Abstract
The newer oral treatments for chronic hepatitis C virus infection are one of the greatest revolutions in modern medicine. These drugs promise to eradicate the infection, showing high cure rates even in difficult to treat populations with very few side effects. Nevertheless, some cases of recurrence and de novo hepatocellular carcinoma after treatment with these drugs have been reported. We describe two cases of patients treated with direct-acting antiviral agents that developed hepatocarcinoma during follow-up post-treatment.
Keywords: Hepatocellular carcinoma, HCV infection, Direct-acting antiviral therapy
Introduction
The use of the direct-acting antiviral agents (DAA) has completely revolutionized the treatment of patients with chronic hepatitis C virus (HCV) infection. The cure rates obtained with this type of treatment, combined with the comfort provided by oral intake and the scarcity of side effects have contributed to the success and high adherence to treatment with these agents. Such benefits are further emphasized taking into account that none of these positive aspects occurred in the era of interferon (IFN) plus ribavirin regimens. Despite this, some cases of recurrence of previously treated hepatocellular carcinomas (HCC) or even de novo diagnosis after treatment for HCV infection with DAA have been described in the literature. Such observation has raised doubts as to whether these drugs may in some way potentiate the appearance of hepatocarcinoma. We describe two cases of patients treated with DAA that developed hepatocellular carcinoma during follow-up post-treatment.
Cases
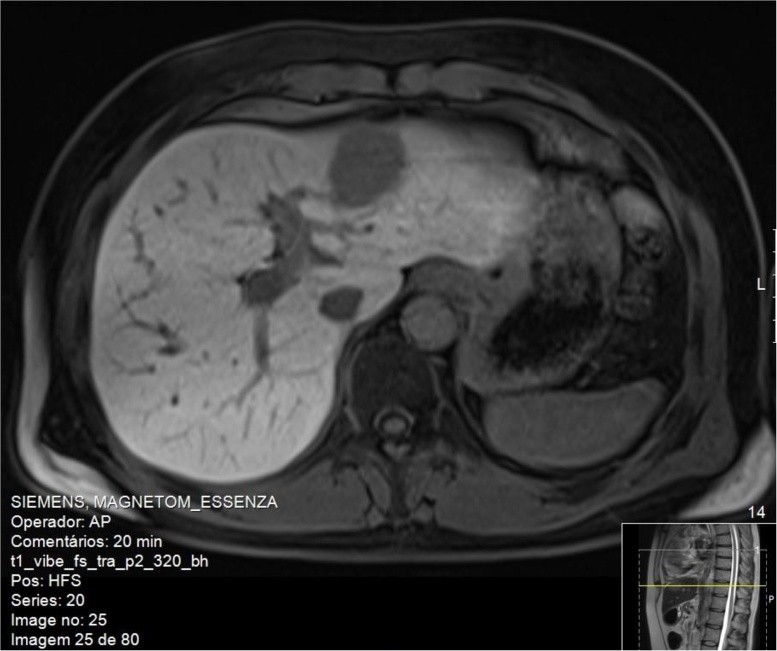
Patient 1 was a 52-year-old man with a history of moderate alcohol consumption, former intravenous drug abuse, vascular risk factors (type 2 diabetes mellitus, arterial hypertension and dyslipidemia) and chronic HCV infection (genotype 3a) diagnosed in 1997. As complications of that infection he presented secundary hemochromatosis and proliferative membranous glomerulonephritis. Hepatic elastography showed values compatible with grade of liver fibrosis METAVIR stage F4. In 2011, he completed 24 weeks of therapy with pegylated interferon and ribavirin, and presented rapid virologic response. Nevertheless, there was always evidence of hepatic cytolysis during follow-up. Approximately three months after the end of therapy, treatment failure was observed. From April to October 2015 he completed 24 weeks of treatment with ledispavir, sofosbuvir and ribavirin, achiving virologic sustained response. At 24 weeks of follow-up, the HCV maintained undetectable but the protocolar upper abdominal ultrasonography showed a heterogeneous nodular lesion in the left lobe with 43 by 32 mm. This lesion was not present in the ultrasonography performed before the beginning of treatment. Additional magnetic resonance imaging suggested a hepatic adenoma (Fig. 1), but hepatic biopsy was suggestive of hepatocellular carcinoma. At that time, the serum alpha-fetoprotein value was 204.20 IU / mL. The patient was submitted to a 2/3 bisegmentectomy. The histological examination revealed the presence of well differentiated hepatocellular carcinoma with images of lymphatic vascular invasion which were 3 mm from the surgical margin (pT2NxR0; TNM stage).
Fig. 1.
Abdominal magnetic resonance cross-section. T1 weighted with contrast showing a nodular image with imaging features suggestive of hepatic adenoma.
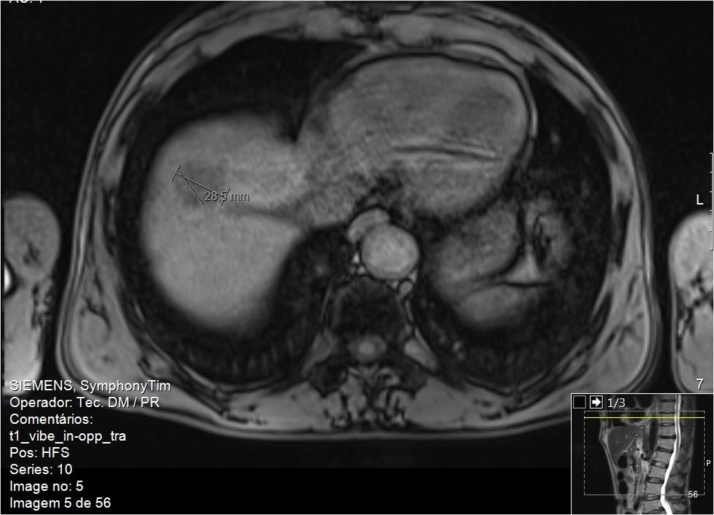
Patient 2 was a 58-year-old man with history of active heavy alcohol abuse, smoker (about 44 pack-years), former drug abuse (consumer of cocaine and intravenous heroin from 14 to 40 years old). HCV infection (genotype 1a) diagnosed in 2013, porphyria cutanea tarda and secondary hemochromatosis under programmed phlebotomies. Hepatic elastography showed values compatible with grade of liver fibrosis METAVIR stage F4. In 2014, on abdominal ultrasound was detected an injury that was imagiologically suspected to be a hemangioma of the right hepatic lobe. The magnetic resonance revealed to be only an area of perfusion alteration of segments VII / VIII. In 2015 he completed 24 weeks of treatment with ledispavir and sofosbuvir. He presented a favorable virological response, with undetectable viral load and cutaneous porphyria resolution. About 13 months after the end of treatment, a 29 mm nodule on segments VII / VIII, suspected of hepatocellular carcinoma, was detected on magnetic resonance imaging (Fig. 2). The histological study was compatible with well differentiated hepatocellular carcinoma, with extensive areas of necrosis, rare images suggestive of intratumoral lymphovascular invasion and absence of evidence of extratumoral lymphovascular invasion; the minimum surgical margin was 5 mm (pT2NxR0).
Fig. 2.
Abdominal magnetic resonance cross-section. T1 weighted showing a nodular image with imaging features suggestive of suspected of hepatocellular carcinoma.
Discussion
Clearly overcoming previous therapies based on pegylated-IFN and ribavirin regimens, drugs currently used for the treatment of HCV infection have cure rates / sustained virological response (SVR) greater than 95% [1]. SVR by itself has demonstrated a significant reduction in liver fibrosis [[2], [3], [4]], as well as an increase in liver-related and overall survival (related largely to prevention of decompensation of liver disease, reducing the need for transplantation and the risk of occurrence or recurrence of HCC) [5,6]. With regard to HCC, this can be easily understood considering that hepatic cirrhosis is the major risk factor for HCC and, in turn, that chronic HCV infection is one of the most frequent etiologies for cirrhosis [7], with approximately 2–8% risk of developing HCC in patients with HCV cirrhosis [8]. Taking into account the previous, it would be expected that the DAA would allow a reduction in the occurrence and recurrence of HCC. However, opinions have diverged since description of cases of HCC recurrence in patients treated with these drugs, generating controversy and even speculation that DAA may contribute to the development of this neoplasia.
This finding can be explained by several aspects. On the one hand, it is known that, even after SVR, the risk of HCC, although lower, persists mainly in patients already with cirrhosis [9], as in the cases presented here. On the other hand, DAA allow the treatment of patients in more advanced stages of disease, who alone may be at higher risk of developing HCC. In pathophysiologic terms it has been proposed that DAA may lead to deregulation of immune surveillance related to the rapid and marked decrease in HCV viral load [[10], [11], [12]]. Through various mechanisms, the absence of interferon activation, reconstitution of innate immunity, abrupt reduction of natural killer cells and their toxicity, among others, may contribute to a more rapid progression of HCC.
It has also been speculated that some biomarkers may predict the risk of HCC after HCV treatment. For example, one study has shown that the vascular endothelial growth factor increased significantly during and after treatment with DAA [13], although others point out that such an increase may only function as a booster in patients with other clinical or analytical predispositions for HCC [14]. Although several studies have been published to date, attempting to assess the existence of a higher risk of occurrence and / or recurrence of HCC after DAA relative to IFN, there is no evidence yet to support a difference between these two groups of patients [15].
In this article we report two cases of patients with HCV infection, with evidence of liver cirrhosis and who were diagnosed HCC soon after treatment of their chronic infection with DAA, although it may well be that small lesions were already be present in dimensions that did not allow its imaging detection and which are later enhanced by the aspects described above.
Conclusion
In the absence of evidence supporting the direct relationship between the use of AADs and the development of HCC, there is a clear benefit in treating all patients with HCV who meet criteria for treatment. However, the continuing appearance of new cases such as those presented here emphasizes the need for further studies, including multicenter and prospective. In particular, a short-term outcome may be the evaluation of potential biological markers that together with clinical aspects may predict which patients are at increased risk of developing HCC after treatment with DAA.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Authors’ contributions
Cátia Dias: Conceptualization, Investigation, Bibliographic research, Project administration, Writing original draft, Review and editing.
Filipa Duarte-Ribeiro: Writing original draft, Bibliographic research.
Sara Pipa: Writing original draft, Bibliographic research.
Ana Rita Barbosa: Writing original draft, Bibliographic research.
Margarida Mota: Conceptualization, Investigation, Project administration, Writing original draft, Review and editing.
Fernando Rosas Vieira: Project administration.
Contributor Information
Cátia Dias, Email: catia.dias@chvng.min-saude.pt.
Filipa Duarte-Ribeiro, Email: filipa.duarte.ribeiro@chvng.min-saude.pt.
Sara Pipa, Email: sara.pipa@chvng.min-saude.pt.
Ana Rita Barbosa, Email: ana.rita.barbosa@chvng.min-saude.pt.
Margarida Mota, Email: mmota@chvng.min-saude.pt.
Fernando Rosas Vieira, Email: rvieira@chvng.min-saude.pt.
References
- 1.Zhang J., Nguyen D., Hu K.Q. Chronic hepatitis C virus infection: a review of current direct-acting antiviral treatment strategies. N Am J Med Sci (Boston) 2016;9:47–54. [PMC free article] [PubMed] [Google Scholar]
- 2.Marcellin P., Boyer N., Gervais A. Long-term histologic improvement and loss of detectable intrahepatic HCV RNA in patients with chronic hepatitis C and sustained response to interferon‐alpha therapy. Ann Intern Med. 1997;127:875–881. doi: 10.7326/0003-4819-127-10-199711150-00003. [DOI] [PubMed] [Google Scholar]
- 3.Shiffman M.L., Hofmann C.M., Thompson E.B. Relationship between biochemical, virological, and histological response during interferon treatment of chronic hepatitis C. Hepatology. 1997;26:780–785. doi: 10.1002/hep.510260335. [DOI] [PubMed] [Google Scholar]
- 4.Shiratori Y., Imazeki F., Moriyama M. Histologic improvement of fibrosis in patients with hepatitis C who have sustained response to interferon therapy. Ann Intern Med. 2000;132:517–524. doi: 10.7326/0003-4819-132-7-200004040-00002. [DOI] [PubMed] [Google Scholar]
- 5.Bruno S., Di Marco V., Iavarone M. Improved survival of patients with hepatocellular carcinoma and compensated hepatitis C virusrelated cirrhosis who attained sustained virological response. Liver Int. 2017;37:1526–1534. doi: 10.1111/liv.13452. [DOI] [PubMed] [Google Scholar]
- 6.Nishiguchi S., Kuroki T., Nakatani S. Randomised trial of effects of interferon-alpha on incidence of hepatocelular carcinoma in chronic active hepatitis C with cirrhosis. Lancet. 1995;346:1051–1055. doi: 10.1016/s0140-6736(95)91739-x. [DOI] [PubMed] [Google Scholar]
- 7.El-Serag H.B. Epidemiology of viral hepatitis and hepatocelular carcinoma. Gastroenterology. 2012;142:1264–1273. doi: 10.1053/j.gastro.2011.12.061. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruix J., Sherman M. American association for the study of liver diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruno S., Di Marco V., Iavarone M. Improved survival of patients with hepatocellular carcinoma and compensated hepatitis C virusrelated cirrhosis who attained sustained virological response. Liver Int. 2017;37:1526–1534. doi: 10.1111/liv.13452. [DOI] [PubMed] [Google Scholar]
- 10.Reig M., Mariño Z., Perelló C. Unexpected high rate of early tumor recurrence in patients with HCV-related HCC undergoing interferon-free therapy. J Hepatol. 2016;65:719–726. doi: 10.1016/j.jhep.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 11.Kanda T., Matsuoka S., Moriyama M. Early occurrence and recurrence of hepatocellular carcinoma in hepatitis C virus-infected patients after sustained virological response. Hepatol Int. 2018;12:90–93. doi: 10.1007/s12072-018-9862-1. [DOI] [PubMed] [Google Scholar]
- 12.Chu P.S., Nakamoto N., Taniki N. On-treatment decrease of NKG2D correlates to early emergence of clinically evident hepatocellular carcinoma after interferon-free therapy for chronic hepatitis C. PLoS One. 2017;12:e0179096. doi: 10.1371/journal.pone.0179096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Villani R., Facciorusso A., Bellanti F. DAAs rapidly reduce inflammation but increase serum VEGF level: a rationale for tumor risk during anti-HCV treatment. PLoS One. 2016;11:e0167934. doi: 10.1371/journal.pone.0167934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Faillaci F., Marzi L., Critelli R. Liver Angiopoietin-2 is a key predictor of de novo or recurrent hepatocelular cancer after HCV direct-acting antivirals. Hepatology. 2018 doi: 10.1002/hep.29911. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guarino M., Sessa A., Cossiga V. On behalf of the Special Interest Group on “hepatocellular carcinoma and new anti-HCV therapies” of the Italian Association for the Study of the Liver. Direct-acting antivirals and hepatocellular carcinoma in chronic hepatitis C: a few lights and many shadows. World J Gastroenterol. 2018;24(24):2582–2595. doi: 10.3748/wjg.v24.i24.2582. [DOI] [PMC free article] [PubMed] [Google Scholar]