Abstract
A 26 year old female presented for recurrent blood tinged sputum during the previous year with development of frank hemoptysis three days prior to admission. Diffuse alveolar hemorrhage (DAH) was confirmed with serial lavages. The patient had no history of autoimmune disease, vascular thrombosis or pregnancy morbidity including miscarriages or pre-eclampsia. High dose steroids were initiated along with noninvasive ventilatory support. Transthoracic echocardiogram showed severe mitral regurgitation and a vegetation on the mitral valve; transesophageal echocardiogram determined the lesion highly suggestive of Libman-Sachs endocarditis. Blood cultures were negative. Immunological evaluation established the patient was negative for: anti-nuclear antibody, anti-double-stranded DNA antibody, rheumatoid factor, anti-smith antibody, anti-cyclic citrullinated peptide, anti-neutrophil cytoplasmic antibodies, anti-glomerular basement membrane antibodies. Further evaluation revealed elevated levels of anticardiolipin immunoglobulin G and anti-beta 2 glycoprotein immunoglobulin G which continued to increase for months after hospitalization. She was diagnosed with DAH secondary to acute mitral regurgitation caused by Libman-Sachs endocarditis in the presence of primary antiphospholipid antibody syndrome.
DAH is an important disease to understand given its high mortality rate. Few case reports relating the presence of Libman-Sachs endocarditis induced by antiphospholipid antibody syndrome leading to DAH have been published. Unique here is the absence of rheumatologic markers thus supporting a diagnosis of primary antiphospholipid antibody syndrome (APS). This patient had no findings associated with rheumatological disorders potentially making this diagnosis easily overlooked. This case further illustrates the importance of evaluating patients with APS presenting with DAH as there are multiple etiologies that lead to this pathology thus different treatment avenues are to be considered during management.
Keywords: Diffuse alveolar hemorrhage, Valves, Antithrombotic therapy, Mitral valve, Pearls
1. Introduction
Diffuse alveolar hemorrhage (DAH) is a life-threatening condition with a high incidence of mortality supporting its need for early diagnosis and treatment. Frank hemoptysis requires evaluation by bronchoalveolar lavage to isolate the source of bleeding within the lung and serial aliquots of bloody lavage return remains the standard in diagnosis of DAH. Because of its association with vasculitis and rheumatologic disease, prompt evaluation for these potential inciting factors is essential for management.
2. Case report
A 26-year-old female with a past medical history of stable idiopathic thrombocytopenic purpura (ITP) diagnosed ten years ago presented with a complaint of intermittent, blood tinged sputum over several months. This evolved to frank hemoptysis two days prior to admission along with hypoxic respiratory failure. She had been treated unsuccessfully for upper and lower respiratory infections with antibiotics and inhalers during the previous year. Current medications included docusate and pantoprazole.
On presentation she was tachycardic with heart rate of 112 beats per minute and tachypneic with a respiratory rate of 25 breaths per minute. She was afebrile and her oxygenation saturation was 95% on Bilevel Positive Airway Pressure (inspiratory pressure of 12 mmHg and positive end-expiratory pressure of 5 mmHg at 60% FiO2). Physical exam findings were remarkable for diminished breath sounds in bilateral bases along with new onset, grade 2 holosystolic murmur.
Initial laboratory evaluation showed stable thrombocytopenia near the patient's baseline at 53 × 103/μl. WBC, hemoglobin, and hematocrit were noted as being 14.5 × 103/cu mm, 8.2 g/dL and 25.9% respectively. Immunological evaluation was significant for elevations of anti-beta2 glycoprotein antibodies at 24.2 SGU U/mL and anticardiolipin immunoglobulin G at 52.3 GPL U/mL. Laboratory findings of the following autoimmune studies were found to be unremarkable: antinuclear antibody (ANA), anti-double-stranded DNA antibody, rheumatoid factor, anti-Smith antibody, anti-cyclic citrullinated peptide, anti-neutrophil cytoplasmic antibodies, anti-glomerular basement membrane antibodies.
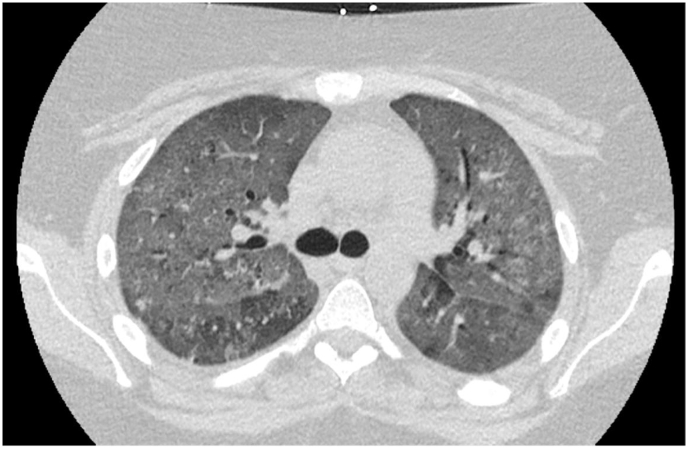
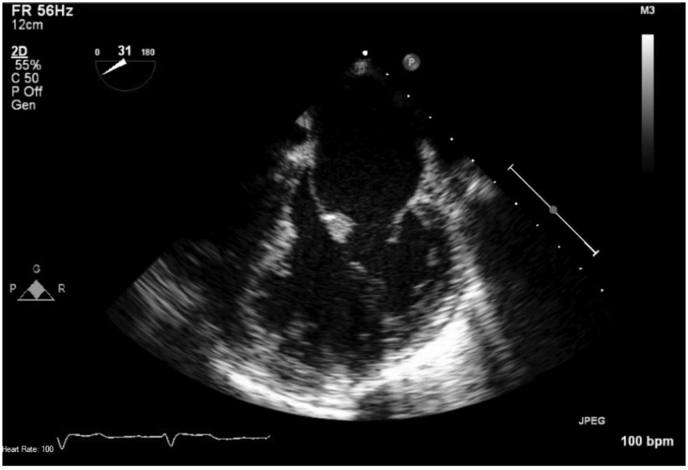
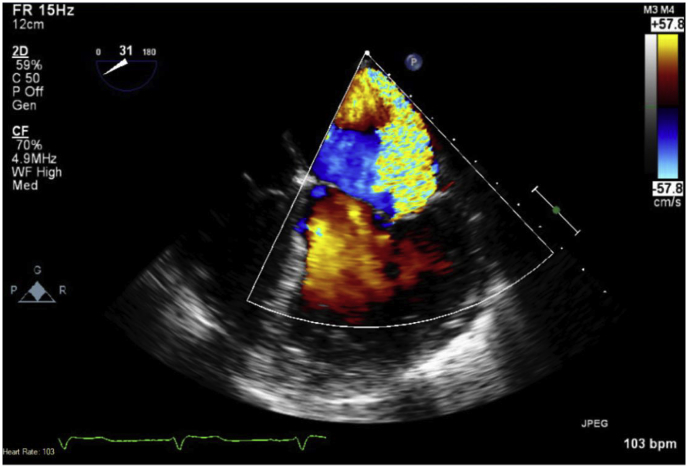
Non-contrasted computed tomography scan of the chest revealed diffuse ground-glass attenuation throughout bilateral lungs (Fig. 1). Due to identification of a new onset murmur on examination, the patient underwent transthoracic echocardiogram followed by transesophageal echocardiogram. Mitral valve vegetations were noted on anterior and posterior leaflets measuring up to 1.5 × 0.4 cm (Fig. 2) with new severe mitral regurgitation with a mean gradient of 20–25 mmHg across the valve (Fig. 3). Bronchoalveolar lavage with serial aliquots demonstrated persistent bloody return. Cultures from bronchoalveolar lavage and venous blood were negative.
Fig. 1.
Non-contrasted CT scan of chest.
Fig. 2.
TEE demonstrating vegetation on atrial side of anterior leaflet.
Fig. 3.
TEE demonstrating the mosaic jet of eccentric flow reaching the posterior left atrium.
The patient's platelet count remained stable with a nadir of 48 thousand/cu mm. Methylprednisolone 1g/day ×3 days was started immediately followed by a one-month prednisone taper starting at 40 mg daily. Given her response to steroids, as observed by improvement of her respiratory symptoms with weaning of Bilevel Positive Airway Pressure to room air along with a persistently stable platelet count, rituximab was not started. Her mitral valve vegetations persisted at follow up although she declined surgical intervention given clinical improvement with medical management. Subsequently she was managed with lifelong anticoagulation and close follow up. There was no recurrence of DAH.
3. Discussion
Pulmonary manifestations of antiphospholipid syndrome (APS) have long been observed. The most common of these manifestations include pulmonary hypertension and thromboembolic disease [1,2]. A less common initial manifestation of APS includes DAH which has been described in the literature as a non-criteria manifestation of APS [1,3,4]. A retrospective study demonstrated that DAH constituted 3.7% of systemic lupus erythematosus (SLE) admissions within a single institution [4]. As such, evaluation of DAH should include diagnostic testing not only for secondary APS but also primary APS as this may be a less common initial manifestation of these disease processes [[1], [2], [3],[5], [6], [7], [8], [9]]. Primary and secondary APS differ in that secondary APS has a concomitant diagnosis of another disease process, often SLE [8]. Because there are distinctly different disease processes potentially leading to a presentation of DAH, understanding of the underlying pathophysiology and their treatment is fundamental.
Both primary and SLE associated APS can lead to Libman-Sacs endocarditis although SLE associated APS has a higher incidence [1,8,10]. Although there can be overlap in clinical presentation among primary and secondary APS the incidence of valvular pathology is more common in those with secondary APS [10]. This form of marantic endocarditis is most commonly associated with mitral valve regurgitation thought to be the result of fibrin, platelet, leukocytes and plasma cells being deposited on the valves themselves [1,3,8]. In the case of mitral regurgitation, the flow of blood can be directed towards the right upper pulmonary vein leading to unilateral pulmonary edema and DAH [11]. In cases where regurgitant flow is contributing to DAH surgical intervention should be considered as this is known to quickly resolve symptoms [11]. Other etiologies need to be considered in the management of patients presenting with DAH in the presence of known or suspected APS.
Both capillaritis and microthrombotic disease contribute to the development of alveolar hemorrhage in APS patients [2,8]. Thrombotic events remain a hallmark of APS; among those presenting with DAH, multiple causes exist due to the presence of both inflammatory and noninflammatory states [9]. Case reports have demonstrated the etiology of DAH among small populations of those diagnosed with primary APS to be that of capillaritis [[3], [4], [5],9]. Inflammation leading to capillaritis is thought to be the result of direct vessel damage from the inflammation itself or because of immune complex deposition [9]. Mechanisms promoting a proinflammatory state include an increase in adhesion of endothelial cells leading to an increased neutrophil response, a process that is a direct result of the APS antibodies [9]. Theoretically, this mechanism would target the respiratory system preferentially due to the system's high surface area needed for gas exchange [9]. Because this is an inflammatory response driving DAH these case reports also suggest withholding anticoagulation as this may result in worsening DAH and recurrent hemoptysis in favor of immunosuppressive therapy including pulse steroids, cyclophosphamide, mycophenolate mofetil and possibly the implementation of intravenous immunoglobulin in refractory cases [3,9].
Microthrombosis leading to DAH can also present among APS patients although the incidence of this may be higher among those with secondary APS [5]. Clinical manifestations, including pulmonary manifestations, as severe as multiorgan failure are thought to be largely secondary to thromboembolic disease [8,9]. Studies found the most consistent organ affected among DAH patients due to secondary APS was the kidneys [4]. Mechanisms that lead to thrombotic events include inhibition of factors such as protein C and antiphospholipid activation of endothelial cells, complement, and platelets [9]. Because of this prothrombotic state anticoagulation must be considered among patients with evident thrombotic disease such as PE or multiorgan dysfunction [6]. Due to thrombotic events driving the development of pathology management can differ among those with multiorgan failure and the literature describes resolution of multiorgan dysfunction is as many as 70% of patients by implementing pulse steroids, anticoagulation, and plasmapheresis or intravenous immunoglobulins [8].
APS antibodies present in both primary and secondary APS lead to overlap in treatment. Despite the variation in etiologies of APS associated DAH, whether primary or secondary, treatment should begin with corticosteroids [2]. However, because there is no confirmed treatment regimen among DAH patients with either form of APS, management can be difficult [7]. There is data to suggest that different immunohistochemical characteristics of those presenting with either form of APS may have different incidences of pathology leading to pulmonary complications. Among primary and secondary APS patients that have elevated lupus anticoagulation antibodies there is an increased tendency for microthrombotic disease inciting pulmonary injury [12,13]. Alveolar inflammation leading to pulmonary manifestations among primary APS patients was found most commonly in those with moderately elevated anti-beta2 glycoprotein IgM while, secondary APS patients had a stronger tendency toward alveolar inflammation with moderately elevated anticardiolipin IgG levels [13]. Due to these subtle differences it could be possible to further develop treatment algorithms for these small populations although further research is needed to substantiate this.
4. Conclusion
Pulmonary manifestations of APS remain difficult to manage. Because APS can result in multiple causes of DAH, management is further complicated. The corner stone of therapy remains high dose corticosteroids although management after this remains controversial. Treatment algorithms must be developed in ordered to appropriately manage this rare pulmonary manifestation of APS. Included in this treatment strategy must be the determination of when to use anticoagulation therapy among those with APS presenting with DAH. Anticoagulation may not be appropriate in those with DAH due to capillaritis whereas microthrombotic events would require this intervention. Further retrospective studies are required to determine the propensity of pathologic processes resulting in DAH among both types of APS patients as this could further aide in the development of future treatment algorithms. Lastly, the role of APS markers must be addressed in future studies as this may provide objective evidence when managing APS patients presenting with DAH.
Conflict of interest
Nothing to declare.
Conflicts of interest
None.
Author contributions
RH, MR, AA, LR, and AA contributed to the content and the writing of the manuscript. AA takes full responsibility for the content of manuscript.
Financial/nonfinancial disclosures
None declared.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.rmcr.2018.08.018.
Contributor Information
Ryan Hyde, Email: Ryan.hyde@medicine.ufl.edu.
Ali Ataya, Email: ali.ataya@medicine.ufl.edu.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Hambly N., Sekhon S., McIvor R.A. Antiphospholipid antibody syndrome: diffuse alveolar hemorrhage and Libman-Sacks endocarditis in the absence of prior thrombotic events. Ulster Med. J. 2014;83(1):47–49. [PMC free article] [PubMed] [Google Scholar]
- 2.Stojanovich L. Pulmonary manifestations in antiphospholipid syndrome. Autoimmun. Rev. 2006;5(5):344–348. doi: 10.1016/j.autrev.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Suzuki A., Asazuma N., Kikuchi E. “Possible primary antiphospholipid syndrome” with concurrent diffuse alveolar hemorrhaging and Libman-Sacks endocarditis mimicking catastrophic antiphospholipid syndrome. Intern. Med. 2012;51(7):813–816. doi: 10.2169/internalmedicine.51.6592. [DOI] [PubMed] [Google Scholar]
- 4.Zamora M.R., Warner M.L., Tuder R., Schwarz M.I. Diffuse alveolar hemorrhage and systemic lupus erythematosus. Clinical presentation, histology, survival, and outcome. Medicine (Baltimore) 1997;76(3):192–202. doi: 10.1097/00005792-199705000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Espinosa G., Cervera R., Font J. [The lung in antiphospholipid syndrome] Arch. Bronconeumol. 2002;38(1):27–32. doi: 10.1016/s0300-2896(02)75143-0. [DOI] [PubMed] [Google Scholar]
- 6.Greaves M. Antiphospholipid syndrome: unusual clinical presentations. Thromb. Res. 2011;127(Suppl 3):S47–S50. doi: 10.1016/S0049-3848(11)70013-5. [DOI] [PubMed] [Google Scholar]
- 7.Tselios K., Urowitz M.B. Cardiovascular and pulmonary manifestations of systemic lupus erythematosus. Curr. Rheumatol. Rev. 2017;13(3):206–218. doi: 10.2174/1573397113666170704102444. [DOI] [PubMed] [Google Scholar]
- 8.Wiedermann F.J., Mayr A., Schobersberger W. Acute respiratory failure associated with catastrophic antiphospholipid syndrome. J. Intern. Med. 2000;247(6):723–730. doi: 10.1046/j.1365-2796.2000.00687.x. [DOI] [PubMed] [Google Scholar]
- 9.Deane K.D., West S.G. Antiphospholipid antibodies as a cause of pulmonary capillaritis and diffuse alveolar hemorrhage: a case series and literature review. Semin. Arthritis Rheum. 2005;35(3):154–165. doi: 10.1016/j.semarthrit.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 10.Vianna J.L., Khamashta M.A., Ordi-Ros J. Comparison of the primary and secondary antiphospholipid syndrome: a European Multicenter Study of 114 patients. Am. J. Med. 1994;96(1):3–9. doi: 10.1016/0002-9343(94)90108-2. [DOI] [PubMed] [Google Scholar]
- 11.Marak C.P., Joy P.S., Gupta P., Bukovskaya Y., Guddati A.K. Diffuse alveolar hemorrhage due to acute mitral valve regurgitation. Case Rep. Pulmonol. 2013;2013 doi: 10.1155/2013/179587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stojanovich L., Djokovic A., Kontic M. Antiphospholipid-mediated thrombosis: interplay between type of antibodies and localisation of lung, and cardiovascular incidences in primary antiphospholipid syndrome. Clin. Exp. Rheumatol. 2015;33(4):531–536. [PubMed] [Google Scholar]
- 13.Stojanovich L., Kontic M., Djokovic A., Ilijevski N., Stanisavljevic N., Marisavljevic D. Pulmonary events in antiphospholipid syndrome: influence of antiphospholipid antibody type and levels. Scand. J. Rheumatol. 2012;41(3):223–226. doi: 10.3109/03009742.2011.641580. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.