Abstract
Background:
Bone marrow aspirates and concentrates are increasingly being used for musculoskeletal regenerative therapies, providing bone and cartilage progenitors. However, the quality of these bone marrow samples remains imprecise within clinical settings. As there is a need for the development of these therapies, a method of counting CD45lowCD271high cells was optimized and tested as an indicator of bone marrow sample quality.
Methods:
Bone marrow aspirates were collected from 54 donors (28 male and 26 female; median age of 48 years). The reagent concentrations were optimized for fast staining, and an acoustic-focusing flow cytometer (Attune) was used to enable automated CD45lowCD271high cell counting in bone marrow aspirates, bone marrow concentrates, and samples loaded onto a collagen scaffold. The CD45lowCD271high cell counts were compared with those obtained using another flow-cytometry-based method (LSR II) and with connective tissue progenitor (CTP) counts quantified using a colony forming unit-fibroblast (CFU-F) assay.
Results:
The optimized method enabled the counting of CD45lowCD271high cells within only 15 minutes. The quantified cell counts (median, 1,520; range, 96 to 20,992 cells/mL of bone marrow) were positively correlated with the CTP counts (p < 0.0001; r = 0.7237). In agreement with CFU-F and LSR II-based assays, the CD45lowCD271high cell counts quantified using the Attune-based method decreased with age in the samples from female but not male donors (p = 0.0015 and p = 0.3877, respectively). A significant increase in CD45lowCD271high cell counts was detected following bone marrow concentration (mean, 5-fold; 95% confidence interval [CI], 3.6 to 7.2-fold). Additionally, the number of CD45lowCD271high cells attached to the collagen scaffold was positively correlated with the number of progenitor cells that survived on the scaffold after 2-week culture (p = 0.0348).
Conclusions:
An assay for counting CD45lowCD271high cells may provide a useful measurement of bone marrow quality. While the specificity of this measurement of CD45lowCD271high cells remained low in our experimental conditions, CD45lowCD271high cell counts were positively and modestly correlated with the prevalence of CTPs.
Clinical Relevance:
A fast and automated assessment of bone marrow aspirate/concentrate quality using CD45lowCD271high cell counting may be a useful tool for improving the quality of regenerative therapy.
The field of regenerative medicine is constantly evolving, with new approaches for cartilage and bone healing dominating clinical and research activities. Targeting the environment of the nonunion of fractures or joint degeneration with biological modifiers, such as progenitor cells and/or growth factors, represents a promising therapeutic strategy1-3. The rationale behind such a strategy is that the repopulation of cartilage and bone defects is possible, as long as the progenitor cells are present. For example, the potential efficacy of the microfracture technique for cartilage repair in osteoarthritis could be related to the effect of the subchondral bone progenitor cells that produce growth factors and tissue matrix4. Furthermore, the use of bone marrow progenitors with or without platelet-rich plasma has been reported to aid bone repair in preclinical and clinical studies of osteochondral defects, metaphyseal bone defects, and femoral head osteonecrosis5-9.
Authors of previous studies have reported the clinical value of bone marrow aspirates or concentrates, showing a positive correlation between the number of applied bone marrow progenitors and favorable clinical outcomes in tibial fracture nonunion10, osteoarthritis11, and osteonecrosis therapy12-15. Despite the advantages of using bone marrow aspirates or concentrates, the quality of these samples remains difficult to assess and is poorly controlled. Furthermore, the number of progenitor cells in bone marrow aspirates is widely variable, depending on the aspiration site, volume, and surgical technique16,17 as well as on donor-related factors such as age and sex18. The determination of the quality of bone marrow samples is crucial in order to optimize clinical outcomes, cost, and time associated with cell-based therapies. The colony forming unit-fibroblast (CFU-F) assay facilitates the counting of connective tissue progenitors (CTPs) and is commonly used as an indicator for bone marrow sample quality19,20; however, it usually takes several days to be informative. CTPs represent the progenitors in native tissues that are able to form colonies in vitro. However, CTP concentration and prevalence can be influenced by bone marrow processing methods. The efficiency of colony formation (the likelihood that a viable CTP will form a colony when placed into a CFU-F assay) is also dependent on culture conditions17,21.
The aim of the current study was to introduce a fast and automated method, with minimum sample processing, that helps to indicate the quality of bone marrow aspirates and concentrates. Bone marrow cells isolated on the basis of the CD45lowCD271high phenotype are known to express CD73, CD90, and CD105, but not hematopoietic lineage markers, and on culture, generate multipotential stromal cells fully consistent with the International Society for Cellular Therapy (ISCT) criteria22-24. Importantly, several groups have reported that no colony-forming cells are present in the CD271-negative fraction of bone marrow cells, and bone marrow colony-forming activity is completely confined to the CD45lowCD271high cells24-30. Therefore, we chose to quantify, by a flow-cytometry-based assay, CD45lowCD271high cell counts, as an indicator of the quality of bone marrow aspirates and concentrates. We hope that our work will contribute to the standardization of therapies and the setting of thresholds between the “success” and “failure” of musculoskeletal regenerative therapies.
Materials and Methods
Bone Marrow Aspirates
Bone marrow samples from 54 donors (28 male and 26 female) were used for this study under ethical approval (06/Q1206/127, National Research Ethics Committee Yorkshire & Humber-Leeds East). The donors were admitted at Leeds General Infirmary for orthopaedic surgery; they did not have any systemic illness, cancer, or metabolic diseases. Donor age ranged from 22 to 80 years (median, 48 years). Two groups of donor samples were used, as described in Table I. All bone marrow aspirates were consistently harvested from the same location (zone 6) of the posterior region of the iliac crest as previously described16,25,31. Each sample analysis was carried out on a single bone marrow sample harvested from a single individual at a single time point.
TABLE I.
Donor Sample Groups
| Group 1 | Group 2 | |
| No. of donors | 39 | 15 |
| Bone marrow aspirate volume (mL) | 8 | 60 |
| Aspiration location | Posterior region of the iliac crest | Posterior region of the iliac crest |
| Assessments performed | Optimization of marker concentration (Fig. 1) Comparison of assays (Figs. 2 and 3) Assessment of CD45lowCD271high cells attached onto collagen scaffold (Fig. 5) |
Assessment of CD45lowCD271high cells in bone marrow concentrates (Fig. 4) |
Using Flow Cytometry for Counting CD45lowCD271high Cells
A 100-μL volume of whole bone marrow was stained using a 3-marker panel containing Vybrant DyeCycle Ruby 2.5-mM solution in dimethyl sulfoxide (DMSO) (Thermo Fisher Scientific), a DNA-selective dye that only labels the nucleated cells, enabling the gating out of red blood cells (RBCs) and platelets. Additionally, the panel contained anti-CD45 antibody (V450, clone HI30, mouse IgG1κ; concentration, 100 μg/mL; BD Biosciences) and anti-CD271 antibody (PE, clone ME20.4-1.H4, mouse IgG1; concentration, 0.75 μg/mL; Miltenyi Biotec). The phenotype indicating bone marrow progenitor cells (CD45lowCD271high cells) was applied as previously described17. The manufacturer recommendation for CD45 and CD271 antibodies was 15 minutes at room temperature and for Vybrant DyeCycle Ruby dye, 15 minutes at 37°. However, the antibody/dye staining was optimized to count CD45lowCD271high cells within the shortest time (see Results). We used the Attune acoustic-focusing flow cytometer (Thermo Fisher Scientific), which allowed automated cell counting. For some experiments, the CD45lowCD271high cell counts were quantified using our previously published flow cytometry-based method17. Briefly, this method involved bone marrow sample staining (using CD90, CD271, and CD45), RBC lysis, and then adding CountBright absolute counting beads (Thermo Fisher Scientific). The data acquisition for this part of the study was performed using an LSR II flow cytometer (BD Biosciences).
CFU-F Assay
The CFU-F assay was employed as previously described17 to count CTPs, whereby the bone marrow samples were added to StemMACS MSC expansion media (Miltenyi Biotec) and then cultured for 14 days. The colonies were visualized using methylene blue and counted manually. Each colony was defined as having at least 50 cells32.
Bone Marrow Concentration
Bone marrow samples (n = 15) were concentrated by means of the gradient centrifugation using the BioCUE device (Zimmer Biomet). The bone marrow aspirates were collected into syringes, washed with anticoagulant acid citrate dextrose (ACD), and loaded into the BioCUE device. From both pre-concentration and post-concentration fractions, aliquots were analyzed for CD45lowCD271high cell and CTP counts using the Attune-based method and CFU-F assays, respectively. The counting of platelets was performed for some samples (n = 10) using an automated hematopoietic cell counter (Sysmex; Sysmex Ltd).
Loading of Bone Marrow Samples on a Collagen Scaffold
The bone marrow aspirates were used to load a collagen scaffold (Bio-Gide; Geistlich Pharma). Samples from pre-loading and post-loading fractions of the bone marrow aspirates were processed to count CD45lowCD271high cells. The bone marrow-loaded scaffolds were cultured for 2 weeks and subsequently processed to quantify bone marrow progenitors that survived on the scaffolds, as previously described21. Briefly, the scaffolds were digested using 0.25% collagenase (Stem Cell Technologies). As the surface expression of CD271 can be reduced on cultured cells24, the extracted cells were stained using CD45, CD90 (BioLegend), and CD73 (Miltenyi Biotec) antibodies and counted using counting beads.
Statistical Analysis
The statistical analysis and graph preparation were performed using Prism software (version 7.0a; GraphPad). The normal distribution of the data was assessed using the Shapiro-Wilk normality test, and the appropriate test for the data analysis was applied accordingly (as specified in the figure legends). A p value of <0.05 was considered significant.
Results
Optimization of Marker Concentration
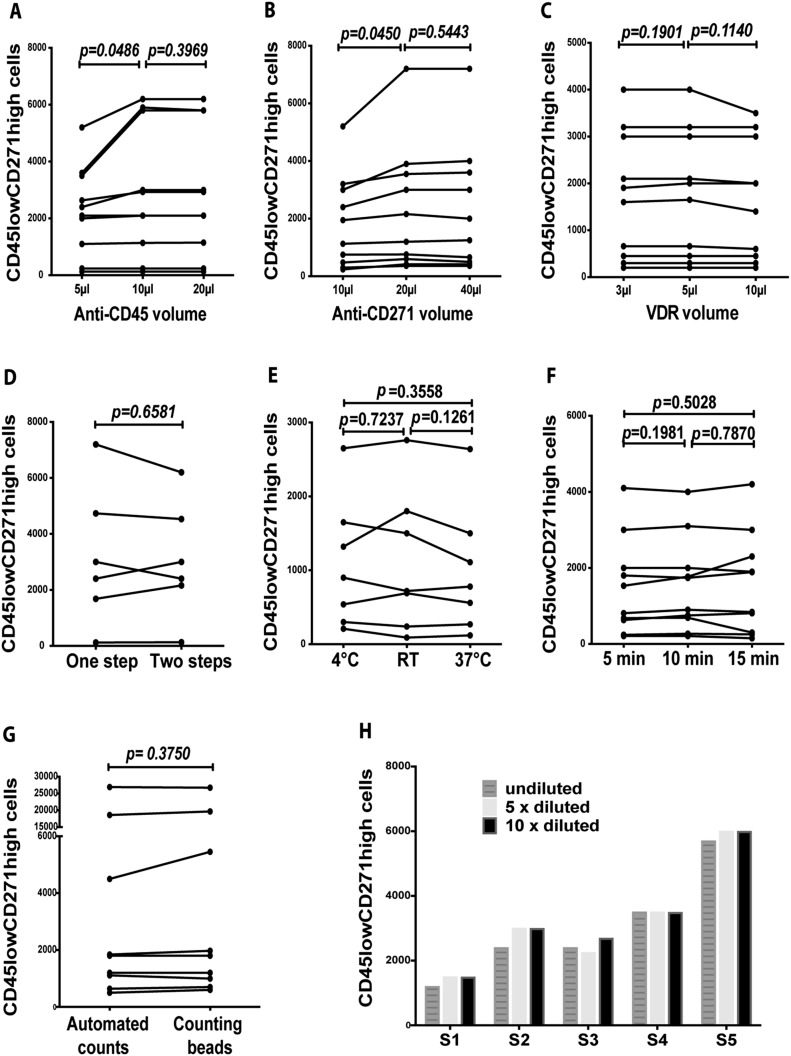
The fast staining of the bone marrow samples was initially optimized. With respect to the anti-CD45 antibody, the number of CD45lowCD271high cells counted using a volume of 10 μL of the antibody was significantly higher compared with the use of 5 μL (p = 0.0486) but did not differ significantly from the use of 20 μL (p = 0.3969) (Fig. 1-A). Regarding the anti-CD271 antibody, the number of CD45lowCD271high cells quantified using a volume of 20 μL of the antibody was significantly higher compared with the use of 10 μL (p = 0.0450) but similar to the number quantified using 40 μL (p = 0.5443) (Fig. 1-B). When 3 different volumes of the Vybrant DyeCycle Ruby dye were used, the CD45lowCD271high cell counts were similar (p = 0.1901 for 3 compared with 5 μL, and p = 0.1140 for 10 compared with 5 μL) (Fig. 1-C).
Fig. 1.
Optimization of the staining and counting of CD45lowCD271high cells using the Attune flow cytometer. Figs. 1-A, 1-B, and 1-C Comparison of CD45lowCD271high cell counts quantified using 3 different volumes of anti-CD45 and CD271 antibodies and Vybrant DyeCycle Ruby (VDR) dye (Student paired t test; n = 10 samples). Figs. 1-D, 1-E, and 1-F Comparison of CD45lowCD271high cell counts quantified using 1-step and 2-step staining (Student paired t test; n = 6 samples), at different staining temperatures (Student paired t test; n = 7 samples; RT = room temperature), and after 5, 10, and 15-minute staining (Student paired t test, n = 10 samples). Fig. 1-G Comparison of CD45lowCD271high cell counts quantified using automated counting or using counting beads, on the Attune flow cytometer (Wilcoxon matched-pairs signed-rank test; n = 9 samples). Fig. 1-H The CD45lowCD271high cell counts were quantified in bone marrow aspirates that were stained undiluted or diluted ×5 or ×10 (n = 5 samples; S = sample).
We next tested the use of CD45 and CD271 antibodies followed by Vybrant DyeCycle Ruby (2-step staining) compared with the addition of all markers in 1 step. The cell counts did not differ significantly between 1-step and 2-step staining methods (p = 0.6581) (Fig. 1-D). The CD45lowCD271high cell counts were also similar using different staining temperatures (p = 0.7237 for 4°C compared with room temperature, p = 0.1261 for 37°C compared with room temperature, and p = 0.3558 for 4°C compared with 37°C) (Fig. 1-E). The CD45lowCD271high cell counts also did not differ significantly for 5-minute staining compared with 10-minute staining (p = 0.1981), 5-minute staining compared with 15-minute staining (p = 0.5028), or 10-minute staining compared with 15-minute staining (p = 0.7870) (Fig. 1-F).
For each bone marrow sample, the acquisition time on the Attune flow cytometer was completed within 10 minutes. An internal control for automated counting was used (the use of counting beads), and automated and bead-dependent quantification were comparable (p = 0.3750) (Fig. 1-G). The data also showed similar cell counts quantified when bone marrow samples were diluted ×5 or ×10 compared with undiluted samples (Fig. 1-H). Overall, we optimized an automated and simple assay of CD45lowCD271high cells within only 15 minutes.
Comparison of Assays
Using the Attune-based method, the median percentage of CD45lowCD271high cells per total bone marrow cells was 0.016% (95% confidence interval [CI], 0.009% to 0.032%). The absolute counts of CD45lowCD271high cells showed a median of 1,520 cells/mL of bone marrow (95% CI, 1,056 to 6,112; range, 96 to 20,992 cells/mL of bone marrow).
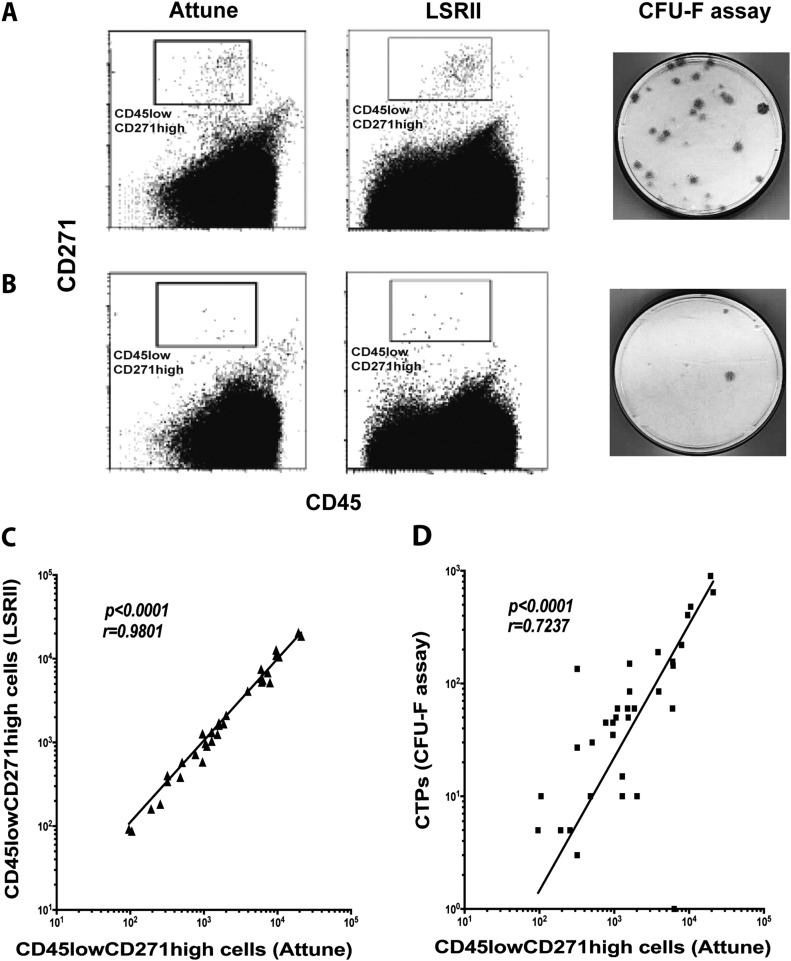
The results obtained by the Attune-based, LSR II-based, and CFU-F assays were consistent, indicating, for example, high or low-quality bone marrow samples (Figs. 2-A and 2-B). The CD45lowCD271high cell counts obtained using the Attune-based method were close to those of the LSR II-based method (median, 1,311; 95% CI, 900 to 5,533; range, 87 to 20,471 cells/mL of bone marrow). However, the CD45lowCD271high cell counts obtained by the Attune-based assay were higher than the counts of CTPs (median 60; 95% CI, 45 to 190; range, 3 to 900 CTPs/mL of bone marrow). The CD45lowCD271high cell counts measured using the Attune-based method were positively correlated with the cell counts from the LSR II-based assay (p < 0.0001; r = 0.9801) (Fig. 2-C) and CTP counts by CFU-F assay (p < 0.0001; r = 0.7237) (Fig. 2-D).
Fig. 2.
The CD45lowCD271high cell counts quantified using the Attune-based method were compared with those obtained by the LSR II-based method and with CTP counts assessed by CFU-F assay. Examples of high-quantity (Fig. 2-A) and low-quantity (Fig. 2-B) bone marrow progenitor samples are shown. The correlation between CD45lowCD271high cell counts quantified using the Attune-based method and the LSR II-based method (n = 33 samples) (Fig. 2-C) or CTP counts (n = 33 samples) (Fig. 2-D) was analyzed. A Spearman r test was used for the correlation analysis.
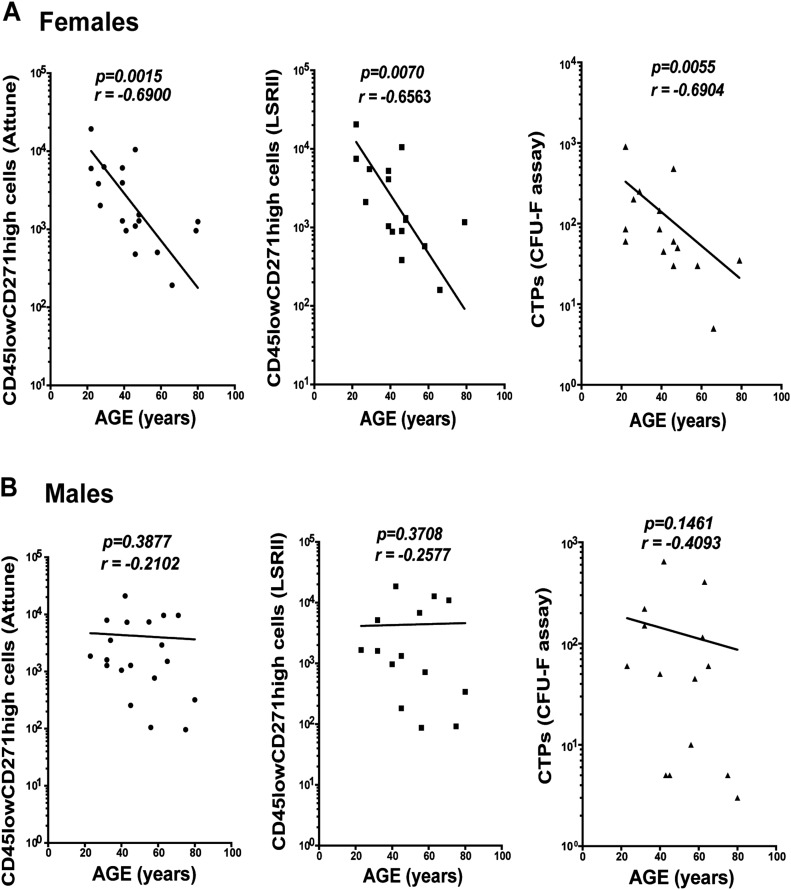
When the data were analyzed in relation to the age and sex of the donors, a clear pattern of a negative correlation between the CD45lowCD271high cell counts and age was observed for the samples from female donors using the Attune-based assay (p = 0.0015; r = −0.6900) (Fig. 3-A, left panel) but not for the samples from male donors (p = 0.3877; r = −0.2102) (Fig. 3-B, left panel). This pattern was also consistently detected using the LSR II-based method and CFU-F assay (LSR II-based method: female, p = 0.0070, and r = −0.6563; male, p = 0.3708, and r = −0.2577 [Figs. 3-A and 3-B, middle panels]; and CFU-F assay: female, p = 0.0055, r = −0.6904; male, p = 0.1461, r = −0.4093 [Figs. 3-A and 3-B, right panels]). Overall, CD45lowCD271high cell counts were comparable between the Attune and LSR II flow-cytometry methods and positively correlated with the CTP counts.
Fig. 3.
The correlation between CD45lowCD271high cell counts and increasing age in samples from female (Fig. 3-A) and male (Fig. 3-B) donors is shown. The results of the 2 methods for the counting of CD45lowCD271high cells (the Attune and LSR II-based methods) and CTP counts using CFU-F assay were compared. For the analysis in female donors (Fig. 3-A), 18, 16, and 15 samples were included, respectively. For the analysis in male donors (Fig. 3-B), 19, 14, and 14 samples were used, respectively. A Spearman r test was used for the correlation analysis.
Assessment of CD45lowCD271high Cells in Bone Marrow Concentrates
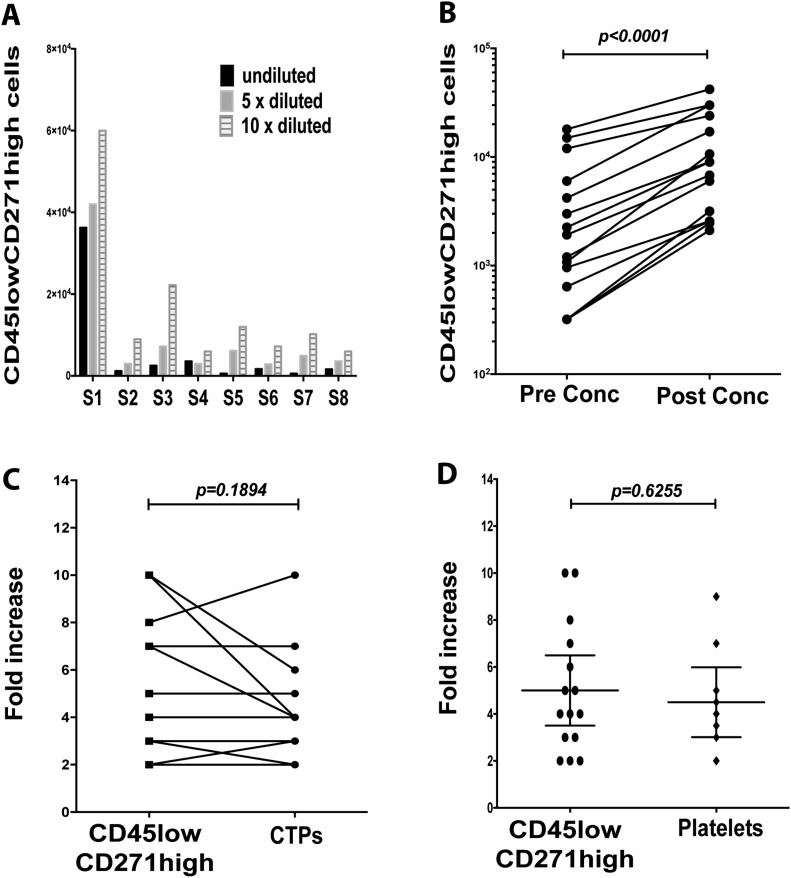
Our optimization results showed that the quantified CD45lowCD271high cell counts in bone marrow concentrates were generally higher after ×10 dilution compared with ×5 dilution and nondilution (Fig. 4-A), and thus, ×10 dilution of bone marrow concentrates is needed to ensure accurate estimation. The CD45lowCD271high cell counts increased significantly after bone marrow concentration (p < 0.0001) (Fig. 4-B). The fold increase of the CD45lowCD271high cell counts (mean, 5-fold; 95% CI, 3.6 to 7.2-fold) and those of CTPs (mean, 4.6-fold; 95% CI, 3.1 to 6-fold) were comparable (p = 0.1894) (Fig. 4-C). The Sysmex results showed an increase in the platelet counts in bone marrow concentrates (p = 0.6255), with a mean increase of 4.5-fold (95% CI, 3 to 6-fold) (Fig. 4-D). In summary, we demonstrated a method of quickly assessing increased CD45lowCD271high cell counts in the bone marrow concentrates.
Fig. 4.
Assessment of CD45lowCD271high cells in bone marrow concentrates. Fig. 4-A The samples of bone marrow concentrates were either undiluted or diluted ×5 or ×10 before the counting of CD45lowCD271high cells (n = 8 samples; S = sample). Fig. 4-B The CD45lowCD271high cell counts were compared between pre-concentration (conc) and post-concentration samples (Wilcoxon matched-pairs signed-rank test; n = 15 samples). Fig. 4-C The fold increase of CD45lowCD271high cell counts was compared with the fold increase in CTPs after bone marrow concentration (Student paired t test; n = 13 samples). Fig. 4-D The mean fold increase (and 95% CI) of CD45lowCD271high cells (calculated using the Attune-based method) and platelets (calculated using the Sysmex cell counter) were compared after bone marrow concentration (unpaired t test; n = 15 samples for CD45lowCD271high cells and n = 10 samples for platelets).
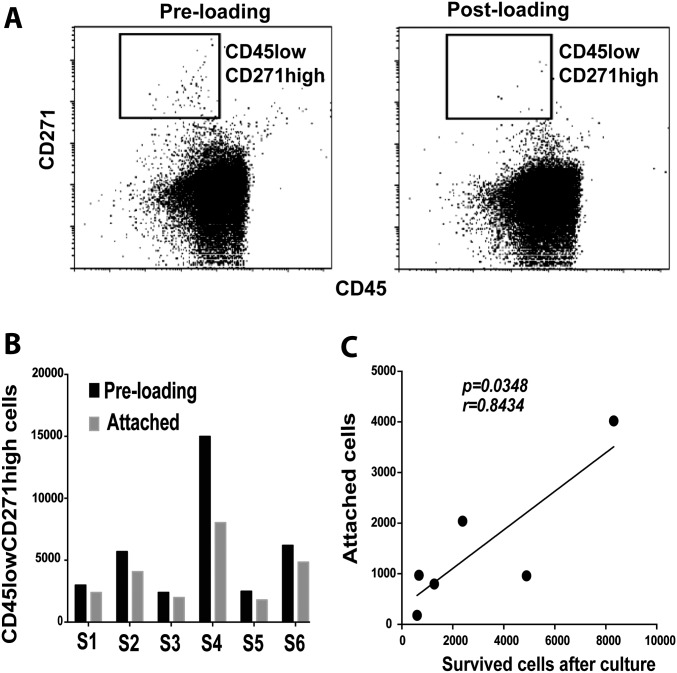
Assessment of CD45lowCD271high Cells Attached to a Collagen Scaffold
We used bone marrow aspirates to load the Bio-Gide scaffold. The number of attached CD45lowCD271high cells was then calculated by counting these cells in the pre-loading and post-loading samples (Fig. 5-A). The number of CD45lowCD271high cells attached to the Bio-Gide scaffold varied between samples but was consistently dependent on the pre-loading cell quantities (Fig. 5-B). Furthermore, the CD45lowCD271high cell count strongly correlated with the number of progenitor cells that survived on the Bio-Gide scaffold (p = 0.0348; r = 0.8434) (Fig. 5-C). The CD45lowCD271high cell assessment helped to detect the donor-related differences in cell attachment onto the scaffold.
Fig. 5.
Assessment of CD45lowCD271high cells attached to the Bio-Gide scaffold. Fig. 5-A The number of CD45lowCD271high cells attached to the Bio-Gide scaffold was calculated by counting the cells in samples from pre-loading and post-loading fractions of the bone marrow aspirates. Fig. 5-B The number of CD45lowCD271high cells on pre-loading and attached to the Bio-Gide scaffold is shown (n = 6 samples; S = sample). Fig. 5-C The correlation between the number of CD45lowCD271high cells attached to Bio-Gide scaffold and the progenitor cells (CD45−CD90+CD73+) surviving on the Bio-Gide scaffold after 2-week culture was analyzed (Pearson r test; n = 6 samples).
Discussion
Bone marrow samples contain CTPs that are potentially useful in treating degenerative musculoskeletal diseases and the nonunion of bone fractures. Processing bone marrow samples helps to concentrate these CTPs. However, the concentration and prevalence of CTPs vary widely between individuals and according to different aspiration locations and techniques16-18. The gold standard CFU-F assay requires at least 6 days18, and thus, clinicians currently have no way of knowing, at the time of the procedure, the quality of the bone marrow sample utilized. It would be desirable, therefore, to have a rapid measurement method that could provide insight into bone marrow quality on the day of the procedure. Here, we introduce a fast and automated quantification of CD45lowCD271high cell counts in bone marrow preparations that may be used to judge the quality of bone marrow samples. This assay was compared with another more time-consuming flow-cytometry assay17 and provided a similar range of CD45lowCD271high cells. Both assays confirmed an age-related decline in CD45lowCD271high cells in samples among females but not among males, as was also previously reported for CTPs18.
The specificity of the CD45lowCD271high cell measurement compared with CTP counts was low (0.05 on average); we noted 20 times more CD45lowCD271high cells with the Attune-based method than CTPs quantified by CFU-F assay. This finding agrees with those of previous studies17,26. However, the CD45lowCD271high counts were positively and modestly correlated with the prevalence of CTPs (r = 0.7237). This low specificity does not prevent the use of this assay for the estimating of aspirate quality; however, it is clear that it does not enable exact measurement of CTP counts. This might be related to the senescence of some CD45lowCD271high cells in culture during CFU-F assay as a result of plating at very low clonal densities. Another possible explanation for this disparity is that CTPs represent only a subset of the CD45lowCD271high population, as suggested recently33. It is possible that, with the addition of more markers, this subpopulation could be defined, allowing increased specificity of the assay. One previous study tested the CD146 marker, but no further enrichment in CTP counts was detected in the CD146+CD271+ fraction compared with CD146−CD271+ fraction26. Subsequently, the same group showed that the majority of CTPs resided in the CD140a−CD271+ fraction28. However, our group did not find such a clear subpopulation34. Others have not yet devised additional, more selective markers, while there is a mutual agreement regarding the value of CD27135.
We believe that the assessment of CD45lowCD271high cells has a very high sensitivity (close to 100%), as other studies have shown that all bone marrow colony-forming activity is confined to CD45lowCD271high cells, and CD271-negative cells have not been shown to have any colony-forming ability24-30. The implications of the Attune-based assay with high sensitivity and relatively low specificity is that no CTPs are missed, while some progenitor cells with potentially lower colony-forming capacity than detected in our experimental conditions can be counted.
The CFU-F assay data can vary depending on patient age and bone marrow aspiration site and volume17,21. This could explain why the CTP counts in this study showed some variability from previous work36. Using various bone marrow processing methods could have an additional effect on the variability of CTP counts. For example, using density gradient centrifugation during bone marrow processing causes CTP loss37. We ensured optimal and consistent culture conditions by using complete and batch-tested media for the CFU-F assays. Thus, the possibility of the underestimation of CTPs is small, but still exists.
Bone marrow aspirates or concentrates loaded onto scaffolds have been reported to enhance cartilage repair in knee or hip osteoarthritis, focal condylar lesions of knee articular cartilage, and talar osteochondral injuries, with promising outcomes9,38-41. The results presented here show that the CD45lowCD271high cell counts can be increased 5-fold after bone marrow concentration. We also demonstrated that platelets were concentrated 4.5-fold, showing an additional value of unfractionated bone marrow concentrates with respect to providing growth factors42. Compared with our data, Dawson et al. demonstrated a 4-fold increase of CTPs in bone marrow concentrates43. Another recent study showed that 2 concentrator devices produced substantially different counts of CTPs and dissimilar levels of growth factors44. Our data also showed that the number of attached CD45lowCD271high cells on the Bio-Gide collagen scaffold varied depending on the initial cell count in the bone marrow samples. Collectively, this further emphasizes the potential value of CD45lowCD271high cell count assessment to indicate the quality of bone marrow samples after concentration or when loaded onto scaffolds.
In conclusion, we report a method that can help to indicate the “potency” or “quality” of the bone marrow sample applied in clinical settings. The quantitative assessment of CD45lowCD271high cells in bone marrow aspirates was performed rapidly, and the CD45lowCD271high cell counts positively correlated with CTP counts. While the specificity of CD45lowCD271high cell assessment was low compared with CFU-F assay, the sensitivity of this method was very high. Since CFU-F data cannot be immediately available on the day of surgery, our findings support the view that an assay measuring CD45lowCD271high cell counts may instead serve as a useful surrogate measure of bone marrow quality. Additional studies on the rapid measurement of CTP prevalence in bone marrow samples with the inclusion of other specific markers are desirable to further enhance the method described here.
Acknowledgments
Note: The authors thank the orthopaedic team at the Academic Unit of Trauma and Orthopaedics at Leeds General Infirmary for help with sample collection. The authors also acknowledge the staff at Leeds Haematological Malignancy Diagnostic Service (HMDS) for access to the Sysmex machine and help in performing platelet counting. The flow cytometry-facility team at the Leeds Institute of Rheumatic and Musculoskeletal Medicine (LIRMM), Adam Davidson and Liz Straszynski, provided support with flow cytometry.
Footnotes
Investigation performed at the Leeds Institute of Rheumatic and Musculoskeletal Medicine, St. James Hospital, University of Leeds, Leeds, United Kingdom
Disclosure: This study was supported by a grant from Zimmer-Biomet, USA, and by NIHR-Leeds Musculoskeletal Biomedical Research Unit (LMBRU). The funding sources did not have any role in the study design, sample collection, or data analysis or interpretation. The Disclosure of Potential Conflicts of Interest forms are provided with the online version of the article (http://links.lww.com/JBJS/D426).
References
- 1.Wyles CC, Houdek MT, Behfar A, Sierra RJ. Mesenchymal stem cell therapy for osteoarthritis: current perspectives. Stem Cells Cloning. 2015. August 28;8:117-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barry F, Murphy M. Mesenchymal stem cells in joint disease and repair. Nat Rev Rheumatol. 2013. October;9(10):584-94. Epub 2013 Jul 23. [DOI] [PubMed] [Google Scholar]
- 3.Watson L, Elliman SJ, Coleman CM. From isolation to implantation: a concise review of mesenchymal stem cell therapy in bone fracture repair. Stem Cell Res Ther. 2014. April 15;5(2):51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steadman JR, Rodkey WG, Rodrigo JJ. Microfracture: surgical technique and rehabilitation to treat chondral defects. Clin Orthop Relat Res. 2001. October;391(Suppl):S362-9. [DOI] [PubMed] [Google Scholar]
- 5.Lau RL, Perruccio AV, Evans HM, Mahomed SR, Mahomed NN, Gandhi R. Stem cell therapy for the treatment of early stage avascular necrosis of the femoral head: a systematic review. BMC Musculoskelet Disord. 2014. May 16;15:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Betsch M, Schneppendahl J, Thuns S, Herten M, Sager M, Jungbluth P, Hakimi M, Wild M. Bone marrow aspiration concentrate and platelet rich plasma for osteochondral repair in a porcine osteochondral defect model. PLoS One. 2013. August 12;8(8):e71602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hakimi M, Grassmann JP, Betsch M, Schneppendahl J, Gehrmann S, Hakimi AR, Kröpil P, Sager M, Herten M, Wild M, Windolf J, Jungbluth P. The composite of bone marrow concentrate and PRP as an alternative to autologous bone grafting. PLoS One. 2014. June 20;9(6):e100143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gobbi A, Chaurasia S, Karnatzikos G, Nakamura N. Matrix-induced autologous chondrocyte implantation versus multipotent stem cells for the treatment of large patellofemoral chondral lesions: a nonrandomized prospective trial. Cartilage. 2015. April;6(2):82-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giannini S, Buda R, Battaglia M, Cavallo M, Ruffilli A, Ramponi L, Pagliazzi G, Vannini F. One-step repair in talar osteochondral lesions: 4-year clinical results and t2-mapping capability in outcome prediction. Am J Sports Med. 2013. March;41(3):511-8. Epub 2012 Dec 5. [DOI] [PubMed] [Google Scholar]
- 10.Hernigou P, Poignard A, Beaujean F, Rouard H. Percutaneous autologous bone-marrow grafting for nonunions. Influence of the number and concentration of progenitor cells. J Bone Joint Surg Am. 2005. July;87(7):1430-7. [DOI] [PubMed] [Google Scholar]
- 11.Centeno CJ, Al-Sayegh H, Bashir J, Goodyear S, Freeman MD. A dose response analysis of a specific bone marrow concentrate treatment protocol for knee osteoarthritis. BMC Musculoskelet Disord. 2015. September 18;16:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hernigou P, Beaujean F. Treatment of osteonecrosis with autologous bone marrow grafting. Clin Orthop Relat Res. 2002. December;405:14-23. [DOI] [PubMed] [Google Scholar]
- 13.Hernigou P, Beaujean F, Lambotte JC. Decrease in the mesenchymal stem-cell pool in the proximal femur in corticosteroid-induced osteonecrosis. J Bone Joint Surg Br. 1999. March;81(2):349-55. [DOI] [PubMed] [Google Scholar]
- 14.Hernigou P, Poignard A, Zilber S, Rouard H. Cell therapy of hip osteonecrosis with autologous bone marrow grafting. Indian J Orthop. 2009. January;43(1):40-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gangji V, De Maertelaer V, Hauzeur JP. Autologous bone marrow cell implantation in the treatment of non-traumatic osteonecrosis of the femoral head: five year follow-up of a prospective controlled study. Bone. 2011. November;49(5):1005-9. Epub 2011 Jul 29. [DOI] [PubMed] [Google Scholar]
- 16.Hernigou P, Homma Y, Flouzat Lachaniette CH, Poignard A, Allain J, Chevallier N, Rouard H. Benefits of small volume and small syringe for bone marrow aspirations of mesenchymal stem cells. Int Orthop. 2013. November;37(11):2279-87. Epub 2013 Jul 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cuthbert R, Boxall SA, Tan HB, Giannoudis PV, McGonagle D, Jones E. Single-platform quality control assay to quantify multipotential stromal cells in bone marrow aspirates prior to bulk manufacture or direct therapeutic use. Cytotherapy. 2012. April;14(4):431-40. Epub 2012 Jan 24. [DOI] [PubMed] [Google Scholar]
- 18.Muschler GF, Nitto H, Boehm CA, Easley KA. Age- and gender-related changes in the cellularity of human bone marrow and the prevalence of osteoblastic progenitors. J Orthop Res. 2001. January;19(1):117-25. [DOI] [PubMed] [Google Scholar]
- 19.Muschler GF, Midura RJ, Nakamoto C. Practical modeling concepts for connective tissue stem cell and progenitor compartment kinetics. J Biomed Biotechnol. 2003;2003(3):170-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caralla T, Boehm C, Hascall V, Muschler G. Hyaluronan as a novel marker for rapid selection of connective tissue progenitors. Ann Biomed Eng. 2012. December;40(12):2559-67. Epub 2012 Jun 15. [DOI] [PubMed] [Google Scholar]
- 21.El-Jawhari JJ, Sanjurjo-Rodríguez C, Jones E, Giannoudis PV. Collagen-containing scaffolds enhance attachment and proliferation of non-cultured bone marrow multipotential stromal cells. J Orthop Res. 2016. April;34(4):597-606. Epub 2015 Dec 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Churchman SM, Ponchel F, Boxall SA, Cuthbert R, Kouroupis D, Roshdy T, Giannoudis PV, Emery P, McGonagle D, Jones EA. Transcriptional profile of native CD271+ multipotential stromal cells: evidence for multiple fates, with prominent osteogenic and Wnt pathway signaling activity. Arthritis Rheum. 2012. August;64(8):2632-43. [DOI] [PubMed] [Google Scholar]
- 23.Cox G, Boxall SA, Giannoudis PV, Buckley CT, Roshdy T, Churchman SM, McGonagle D, Jones E. High abundance of CD271(+) multipotential stromal cells (MSCs) in intramedullary cavities of long bones. Bone. 2012. February;50(2):510-7. Epub 2011 Jul 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Battula VL, Treml S, Bareiss PM, Gieseke F, Roelofs H, de Zwart P, Müller I, Schewe B, Skutella T, Fibbe WE, Kanz L, Bühring HJ. Isolation of functionally distinct mesenchymal stem cell subsets using antibodies against CD56, CD271, and mesenchymal stem cell antigen-1. Haematologica. 2009. February;94(2):173-84. Epub 2008 Dec 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cuthbert RJ, Giannoudis PV, Wang XN, Nicholson L, Pawson D, Lubenko A, Tan HB, Dickinson A, McGonagle D, Jones E. Examining the feasibility of clinical grade CD271+ enrichment of mesenchymal stromal cells for bone regeneration. PLoS One. 2015. March 11;10(3):e0117855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tormin A, Li O, Brune JC, Walsh S, Schütz B, Ehinger M, Ditzel N, Kassem M, Scheding S. CD146 expression on primary nonhematopoietic bone marrow stem cells is correlated with in situ localization. Blood. 2011. May 12;117(19):5067-77. Epub 2011 Mar 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quirici N, Soligo D, Bossolasco P, Servida F, Lumini C, Deliliers GL. Isolation of bone marrow mesenchymal stem cells by anti-nerve growth factor receptor antibodies. Exp Hematol. 2002. July;30(7):783-91. [DOI] [PubMed] [Google Scholar]
- 28.Li H, Ghazanfari R, Zacharaki D, Ditzel N, Isern J, Ekblom M, Méndez-Ferrer S, Kassem M, Scheding S. Low/negative expression of PDGFR-α identifies the candidate primary mesenchymal stromal cells in adult human bone marrow. Stem Cell Reports. 2014. December 9;3(6):965-74. Epub 2014 Oct 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones E, English A, Churchman SM, Kouroupis D, Boxall SA, Kinsey S, Giannoudis PG, Emery P, McGonagle D. Large-scale extraction and characterization of CD271+ multipotential stromal cells from trabecular bone in health and osteoarthritis: implications for bone regeneration strategies based on uncultured or minimally cultured multipotential stromal cells. Arthritis Rheum. 2010. July;62(7):1944-54. [DOI] [PubMed] [Google Scholar]
- 30.Bühring HJ, Battula VL, Treml S, Schewe B, Kanz L, Vogel W. Novel markers for the prospective isolation of human MSC. Ann N Y Acad Sci. 2007. June;1106:262-71. Epub 2007 Mar 29. [DOI] [PubMed] [Google Scholar]
- 31.Hernigou J, Picard L, Alves A, Silvera J, Homma Y, Hernigou P. Understanding bone safety zones during bone marrow aspiration from the iliac crest: the sector rule. Int Orthop. 2014. November;38(11):2377-84. Epub 2014 May 3. [DOI] [PubMed] [Google Scholar]
- 32.Kuznetsov SA, Mankani MH, Bianco P, Pamela G. Enumeration of the colony-forming units-fibroblast from mouse and human bone marrow in normal and pathological conditions. Stem Cell Res. 2009. January;2(1):83-94. Epub 2008 Aug 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ghazanfari R, Li H, Zacharaki D, Lim HC, Scheding S. Human non-hematopoietic CD271(pos)/CD140a(low/neg) bone marrow stroma cells fulfill stringent stem cell criteria in serial transplantations. Stem Cells Dev. 2016. September 19 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tan HB, Giannoudis PV, Boxall SA, McGonagle D, Jones E. The systemic influence of platelet-derived growth factors on bone marrow mesenchymal stem cells in fracture patients. BMC Med. 2015. January 13;13:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harichandan A, Bühring HJ. Prospective isolation of human MSC. Best Pract Res Clin Haematol. 2011. March;24(1):25-36. Epub 2011 Feb 23. [DOI] [PubMed] [Google Scholar]
- 36.Muschler GF, Boehm C, Easley K. Aspiration to obtain osteoblast progenitor cells from human bone marrow: the influence of aspiration volume. J Bone Joint Surg Am. 1997. November;79(11):1699-709. [DOI] [PubMed] [Google Scholar]
- 37.Dal Pozzo S, Urbani S, Mazzanti B, Luciani P, Deledda C, Lombardini L, Benvenuti S, Peri A, Bosi A, Saccardi R. High recovery of mesenchymal progenitor cells with non-density gradient separation of human bone marrow. Cytotherapy. 2010. September;12(5):579-86. [DOI] [PubMed] [Google Scholar]
- 38.Hauser RA, Orlofsky A. Regenerative injection therapy with whole bone marrow aspirate for degenerative joint disease: a case series. Clin Med Insights Arthritis Musculoskelet Disord. 2013. September 4;6:65-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Centeno C, Pitts J, Al-Sayegh H, Freeman M. Efficacy of autologous bone marrow concentrate for knee osteoarthritis with and without adipose graft. Biomed Res Int. 2014;2014:370621 Epub 2014 Sep 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Veronesi F, Giavaresi G, Tschon M, Borsari V, Nicoli Aldini N, Fini M. Clinical use of bone marrow, bone marrow concentrate, and expanded bone marrow mesenchymal stem cells in cartilage disease. Stem Cells Dev. 2013. January 15;22(2):181-92. Epub 2012 Nov 16. [DOI] [PubMed] [Google Scholar]
- 41.Enea D, Cecconi S, Calcagno S, Busilacchi A, Manzotti S, Gigante A. One-step cartilage repair in the knee: collagen-covered microfracture and autologous bone marrow concentrate. A pilot study. Knee. 2015. January;22(1):30-5. Epub 2014 Nov 20. [DOI] [PubMed] [Google Scholar]
- 42.Civinini R, Nistri L, Martini C, Redl B, Ristori G, Innocenti M. Growth factors in the treatment of early osteoarthritis. Clin Cases Miner Bone Metab. 2013. January;10(1):26-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dawson JI, Smith JO, Aarvold A, Ridgway JN, Curran SJ, Dunlop DG, Oreffo RO. Enhancing the osteogenic efficacy of human bone marrow aspirate: concentrating osteoprogenitors using wave-assisted filtration. Cytotherapy. 2013. February;15(2):242-52. Epub 2012 Dec 12. [DOI] [PubMed] [Google Scholar]
- 44.Cassano JM, Kennedy JG, Ross KA, Fraser EJ, Goodale MB, Fortier LA. Bone marrow concentrate and platelet-rich plasma differ in cell distribution and interleukin 1 receptor antagonist protein concentration. Knee Surg Sports Traumatol Arthrosc. 2016. February 1 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]