Abstract
A 51-year-old previously healthy woman presenting with two-weeks of fever, flu-like symptoms, jaundice, and abdominal pain was found to have pancytopenia, transaminitis, and significantly elevated ferritin in the setting of an Epstein-Barr Virus (EBV) infection. Bone marrow biopsy revealed phagocytic macrophages consistent with findings of hemophagocytic lymphohistiocytosis (HLH). Given bone marrow findings and that the patient had five of the eight clinical criteria supporting the diagnosis of HLH, chemotherapy was initiated as per the HLH-94 protocol with initial improvement in patient’s symptoms and overall functional status. This case demonstrates a classic presentation of HLH and displays the importance of correct diagnosis and prompt treatment.
Keywords: HLH, Acute EBV infection, Acute liver failure, Cytokine storm
Introduction
Since its first description in 1952 as a “familial hemophagocytic reticulosis”, hemophagocytic lymphohistiocytosis (HLH) has emerged as a novel and rare disorder with two primary types—familial and secondary or acquired. Acquired HLH is extremely rare with an incidence of 1.2 per 1,000,000 individuals. Characterized as a cytokine storm disorder, secondary HLH is believed to develop following a “two-hit” model. As an extremely heterogeneous disorder, many conditions and exposures can lead to HLH—infectious, malignant, metabolic, rheumatologic, or autoimmune. We present a case of a 51-year-old woman with a history of lymphopenia (first hit) that developed HLH following acute Epstein-Barr Virus (EBV) infection (second hit).
Case presentation
A 51-year-old woman with a past medical history significant for osteoporosis and a one-year history of lymphopenia presented with two-weeks of flu-like symptoms and fevers. The lymphopenia had been monitored with serial complete blood counts for one year, although no bone marrow biopsy was done at that time. The patient also had a family history of hemochromatosis (maternal grandmother). She presented to a tertiary center as a hospital transfer for further management of acute EBV viral infection with transaminitis, fever, and pancytopenia. Prior to presentation, she noted two-weeks of symptoms of lethargy, flu-like symptoms, fever, loss of appetite (fifteen-pound weight loss), nausea and vomiting (non-bilious, non-bloody), and mid-epigastric and dull right upper quadrant pain. The patient had no sick contacts, no history of injection drug use, and was in a heterosexual monogamous relationship. She had recent travel to the Bahamas on a cruise three-months prior to illness. Upon further discussion, the patient also revealed that she had seen a hematologist as an outpatient one-year prior for lymphopenia without any reported diagnosis or findings. She had never been a consumer of tobacco and consumed alcohol rarely in social settings. The patient lived in an urban part of the Southeastern United States. She reported no history of rashes or dermatologic conditions. With development and progression of symptoms, memory loss and cognitive slowing were also noted.
The patient was admitted for further evaluation of acute liver failure. At the time of admission, she was afebrile (36.6°C), noticeably jaundiced with scleral icterus. She had no other significant findings on physical examination. She was found to be pancytopenic (WBCs of 1.4 × 103/mm3, (reference range: 4–10 × 103/mm3), hemoglobin of 10.3 g/dL (reference range: 12–16 g/dL), and platelets of 68 × 103/mm3 (reference range: 150–400 × 103/mm3)) with grossly elevated ferritin (51,750 ng/mL, reference range: 11–307 ng/mL), elevated triglycerides (427 ng/mL, reference range: 55–149 ng/mL), transaminitis (AST/ALT of 647/194 IU/L, reference range: 0–37/0-35 IU/L), hyperbilirubinemia (direct/total of 7.9/10.1 mg/dL, reference range: 0.0-0.2/0.0–1.0 mg/dL) and with negative hepatitis A IgM, hepatitis B core antibody, hepatitis B surface antibody, hepatitis B surface antigen, and hepatitis C antibody and viral PCR. Ebstein Barr virus (EBV) viral capsid antigen IgG was positive and EBV nuclear antigen antibody was negative. An EBV DNA PCR was also positive at 39 copies/100 × 103 WBCs. These findings confirmed the presence of acute EBV infection. Interleukin-2 (CD25) was elevated (15,790 pg/mL, reference range: <1033 pg/mL). See Fig. 1 for further relevant laboratory studies.
Fig. 1.
Relevant Laboratory Studies.
HLH was considered the leading diagnosis with the findings of extremely elevated ferritin level, hypertriglyceridemia, and pancytopenia. Bone marrow biopsy was performed and preliminary results demonstrated hypercellular marrow (80–90% cellularity), decreased presence of T-cells, and increased numbers of macrophages with evidence of hemophagocytosis. Given the high clinical suspicion for HLH and the urgency for early treatment for this condition, the patient underwent treatment protocol as per the HLH-94 guideline without delay [1].The regimen consisted of etoposide and dexamethasone with eight cycles of therapy. Etoposide dosing had to be adjusted due to transaminitis. Final bone marrow results showed typical findings of HLH, most notably multiple macrophages engulfing and phagocytizing red blood cells (Fig. 2, Fig. 4, Fig. 5) [2]. After treatment was initiated, the patient’s repeat bone marrow biopsy showed continued presence of phagocytic macrophages. As a result, allogenic bone marrow transplant with alemtuzumab (a monoclonal antibody that binds CD52, a protein present on mature lymphocytes) as a bridge was considered. However, due to her clinical deterioration and repeated hospitalizations, she was not considered a suitable bone marrow transplant candidate and was provided palliative care as per the family’s wishes.
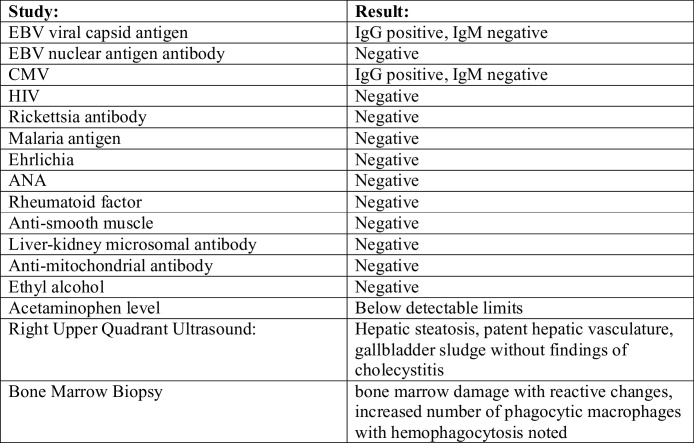
Fig. 2.
Bone marrow aspirate smear showing hemophagocytosis, macrophage is engulfing nucleated Red blood cell (BMA, 100×).
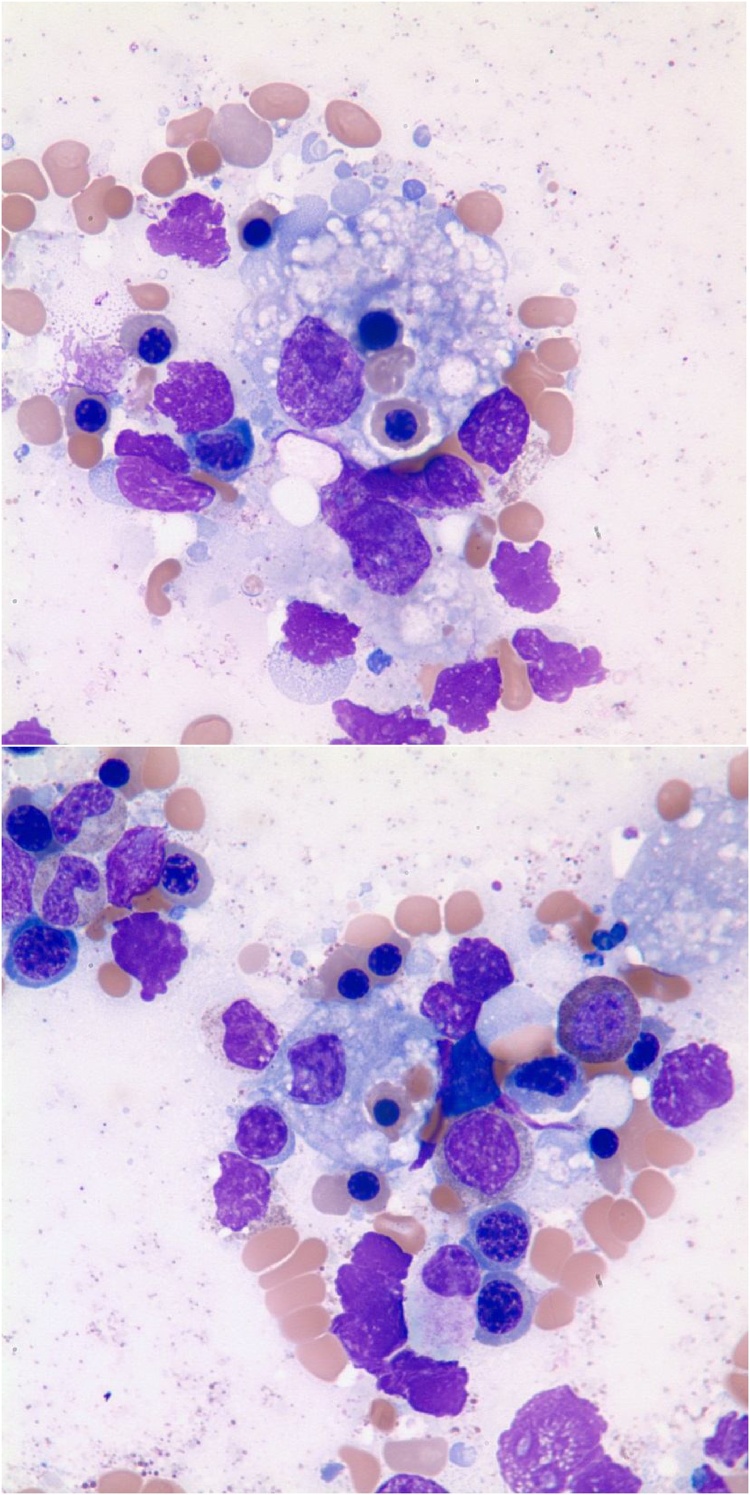
Fig. 4.
Bone marrow biopsy showing CD68 positive macrophages engulfing nucleated red blood cells (CD68, 60×).
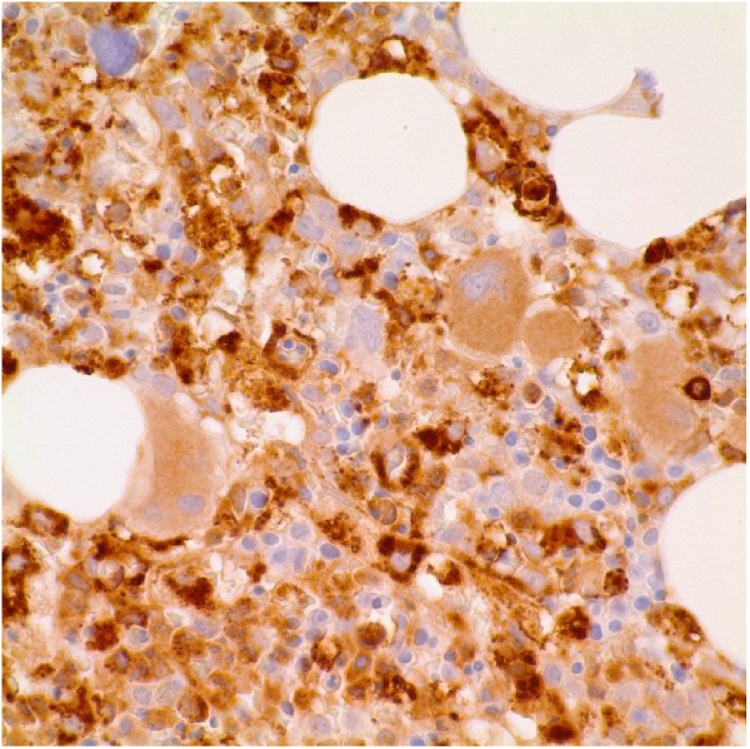
Fig. 5.
Bone marrow biopsy image showing trilineage hematopoiesis and increased macrophages (H&E, 10×).
Discussion
HLH is a rare condition that is often difficult to recognize early enough to provide effective treatment. It presents with non-specific findings of systemic inflammatory response. The syndrome typically is a consequence of an inciting factor that produces significant immune response—infectious, malignant, or autoimmune. Fundamentally, HLH is driven by out of control immune activation and subsequent tissue damage, in what is classified as a cytokine storm disorder [3]. Similar to many hematologic-oncologic pathologies, there is believed to be a “two-hit” mechanism to the development of HLH. In this particular case, the development of HLH was thought to be secondary to a first hit of lymphopenia followed by an acute EBV infection. EBV and HIV are the leading infectious causes of HLH [4]. Acute EBV infection was confirmed by positive EBV DNA PCR, EBV viral capsid antigen IgG but negative nuclear antigen IgG, confirming acute infection of less than three-months. If diagnosis is not recognized and prompt treatment not initiated, progressive multiple organ system failure and death results within months. However, with the chemotherapeutic standard of care, 54% of patients were shown to survive at a median follow-up of six-years. This data was gathered from the largest study to date, representing a study population of 249-patients, 227 of which were followed for a minimum of 5-years [5].
Conclusion
It is important for general medicine providers and specialists alike to consider HLH in the differential diagnosis of patients presenting with findings similar to our patient. In particular, HLH should be suspected and hematology oncology promptly consulted in patients presenting with cytopenia in at least two cell lines, with hypertriglyceridemia, and an extremely elevated ferritin [6,7]. Diagnosis is confirmed by bone marrow biopsy revealing phagocytic macrophages with hemophagocytosis. Typical precipitators of acquired HLH are infection (EBV, CMV, HSV), malignancy, or autoimmune disorders [8]. The mainstay of treatment is multiple cycles of chemotherapy with survival at five-years of approximately 50%.
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
References
- 1.Henter J.I., Samuelsson-Horne A., Aricò M., Egeler R.M., Elinder G., Filipovich A.H. Treatment of hemophagocytic lymphohistiocytosis with HLH-94 immunochemotherapy and bone marrow transplantation. Blood. 2002;100(October (7)):2367–2373. doi: 10.1182/blood-2002-01-0172. PubMed PMID: 12239144. [DOI] [PubMed] [Google Scholar]
- 2.Larroche C. Hemophagocytic lymphohistiocytosis in adults: diagnosis and treatment. Jt Bone Spine. 2012;79(4):356–361. doi: 10.1016/j.jbspin.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 3.Trottestam H., Horne A., Aricò M., Egeler R.M., Filipovich A.H., Gadner H. Chemoimmunotherapy for hemophagocytic lymphohistiocytosis: long-term results of the HLH-94 treatment protocol. Blood. 2011;118(October (17)):4577–4584. doi: 10.1182/blood-2011-06-356261. Epub 2011 Sep 6. PubMed PMID: 21900192; PubMed Central PMCID: PMC3208276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Filipovich A., Mcclain K., Grom A. Histiocytic disorders: recent insights into pathophysiology and practical guidelines. Biol Blood Marrow Transpl. 2010;16(1) doi: 10.1016/j.bbmt.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 5.Egeler R.M., Shapiro R., Loechelt B., Filipovich A. Characteristic immune abnormalities in hemophagocytic lymphohistiocytosis. J Pediatr Hematol Oncol. 1996;18(4):340–345. doi: 10.1097/00043426-199611000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Miranda R.N., Khoury J.D., Medeiros L.J. Atlas lymph node pathology. 2013. Hemophagocytic lymphohistiocytosis/hemophagocytic syndromes; pp. 133–137. [Google Scholar]
- 7.Otrock Z.K., Eby C.S. Clinical characteristics, prognostic factors, and outcomes of adult patients with hemophagocytic lymphohistiocytosis. Am J Hematol. 2015;90(3):220–224. doi: 10.1002/ajh.23911. [DOI] [PubMed] [Google Scholar]
- 8.Hashemi-Sadraei N., Vejpongsa P., Baljevic M., Chen L., Idowu M. Epstein-Barr virus-related hemophagocytic lymphohistiocytosis: hematologic emergency in the critical care setting. Case Rep Hematol. 2015;2015:1–6. doi: 10.1155/2015/491567. [DOI] [PMC free article] [PubMed] [Google Scholar]