and sexual dimorphism, and here we outline how hybridization between species with heteromorphic sex chromosomes may influence these factors. In addition, we discuss how hybridization between species with different sex-determination genes can lead to sex-chromosome turnover and affect sexual conflict.
Almost a century ago Haldane [10] noted that ‘when in the F1 offspring of two different animal races one sex is absent, rare or sterile, that sex is the heterozygous sex’ (Haldane's rule; Glossary). A closely related observation is the so-called ‘large X(Z) effect’ (Glossary), pertaining to the disproportionate contribution of the X/Z-chromosome in causing the reduced fitness of heterogametic hybrids [23]. The principal cause of both patterns is thought to be recessive alleles with deleterious effects in hybrids having a stronger impact on the heterogametic relative to the homogametic sex, due to hemizygous expression [24]. In taxa with well-differentiated sex chromosomes, Haldane's rule has shown to be close to universal, and heteromorphic sex chromosomes show reduced introgression on the X in XY (in mammals [25]; flies [26]) and the Z in ZW systems (Lepidoptera [27]; birds [28,29]).
While ‘Haldane's rule’ and the ‘large X(Z) effect’ both consider alleles with the same fitness effects in males and females, sex chromosomes are expected to accumulate disproportionate numbers of sexually antagonistic alleles. This follows from their sexually asymmetric inheritance resulting in the relative effect of male- and female-specific selection acting on the sex chromosomes becoming unbalanced [30]. Dominant alleles coding for sexually antagonistic traits that benefit the homogametic sex are expected to accumulate on the X chromosome in XY systems (female-benefitting alleles) and on the Z chromosome in ZW systems (male-benefitting alleles). This is because they spend two-thirds of their evolutionary time in the homogametic sex which has two copies of that sex chromosome. Recessive alleles that favour the heterogametic sex are expected to accumulate on the X chromosome in XY systems and on the Z chromosome in ZW systems because they are rarely exposed to antagonistic selection in the homogametic sex. Modifiers that lead to reduced gene expression in the sex with lower fitness or increased expression in the sex with higher fitness are expected to subsequently evolve and accumulate [31,32].
While these properties and patterns of sex-chromosome evolution have been extensively reviewed elsewhere [30,32,33], their implications for sex-specific local adaptation in hybrid populations remain poorly understood. The lower viability of the heterogametic sex may lead to biased sex ratios in hybrid populations in laboratory settings, e.g. in Drosophila [23], but also in the wild, e.g. in flycatcher hybrids [34]. Sex-linked gene regulation may become disrupted in hybrids resulting in abnormal gene expression. Male sterility due to disrupted sex-linked gene regulation has been observed, e.g. in Drosophila [35,36] and hybrids between Mus musculus and M. domesticus [37]. This may potentially cause sex-specific sterility, inviability or phenotypic differences influencing sexual dimorphism.
‘Haldane's rule’ and the ‘large X(Z) effect’ are less important in taxa with sex chromosomes that are not strongly differentiated and in taxa without reduced recombination rates in the sex-linked chromosome. In many taxa, genetic sex determination differs even between closely related species (e.g. in fishes [38–40], geckos [41] and Drosophila [42]). Hybridization between species with different sex-determining regions may result in biased sex ratios [38,43] and modified interactions between sex determination and sexually antagonistic alleles. Theoretical models suggest that selection against biased sex ratio or sexual conflict may lead to turnover of sex-determination genes [44–47] with some support from empirical studies, e.g. in frogs where the authors find introgression of sex chromosome due to selection against biased sex ratio [48], guppies [49] and cichlids [40,50,51]. If a sex determination or modifier gene of one species is more closely linked to sexually antagonistic genes than the sex determiners of the other species, it may introgress into the other species as a result of reduced sexual conflict. Sexually antagonistic alleles linked to the sex determiner may introgress in concert increasing the fitness in hybrids of both sexes. Hybridization between species with different sex determiners may also modify sexual dimorphism as has been shown for strawberry hybrids [52].
(b). Cytonuclear incompatibilities
It is increasingly clear that cytonuclear incompatibilities often affect hybrid fitness, but their effects on sex-specific survival, sexual antagonism and sexual dimorphism have rarely been discussed. Below we outline how such consequences may arise.
Cytonuclear incompatibilities arise as the mitochondrial genome encodes specific components of the oxidative phosphorylation system used for aerobic respiration [53], and there is hence strong selection for compatibility between the mitochondrial (mtDNA) and the nuclear (nuDNA) genome [11]. The mitochondrial genome is transmitted through the maternal lineage in most species [54]. Consequently, a male–female asymmetry in the fitness effects of mitochondrial mutations can arise [55] as mtDNA mutations that affect only males detrimentally will be less easily removed by natural selection than mutations that are also or only detrimental to females. The resulting accumulation of mutations that are disadvantageous to males but benign to females is coined ‘mother's curse’ (Glossary) [56]. This is supported by evidence for cytoplasmic variants beneficial to females being disadvantageous to males with consequences, e.g. disruption of production of cytochrome c oxidase [57,58]. Effects of mtDNA mutations in the form of male-biased fitness costs include reduced male fertility and increased rates of male ageing, e.g. in Drosophila melanogaster strains with introgressed mitochondria [55,59,60]. However, compensatory nuclear adaptations may evolve after a lag time [61]. Negative effects associated with disruption of co-evolved mitonuclear complexes, e.g. on ageing [60,62] and fertility [59,62], support the existence of such compensatory genetic variants. Cytonuclear incompatibilities arising from hybridization between diverged taxa are found in a range of taxa, e.g. in birds [63–65], carnivorous mice [66], flat worms [67] and plants [68,69]. Suboptimal respiration is one of the fitness costs to hybrids in flycatchers [64], carnivorous mice [66], voles [70] and chickadees [71], likely due to mitonuclear incompatibilities. Mitonuclear incompatibilities have also been shown to distort sex ratios, e.g. in experimental mitonuclear introgression lines of D. pseudoobscura [72]. In a recent modelling study, the authors found that strong selection on males or nonlinear fitness effects of mitochondria resulted in paternal leakage [73]. Consistent with this scenario, heteroplasmy found in hybrids across a wide range of taxa, including mussels [74], wheat [75], birds [63,65] and Drosophila [76] could potentially be due to selection for paternal leakage to counteract negative fitness effects of matrilinearily inherited mitochondria.
Interactions between mtDNA and nuDNA can lead to sex-specific global transcript responses [77]. In experimental trials in D. melanogaster mitochondrial polymorphism had major effects in males, modifying almost 10 per cent of nuclear transcripts. For most transcripts expression was upregulated in males, while effects on females were small. Expression differences were most pronounced in the testes and accessory glands [78], suggesting a cost to males and potentially reducing male ability for sexual coercion. Sex-specific expression alterations could either increase or decrease sexual dimorphism, contingent on whether the expression patterns of individuals with introgressed mitochondria are more similar among sexes or not. Finally, introgression of heterospecific mitochondrial variants could also have direct positive effects on population fitness. Introgressed mitochondria could replace mitochondrial genomes that have accumulated mutations with negative fitness effects, e.g. through genetic drift (e.g. due to Muller's ratchet [79]; see Glossary) as suggested in Llopart et al. [80]. Moreover, introgression of mitochondria with allelic variants that are well adapted to e.g. the local climate could improve population fitness. An example of this is the eastern yellow robin Eopsaltria australis, where mitochondrial DNA variants suited to coastal and inland climates covary with climate rather than nuclear genome origin, creating perpendicular axes of nuDNA and mtDNA differentiation [81].
Cytonuclear incompatibilities are also found in plants where chloroplast-driven incompatibilities cause reduced hybrid fitness [82,83], which can be remedied by biparental chloroplast inheritance, as found in Campanulastrum americanum where biparental inheritance leads to increased fitness of F1 hybrids and recovery in the F2 generation [84].
(c). Sex-biased introgression and meiotic drive
In this section, we discuss how sex-biased introgression and meiotic drive can affect patterns of sexual dimorphism, sexual conflict and sex ratio. Rates of introgression may differ between the sexes due to interspecific differences in mate preferences [85]. Additionally, sex-biased dispersal [86] may lead to increased introgression via the more dispersive sex. Unidirectional hybridization may thus contribute to differential introgression between sex-linked genes and bi-parentally inherited genes [87]. Reduced or no recombination in sex-limited chromosomes (Y or W) may additionally alter their introgression rates compared autosomes. In the absence of recombination, the combined effects of selection against introgression on multiple loci will lead to purging of entire introgressed sex chromosomes and as beneficial alleles cannot recombine away from incompatibilities, they cannot introgress [88]. Differential introgression of sex-linked genes and nuclear genes may alter sexual conflict.
In many species, one sex shows strongly reduced (heterochiasmy, e.g. some frogs, many fishes [89]) or no recombination (achiasmy, e.g. Drosophila, butterflies, copepods; see Glossary). We expect that in these species, alleles that are beneficial mostly to the non-recombining sex cannot introgress as easily as alleles beneficial to the recombining sex, thus potentially shifting the balance of sexual conflict. In addition, crosses between Tigriopus copepod populations suggest that if multiple loci on the same chromosome jointly cause Dobzhansky–Müller (DM) incompatibilities with other loci, they are most detrimental in backcrosses of the non-recombining sex (hybrid female, non-recombining, crossed with parental male) [90].
Finally, meiotic drive (Glossary) can manipulate the meiotic process to distort the allelic segregation away from expected Mendelian ratios [91]. The resulting reduced fecundity favours the evolution of drive suppressors [92], and the breaking-up of these associations may affect hybrid fertility and viability [91]. Avoidance of meiotic drive has been shown to drive female preference for larger eye-span, a sexually dimorphic ornament, in stalk-eyed flies Teleopsis dalmanni as short eye-span is coupled to the X-linked region causing the drive [93].
(d). Transgressive sorting of sexually antagonistic variation
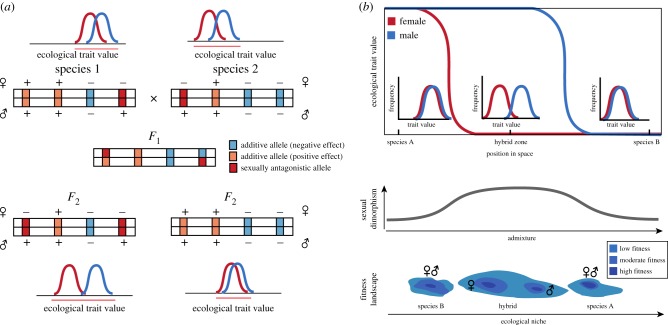
Hybridization may reshuffle sexually antagonistic alleles [12], leading to transgressive segregation (Glossary) of phenotypic sex differences. This may, in turn, generate early generation hybrid populations with extreme sexual dimorphism (figure 2a). When sexually antagonistic alleles are fixed at different loci in the hybridizing species, hybrids could either eliminate all sources of sexual antagonism or fix sexually antagonistic alleles at several loci through recombination. The latter scenario could enable hybridizing species to evolve stronger sexual dimorphism. Sexual dimorphism may, in turn, increase the carrying capacity of hybrid populations through intersexual niche partitioning [94], and may even allow hybrid species to colonize habitats that are unsuitable for their parent species. Such transgression in terms of ecological niche is well documented in both Helianthus and Cottus [20,95], but it has yet to be investigated from a sexual dimorphism perspective. Strongly sexually dimorphic hybrid lineages may also be able to adapt to environments with otherwise constraining levels of sex-specific selection. For instance, Saino & Bernardi [96] found that the extent of sexual size dimorphism varied across a crow hybrid zone. Moreover, the sexual dimorphism was significantly correlated both to sex-specific selection on males and altitude [96].
Figure 2.
Mechanisms through which hybridization can enhance or reduce sexual dimorphism and, in turn, affect local adaptation. (a) Transgressive segregation of sexually antagonistic alleles which have become fixed at different loci in two hybridizing species. These are quantitative trait loci for a trait involved in niche use (e.g. beak shape in birds). After initial hybridization, recombination may lead to different phenotypic outcomes (females above and males below each locus) where sexual dimorphism is either enhanced (left lower panel) or dampened (right lower panel). This may, in turn, have consequences on intersexual niche partitioning and local adaptation. (b) Non-coincident geographical clines between sexes for ecological traits in a hybrid zone. In admixed populations, enhanced sexual dimorphism, due to sex-specific differences in geographical clines (upper panel), may promote the occupation of novel ecological niches. Parent species may be incapable of colonizing this novel ecological niche, not because of morphospace constraints, but simply as a result of decreased mean population fitness due to intersexual competition and costly gender load (lower panel).
3. How hybridization may affect local adaptation via alteration of sexual dimorphism, sex ratio and sexual conflict
In this section, we outline how effects of hybridization on sexual dimorphism, sex ratio and sexual conflict may affect local adaptation. While these three phenomena are interrelated, we present them separately as they have different implications for local adaptation.
(a). Effects of hybridization-altered sexual dimorphism on local adaptation
Here, we discuss how patterns of sexual dimorphism altered by hybridization may affect local adaptation. As explained above, hybridization may affect sexual dimorphism and could hence potentially increase the ability of males and females to exploit different niches, adding to other selection pressures and mechanisms that enable the sexes to use different niches. Additionally, we argue that hybridization may affect the genetic architecture of traits in such a way that hybrid males and females reach their maximum intrinsic fitness at different levels of genome-wide admixture (for instance, at different points along a hybrid zone (figure 2), due to cytonuclear and/or sex-linked genetic incompatibilities). In hybrid zones, this may be reflected by non-coincident genomic clines (Glossary) for sex-specific genetic markers [97]. Along the hybrid zone, geographical clines of ecological traits may thus also become decoupled and displaced between males and females (figure 2b), especially if sex-biased genotype by environment interactions are directly affected by hybridization [98]. This could lead to a situation where sexual dimorphism increases in the centre of the hybrid zone, enhancing intersexual niche partitioning (Glossary) and mean population fitness, as both sexes then are better adapted to local conditions. For two species with weak sexual dimorphism and high gender load (Glossary), i.e. where the sexes have different optima but have not been able to develop sexual dimorphism to better match these optima, hybridization could thus potentially dampen sexual conflict through formation of hybrid lineages. Sexual conflict could partially or fully be resolved in hybrid lineages through transgressive sorting of variants that enable sexually sex-specific expression of traits (cf. [12]). Such sorting where variants underlying sexual dimorphism from both lineages are favoured and accumulate in a hybrid lineage would result in elevated mean population fitness, and could potentially allow for the colonization of habitats where parental species would not be able to survive (cf. [99]), although no empirical examples have yet been identified to our knowledge. Increased sexual dimorphism allows a population to explore a wider phenotypic space around the local fitness peak, potentially facilitating climbing alternative fitness peaks [100], increasing prospects for local adaptation.
Finally, the impact of hybridization on sexual dimorphism could be directly involved in range shift processes (Glossary) and species range dynamics. Theory predicts that sex-specific maladaptation should increase at range margins [1]. The probability for hybridization might also increase at range margins though. Fitness asymmetries between sexes and maladaptation could thus be reduced following interspecific gene flow, and improve the viability of range margin populations by alleviating gender load and increasing fitness of the maladapted sex through introgression of beneficial alleles.
(b). Sex-ratio distortion
Here, we introduce how sex-ratio distortions due to hybridization may facilitate or hamper local adaptation. Sex-specific viability following hybridization may result in skewed sex ratios. The operational sex ratio (OSR; Glossary) may affect intrasexual mating competition [101], but empirical evidence for an effect of OSR on mating competition is mixed [102] because skewed sex ratios might also increase the cost of mate guarding [103]. A recent meta-analysis concluded that there is compelling evidence that OSR predicts strength of sexual selection in males, but not females [104]. Sexual selection can both promote and inhibit local adaptation (reviewed in [105]). When sexual selection inhibits local adaptation, e.g. through pushing the population off the fitness optimum [106,107], a relaxation in sexual selection is likely to increase the prospects for local adaptation. Hence, altered OSR could potentially reduce sexual selection on males, enabling populations to match the ecological optimum closer in cases where sexual selection opposes natural selection.
Sex ratio is also important for the ability of populations to survive and adapt as the number of females in the population determines the reproductive output (e.g. [108]) and strongly biased sex ratios may lead to inbreeding depression as found, e.g. in the gypsy moth Lymantria dispar [109]. Biased sex ratios may hence also hamper local adaptation.
(c). Effects of hybridization on local adaptation via modulation of sexual conflict
A shift in the balance between male harming and female harming antagonistic variants can lead to sex-ratio distortion, which may impact local adaptation, as outlined above. In addition, a reduction of sexual conflict, e.g. due to introgression of a sex modifier increasing sex-linkage of a sexually antagonistic gene [44] or of a sex chromosome harbouring sexually antagonistic genes [99], may facilitate local adaptation by allowing for greater sexual dimorphism in ecology. Such sexual dimorphism could allow the sexes to better track their respective adaptive optima, and hence add to local adaptation of the population.
4. Testing for effects of hybridization on sex-specific local adaptation
Many of the interactions between hybridization and local adaptation via modulation of sex ratio, sexual dimorphism and sexual conflict, which we have proposed above, lack empirical examples and theoretical studies. In this section, we suggest approaches to study some of these interactions.
Sex-specific viability in early generation hybrids may result from the greater impact of deleterious recessive alleles on hybrids of the heterogametic sex, i.e. the faster X/Z theory and mitonuclear incompatibilities. This may lead to a biased sex ratio affecting sexual conflict and sex-specific adaptation as outlined above. Meta-analyses of sex ratios in young hybrid populations or in hybrid zones would allow testing of this hypothesis, especially given such data must have been already collected and should be available from the numerous field studies of hybrid zones published over the years. Another interesting comparison would be one of sex ratios between young hybrid taxa or hybrid swarms and old, stabilized hybrid taxa. Taking advantage of the fact that nucleotide diversity on the Y/W chromosome depends only on the effective population size of the heterogametic sex, while the nucleotide diversity of the other sex chromosome depends on effective population sizes of both sexes, it is possible to tentatively infer past sex ratios. Comparing the relative effective population sizes of the two heteromorphic sex chromosomes in hybrid taxa and parental taxa (where other factors affecting this ratio, such as mating systems should be very similar) could hence be informative of differences in sex-specific survival between these taxa. Sex-specific viability may affect local adaptation by relaxing sexual selection, and by increasing the probability of population persistence through female-skewed sex ratios (see above). To address whether these mechanisms take place in hybrid populations, it may be possible to compare the relative strength of sexual selection in hybrid taxa or hybrid zones with that of the parental taxa.
Several specific predictions can be made based on the current knowledge of mitonuclear incompatibilities. First, hybrids with foreign mitochondria are expected to have suboptimal respiration and a higher incidence of sterility. Moreover, when hybrid populations differ in parental contributions, e.g. as in the hybrid species Italian sparrow (Passer italiae, cf. [65]), populations with larger parts of their genomes matching the mitochondrial ancestry are expected to have a more well-functioning respiration. In addition, males are expected to be disproportionately affected by mitonuclear incompatibilities in species with XY systems where mitochondria are not selected to be compatible with the Y chromosome due to female inheritance. These predictions can be tested by comparing, e.g. cost of respiration or basal metabolic rate in the two sexes in young hybrid taxa and stabilized hybrid taxa [70]. Moreover, meta-studies addressing whether taxa with heterospecific introgressed mitochondria have obtained these from taxa adapted to the climate in their current distribution, e.g. as in the eastern yellow robin [81], could be interesting.
The consequences of hybridization on sexual dimorphism and local adaptation have been poorly studied, as much empirical work on hybridization often only considers one sex (e.g. [110]) or controls for sexual dimorphism at the phenotypic level (e.g. [96]) without making it a specific focus. However, we argue that our hypotheses warrant reanalyses of the data on hybrid zones and hybrid species. To understand how hybridization affects sexual dimorphism in ecological traits and niche partitioning, we suggest a more systematic investigation of whether sexual dimorphism is greater in hybrid species than in parent species. This would be predicted if transgressive sorting of sexually antagonistic alleles could increase beneficial dimorphism. Consistent testing of variation in sexual dimorphism across hybrid zones would also shed light on the effects of hybridization on sexual dimorphism. Another interesting possibility is to use hybrid zones as natural experiments, and test if genomic clines and geographical clines differ between sexes. If hybrid zone clines of ecological traits are shifted between the sexes, it implies that males and females have different ecological fitness optima (figure 2b).
In some taxa, clades with the strongest sexual dimorphism show particularly high rates of hybridization and turnover in sex-determination genes, potentially to reduce sexual conflict (e.g. in cichlids [40] and jumping spiders [111,112]). Investigating the role of introgression in sex chromosome turnover in these systems and performing meta-analyses investigating the generality of these findings would be a promising avenue. Little if anything is known about how the phenomena we have discussed above differ between early generation hybrids and stabilized hybrid taxa. Investigating this may give insights into the selection for compatibility of hybrid genomes [65,113] and the balance between selection for compatibility and selection for local adaptation [114]. We argue that the study of hybridization should move beyond classical approaches and also focus on the study of how hybridization and sex-specific selection pressures interact and affect, e.g. sexual dimorphism, sex differences in viability and sexual conflict. Much remains to be done to assess the generality of the impact of hybridization on local adaptation via modulation of sexual conflict, sex ratio and sexual dimorphism.
Data accessibility
This article has no additional data.
Authors' contributions
A.R. and F.E. conceived of the idea. A.R. wrote the introduction, §§2b, 3b, 3c and 4. J.I.M. prepared figure 1, wrote §2c and wrote §2a together with J.S.H. and provided critical feedback on the other sections. F.E. and J.S.H. prepared figure 2 and wrote §§2d and 3a. A.N.B. prepared the Glossary. All authors discussed ideas and commented on the manuscript. All authors gave final approval for publication.
Competing interests
We declare no competing interests.
Funding
This work was funded by a Wenner-Gren Fellowship to A.R.
Glossary
- Achiasmy
Absence of recombination in one sex.
- Dobzhansky–Müller incompatibilities
Genetic incompatibilities arising from fixation of alternative alleles at two or more loci in the parental species that if brought together in hybrids are incompatible and decrease fitness.
- Gender load
The reduction of fitness resulting from sexual conflict.
- Genomic cline
Analysis that compares allele or genotype frequencies of each locus to a genome-wide average.
- Haldane's rule
If only one sex is inviable or sterile in a species hybrid, that sex is more likely to be the heterogametic sex.
- Heterochiasmy
Differential recombination rates between sexes.
- Interlocus sexual conflict
Displacement of the phenotypic optimum due to selection on the opposite sex, and by interactions between sexually antagonistic alleles at different loci.
- Intersexual niche partitioning
The divergence in the niche space between the sexes.
- Large X(Z) effect
Sex chromosomes (X or Z) have a disproportionate impact in adaptive evolution.
- Meiotic drive
When a gene is passed to the offspring more frequently than expected due to manipulation of the meiotic process.
- Mother's curse
Accumulation of mitochondrial mutations which are deleterious to males but not to females because, due to the matrilineal inheritance, they are less easily removed by selection than mutations that are also deleterious to females.
- Muller's ratchet
Irreversible accumulation of deleterious mutations in the genomes of asexual populations.
- Niche packing
The resulting narrower (i.e. more specialized) niches of species occurring in biologically diverse communities relative to similar species in less biologically diverse communities as a consequence of increased interspecific competition in diverse communities.
- Operational sex ratio
The ratio of fertilizable females to sexually active males at any given time.
- Range shift processes
The processes that might shift species' ranges, such as climatic factors, dispersal capacity and population persistence.
- Transgressive segregation
Hybrid offspring trait values that fall outside the range of both parentals.
References
- 1.Connallon T. 2015. The geography of sex-specific selection, local adaptation, and sexual dimorphism. Evolution 69, 2333–2344. ( 10.1111/evo.12737) [DOI] [PubMed] [Google Scholar]
- 2.Bolnick DI, Doebeli M. 2003. Sexual dimorphism and adaptive speciation: two sides of the same ecological coin. Evolution 57, 2433–2518. ( 10.1554/02-595) [DOI] [PubMed] [Google Scholar]
- 3.Cooper IA, Gilman RT, Boughman JW. 2011. Sexual dimorphism and speciation on two ecological coins: patterns from nature and theoretical predictions. Evolution 65, 2553–2571. ( 10.1111/j.1558-5646.2011.01332.x) [DOI] [PubMed] [Google Scholar]
- 4.Shine R. 1989. Ecological causes for the evolution of sexual dimorphism: a review of the evidence. Q. Rev. Biol. 64, 419–461. ( 10.1086/416458) [DOI] [PubMed] [Google Scholar]
- 5.Moorehouse RJ. 1996. The extraordinary bill dimorphism of the Huia (Heteralocha acutirostris): sexual selection or intrasexual competition. Notornis 43, 19–34. [Google Scholar]
- 6.Radford AN, Plessis DMA. 2003. Bill dimorphism and foraging niche partitioning in the green woodhoopoe. J. Anim. Ecol. 72, 258–269. ( 10.1046/j.1365-2656.2003.00697.x) [DOI] [Google Scholar]
- 7.Furtado MFD, Travaglia-Cardoso SR, Rocha MMT. 2006. Sexual dimorphism in venom of Bothrops jararaca (Serpentes: Viperidae). Toxicon 48, 401–410. ( 10.1016/j.toxicon.2006.06.005) [DOI] [PubMed] [Google Scholar]
- 8.Sakashita H. 1992. Sexual dimorphism and food habits of the clingfish, Diademichthys lineatus, and its dependence on host sea urchin. Environ. Biol. Fishes 34, 95–101. ( 10.1007/BF00004787) [DOI] [Google Scholar]
- 9.Rice WR, Chippindale AK. 2008. Intersexual ontogenetic conflict. J. Evol. Biol . 14, 685–693. ( 10.1046/j.1420-9101.2001.00319.x) [DOI] [Google Scholar]
- 10.Haldane JBS. 1922. Sex ratio and unisexual sterility in hybrid animals. J. Genet. 12, 101–109. ( 10.1007/BF02983075) [DOI] [Google Scholar]
- 11.Hill GE. 2016. Mitonuclear coevolution as the genesis of speciation and the mitochondrial DNA barcode gap. Ecol. Evol. 6, 5831–5842. ( 10.1002/ece3.2338) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parsons KJ, Son YH, Albertson RC. 2011. Hybridization promotes evolvability in African cichlids: connections between transgressive segregation and phenotypic integration. Evol. Biol. 38, 306–315. ( 10.1007/s11692-011-9126-7) [DOI] [Google Scholar]
- 13.Abbott R, et al. 2013. Hybridization and speciation. J. Evol. Biol . 26, 229–246. ( 10.1111/j.1420-9101.2012.02599.x) [DOI] [PubMed] [Google Scholar]
- 14.Andersson E, Stebbins GL. 1954. Hybridization as an evolutionary stimulus. Evolution 4, 378–388. ( 10.1111/j.1558-5646.1954.tb01504.x) [DOI] [Google Scholar]
- 15.Anderson E. 1949. Introgressive hybridization. New York, NY: Wiley. [Google Scholar]
- 16.Mayr E. 1963. Animal species and evolution. Cambridge, MA: Harvard University Press. [Google Scholar]
- 17.Pennisi E. 2016. Shaking up the tree of life. Science 354, 817–821. ( 10.1126/science.354.6314.817) [DOI] [PubMed] [Google Scholar]
- 18.Mallet J. 2005. Hybridization as an invasion of the genome. Trends Ecol. Evol. 20, 229–237. ( 10.1016/j.tree.2005.02.010) [DOI] [PubMed] [Google Scholar]
- 19.Mallet J, Besansky N, Hahn MW. 2015. How reticulated are species? Bioessays 38, 140–149. ( 10.1002/bies.201500149) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rieseberg LH. 2003. Major ecological transitions in wild sunflowers facilitated by hybridization. Science 301, 1211–1216. ( 10.1126/science.1086949) [DOI] [PubMed] [Google Scholar]
- 21.Kagawa K, Takimoto G. 2017. Hybridization can promote adaptive radiation by means of transgressive segregation. Ecol. Lett. 21, 264–274. ( 10.1111/ele.12891) [DOI] [PubMed] [Google Scholar]
- 22.Arnold BJ, Lahner B, DaCosta JM, Weisman CM, Hollister JD, Salt DE, Bomblies K, Yant L. 2016. Borrowed alleles and convergence in serpentine adaptation. Proc. Natl Acad. Sci. USA 113, 8320–8325. ( 10.1073/pnas.1600405113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coyne JA, Orr HA. 1989. Patterns of speciation in Drosophila. Evolution 43, 362 ( 10.2307/2409213) [DOI] [PubMed] [Google Scholar]
- 24.Turelli M, Orr HA. 1995. The dominance theory of Haldane's rule. Genetics 140, 389–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Janousek V, et al. 2012. Genome-wide architecture of reproductive isolation in a naturally occurring hybrid zone between Mus musculus musculus and M. m. domesticus. Mol. Ecol . 21, 3032–3047. ( 10.1111/j.1365-294X.2012.05583.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garrigan D, Kingan SB, Geneva AJ, Andolfatto P, Clark AG, Thornton KR, Presgraves DC. 2012. Genome sequencing reveals complex speciation in the Drosophila simulans clade. Genome Res. 22, 1499–1511. ( 10.1101/gr.130922.111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin SH, et al. 2013. Genome-wide evidence for speciation with gene flow in Heliconius butterflies. Genome Res. 23, 1817–1828. ( 10.1101/gr.159426.113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saetre GP, Borge T, Lindroos K, Haavie J, Sheldon BC, Primmer C, Syvanen AC. 2003. Sex chromosome evolution and speciation in Ficedula flycatchers. Proc. R. Soc. Lond. B 270, 53–59. ( 10.1098/rspb.2002.2204) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ellegren H, et al. 2012. The genomic landscape of species divergence in Ficedula flycatchers. Nature 491, 756–760. ( 10.1038/nature11584) [DOI] [PubMed] [Google Scholar]
- 30.Irwin DE. 2018. Sex chromosomes and speciation in birds and other ZW systems. Mol. Ecol . Special Issue, 1–24. ( 10.1111/mec.14537) [DOI] [PubMed] [Google Scholar]
- 31.Rice WR. 1984. Sex chromosomes and the evolution of sexual dimorphism. Evolution 38, 735–742. ( 10.1111/j.1558-5646.1984.tb00346.x) [DOI] [PubMed] [Google Scholar]
- 32.Qvarnström A, Bailey RI. 2008. Speciation through evolution of sex-linked genes. Heredity 102, 4–15. ( 10.1038/hdy.2008.93) [DOI] [PubMed] [Google Scholar]
- 33.Schilthuizen M, Giesbers MCWG, Beukeboom LW. 2011. Haldane's rule in the 21st century. Heredity 107, 95–102. ( 10.1038/hdy.2010.170) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Veen T, Borge T, Griffith SC, Saetre GP, Bures S, Gustafsson L, Sheldon BC. 2001. Hybridization and adaptive mate choice in flycatchers. Nature 411, 45–50. ( 10.1038/35075000) [DOI] [PubMed] [Google Scholar]
- 35.Michalak P, Noor MAF. 2003. Genome-wide patterns of expression in Drosophila pure species and hybrid males. Mol. Biol. Evol. 20, 1070–1076. ( 10.1093/molbev/msg119) [DOI] [PubMed] [Google Scholar]
- 36.Moehring AJ, Teeter KC, Noor MA. F. 2006. Genome-wide patterns of expression in Drosophila pure species and hybrid males. II. Examination of multiple-species hybridizations, platforms, and life cycle stages. Mol. Biol. Evol. 24, 137–145. ( 10.1093/molbev/msl142) [DOI] [PubMed] [Google Scholar]
- 37.Good JM, Giger T, Dean MD, Nachman MW. 2010. Widespread over-expression of the X chromosome in sterile F1 hybrid mice. PLoS Genet. 6, e1001148 ( 10.1371/journal.pgen.1001148) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ser JR, Roberts RB, Kocher TD. 2010. Multiple interacting loci control sex determination in Lake Malawi cichlid fish. Evolution 64, 486–501. ( 10.1111/j.1558-5646.2009.00871.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Woram RA, et al. 2003. Comparative genome analysis of the primary sex-determining locus in salmonid fishes. Genome Res. 13, 272–280. ( 10.1101/gr.578503) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seehausen OJJM, Lande R. 1999. Color polymorphism and sex ratio distortion in a cichlid fish as an incipient stage in sympatric speciation by sexual selection. Ecol Lett. 2, 367–378. ( 10.1046/j.1461-0248.1999.00098.x) [DOI] [Google Scholar]
- 41.Gamble T, Coryell J, Ezaz T, Lynch J, Scantlebury DP, Zarkower D. 2015. Restriction site-associated DNA sequencing (RAD-seq) reveals an extraordinary number of transitions among gecko sex-determining systems. Mol. Biol. Evol. 32, 1296–1309. ( 10.1093/molbev/msv023) [DOI] [PubMed] [Google Scholar]
- 42.Vicoso B, Bachtrog D. 2015. Numerous transitions of sex chromosomes in Diptera. PLoS Biol. 13, e1002078 ( 10.1371/journal.pbio.1002078) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miura I. 2017. Sex determination and sex chromosomes in Amphibia. Sex Dev. 11, 298–306. ( 10.1159/000485270) [DOI] [PubMed] [Google Scholar]
- 44.Vuilleumier S, Lande R, van Alphen JJM, Seehausen O. 2007. Invasion and fixation of sex-reversal genes. J. Evol. Biol . 20, 913–920. ( 10.1111/j.1420-9101.2007.01311.x) [DOI] [PubMed] [Google Scholar]
- 45.van Doorn GS, Kirkpatrick M. 2007. Turnover of sex chromosomes induced by sexual conflict. Nature 449, 909–912. ( 10.1038/nature06178) [DOI] [PubMed] [Google Scholar]
- 46.van Doorn GS, Kirkpatrick M. 2010. Transitions between male and female heterogamety caused by sex-antagonistic selection. Genetics 186, 629–645. ( 10.1534/genetics.110.118596) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Veltsos P, Keller I, Nichols RA. 2008. The inexorable spread of a newly arisen neo-Y chromosome. PLoS Genet. 4, e1000082 ( 10.1371/journal.pgen.1000082) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ogata M, Ohtani H, Igarashi T, Hasegawa Y, Ichikawa Y, Miura I. 2003. Change of the heterogametic sex from male to female in the frog. Genetics 164, 613–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Volff JN, Schartl M. 2001. Variability of genetic sex determination in poeciliid fishes. Genetica 111, 101–110. ( 10.1023/A:1013795415808) [DOI] [PubMed] [Google Scholar]
- 50.Parnell NF, Streelman JT. 2013. Genetic interactions controlling sex and color establish the potential for sexual conflict in Lake Malawi cichlid fishes. Heredity 110, 239–246. ( 10.1038/hdy.2012.73) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roberts RB, Ser JR, Kocher TD. 2009. Sexual conflict resolved by invasion of a novel sex determiner in Lake Malawi cichlid fishes. Science 326, 998–1001. ( 10.1126/science.1174705) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Govindarajulu R, Liston A, Ashman T-L. 2012. Sex-determining chromosomes and sexual dimorphism: insights from genetic mapping of sex expression in a natural hybrid Fragaria×ananassa subsp. cuneifolia. Heredity 110, 430–438. ( 10.1038/hdy.2012.96) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Björkholm P, Harish A, Hagström E, Ernst AM, Andersson SG. E. 2015. Mitochondrial genomes are retained by selective constraints on protein targeting. Proc. Natl Acad. Sci. USA 112, 10 154–10 161. ( 10.1073/pnas.1421372112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Birky CW. 1995. Uniparental inheritance of mitochondrial. Proc. Natl Acad. Sci. USA 92, 11 331–11 338. ( 10.1073/pnas.92.25.11331) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Beekman M, Dowling DK, Aanen DK. 2014. The costs of being male: are there sex-specific effects of uniparental mitochondrial inheritance? Phil. Trans. R. Soc. B 369, 20130440 ( 10.1098/rstb.2013.0440) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gemmell NJ, Metcalf VJ, Allendorf FW. 2004. Mother's curse: the effect of mtDNA on individual fitness and population viability. Trends Ecol. Evol . 19, 238–244. ( 10.1016/j.tree.2004.02.002) [DOI] [PubMed] [Google Scholar]
- 57.Rand DM, Fry A, Sheldahl L. 2005. Nuclear-mitochondrial epistasis and Drosophila aging: introgression of Drosophila simulans mtDNA modifies longevity in D. melanogaster nuclear backgrounds. Genetics 172, 329–341. ( 10.1534/genetics.105.046698) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sackton TB, Haney RA, Rand DM. 2003. Cytonuclear coadapation in Drosophila: disruption of cytochrome c oxidase in backcross genotypes. Evolution 57, 2315–2325. ( 10.1111/j.0014-3820.2003.tb00243.x) [DOI] [PubMed] [Google Scholar]
- 59.Patel MR, et al. 2016. A mitochondrial DNA hypomorph of cytochrome oxidase specifically impairs male fertility in Drosophila melanogaster. eLife 5, 1144 ( 10.7554/eLife.16923) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Camus MF, Clancy DJ, Dowling DK. 2012. Mitochondria, maternal inheritance, and male aging. Curr. Biol . 22, 1717–1721. ( 10.1016/j.cub.2012.07.018) [DOI] [PubMed] [Google Scholar]
- 61.Connallon T, Camus MF, Morrow EH, Dowling DK. 2018. Coadaptation of mitochondrial and nuclear genes, and the cost of mother's curse. Proc. R. Soc. B 285, 20172257 ( 10.1098/rspb.2017.2257) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Camus MF, Wolf JBW, Morrow EH, Dowling DK. 2015. Single nucleotides in the mtDNA sequence modify mitochondrial molecular function and are associated with sex-specific effects on fertility and aging. Curr. Biol . 25, 2717–2722. ( 10.1016/j.cub.2015.09.012) [DOI] [PubMed] [Google Scholar]
- 63.Trier CN, Hermansen JS, Sætre G-P, Bailey RI. 2014. Evidence for mito-nuclear and sex-linked reproductive barriers between the hybrid Italian sparrow and its parent species. PLoS Genet. 10, e1004075 ( 10.1371/journal.pgen.1004075) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McFarlane SE, Sirkiä PM, Ålund M, Qvarnström A. 2016. Hybrid dysfunction expressed as elevated metabolic rate in male Ficedula flycatchers. PLoS ONE 11, e0161547 ( 10.1371/journal.pone.0161547) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Runemark A, Trier CN, Eroukhmanoff F, Hermansen JS, Matschiner M, Ravinet M, Elgvin TO, Sætre G-P. 2018. Variation and constraints in hybrid genome formation. Nat. Ecol. Evol . 2, 549–556. ( 10.1038/s41559-017-0437-7) [DOI] [PubMed] [Google Scholar]
- 66.Shipley JR, Campbell P, Searle JB, Pasch B. 2016. Asymmetric energetic costs in reciprocal-cross hybrids between carnivorous mice (Onychomys). J. Exp. Biol . 219, 3803–3809. ( 10.1242/jeb.148890) [DOI] [PubMed] [Google Scholar]
- 67.Chang C-C, Rodriguez J, Ross J. 2016. Mitochondrial–nuclear epistasis impacts fitness and mitochondrial physiology of interpopulation Caenorhabditis briggsae hybrids. Genes Genomes Genet. 6, 209–219. ( 10.1534/g3.115.022970) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Barr CM, Fishman L. 2010. The nuclear component of a cytonuclear hybrid incompatibility in Mimulus maps to a cluster of pentatricopeptide repeat genes. Genetics 184, 455–465. ( 10.1534/genetics.109.108175) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gaborieau L, Brown GG, Mireau H. 2016. The propensity of pentatricopeptide repeat genes to evolve into restorers of cytoplasmic male sterility. Front. Plant. Sci . 7, 1816 ( 10.3389/fpls.2016.01816) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Boratyński Z, Ketola T, Koskela E, Mappes T. 2015. The sex specific genetic variation of energetics in bank voles, consequences of introgression? Evol. Biol . 43, 37–47. ( 10.1007/s11692-015-9347-2) [DOI] [Google Scholar]
- 71.Olson JR, Cooper SJ, Swanson DL. 2010. The relationship of metabolic performance and distribution in black-capped and Carolina chickadees. Physiol. Biochem. Zool . 83, 263–275. ( 10.1086/648395) [DOI] [PubMed] [Google Scholar]
- 72.Jelić M, Arnqvist G, Novičić ZK, Kenig B, Tanasković M, Anđelković M, Stamenković-Radak M. 2015. Sex-specific effects of sympatric mitonuclear variation on fitness in Drosophila subobscura. BMC Evol. Biol . 15, 135 ( 10.1186/s12862-015-0421-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kuijper B, Lane N, Pomiankowski A. 2015. Can paternal leakage maintain sexually antagonistic polymorphism in the cytoplasm? J. Evol. Biol . 28, 468–480. ( 10.1111/jeb.12582) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Śmietanka B, Burzyński A. 2017. Disruption of doubly uniparental inheritance of mitochondrial DNA associated with hybridization area of European Mytilus edulis and Mytilus trossulusin Norway. Mar. Biol . 164, 209 ( 10.1007/s00227-017-3235-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Aksyonova E, Sinyavskaya M, Danilenko N, Pershina L, Nakamura C, Davydenko O. 2005. Heteroplasmy and paternally oriented shift of the organellar DNA composition in barley–wheat hybrids during backcrosses with wheat parents. Genome 48, 761–769. ( 10.1139/g05-049) [DOI] [PubMed] [Google Scholar]
- 76.Dokianakis E, Ladoukakis ED. 2014. Different degree of paternal mtDNA leakage between male and female progeny in interspecific Drosophila crosses. Ecol. Evol . 4, 2633–2641. ( 10.1002/ece3.1069) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mossman JA, Tross JG, Li N, Wu Z, Rand DM. 2016. Mitochondrial-nuclear interactions mediate sex-specific transcriptional profiles in Drosophila. Genetics 204, 613–630. ( 10.1534/genetics.116.192328) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Innocenti P, Morrow EH, Dowling DK. 2011. Experimental evidence supports a sex-specific selective sieve in mitochondrial genome evolution. Science 332, 845–848. ( 10.1126/science.1201157) [DOI] [PubMed] [Google Scholar]
- 79.Muller HJ. 1932. Some genetic aspects of sex. Am. Nat . 66, 118–138. ( 10.1086/280418) [DOI] [Google Scholar]
- 80.Llopart A, Herrig D, Brud E, Stecklein Z. 2014. Sequential adaptive introgression of the mitochondrial genome in Drosophila yakuba and Drosophila santomea. Mol. Ecol . 23, 1124–1136. ( 10.1111/mec.12678) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Morales HE, Sunnucks P, Joseph L, Pavlova A. 2017. Perpendicular axes of differentiation generated by mitochondrial introgression. Mol. Ecol . 26, 3241–3255. ( 10.1111/mec.14114) [DOI] [PubMed] [Google Scholar]
- 82.Barnard-Kubow KB, So N, Galloway LF. 2016. Cytonuclear incompatibility contributes to the early stages of speciation. Evolution 70, 2752–2766. ( 10.1111/evo.13075) [DOI] [PubMed] [Google Scholar]
- 83.Zeng Y-F, Zhang J-G, Duan A-G, Abuduhamiti B. 2016. Genetic structure of Populus hybrid zone along the Irtysh River provides insight into plastid-nuclear incompatibility. Sci. Rep. 6, 28043 ( 10.1038/srep28043) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Barnard-Kubow KB, McCoy MA, Galloway LF. 2017. Biparental chloroplast inheritance leads to rescue from cytonuclear incompatibility. New Phytol. 213, 1466–1476. ( 10.1111/nph.14222) [DOI] [PubMed] [Google Scholar]
- 85.Peters KJ, Myers SA, Dudaniec RY, O'Connor JA, Kleindorfer S. 2017. Females drive asymmetrical introgression from rare to common species in Darwin's tree finches. J. Evol. Biol . 30, 1940–1952. ( 10.1111/jeb.13167) [DOI] [PubMed] [Google Scholar]
- 86.Greenwood PJ. 1980. Mating systems, philopatry and dispersal in birds and mammals. Anim. Behav . 28, 1140–1162. ( 10.1016/S0003-3472(80)80103-5) [DOI] [Google Scholar]
- 87.Wirtz P. 1999. Mother species-father species: unidirectional hybridization in animals with female choice. Anim. Behav . 58, 1–12. ( 10.1006/anbe.1999.1144) [DOI] [PubMed] [Google Scholar]
- 88.Martin SH, Jiggins CD. 2017. Interpreting the genomic landscape of introgression. Curr. Opin. Genet. Dev . 47, 69–74. ( 10.1016/j.gde.2017.08.007) [DOI] [PubMed] [Google Scholar]
- 89.Lenormand T, Dutheil J. 2005. Recombination difference between sexes: a role for haploid selection. PLoS Biol. 3, e63 ( 10.1371/journal.pbio.0030063) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Willett CS, Lima TG, Kovaleva I, Hatfield L. 2016. Chromosome-wide impacts on the expression of incompatibilities in hybrids of Tigriopus californicus. Genes Genomes Genet. 6, 1739–1749. ( 10.1534/g3.116.028050) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.McDermott SR, Noor MAF. 2010. The role of meiotic drive in hybrid male sterility. Phil. Trans. R. Soc. B 365, 1265–1272. ( 10.1098/rstb.2009.0264) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Larracuente AM, Presgraves DC. 2012. The selfish segregation distorter gene complex of Drosophila melanogaster. Genetics 192, 33–53. ( 10.1534/genetics.112.141390) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cotton AJ, Földvári M, Cotton S, Pomiankowski A. 2014. Male eyespan size is associated with meiotic drive in wild stalk-eyed flies (Teleopsis dalmanni). Heredity 112, 363–369. ( 10.1038/hdy.2013.131) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Butler MA, Sawyer SA, Losos JB. 2007. Sexual dimorphism and adaptive radiation in Anolis lizards. Nature 447, 202–205. ( 10.1038/nature05774) [DOI] [PubMed] [Google Scholar]
- 95.Nolte AW, Freyhof J, Stemshorn KC, Tautz D. 2005. An invasive lineage of sculpins, Cottus sp. (Pisces, Teleostei) in the Rhine with new habitat adaptations has originated from hybridization between old phylogeographic groups. Proc. R. Soc. B 272, 2379–2387. ( 10.1038/rspb.2005.3231) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Saino N, Bernardi FD. 1994. Geographic variation in size and sexual dimorphism across a hybrid zone between carrion crows (Corvus corone corone) and hooded crows (C. c. cornix). Can. J. Zool . 72, 1543–1550. ( 10.1139/z94-205) [DOI] [Google Scholar]
- 97.Jaarola M, Tegelstrom H, Fredga K. 1997. A contact zone with noncoincident clines for sex-specific markers in the field vole (Microtus agrestis). Evolution 51, 241 ( 10.2307/2410977) [DOI] [PubMed] [Google Scholar]
- 98.Benvenuto C, Cheyppe-Buchmann S, Bermond G, Ris N, Fauvergue X. 2012. Intraspecific hybridization, life history strategies and potential invasion success in a parasitoid wasp. Evol. Ecol . 26, 1311–1329. ( 10.1007/s10682-011-9553-z) [DOI] [Google Scholar]
- 99.Kunte K, Shea C, Aardema ML, Scriber JM, Juenger TE, Gilbert LE, Kronforst MR. 2011. Sex chromosome mosaicism and hybrid speciation among tiger swallowtail butterflies. PLoS Genet. 7, e1002274 ( 10.1371/journal.pgen.1002274) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bonduriansky R. 2011. Sexual selection and conflict as engines of ecological diversification. Am. Nat . 178, 729–745. ( 10.1086/662665) [DOI] [PubMed] [Google Scholar]
- 101.Emlen ST, Oring LW. 1977. Ecology, sexual selection, and the evolution of mating systems. Science 197, 215–223. ( 10.1126/science.327542) [DOI] [PubMed] [Google Scholar]
- 102.Rios MR, Peixoto PEC. 2013. The effect of operational sex ratio on the opportunity for sexual selection: a meta-analysis. Anim. Behav . 86, 675–683. ( 10.1016/j.anbehav.2013.07.002) [DOI] [Google Scholar]
- 103.Klug H, Heuschele J, Jennions MD, Kokko H. 2010. The mismeasurement of sexual selection. J. Evol. Biol . 23, 447–462. ( 10.1111/j.1420-9101.2009.01921.x) [DOI] [PubMed] [Google Scholar]
- 104.Janicke T, Morrow EH. 2018. Operational sex ratio predicts the opportunity and direction of sexual selection across animals. Ecol. Lett. 21, 384–391. ( 10.1111/ele.12907) [DOI] [PubMed] [Google Scholar]
- 105.Servedio MR, Boughman JW. 2017. The role of sexual selection in local adaptation and speciation. Annu. Rev. Ecol. Evol. Syst . 48, 85–109. ( 10.1146/annurev-ecolsys-110316-022905) [DOI] [Google Scholar]
- 106.Kirkpatrick M. 1982. Sexual selection and the evolution of female choice. Evolution 36, 1 ( 10.2307/2407961) [DOI] [PubMed] [Google Scholar]
- 107.Lande R. 1981. Models of speciation by sexual selection on polygenic traits. Proc. Natl Acad. Sci. USA 78, 3721–3725. ( 10.1073/pnas.78.6.3721) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Harts AMF, Schwanz LE, Kokko H. 2014. Demography can favour female-advantageous alleles. Proc. R. Soc. B 281, 20140005 ( 10.1098/rspb.2014.0005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Higashiura Y, Ishihara M, Schaefer PW. 1999. Sex ratio distortion and severe inbreeding depression in the gypsy moth Lymantria dispar L. in Hokkaido, Japan. Heredity 83, 290–297. ( 10.1038/sj.hdy.6885590) [DOI] [PubMed] [Google Scholar]
- 110.Bailey RI, Tesaker MR, Trier CN, Saetre GP. 2015. Strong selection on male plumage in a hybrid zone between a hybrid bird species and one of its parents. J. Evol. Biol . 28, 1257–1269. ( 10.1111/jeb.12652) [DOI] [PubMed] [Google Scholar]
- 111.Leduc-Robert G, Maddison WP. 2018. Phylogeny with introgression in Habronattus jumping spiders (Araneae: Salticidae). BMC Evol. Biol . 18, 24 ( 10.1186/s12862-018-1137-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Maddison WP, Leduc-Robert G. 2013. Multiple origins of sex chromosome fusions correlated with chiasma localization in Habronattus jumping spiders (Araneae: Salticidae). Evolution 67, 2258–2272. ( 10.1111/evo.12109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Eroukhmanoff F, Bailey RI, Elgvin TO, Hermansen JS, Runemark A, Trier CN, Sætre G-P. 2017. Resolution of conflict between parental genomes in a hybrid species. bioRxiv online, 102970 ( 10.1101/102970) [DOI] [Google Scholar]
- 114.Runemark A, Piñeiro L, Eroukhmanoff F, Sætre G-P. 2018. Genomic contingencies and the potential for local adaptation in a hybrid species. Am. Nat . 192, 10–22. ( 10.1086/697563) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.