Abstract
Early joint damage in patients with haemarthrosis often escapes diagnosis because of insufficient investigation of biomechanical changes. Arthropathy in haemophilia requires complex assessment with several tools. Considering the increased emphasis on an integrated approach to musculoskeletal (MSK) outcomes, re-evaluation of MSK assessment to address individual patient needs is warranted. To advise on the optimal use of current assessment tools and strategies for tailored MSK evaluation in patients with haemophilia. A panel of experts in haemophilic arthropathy evaluated internationally recognized assessment tools through published literature and personal expertise. Each tool was considered, scored and ranked for their utility in the clinical assessment of MSK damage. Subsequently, a patient evaluation table detailing advice on type and frequency of assessments for different patient populations was constructed. To obtain a complete MSK assessment, multiple tools must be used to ensure each criterion is evaluated. For patients with haemophilia, clinical examination of the joint, disease-specific structure/function scores, and activity/participation scores including quality of life are important, and should be performed on a regular basis according to age and clinical condition. Joint imaging is recommended in the prevention, diagnosis and follow-up of haemophilic arthropathy and should be used in conjunction with joint structure and function scores. An integrated approach to MSK assessment using combinations of tools will allow earlier management of dysfunction and may improve long-term outcomes. This approach could be used in long-term follow-up of all patients independent of age and disease stage, especially in children to prevent arthropathy.
Keywords: functional assessment, haemophilia, measurement tools, musculoskeletal, scores, structural assessment
Introduction
Bleeding episodes in patients with haemophilia mainly occur into joints, that is, haemarthroses [1]. When recurrent haemarthroses are not properly treated, synovitis occurs resulting in cartilage erosions, which ultimately leads to irreversible bone damage and impaired joint function. However, early joint damage often escapes clinical diagnosis because many biomechanical mechanisms occurring at this early stage, such as secondary changes in muscle contraction patterns, are still insufficiently investigated in the context of haemophilia [2].
Joint degeneration is a long process, and structural damage is often preceded by joint dysfunction, which is attributed to abnormal feedback pathways triggered by inflammation [3–5]. In fact, the more joint function is disturbed by ligament overload, inflammation and/or synovial hypertrophy, the higher is the likelihood of further haemarthroses creating a vicious cycle of bleeding, abnormal joint motion and inflammation. Hence, joint involvement in haemophilia pertains to both structure and function, which unfortunately cannot be assessed at the same time with a single tool. The slowly changing structure can be analyzed according to well recognized benchmarks, but function is strongly influenced by motoric experience, emotional inputs and neural control, which vary between individuals and even in the same individual over time.
Recently, a more integrated approach to the assessment of musculoskeletal (MSK) outcomes, combining the impact of the disease on both body structure and function and including activity and participation, has been proposed by the WHO in the frame of the International Classification of Functioning, Disability and Health (ICF) [6]. Indeed, in patients with haemophilia, a correlation between joint damage and deterioration in health-related quality of life (HRQoL) has been shown [7]. In this light, the evaluation of joint function in persons with haemophilia often includes haemophilia-specific QoL-assessment tools.
To simplify the complex assessment of joint damage, it may be beneficial to re-evaluate how tools and strategies can be used in individual patients to address their personal needs. This article provides advice on using an integrated approach, with different tools and strategies, to enable tailored MSK evaluation in patients across the spectrum of joint disease severity.
Methods
A group of five experts in the field of haemophilic arthropathy were recruited in the frame of the Novo Nordisk Haemophilia Foundation. The group consisted of one orthopaedic surgeon (A.S.), one haematologist (C.N.), one paediatric haematologist (C.D.K.) and two rehabilitation specialists (L.H., Ad.Sa.). The expert panel held a total of four meetings to discuss ratings for the various tools and prepare and agree on the resulting advice for clinicians.
A literature search was performed on PubMed and the Novo Nordisk GLIA database to identify internationally recognized clinimetric instruments and evaluation tools for joint assessment in haemophilia. In an attempt to optimize the capture of relevant literature, the search used the terms ‘haemophil∗/hemophil∗’ and additional keywords to narrow the focus to joint assessment, for example, ‘musculoskeletal,’ ‘physical assessment,’ ‘symptoms,’ ‘activity,’ and so forth. Additional literature and particularly articles on biomechanical evaluation methods were retrieved from members of the expert panel, per their knowledge and experience.
Assessment tools for joint structure and function included those suggested by the Integrative Model of Joint Function, which includes stability and movement factors [8–10] (Fig. 1). Additionally, the group agreed upon the inclusion of activity and participation assessment tools to enhance functional insight.
Fig. 1.
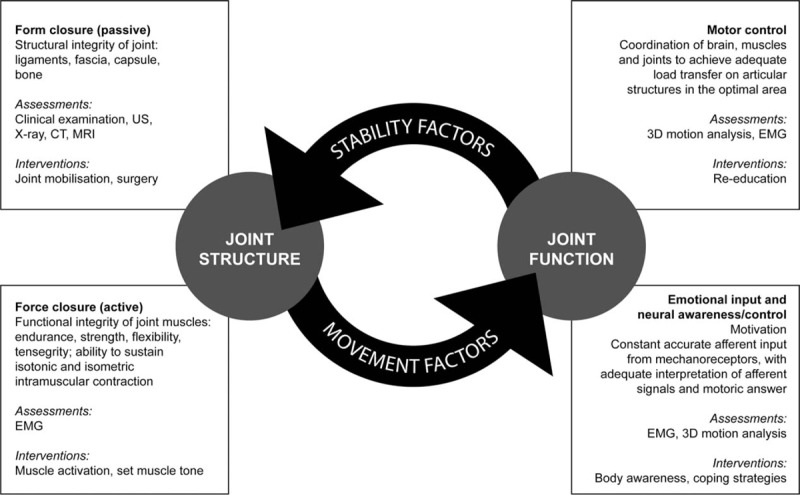
Integrative Model of Joint Function (adapted from [8] and [10]). CT, computed tomography; EMG, electromyography; US, ultrasound.
The selected tools were divided into six clinimetric groups for rating: clinical/physical examination; tools assessing structure/function; imaging techniques; tools assessing activity (i.e. the ability to perform a specific task); tools assessing participation (i.e. the functioning of a person as a member of society); and objective functional measurements (i.e. objective measurement of the performance of a specific joint or muscle). This clear depiction of the tools allowed them to be considered and ranked by the expert panel for use in MSK assessment.
Clinimetric tools were rated according to their ability to detect early structural and functional changes according to the parameters shown in Table 1. The group considered the potential and/or suitability of each assessment tool for clinical use, research, or quality control and the suitability of such tools for the use in children aged 3 years or older was also evaluated. Where appropriate, the utility of each assessment tool was evaluated separately for use in preclinical, mild-to-moderate, or severe arthropathy. We considered preclinical arthropathy to include Arnold-Hilgartner Stage 0 disease (normal), as may be present in patients with initial acute haemarthrosis, mild-to-moderate arthropathy to include Arnold-Hilgartner Stage I–III disease (soft-tissue swelling, overgrowth of epiphysis, squaring of joint ends, narrowing of joint space but preservation of cartilage), as might be found in target joints of patients with subacute haemarthroses, and severe arthropathy to include Arnold-Hilgartner Stage IV and V (narrowed or lost cartilage space, extensive epiphyseal enlargement, substantial joint disorganization), as might be found in patients with chronic arthropathy [11–13].
Table 1.
Clinimetric tool assessment parameters
| Parameter | Description | Scoring system |
| Clinical relevance | Suitability/accuracy of the tool in a clinical setting | 0 (no) to 3 (high) suitability to assess preclinical, moderate and severe arthroses |
| Content validity | Elements assessed by the content of the tool (the greater the number of elements assessed, the greater the validity of the instrument) | |
| Sensitivity to change | Assessment of individual response and responsiveness over time (important for patient follow-up) | |
| Disease specificity | Disease-specific costs and training required to perform the assessment | Yes/no |
| Feasibility | Speed and simplicity of the assessment | 0 (slow/difficult) to 3 (rapid/easy) |
| Suitable for child more than 3 years | – | Yes/no |
| Structural assessment | Tool capable of assessing joint structure | Yes/no |
| Force closure | Tool capable of single muscle assessment | 0 (no) to 3 (high) suitability to assess preclinical, moderate and severe arthroses |
| Motor control | Tool capable of assessment of muscular interaction (muscle chain) | |
| Neural control | Tool capable of assessing quality of motion and awareness | |
| Activity | Quality-of-life activity score | 0 (low) to 3 (high) suitability for activity assessment |
| Participation | Quality-of-life participation score | 0 (low) to 3 (high) suitability for participation assessment |
| Uses | Use of the tool in clinical, quality control and research situations | 0 (low) to 3 (high) suitability in each situation |
The impact/strength of each tool in terms of the assessment parameters described in Table 1 was scored as follows: 0 for none, 1 for low, 2 for medium and 3 for high. Each member of the expert panel scored all the tools according to available literature and their own personal expertise. To ascertain consensus, the content validity ratio (CVR; +1 to −1) was used as described previously [14]. CVR is a tool for structured consensus finding and follows the formula CVR = (ne − N/2)/(N/2), where ne = the number of agreeing votes and N = the total number of voters. No other statistical analyses were required.
Finally, based on the evaluation and scoring of all assessment tools, a patient evaluation table was designed to provide treating clinicians with a comprehensive and user-friendly overview of available and suitable tools to assess joint status in individual patients.
Results
A wide variety of assessment tools were identified. Tools selected for rating and discussion (Table 2) were frequently cited and included those identified in the literature search and those fitting the Integrative Model of Joint Function [15]. Upon rating completion, the CVR was +1, reflecting a high degree of agreement among the expert panel.
Table 2.
Assessment tool scores for the detection of early structural and functional joint changes
| Physical examination | Structure and function scores | Imaging | Activity and QoL | Participation and QoL | Functional measurement | ||||||||||||
| Parameter | Circ | Pain/ VAS | Gonio | Silent | Gilbert | Colorado | HJHS | Pett | MRI | US | HAL | FISH | SF-36 | HRQoL | MA | EMG | |
| Clinical relevance | Preclinical | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 0 | 3 | 3 | 0 | 0 | 1 | 1 | 3 | 3 |
| Moderate | 1 | 2 | 2 | 2 | 1 | 2 | 2 | 1 | 3 | 3 | 2 | 1 | 2 | 2 | 3 | 3 | |
| Severe | 0 | 2 | 2 | 2 | 2 | 1 | 1 | 2 | 2 | 2 | 3 | 3 | 2 | 2 | 2 | 2 | |
| Content validity | Preclinical | x | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 2 | 2 | 0 | 0 | 0 | 0 | 2 | 2 |
| Moderate | 1 | 1 | 3 | 3 | 1 | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | |
| Severe | x | 1 | 3 | 3 | 2 | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | |
| Sensitivity to change | Preclinical | x | 1 | 0 | 3 | 0 | 0 | 0 | 0 | 3 | 2 | 0 | 0 | 0 | 0 | 3 | 3 |
| Moderate | 1 | 2 | 2 | 3 | 1 | 2 | 2 | 2 | 3 | 2 | 2 | 2 | 2 | 2 | 3 | 3 | |
| Severe | x | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 3 | 1 | 2 | 2 | 2 | 2 | 3 | 3 | |
| Disease specificity | n | n | n | n | y | y | Y | n | n | n | y | y | n | y | n | n | |
| Feasibility | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 2 | 0 | 2 | 3 | 3 | 3 | 3 | 0 | 2 | |
| Suitable for child >3 years | 3 | 1 | 3 | 3 | 1 | 2 | 2 | 2 | 2 | 3 | 2 | 1 | 1 | 1 | 3 | 3 | |
| Structural assessment | y | y | y | y | y | y | Y | y | y | y | n | n | n | n | n | n | |
| Force closure | Preclinical | x | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 2 | 1 | 1 | 0 | 0 | 2 | 3 |
| Moderate | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 2 | 1 | 2 | 0 | 0 | 2 | 3 | |
| Severe | x | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 2 | 1 | 2 | 0 | 0 | 2 | 3 | |
| Motor control | Preclinical | x | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 3 | 3 |
| Moderate | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | 3 | 3 | |
| Severe | x | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | 3 | 3 | |
| Neural control | Preclinical | x | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 3 | 2 |
| Moderate | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 2 | 3 | 2 | |
| Severe | x | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 2 | 3 | 2 | |
| Activity | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 3 | 1 | 1 | 0 | 0 | |
| Participation | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 2 | 3 | 0 | 0 | |
| Uses | Clinical | 2 | 2 | 2 | 3 | 2 | 2 | 2 | 1 | 3 | 3 | 1 | 3 | 2 | 2 | 3 | 3 |
| Quality control | 0 | 2 | 1 | 2 | 1 | 1 | 2 | 1 | 3 | 3 | 1 | 2 | 2 | 2 | 3 | 3 | |
| Research | 0 | 1 | 0 | 1 | 2 | 2 | 2 | 2 | 3 | 3 | 1 | 2 | 2 | 2 | 3 | 3 | |
| Sum of severity scores | Preclinical | 0 | 1 | 1 | 8 | 0 | 0 | 0 | 0 | 8 | 9 | 2 | 3 | 1 | 1 | 16 | 16 |
| Moderate | 3 | 5 | 7 | 8 | 3 | 6 | 6 | 4 | 8 | 9 | 8 | 10 | 8 | 8 | 16 | 16 | |
| Severe | 0 | 5 | 7 | 7 | 6 | 5 | 5 | 5 | 7 | 7 | 9 | 12 | 8 | 8 | 15 | 15 | |
| Total | 11 | 20 | 24 | 35 | 21 | 24 | 28 | 17 | 34 | 39 | 31 | 39 | 30 | 31 | 59 | 61 | |
Data from [15,17,18,23,26,29,31,43,52,55–78]. The scoring system is described in Table 1; the total score for each tool gives a reflection of its utility across all assessment criteria. Preclinical, moderate and severe relate to stages of arthropathy. Circ, joint circumference; Colorado, Colorado score; EMG, electromyography; FISH, Functional Independence Score in Haemophilia; Gilbert, Gilbert score; Gonio, goniometer; HAL, Haemophilia Activities List; HJHS, Haemophilia Joint Health Score; HRQoL, haemophilia-related quality of life; MA, motion analysis; Pett, Pettersson score; SF-36, 36-item short-form health survey; Silent, Silent symptoms; US, ultrasound; VAS, visual analogue scale.
Review of clinimetric tool categories
Physical examination
Clinical assessment is used to not only identify obvious signs of arthropathy but also subclinical joint damage, for instance, tender points on ligaments that indicate overloading. If ligaments are stretched beyond their normal capacity through abnormal movement, afferent signals are sent to the motoric cortex to optimize the abnormal movement by adapting muscle tone and de-loading the ligament [16]. Intensive abnormal movements can cause ligament microtrauma, local inflammation and intrinsic pressure through oedema and cell migration, resulting in permanent stimulation of mechanoreceptors without functional disturbance; false information for muscle twitch results in chronic and intense abnormal movements and ligament inflammation [5].
In a study of orthopaedic assessment results, including knee motion analysis, from 273 children (median age 9.8 years) with haemophilia A, B or von Willebrand disease, compared with 200 age-matched and sex-matched controls, 90% of children studied did not complain of any acute pain before assessment. Of 195 children with haemophilia who declared no pain before assessment, 83% displayed evidence of subclinical MSK changes in the lower extremities, with a mean of above five subclinical findings per child, compared with less than one finding per child in the control group. Tender points were identified in both the knee (38.2% of children), and ankle (60% of children) areas, with 80% of tender points located in the capsule and ligaments despite the absence of clinical abnormalities [3].
Physical examination of the whole body, including, for example, joint circumference, muscle strength, joint effusion, joint angle, pain with a visual analogue scale (VAS) and silent symptoms, should be considered an absolute necessity, and must always be performed at each follow-up. Bleeding history, as a measure of haemophilia treatment efficacy, should also be monitored.
Tools for structure and function
These tools allow systematization of MSK assessment and address several aspects of joint structure and function. The Hemophilia Joint Health Score (HJHS), which was recently developed by a consensus of experts, is sensitive to early changes such as mild stage arthropathy [17]. Although originally created for the paediatric population, it has been used in adults, although this use has not been validated [18–20]. The HJHS has demonstrated excellent inter-rater and intra-rater reliability, even in inexperienced assessors in a country with limited access to haemophilia services [21,22]. The HJHS is designed to detect mild stage arthropathy in 4–18-year-olds, whereas the Colorado scale is designed to identify earlier signs of joint degeneration and function in young children [17,23].
Imaging techniques
In the diagnosis and follow-up of arthropathy, the use of imaging either by MRI, ultrasound and/or X-ray is advised. Ultrasound can be used to differentiate between a bleed, effusion and synovitis and is especially useful to detect silent synovitis in children with no clinical signs [24]. Ultrasound is preferred in children, but if inconclusive, or if a patient needs preparation for surgery, then an MRI scan should be performed. It is of note that some abnormalities are better detected with MRI, such as subchondral cysts, cartilage loss and haemosiderin deposits [25]. To improve consistency in determining joint status, simplified innovative protocols and scoring techniques have been developed for both ultrasound and MRI, that is, the Haemophilia Early Arthropathy Detection with Ultrasound (HEAD-US) and the compatible additive MRI scale, respectively [26,27]. In addition, X-rays scored using the Pettersson scale can still be informative for patients with haemophilia and established joint damage [28].
Joint function assessment
Measuring joint function is supported at all stages of haemophilic arthropathy and in all age groups. Joint function can be measured simply by physical assessment or goniometer for range of motion (ROM), or with measurements such as kinematic analysis and kinetic superficial electromyography (EMG), which determine initial changes in the MSK system and musculature, respectively [2,29]. However, if kinetic or kinematic modalities are not available, other measurement scores that reflect impairment in function, such as those assessing independence/activities, can be used as a surrogate [e.g. HJHS, HAL or Functional Independence Score in Haemophilia (FISH)].
Quality of life and activity/participation assessment
The number of HRQoL tools identified was too great to individually assess, and such an assessment is outside the scope of this analysis. Therefore, HRQoL tools were used to provide an umbrella assessment for disease-specific QoL, and the 36-Item Short-Form Health Survey (SF-36) was used as a general QoL tool. Consequently, tools such as HAEMO-QoL, Canadian Hemophilia Outcomes-Kids Life Assessment Tool and IPAQ were not specifically included. Similarly, for patient-reported outcomes, we used the Paediatric Haemophilia Activities List (PedHAL/HAL) as a representative tool. Following our assessment, we suggest that patients be scored for HRQoL and activity/participation levels, in accordance with the ICF guidelines. To measure activity and participation, disease-specific tools, such as the PedHAL/HAL, and the FISH, can be applied [30,31].
Building a comprehensive musculoskeletal assessment from the currently available tools
Following our assessment, we found that no single tool fulfilled all assessment criteria (Tables 1 and 2). The total score for each tool (Table 2) gives a reflection of its utility across all assessment criteria. The highest scoring tools, deemed suitable across the most assessment criteria were the biomechanical evaluation systems including kinematic motion analysis and EMG, scoring 59 and 61, respectively, whereas the lowest scoring tool was joint circumference (scoring 11), which was deemed most useful in clinical assessment of moderate arthropathy.
Given that none of the assessment tools satisfy all criteria within a given clinimetric group, the use of multiple tools is required to fulfil a comprehensive MSK assessment. Table 3 summarizes our advice for choosing from the available tools to construct a comprehensive assessment. It categorizes patients into groups based on age and disease severity, and advises on the frequency that these tests should be performed for clinical follow-up and research purposes.
Table 3.
Patient evaluation table with recommendations on type and frequency of assessments
| Patient category | Defined by presence or absence of MSK pain and/or synovitis | Children | Adults | Multiple joint involvement with endstage haemophilic arthropathy |
| Initial assessment (every visit) | Clinical examination | In all cases | In all cases | ✓ |
| Silent symptom assessment only applicable for moderate, severe or endstage haemophilia arthropathy | ||||
| Extended assessment tools to be performed as required | Structure and function scores | In all cases | In all cases | ✓ |
| Scores of activity or participation level | In all cases | In all cases | ✓ | |
| Quality of life | Evaluate in patients with medium or severe MSK symptoms | Evaluate in patients with medium or severe MSK symptoms | ✓ | |
| Imaging | In all cases | In all cases | ✓ | |
| Pettersson score not applicable in children or adults with mild MSK symptoms Ultrasound assessment only in children or adults with moderate or no MSK symptoms to assess subclinical synovitis, or in acute bleeds MRI only in children with moderate MSK symptoms in preparation for surgery, or in adults with severe MSK symptoms or endstage arthropathy in preparation for surgery | ||||
| Functional measurement | Evaluate in patients with no or medium MSK symptoms, not in those with severe symptoms | Evaluate in patients with no or medium MSK symptoms; not in those with severe symptoms | ͯ | |
| Early detection of arthropathy and planning individual therapy | ||||
| Results and therapy | ||||
| Physiotherapy | According to findings | According to findings | According to findings | |
| Follow-up | Clinical examination | Maximum twice/year if negative findings (i.e. in patients with no MSK symptoms); every 3 months otherwise | At least once/year | At least once/year |
| Structure and function scores | In patients with no MSK symptoms, maximum twice/year; in those with medium symptoms, at least once/year and if problem joint evident, follow-up until resolved; N/A for patients with severe symptoms | In patients with no or medium MSK symptoms, maximum twice/year; in those with severe symptoms, once/year | N/A | |
| Scores of activity or participation level | Maximum twice/year | In patients with no or medium MSK symptoms, maximum twice/year; in those with severe symptoms, once/year | Once/year | |
| Imaging | In patients with no MSK symptoms, on follow-up as needed; in those with medium symptoms, use to assess subclinical synovitis or in acute bleeds; in those with severe symptoms, X-ray prior to intervention | In patients with no or medium MSK symptoms, use to assess subclinical synovitis or in acute bleeds; in those with severe symptoms, use in preparation for surgery | In preparation for surgery | |
| Functional measurement | In patients with no MSK symptoms, every 3/6/12 months; or as needed, for example, starting a new sport; in those with medium symptoms, every 3/6/12 months; in those with severe symptoms, N/A or once/year | In patients with no or medium MSK symptoms, every 3/6/12 months; in those with severe symptoms, N/A or once/year | N/A or once/year | |
| Frequency of assessment for research purposes (minimum requirement to generate useful data for analysis) | Clinical examination | Every 3 months | At least once/year | At least once/year |
| Structure and function scores | In patients with no or medium MSK symptoms, maximum twice/year; N/A for those with severe symptoms | Maximum twice/year | Maximum once/year | |
| Scores of activity and participation level | Maximum twice/year | In patients with no or medium MSK symptoms, maximum twice/year; in those with severe symptoms, maximum once/year | Maximum once/year | |
| Imaging | In patients with no or medium MSK symptoms, on follow-up as needed, ultrasound on each clinical examination; in those with severe symptoms, X-ray to allow population description | In patients with no MSK symptoms, on follow-up as needed, ultrasound on each clinical examination, MRI maximum once/year; in those with medium symptoms, on follow-up as needed, ultrasound on each clinical examination; as necessary in patients with severe symptoms | X-ray to allow population description | |
| Functional measurement | Maximum of four times/year | In patients with no or medium MSK symptoms, maximum of four times/year; in those with severe symptoms, evaluate as necessary | N/A | |
EMG, electromyography; FISH, Functional Independence Score in Haemophilia; HJHS, Haemophilia Joint Health Score; MSK, musculoskeletal; N/A, not applicable; PedHAL/HAL, Paediatric Haemophilia Activities List; VAS, visual analogue scale.
Where access to different MSK assessment tools is limited or restricted, clinicians can use Tables 2 and 3 to identify, which areas of the comprehensive MSK assessment are most important for their patients and prioritize accordingly; using these tables, clinicians can build a plan to provide the most comprehensive MSK assessment possible using the tools available. Figure 2 shows two possible MSK assessment plans in two specific clinical scenarios as the assessment of children and the assessment of a patient on the occasion of an acute bleed.
Fig. 2.
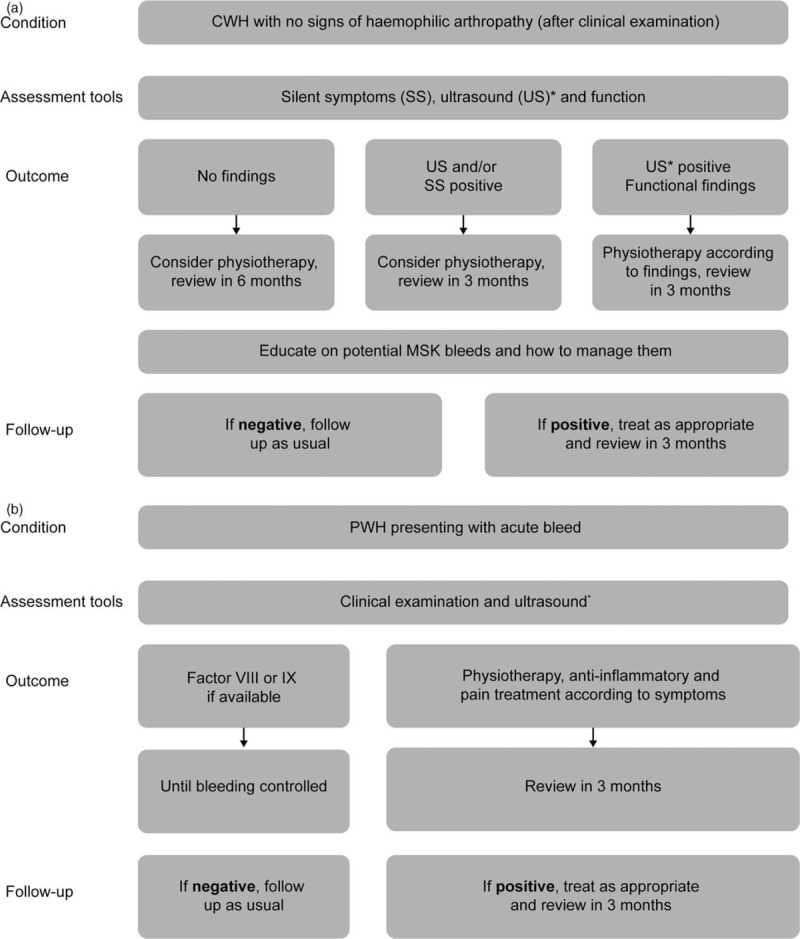
Examples of musculoskeletal assessments in (a) children with haemophilia presenting with subclinical arthropathy and (b) patients with haemophilia who are presenting with an acute bleed. ∗Ultrasound is used to show early structural changes such as low-degree synovitis, chondral changes and bony changes (not central). Immediate ultrasound can detect a bleed or effusion, which will impact on Factor VIII therapy and could lead to additional treatment, such as lymphatic drainage, in the physiotherapy rehabilitation plan. However, ultrasound would not be available in a home-treatment setting. CWH, children with haemophilia; MSK, musculoskeletal; PWH, patients with haemophilia.
Discussion
The summary of assessment tool analysis (Table 2) provides a convenient way for the clinician to combine new approaches (such as ultrasound or kinematic analysis), with established tools to assess structure (such as the HJHS). This multitool approach should enable more sensitive detection of early joint deterioration, analysis of MSK damage and inform appropriate intervention. In line with recommendations for the collection of comparative data, only internationally recognized tools were assessed. The feasibility of using various tools is an important factor for the clinician to consider when contemplating the practical approach to patient evaluation, and an indication of feasibility in terms of the speed and simplicity of the assessment is given for each of the tools described in Table 2. While, for instance, physical examination is not associated with high costs, can be relatively easy to perform and requires little training, other tools, such as imaging via MRI, as considered further below, are associated with higher costs, are more specialized and may not necessarily be readily available.
Our assessment of MSK assessment tools (Table 2) specifically focussed on the ability/strength of currently available tools to detect early structural or functional deficits. The tools do not provide early enough feedback on joint dysfunction nor sufficient accuracy when used in isolation, therefore, we advise that a combination of different tools are used to better identify joint dysfunction early so that appropriate treatment can be administered to preserve joint health.
During clinical examination, a disease-specific joint structure and function score is considered essential. To date, HJHS is the internationally recognized tool for assessing joint structure and function. A recent study demonstrated agreement between the HJHS and MRI scores, indicating that the HJHS may be used safely as a first-line tool to evaluate structure [32], and a study in China has proven the reliability, internal consistency and global transferability of HJHS version 2.1, even for administration by physiotherapists and physicians with limited haemophilic experience [22]. However, other studies have noted that discrepancies between physiotherapists in routine HJHS can hamper comparison of scores between treatment regimens [33]. These discrepancies may reflect a difference in study conditions and/or experience of the healthcare professionals. In the small Chinese study/training exercise, conditions were carefully monitored with experienced physiotherapists and physicians, ensuring protocol adherence and completion of relevant score sheets of eight children [22]. In contrast, Nijdam et al.[33] reports on an international retrospective observational study comparing routine HJHS of 127 children between 1995 and 2005. In this study, interphysiotherapist discrepancies were considered to reflect differences in training and expertise, and not the reliability of the HJHS, hence experienced physiotherapists are necessary for the reliable implementation of the HJHS.
Although useful, clinical scoring may lack sensitivity for detecting joint changes in patients without clinically evident arthropathy, and additional clinical examination may prove informative. A recent study involving two phenotypically similar groups of children – one group constituting patients with haemophilia who had no clinically evident bleeding, and one age-matched group of children with no bleeding disorders – demonstrated that although the two groups could not be differentiated by clinical scoring via the HJHS, statistically significant differences between the two groups were apparent when assessed by silent symptoms and infrared thermography [34]. Although the thermographic data were preliminary, the study highlights the value of clinical examination for detecting early symptoms, with early local inflammation affecting ligaments, tendons and joint capsules in patients with haemophilia. We advise that clinical examination be conducted at all routine follow-ups.
Although not essential, imaging of the joint is advised. MRI is currently the gold standard for imaging and the compatible additive MRI scale is the principal predefined MRI assessment tool [35]. However, MRI has several disadvantages including lack of feasibility in very young children, access to expensive technology and the time to evaluate each joint [36]. In many countries with limited resources, patients may not undergo MRI because of a lack of availability. Ultrasound is less expensive than MRI, globally available and more convenient and can be used to detect and monitor early synovial hypertrophy and osteochondral changes [37]. However, ultrasound can be observer-dependent and cannot visualize deep structures, so is not ideal for detecting subchondral cysts, loss of cartilage or haemosiderin deposits [38]. A new, standardized procedure, HEAD-US [39], aims to increase inter-rater reliability of ultrasonic evaluation of joints, although this score still requires validation. As with other assessment tools, the age of the patient and severity of MSK dysfunction should be considered when selecting an appropriate imaging technique. Both ultrasound and MRI have been shown to identify pathological changes in joints that appear normal on X-ray imaging [40]. However, X-rays using the Pettersson score may have greater utility in patients with more advanced arthropathy, especially as X-rays are more readily available and economical than MRIs.
Due to low sensitivity in detecting early structural joint involvement (e.g. X-rays and clinical scores) or age limitations (e.g. MRI) [41], there is no single structural assessment tool that is applicable to all patients. Furthermore, it has long been known that subclinical bleeds may account for a certain degree of joint damage in the long-term, even in patients receiving prophylaxis [41]. Therefore, it is important to detect early/silent symptoms of joint involvement through functional assessment.
Functional changes can be detected before their clinical effects are evident. For example, in a recent two-year prospective study involving patients in Costa Rica who had haemophilia with joint involvement, kinetic superficial EMG showed significant functional deterioration, whereas the HJHS showed no structural change over the same time period [42]. However, it should be noted that functional changes are highly individual and require specific functional tools, such as EMG/kinematic motion analysis. Indeed, these are the highest rated tools in Table 2, although neither may be routinely implemented in current haemophilia patient care. However, with an increasing number of publications describing their use in haemophilia [2,29,42,43], they are likely to become increasingly utilized in the near future. Kinetic superficial EMG may be the more available tool, being less expensive, as well as easier to learn and apply (for example, as in the study involving patients from Costa Rica described above [42]), with an immediate impact on physical therapy. Motion analysis may be a tool for more specialized centres, where it might also be used in other indications, such as juvenile rheumatoid arthritis or cerebral palsy.
With new drugs becoming available, there is a requirement for more sensitivity to diagnose early symptoms in patients with haemophilia who appear otherwise bleed free. Indeed, early detection of MSK dysfunction can allow for implementation of preventive treatment options and early planning of individual therapy. For example, kinematic analysis can discover early changes in the MSK system at a functional level (e.g. additional accelerations, and loss of gait rhythm and regularity) allowing the implementation of gait training, and kinetic EMG measures preclinical changes in the musculature allowing for preventive treatment options, such as strengthening and stretching of individual muscles. Overall, functional disturbances can be effectively reversed with physiotherapy [44], and greater emphasis should be placed on functional correction in patient treatment plans, if the relevant resources are available.
Decline in joint function negatively impacts HRQoL through decreased activity and participation [45,46], and deterioration in joint scores confirm the importance of lifelong prophylactic clotting factor replacement therapy [47]. Improvements in joint strength and ROM in patients with haemophilia, such as those observed through exercise therapy [45,48], have been linked with improvements in daily functioning and HRQoL [49,50]. Both generic (SF-36) and disease-specific HRQoL tools can be used to assess patients with haemophilia, dependent on the QoL domains being evaluated [49]. Therefore, choosing the appropriate questionnaire for the patient and desired outcome measure is just as important as assessing their QoL.
The Integrative Model of Joint Function, developed over the past 12 years to describe all aspects of joint function, was included as an essential criterion for tools assessing joint function (Fig. 1). In addition to structural components of the joint, the model also focusses on functional components as both are essential for optimal joint health [8–10,51]. In patients with haemophilia, joint function is affected by biomechanical factors including a passive component (e.g. reduced joint mobility), and an active component (e.g. muscle weakness and atrophy), as well as by neural components such as motor control (e.g. patterns of muscle activation), and emotions/awareness (e.g. negative emotions such as fear and pain) on motor control and muscle activation [10]. This integrated approach to joint function should be reflected in the choice of assessments and hence treatment options (e.g. prophylaxis, physiotherapy or surgery). However, if structural and functional aspects of joint health are summarized in a single assessment tool, there is a risk that scoring highly in one component of the tool could mask deficits in another component, affecting overall sensitivity of the tool, resulting in under-detection of arthropathy. For instance, a highly functioning joint may compensate for a related poorly functioning one (e.g. a functioning knee compensating for a malfunctioning hip), masking structural decline in the affected joint [2], and resulting in no early change being detected by the measurement tool. Furthermore, correlation between structural assessment (i.e. extent of haemarthrosis/synovitis) and functional assessment is not predictable [52], with structural measurements being less sensitive than functional ones [2,52]. Consequently, scoring systems may lack sensitivity as a result of combining functional and structural domains.
The summary of assessment tool analysis (Table 2) is intended to be used with the patient evaluation table (Table 3), which provides advice for choosing suitable assessments based on patient characteristics. As an educational tool, the patient evaluation table should allow easy confirmation of appropriate assessments for each patient group at initial presentation and throughout follow-up. Furthermore, these tables may support consistency in assessments throughout therapy and hence aid in treatment evaluation. Currently, our guidance on choice of examinations is dependent on the severity of MSK symptoms, age of the patient and availability of resources. Hence, some methods of examination may be required more or less frequently than others, or may not be required at all. For example, the VAS pain assessment can be used in patients of all ages and severity and should be administered upon each visit, whereas ultrasound is best suited to patients with less severe MSK symptoms.
In all patients, early detection of joint dysfunction is imperative to employ timely intervention with appropriate treatment strategies. Ideally, treatment intervention should be started in the subclinical phases of joint deterioration. However, even in countries with high treatment standards, deterioration of joints, especially the ankle, may only be recognized at the beginning of adulthood when it is too late to correct joint development. In our expert opinion, countries with limited resources encounter this same issue but in much younger patients than more developed countries, possibly because of limited use of prophylaxis [53]. As physiotherapy is less expensive than factor replacement, rehabilitation and muscle reinforcement are often used to compensate for lack of treatment. However, low and very-low dose prophylaxis provided on an individual basis according to affordability for a given patient or nation, is proving beneficial to patients in economically constrained environments by improving number of bleeds, HRQoL and functional participation in society [54].
Conclusion
Our advice aims to help physicians provide comprehensive, individualized assessments of patients with haemophilia and joint deterioration. Joint assessment should be performed using a multimodal approach under the guidance of a person experienced in the field of assessment (e.g. a biomechanist for kinematic motion analysis). To optimize reliability and standardize measurement procedures, sufficient training should be provided.
To date, literature does not extend beyond the existing consortium of assessment tools. If we wish to optimize joint evaluation, we need to broaden our understanding of the data we already collect in a holistic context. Increased availability of reliable tools that are sensitive to early biochemical and biomechanical changes in joint structure and function, and applicable to a wide range of patients with haemophilia, would be ideal. Individualizing treatment based on improved evaluation could, in turn, lead to improved MSK outcomes and HRQoL.
We hope that our advice will help improve MSK assessment by unifying and structuring treatment practices, increasing consistency of measurement outcomes, encouraging use of several assessment tools, engaging patients in therapy, emphasizing the importance of holistic approaches to joint health, and providing confidence that the appropriate tools are being used in each patient's MSK assessment. A standardized system for assessing individual patients’ MSK status in future data collection would also enable better consolidation of datasets to facilitate continuing research or discussions with health authorities, healthcare providers, payers and health insurers.
Acknowledgements
The authors would like to express their sincere and lasting gratitude for the significant contribution of Lily Heijnen to all aspects of this work, until she sadly passed away before the manuscript was submitted.
Medical writing support, funded by the Novo Nordisk Haemophilia Foundation, was provided by Martin Quinn, PhD, CMPP, of Bioscript Medical (Macclesfield, UK).
Author contributions: A.S. provided the original assessment of MSK tools, which was reviewed and discussed by all authors. All authors contributed to the content of the manuscript and approved it for submission.
Conflicts of interest
C.N. has received honorarium and/or funding for research from Alnylam, Baxalta/Shire, Bayer, CSL Behring, LFB, Novo Nordisk, Octapharma, Pfizer, Roche and Sobi. A.S., Ad.Sa. and C.D.K. stated that they had no interests, which might be perceived as posing a conflict or bias.
References
- 1.Mannucci PM, Tuddenham EG. The hemophilias–from royal genes to gene therapy. N Engl J Med 2001; 344:1773–1779. [DOI] [PubMed] [Google Scholar]
- 2.Lobet S, Detrembleur C, Massaad F, Hermans C. Three-dimensional gait analysis can shed new light on walking in patients with haemophilia. Sci World J 2013; 2013:284358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seuser A, Bohm P, Wermes C. Early orthopaedic challenges in haemophilia patients and therapeutic approach. Thromb Res 2014; 134 suppl 1:S61–67. [DOI] [PubMed] [Google Scholar]
- 4.Andia I, Sanchez M, Maffulli N. Joint pathology and platelet-rich plasma therapies. Expert Opin Biol Ther 2012; 12:7–22. [DOI] [PubMed] [Google Scholar]
- 5.Thornton GM, Shrive NG, Frank CB. Altering ligament water content affects ligament prestress and creep behaviour. J Orthop Res 2001; 19:845–851. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization (WHO). International Classification of Functioning, Disability and Health (ICF). Geneva, 2002. [Google Scholar]
- 7.Poonnoose PM, van der Net J. Musculoskeletal outcome in hemophilia: bleeds, joint structure and function, activity, and health-related fitness. Semin Thromb Hemost 2015; 41:872–879. [DOI] [PubMed] [Google Scholar]
- 8.Proceedings of the 4th Interdisciplinary World Congress on Low Back and Pelvic Pain. The integrated model of joint function and its clinical application. Montreal, 8–10 November. [Google Scholar]
- 9.Panjabi MM. The stabilizing system of the spine. Part I. Function, dysfunction, adaptation, and enhancement. J Spinal Disord 1992; 5:383–389. [DOI] [PubMed] [Google Scholar]
- 10.Herbsleb M, Puta C, Hilberg T. Scharrer I, Schramm W. Hemophilia and Exercise Project (HEP) conception and contents of a ‘Programmed Sports Therapy’ for hemophilic patients. 37th hemophilia symposium. Berlin, Heidelberg: Springer; 2008. 45–59. [Google Scholar]
- 11.Arnold WD, Hilgartner MW. Hemophilic arthropathy. Current concepts of pathogenesis and management. J Bone Joint Surg Am 1977; 59:287–305. [PubMed] [Google Scholar]
- 12.Lobet S, Hermans C, Lambert C. Optimal management of hemophilic arthropathy and hematomas. J Blood Med 2014; 5:207–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silva M, Luck JV, Jr, Llinás A. Chronic hemophilic synovitis: the role of radiosynovectomy. Jr World Federation of Hemophilia, 2004. [Google Scholar]
- 14.Lawshe CH. A quantitative approach to content validity. Pers Psychol 1975; 28:563–575. [Google Scholar]
- 15.Aznar JA, Magallon M, Querol F, Gorina E, Tusell JM. The orthopaedic status of severe haemophiliacs in Spain. Haemophilia 2000; 6:170–176. [DOI] [PubMed] [Google Scholar]
- 16.Krauspe R, Schmidt M, Schaible HG. Sensory innervation of the anterior cruciate ligament. An electrophysiological study of the response properties of single identified mechanoreceptors in the cat. J Bone Joint Surg Am 1992; 74:390–397. [PubMed] [Google Scholar]
- 17.Feldman BM, Funk SM, Bergstrom BM, Zourikian N, Hilliard P, van der Net J, et al. Validation of a new pediatric joint scoring system from the International Hemophilia Prophylaxis Study Group: validity of the hemophilia joint health score. Arthritis Care Res (Hoboken) 2011; 63:223–230. [DOI] [PubMed] [Google Scholar]
- 18.Tagliaferri A, Feola G, Molinari AC, Santoro C, Rivolta GF, Cultrera DB, et al. Benefits of prophylaxis versus on-demand treatment in adolescents and adults with severe haemophilia A: the POTTER study. Thromb Haemost 2015; 114:35–45. [DOI] [PubMed] [Google Scholar]
- 19.Khawaji M, Astermark J, Berntorp E. Lifelong prophylaxis in a large cohort of adult patients with severe haemophilia: a beneficial effect on orthopaedic outcome and quality of life. Eur J Haematol 2012; 88:329–335. [DOI] [PubMed] [Google Scholar]
- 20.Sluiter D, Foppen W, de Kleijn P, Fischer K. Haemophilia Joint Health Score in healthy adults playing sports. Haemophilia 2014; 20:282–286. [DOI] [PubMed] [Google Scholar]
- 21.Hilliard P, Funk S, Zourikian N, Bergstrom BM, Bradley CS, McLimont M, et al. Hemophilia joint health score reliability study. Haemophilia 2006; 12:518–525. [DOI] [PubMed] [Google Scholar]
- 22.Sun J, Hilliard PE, Feldman BM, Zourikian N, Chen L, Blanchette VS, et al. Chinese Hemophilia Joint Health Score 2.1 reliability study. Haemophilia 2014; 20:435–440. [DOI] [PubMed] [Google Scholar]
- 23.Hacker MR, Funk SM, Manco-Johnson MJ. The Colorado Haemophilia Paediatric Joint Physical Examination Scale: normal values and interrater reliability. Haemophilia 2007; 13:71–78. [DOI] [PubMed] [Google Scholar]
- 24.Sigl-Kraetzig M, Bauerfeindt S, Wildner A, Seuser A. Standardized ultrasonography (HEAD-US) of joints and first correlation with function in haemophilic arthropathy: results of a clinical trial and potential importance of joint-ultrasound for an individualized prophylaxis in haemophiliacs. Blood 2015; 126:3541. [Google Scholar]
- 25.Dale TM, Saucedo JM, Rodriguez-Merchan EC. Hemophilic arthropathy of the elbow: prophylaxis, imaging, and the role of invasive management. J Shoulder Elbow Surg 2015; 24:1669–1678. [DOI] [PubMed] [Google Scholar]
- 26.Martinoli C, Della Casa Alberighi O, Di Minno G, Graziano E, Molinari AC, Pasta G, et al. Development and definition of a simplified scanning procedure and scoring method for Haemophilia Early Arthropathy Detection with Ultrasound (HEAD-US). Thromb Haemost 2013; 109:1170–1179. [DOI] [PubMed] [Google Scholar]
- 27.Lundin B, Manco-Johnson ML, Ignas DM, Moineddin R, Blanchette VS, Dunn AL, et al. International Prophylaxis Study Group. An MRI scale for assessment of haemophilic arthropathy from the International Prophylaxis Study Group. Haemophilia 2012; 18:962–970. [DOI] [PubMed] [Google Scholar]
- 28.Pettersson H, Ahlberg A, Nilsson IM. A radiologic classification of hemophilic arthropathy. Clin Orthop Relat Res 1980; 149:153–159. [PubMed] [Google Scholar]
- 29.Seuser A, Wendel M, Navarrete-Duran M, Fink D, Auerswald G, Bohm P. Analysis of muscle function with kinetic superficial EMG in children with haemophilia - recognizing subclinical changes, establishing individual therapy, quality control. Hamostaseologie 2011; 31 suppl 1:S38–45. [PubMed] [Google Scholar]
- 30.Groen WG, van der Net J, Helders PJ, Fischer K. Development and preliminary testing of a Paediatric Version of the Haemophilia Activities List (pedhal). Haemophilia 2010; 16:281–289. [DOI] [PubMed] [Google Scholar]
- 31.Poonnoose PM, Manigandan C, Thomas R, Shyamkumar NK, Kavitha ML, Bhattacharji S, Srivastava A. Functional independence score in haemophilia: a new performance-based instrument to measure disability. Haemophilia 2005; 11:598–602. [DOI] [PubMed] [Google Scholar]
- 32.Oymak Y, Yildirim AT, Yaman Y, Gurcinar M, Firat A, Cubuckcu D< ET-AL>. The effectiveness of tools for monitoring hemophilic arthropathy. J Pediatr Hematol Oncol 2015; 37:e80–e85. [DOI] [PubMed] [Google Scholar]
- 33.Nijdam A, Bladen M, Hubert N, Pettersson M, Bartels B, van der Net J, et al. Using routine Haemophilia Joint Health Score for international comparisons of haemophilia outcome: standardization is needed. Haemophilia 2016; 22:142–147. [DOI] [PubMed] [Google Scholar]
- 34.Seuser A, Kurnik K, Mahlein AK. Infrared thermography as a noninvasive tool to explore differences in the musculoskeletal system of children with hemophilia compared to an age-matched healthy group. Sensors (Basel) 2018; 18:518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oldenburg J, Zimmermann R, Katsarou O, Theodossiades G, Zanon E, Niemann B, et al. Controlled, cross-sectional MRI evaluation of joint status in severe haemophilia A patients treated with prophylaxis vs. on demand. Haemophilia 2015; 21:171–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keshava SN, Gibikote S, Doria AS. Imaging evaluation of hemophilia: musculoskeletal approach. Semin Thromb Hemost 2015; 41:880–893. [DOI] [PubMed] [Google Scholar]
- 37.Di Minno MN, Ambrosino P, Quintavalle G, Coppola A, Tagliaferri A, Martinoli C, Rivolta GF. Assessment of hemophilic arthropathy by ultrasound: where do we stand? Semin Thromb Hemost 2016; 42:541–549. [DOI] [PubMed] [Google Scholar]
- 38.Sierra Aisa C, Lucia Cuesta JF, Rubio Martinez A, Fernández Mosteirín N, Iborra Muñoz A, Abío Calvete M, et al. Comparison of ultrasound and magnetic resonance imaging for diagnosis and follow-up of joint lesions in patients with haemophilia. Haemophilia 2014; 20:e51–e57. [DOI] [PubMed] [Google Scholar]
- 39.Altisent C, Martorell M, Crespo A, Casas L, Torrents C, Parra R. Early prophylaxis in children with severe haemophilia A: clinical and ultrasound imaging outcomes. Haemophilia 2015; 22:218–224. [DOI] [PubMed] [Google Scholar]
- 40.Poonnoose PM, Hilliard P, Doria AS, Keshava SN, Gibikote S, Kavitha ML< ET-AL>. Correlating clinical and radiological assessment of joints in haemophilia: results of a cross sectional study. Haemophilia 2016; 22:925–933. [DOI] [PubMed] [Google Scholar]
- 41.Manco-Johnson MJ, Abshire TC, Shapiro AD, Riske B, Hacker MR, Kilcoyne R, et al. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N Engl J Med 2007; 357:535–544. [DOI] [PubMed] [Google Scholar]
- 42.Seuser A, Navarrete-Duran M, Auerswald G, Mancuso ME. Muscle function deterioration in patients with haemophilia: Prospective experience from Costa Rica. Haemophilia 2018. 1–12. DOI: 10.1111/hae.13455. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 43.Bittscheidt W, Hofmann P, Schumpe G. Electromyographic examination of the femoral muscles in hemophilic effusion in the knee-joint and in irritated conditions of the knee-joint (author's transl). Z Orthop Ihre Grenzgeb 1978; 116:56–60. [PubMed] [Google Scholar]
- 44.Negrier C, Seuser A, Forsyth A, Lobet S, Llinas A, Rosas M, Heijnen L. The benefits of exercise for patients with haemophilia and recommendations for safe and effective physical activity. Haemophilia 2013; 19:487–498. [DOI] [PubMed] [Google Scholar]
- 45.Seuser A, Boehm P, Ochs S, Trunz-Carlisi E, Halimeh S, Klamroth R. How fit are children and adolescents with haemophilia in Germany? Results of a prospective study assessing the sport-specific motor performance by means of modern test procedures of sports science. Haemophilia 2015; 21:523–529. [DOI] [PubMed] [Google Scholar]
- 46.Young NL, Wakefield C, Burke TA, Ray R, McCusker PJ, Blanchette V. Updating the Canadian Hemophilia Outcomes-Kids Life Assessment Tool (CHO-KLAT Version2.0). Value Health 2013; 16:837–841. [DOI] [PubMed] [Google Scholar]
- 47.Kramer EL. Retrospektive studie zu den auswirkungen der langzeitprophylaxe mit faktor VIII-konzentrat bei patienten mit schwerer hämophilie A auf den gelenkstatus von kniegelenk, oberen sprunggelenk und ellenbogengelenk. Dissertation for the Universität of Bonn. 2013. [Google Scholar]
- 48.Czepa D, von Mackensen S, Hilberg T. Haemophilia & Exercise Project (HEP): the impact of 1-year sports therapy programme on physical performance in adult haemophilia patients. Haemophilia 2013; 19:194–199. [DOI] [PubMed] [Google Scholar]
- 49.St-Louis J, Urajnik DJ, Menard F, Cloutier S, Klaassen RJ, Ritchie B, et al. Generic and disease-specific quality of life among youth and young men with hemophilia in Canada. BMC Hematol 2016; 16:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kargarfard M, Dehghadani M, Ghias R. The effect of aquatic exercise therapy on muscle strength and joint's range of motion in hemophilia patients. Int J Prev Med 2013; 4:50–56. [PMC free article] [PubMed] [Google Scholar]
- 51.Hoffman J, Gabel P. Expanding Panjabi's stability model to express movement: a theoretical model. Med Hypotheses 2013; 80:692–697. [DOI] [PubMed] [Google Scholar]
- 52.Lobet S, Hermans C, Pasta G, Detrembleur C. Body structure versus body function in haemophilia: the case of haemophilic ankle arthropathy. Haemophilia 2011; 17:508–515. [DOI] [PubMed] [Google Scholar]
- 53.Seuser A, Duran MN, Auerswald G, Wendel M, Chaverri P. Abstracts of the XXIXth International Congress of the World Federation of Hemophilia. Buenos Aires, Argentina. July 10-14, 2010. Haemophilia 2010; 16 suppl 4:1–158. [DOI] [PubMed] [Google Scholar]
- 54.Poon MC, Lee A. Individualized prophylaxis for optimizing hemophilia care: can we apply this to both developed and developing nations? Thromb J 2016; 14 suppl 1:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Albrecht GL, Devlieger PJ. The disability paradox: high quality of life against all odds. Soc Sci Med 1999; 48:977–988. [DOI] [PubMed] [Google Scholar]
- 56.Aledort LM, Haschmeyer RH, Pettersson H. A longitudinal study of orthopaedic outcomes for severe factor-VIII-deficient haemophiliacs. The Orthopaedic Outcome Study Group. J Intern Med 1994; 236:391–399. [DOI] [PubMed] [Google Scholar]
- 57.Beeton K. Evaluation of outcome of care in patients with haemophilia. Haemophilia 2002; 8:428–434. [DOI] [PubMed] [Google Scholar]
- 58.Bullinger M, Globe D, Wasserman J, Young NL, von Mackensen S. Challenges of patient-reported outcome assessment in hemophilia care-a state of the art review. Value Health 2009; 12:808–820. [DOI] [PubMed] [Google Scholar]
- 59.de Kleijn P, van Genderen FR, van Meeteren NL. Assessing functional health status in adults with haemophilia: towards a preliminary core set of clinimetric instruments based on a literature search in rheumatoid arthritis and osteoarthritis. Haemophilia 2005; 11:308–318. [DOI] [PubMed] [Google Scholar]
- 60.Di Minno M, Cimino E, Russolillo A, Coppola A, Marrone E, Cerbone A. Abstracts of the XXIXth International Congress of the World Federation of Hemophilia. Buenos Aires, Argentina July 10-14, 2010. Haemophilia 2010; 16 suppl 4:1–158. [DOI] [PubMed] [Google Scholar]
- 61.Fischer K, van Hout BA, van der Bom JG, Grobbee DE, van den Berg HM. Association between joint bleeds and Pettersson scores in severe haemophilia. Acta Radiol 2002; 43:528–532. [PubMed] [Google Scholar]
- 62.Fischer K, Bom JG, Mauser-Bunschoten EP, Roosendaal G, Berg HM. Effects of haemophilic arthropathy on health-related quality of life and socio-economic parameters. Haemophilia 2005; 11:43–48. [DOI] [PubMed] [Google Scholar]
- 63.Gilbert MS. Prophylaxis: musculoskeletal evaluation. Semin Hematol 1993; 30:3–6. [PubMed] [Google Scholar]
- 64.Guyatt G, Walter S, Norman G. Measuring change over time: assessing the usefulness of evaluative instruments. J Chronic Dis 1987; 40:171–178. [DOI] [PubMed] [Google Scholar]
- 65.Hermans C, De Moerloose P, Fischer K, Holstein K, Klamroth R, Lambert T, et al. European Haemophilia Therapy Standardisation Board. Management of acute haemarthrosis in haemophilia A without inhibitors: literature review, European survey and recommendations. Haemophilia 2011; 17:383–392. [DOI] [PubMed] [Google Scholar]
- 66.Kandel ER, Schwartz JH, Jessel TM. Principles of neural science. 4th edNew York: McGraw-Hill; 2000. [Google Scholar]
- 67.Petrini P, Seuser A. Haemophilia care in adolescents–compliance and lifestyle issues. Haemophilia 2009; 15 suppl 1:15–19. [DOI] [PubMed] [Google Scholar]
- 68.Roosendaal G, Lafeber FP. Pathogenesis of haemophilic arthropathy. Haemophilia 2006; 12 suppl 3:117–121. [DOI] [PubMed] [Google Scholar]
- 69.Seuser A, Boehm P, Kurme A, Schumpe G, Kurnik K. Orthopaedic issues in sports for persons with haemophilia. Haemophilia 2007; 13 suppl 2:47–52. [DOI] [PubMed] [Google Scholar]
- 70.Seuser A, Berdel P, Oldenburg J. Rehabilitation of synovitis in patients with haemophilia. Haemophilia 2007; 13 suppl 3:26–31. [DOI] [PubMed] [Google Scholar]
- 71.Seuser A, Wallny T, Kurth A, Berdel P. Conservative treatment in haemophilia - improving effectivity and establishing standards. Hamostaseologie 2010; 30 suppl 1:S81–88. [PubMed] [Google Scholar]
- 72.Seuser A. Haemophilia and cartilage-the role of movement. Hamostaseologie 2012; 32:S52–S61. [PubMed] [Google Scholar]
- 73.World Federation of Hemophilia (WFH) World Congress. How to evaluate the state and the progress of haemophilic joints with no or only mild haemarthropathy in routine and research. Poster. Melbourne, 11–15 May. [Google Scholar]
- 74.Stephensen D, Tait RC, Brodie N, Collins P, Cheal R, Keeling D, et al. Changing patterns of bleeding in patients with severe haemophilia A. Haemophilia 2009; 15:1210–1214. [DOI] [PubMed] [Google Scholar]
- 75.Stephensen D, Drechsler WI, Scott OM. Outcome measures monitoring physical function in children with haemophilia: a systematic review. Haemophilia 2014; 20:306–321. [DOI] [PubMed] [Google Scholar]
- 76.Torry MR, Decker MJ, Viola RW, O’Connor DD, Steadman JR. Intra-articular knee joint effusion induces quadriceps avoidance gait patterns. Clin Biomech (Bristol, Avon) 2000; 15:147–159. [DOI] [PubMed] [Google Scholar]
- 77.van Genderen FR, Westers P, Heijnen L, de Kleijn P, van den Berg HM, Helders PJ, van Meeteren NL. Measuring patients’ perceptions on their functional abilities: validation of the Haemophilia Activities List. Haemophilia 2006; 12:36–46. [DOI] [PubMed] [Google Scholar]
- 78.Wallny T, Lahaye L, Brackmann HH, Hess L, Seuser A, Kraft CN. Clinical and radiographic scores in haemophilic arthropathies: how well do these correlate to subjective pain status and daily activities? Haemophilia 2002; 8:802–808. [DOI] [PubMed] [Google Scholar]


