Abstract
Lentiviral vectors have emerged as an efficient, safe therapeutic tool for gene therapy based on hematopoietic stem cells (HSCs) or T cells. However, the monitoring of transduced cells in preclinical models remains challenging because of the inefficient transduction of murine primary T cells with lentiviral vectors, in contrast to gammaretroviral vectors. The use of this later in preclinical proof of concept is not considered as relevant when a lentiviral vector will be used in a clinical trial. Hence, there is an urgent need to develop an efficient transduction protocol for murine cells with lentiviral vectors. Here, we describe an optimized protocol in which a nontoxic transduction enhancer (Lentiboost) enables the efficient transduction of primary murine T cells with lentiviral vectors. The optimized protocol combines low toxicity and high transduction efficiency. We achieved a high-level transduction of murine CD4+ and CD8+ T cells with a VSV-G-pseudotyped lentiviral vector with no changes in the phenotypes of transduced T cells, which were stable and long-lived in culture. This enhancer also increased the transduction of murine HSCs. Hence, use of this new transduction enhancer overcomes the limitations of lentiviral vectors in preclinical experiments and should facilitate the translation of strategies based on lentiviral vectors from the bench to the clinic.
Keywords: murine CD4+ T cells, murine CD8+ T cells, murine Sca1+ cells, lentiviral transduction, Lentiboost, gene therapy
Introduction
Gene modification of human T cells has provided remarkable clinical outcomes in cancer immunotherapy.1 Retargeting the specificity of T cells via the expression of a chimeric antigen receptor has drastically improved the prognosis for patients with malignant diseases.2, 3 In this regard, lentiviral vectors are suitable tools for achieving efficient gene transfer. These vectors are able to transduce a broad range of non-dividing cells.4, 5 Improved safety features, such as the deletion of 3′ viral long terminal repeats6 and the production of third-generation constructs,7 have made lentiviral vectors safe virus-based gene delivery tools.
Recently published preclinical data suggest that lentiviral vector-based T cell gene therapy might be applicable to other inherited and acquired T lymphocyte diseases, such as familial hemophagocytic lymphohistiocytosis types 28 and 39 immunodysregulation, polyendocrinopathy, enteropathy, X-linked syndrome,10 and HIV infection.11 A prerequisite for the efficient achievement of this goal in the clinic is a better understanding of the in vivo cell biology of gene-modified T cells. The efficacy of gene transfer in human T cells can be evaluated mostly through in vitro test, while an in vivo model, like xenograft, in immunodeficient animals does not allow a comprehensive evaluation of long-term efficacy and toxicity. Therefore, appropriate preclinical murine models are often required to validate the safety and efficacy of lentiviral vectors. However, in contrast to the situation with human T cells, lentiviral vector-based gene transfer into murine T cells is hampered by low transduction efficiency and inadequate expansion of the transduced T cells ex vivo.12, 13 The latter problem is due (at least in part) to post-entry blockade at and after the reverse transcription step in murine T cells.12 In an effort to bypass these obstacles, some researchers have transduced murine T cells with gamma retroviral vectors.13, 14, 15, 16 Although this type of transduction is relatively efficient, its use in preclinical proof-of-concept studies is not relevant when a lentiviral vector will be used in a clinical trial, based on the fact that these vectors do not have the same efficacy and integration profiles.
An alternative strategy for efficient gene transfer into target cells involves the addition of transduction-promoting polycations, such as polybrene and protamine sulfate (PS).17, 18 However most of these enhancers have toxic effects that limit their use. For example, polybrene is a widely used polycationic enhancer for lentiviral transduction but disrupts the transmembrane potential in some sensitive cells.19 PS is less toxic than polybrene but does not enhance the lentiviral vector-based transduction of primary murine T cells.13 However, the non-ionic, amphiphilic poloxamer synperonic F108 (Lentiboost) has been shown to outperform polybrene in cell transduction experiments with lentiviral vectors.20 By interacting with lipid membranes, poloxamers decrease membrane microviscosity, increase lipid exchange, and enhance transmembrane transport.21, 22 On this basis, we wondered whether the addition of Lentiboost to the cell culture would overcome the lentiviral vectors’ poor ability to transduce murine T cells. Here, we assess the efficiency of Lentiboost versus PS.
Use of Lentiboost resulted in high-level transduction of murine T cells and consistently efficient transgene expression, with no signs of toxicity. Since gene transfer studies are also classically performed with hematopoietic stem cells (HSCs), we looked at whether Lentiboost could enhance the lentiviral vectors’ ability to transduce murine HSCs.
Results
Lentiboost Enables the Efficient Transduction of Primary CD4+ and CD8+ Murine T Cells
We generated a vesicular stomatitis virus G (VSV-G)-pseudotyped lentiviral vector expressing a truncated version of low-affinity nerve growth factor (ΔLNGFR) under the control of a phosphoglycerate kinase 1 (PGK) promoter (Figure 1). Next, we compared three different doses of Lentiboost (0.1, 0.25, and 0.5 mg/mL) and the optimal dose to enhance the transduction of primary murine T cells of a conventional reagent (PS 4 μg/mL).
Figure 1.
The Vector Map
A self-inactivated (SIN) lentiviral vector was designed using a pCCL backbone, allowing the expression of a reporter protein, ΔLNGFR, a truncated form of LNGFR, under the control of PGK promoter and in the presence of a woodchuck hepatitis virus post-transcriptional regulatory element (WPRE).
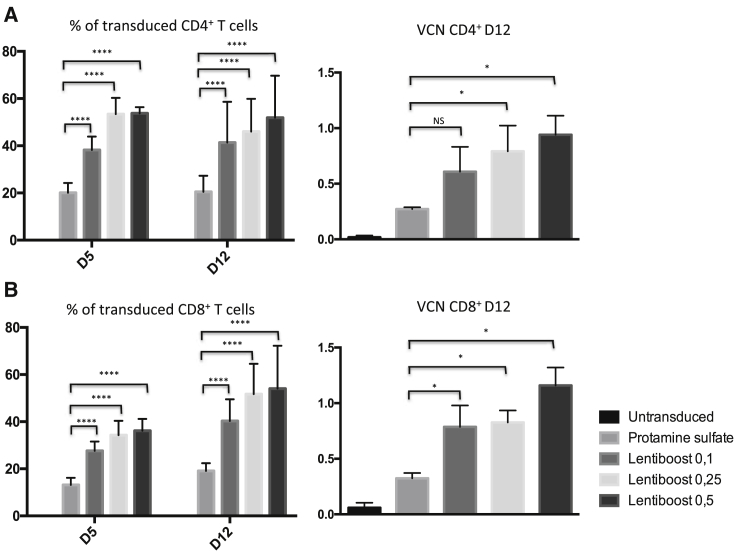
Freshly isolated murine CD4+ and CD8+ T cells from the spleen of C57BL/6 mice were stimulated and concomitantly transduced with LNGFR-lentiviral vector at a multiplicity of infection (MOI) of 10. Transduction efficiency was evaluated in three independent experiments on day 5 and again on day 12 post-transduction. As expected, the mean ± SEM proportion of transduced cells in the presence of PS was low and did not exceed 20 ± 4% for CD4+ T cells and 13 ± 3% for CD8+ T cells on day 5 (Figures 2A and 2B, left graphs). In contrast, Lentiboost outperformed PS by enhancing the transduction of both CD4+ and CD8+ cells—even at the lowest dose (0.1 mg/mL). The transduction efficiency rose 54 ± 3% in CD4+ cells and 36 ± 5% in CD8+ cells in the presence of 0.5 mg/mL Lentiboost on day 5. The more efficient transduction translated into a significantly greater total number of transduced cells on day 5 with Lentiboost at doses of 0.25 mg/mL (2.7-fold higher than with PS) and 0.5 mg/mL (2.7-fold higher than with PS) (Figures S1A and SB). The superiority of Lentiboost was confirmed by calculating the odds ratio (OR) for transduction; the highest ORs were 4.6 for 0.25 mg/mL Lentiboost in CD4+ T cells and 3.6 for 0.25 and 0.5 mg/mL Lentiboost in CD8+ T cells.
Figure 2.
Optimization of Lentiviral Gene Transfer in CD4+ and CD8+ T Cells
(A and B) Transduction efficiency was evaluated on days 5 (D5) and 12 (D12). The transduction efficiency for CD4+ (A) and CD8+ (B) T lymphocytes are shown, with the percentage of transduced cells in the left-hand panels and the VCN on day 12 in the right-hand panels. The data correspond to three independent experiments and are represented as the mean ± SEM. **** p < 0.0001, calculated using a mixed logistic regression model, compared to PS for transduction percentage; *p < 0.05, calculated with a permutation model.
Importantly, Lentiboost did not induce any cell toxicity or mortality at the three tested doses; fewer than 5% of the cells were 7-aminoactinomycine D (7-AAD)+ on days 5 and 12 post-transduction (data not shown). Transduced cells survived in culture for up to 12 days post-transduction, and the proportion of live cells was always higher in Lentiboost experiments than in PS experiments.
The Lentiboost-induced increase in the percentage of transduced cells was accompanied by an increase in the integrated vector copy number (VCN), which was 0.3 for CD4+ and CD8+ cells with PS, and significantly higher with 0.5 mg/mL Lentiboost with 0.9 for CD4+ cells, and 1.2 for CD8+ (p = 0.048 in a permutation test; Figures 2A and 2B, right graphs).
Addition of Lentiboost to the Culture Medium Did Not Change the T Cells’ Phenotype
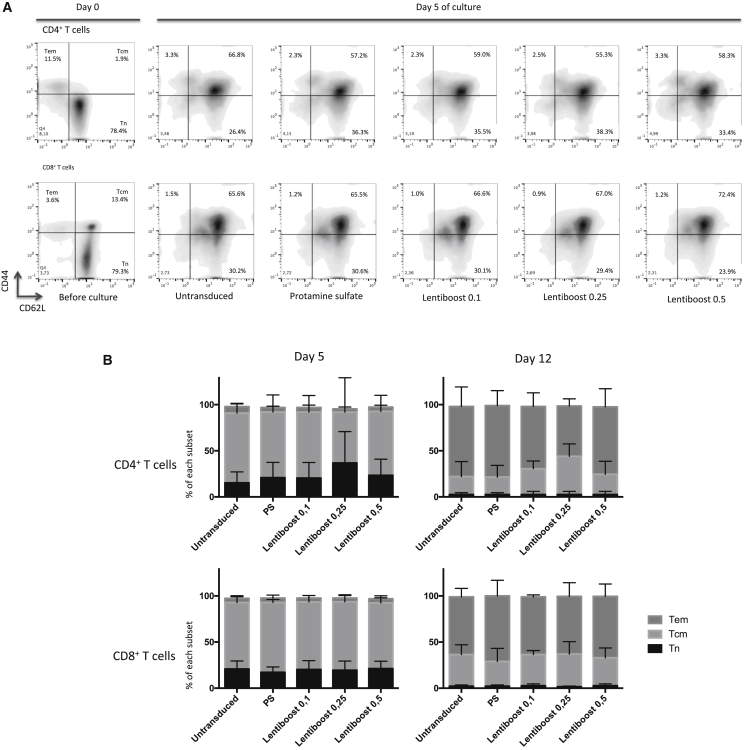
We next checked whether the addition of transduction enhancers to the culture medium induced changes in the naïve and memory phenotypes of transduced and non-transduced cells. The purified populations of both CD4+ and CD8+ T cells from the spleen were mainly constituted of naive and stem memory T cells (TN/SCM, CD62Lhi/CD44lo) (Figure 3A, day 0). Five days after transduction, most of the TN/SCM had differentiated into central memory T cells (TCM, CD62Lhi/CD44hi). There was no difference between the non-transduced and the Lentiboost or PS culture conditions in this respect. Interestingly, a small proportion of TN/SCM could still be detected after the transduction step (Figures 3A and 3B). After prolonged culture (up to day 12), an increase in the frequency of the effector memory phenotype (effector memory T cell [TEM], CD62Llo/CD44hi) was observed under all conditions (Figure 3B, right panel). Thus, transduction in the presence of Lentiboost did not change the T cells’ phenotype relative to non-transduced cells.
Figure 3.
Characterization of CD4+ and CD8+ T Lymphocytes
(A) shows the CD4+ and CD8+ T cells’ phenotype before culture and on day 5 in all five transduction settings on representative fluorescence-activated cell sorting (FACS) plot. Proportion of naive T cells (Tn), effector memory T cells (Tem), and central memory T cells (Tcm) were analyzed using the CD44 and CD62L markers. (B) The proportions of the different CD4+ and CD8+ subpopulations in all three experiments are shown after 5 days (left panels) or 12 days (right panels) of culture. No statistically significant difference was observed in the percentages of different T cell subpopulations between different conditions. We analyzed the following subpopulation: CD62L+CD44− T cells corresponding to TN/SCM for naive and stem memory T cell; CD62L+CD44+ corresponding to TCM for central memory T cell; and CD62L−CD44+ corresponding to TEM for effector memory T cell.
Lentiboost Enables the Highly Efficient Transduction of Murine Sca1+ Progenitor Cells
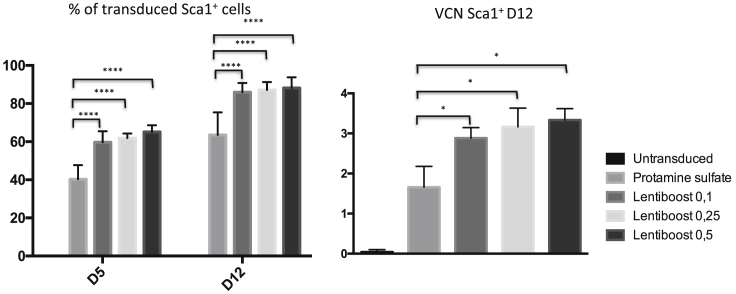
Murine Sca1+ hematopoietic stem and progenitor cells are often transduced as part of proof-of-concept studies for HSC-based gene therapy. Although these Sca1+ cells are more lentiviral vector-permissive than murine T cells, we decided to test Lentiboost’s ability to enhance gene transfer into this progenitor population. To this end, we transduced sorted, bone-marrow-derived Sca1+ cells from wild-type mice in three independent experiments with a LNGFR-lentiviral vector (MOI = 100) after 24 hours of cell activation. As shown in Figure 4, we observed a significant increase in transduction efficiency in the presence of Lentiboost, with a value of 65% at 0.5 mg/mL, compared to PS, with 40%, at day 5. When combined with the greater proportion of transduced cells, this enhancement yielded a significantly greater total number of transduced cells on day 5 with Lentiboost at all doses (Figure S1C). The highest OR (2.6) was achieved with 0.5 mg/mL Lentiboost. There were no significant differences between the three doses tested. Consistently, the mean ± SEM was not significantly different. VCN was significantly higher for Lentiboost (2.9 ± 0.3 at 0.1 mg/mL, 3.2 ± 0.5 at 0.25 mg/mL, and 3.3 ± 0.3 at 0.5 mg/mL) than for PS (1.7 ± 0.5) (p = 0.048 in a permutation test). The cell viability was above 80% under all conditions (data not shown). We conclude that exposure to Lentiboost during the transduction of Sca1+ murine progenitors enhances gene transfer efficacy.
Figure 4.
Optimization of Lentiviral Gene Transfer in Sca1+ Cells
Transduction efficiency was evaluated on days 5 and 12, with the proportion of transduced cells in the left-hand panel and the VCN on day 12 in the right-hand panel. The data correspond to three independent experiments and are represented as the mean ± SEM. ****p < 0.0001, calculated using a mixed logistic regression model, compared to PS for transduction percentage; *p > 0.05, calculated with a permutation model.
Discussion
The inefficient targeting of murine T cells by lentiviral vectors has hindered preclinical proof-of-concept studies in vivo of T cell-based gene therapy. Our present results demonstrate that the addition of Lentiboost enhances the lentiviral transduction of both murine CD4+ and CD8+ T cells in terms of both the proportion of targeted cells and the integrated VCN. Furthermore, the presence of Lentiboost did not alter the T cell’s viability, expansion, or phenotype, indicating a lack of toxicity for these cells. The addition of Lentiboost even enhanced the transduction efficiency (again in terms of the proportion of ΔLNGFR-expressing cells and the integrated VCN) for Sca1+ hematopoietic progenitor cells where baseline levels of transduction may already appear to be sufficient. Although the present study focused on gene delivery into T cells, we anticipate that Lentiboost will enhance many other gene transfer applications in human and murine settings.
As with murine T cells, human T cells were also found to be refractory to VSV-G lentiviral vector transduction, especially when in a quiescent state. This problem has been solved by engineering alternative lentiviral envelopes, such as human measles virus hemagglutinin and fusion (H/F) glycoprotein-pseudotyped lentiviral vectors.23, 24, 25, 26 However, our unsuccessful experiments with an H/F-pseudotyped lentiviral vector (data not shown) suggest that the latter cannot target murine cells.
The efficiency of primary cell transduction with viral vectors is partly determined by the expression levels of the corresponding cell surface receptor. Recently, the low-density lipoprotein receptor (LDL-R) has been identified as the human cell receptor for VSV-G.27 It has been shown that the expression level of LDL-R directly impacts the transduction efficiency for human T lymphocytes using a VSV-G lentiviral vector. The expression level of LDL-R on murine T cells has yet to be unambiguously established, and it remains to be shown that VSV-G-pseudotyped vectors also use the LDL-R on murine cells; it is possible that the VSV-G vector uses a different entry port on murine cells. Independently of the entry mechanism, the intercalation of Lentiboost monomers into the cell membrane may increase membrane permeability and thus facilitate the membrane transport of viral particles, as previously demonstrated by Höfig et al.20
Moreover, differences downstream in the lentiviral vector transduction pathway after the initial binding of the envelope protein to its cognate receptor may account for the inefficient targeting of murine lymphocytes. It has been shown that the amount of late transcription product, the nuclear transfer of pre-integration complexes of HIV-1 virus, and virus integration are significantly lower in murine T cells than in murine fibroblasts.28, 29 This blockade was not saturable at high virus concentrations.12 Based on our observation of successful lentiviral transduction and integration in the presence of Lentiboost, we hypothesize that this enhancer protects viral complexes, even at post-entry steps (e.g., nuclear transfer and integration).
Like Lentiboost, other cationic additives (such as polybrene and PS) enhance transduction efficiency by neutralizing the target cell’s membrane charges and thus promoting vector adhesion.17, 18 Polybrene is toxic for cell proliferation, and our present results demonstrate that Lentiboost outperforms PS in murine T lymphocyte transduction.
The only previously reported protocol for efficient lentiviral transduction in murine T cells featured a 48-hr activation prior to transduction with spinoculation step.30 Although this strategy has not been further validated in preclinical (murine) T cell gene transfer studies, spinoculation may increase the transduction efficiency. However, spinoculation is likely to dramatically decrease the survival of sensitive T cells. Furthermore, combining the transduction and activation steps, as suggested here, shortens the culture period prior to the use of transduced cells in in vivo studies and thus may better preserve the TN/SCM population.
Another advantage of using Lentiboost is its good aqueous solubility, which would facilitate its use in a fully automated transduction process under good manufacturing practice (GMP) conditions. Moreover, poloxamer complexes for therapeutic drug delivery have already been studied in animal models; they display low toxicity when injected intravenously, intraperitoneally, or subcutaneously.31 Accordingly, we suggest that the use of Lentiboost in preclinical mouse models is clinically relevant.
In conclusion, we established a clinically relevant protocol for the stable transduction of murine T lymphocytes ex vivo. This step should facilitate the translation of T cell-based gene therapy strategies from the bench to the clinic. Furthermore, our results suggest that the use of Lentiboost can be extended to other primary cell types (such as HSCs) and might improve the performance level of lentiviral gene transfer strategies.
Materials and Methods
Lentiviral Construction
The cDNA for a truncated human codon-optimized ΔLNGFR was cloned into a self-inactivating lentiviral vector on a pCCL backbone, downstream of a PGK promoter.
Primary T Cell Culture
Splenocytes were collected from C57/BL6J mice (Jackson Laboratory). CD4+ and CD8+ T lymphocytes were prepared using magnetic sorting (CD4+ T cells, CD8+ T cells, Miltenyi Biotec) CD4+ and CD8+ T cells were cultured in RPMI media supplemented with Glutamax (Invitrogen), 10% fetal bovine serum (GIBCO, Thermo Fisher Scientific), 1% penicillin-streptomycin (GIBCO, Thermo Fisher Scientific), 0.1% β-mercaptoethanol (GIBCO, Thermo Fisher Scientific), and recombinant murine interleukin-2 (IL-2; Peprotech) at a concentration of 100 U/mL.
T Cell Transduction
CD4+ and CD8+ T cells were activated and expanded using anti-CD3/CD28 mouse Dynabeads (GIBCO, Thermo Fisher Scientific) at a 1:1 ratio on day 0 and day 9 of culture. Transduction was performed overnight at a MOI of 10 concomitantly with activation. The transduction medium was complete RPMI supplemented with 4 μg/mL PS (Sanofi-Aventis) or Lentiboost (Sirion Biotech) at a concentration of 0.10, 0.25, or 0.50 mg/mL. Transduction was stopped by adding fresh culture medium.
Sca1+ HSC Sorting and Transduction
Bone marrow cells were harvested from femurs and tibias. Sca1+ progenitor cells were isolated using an anti-PE Microbeads kit (Miltenyi Biotec). The Sca1+ progenitors were prestimulated overnight in StemSpan SFEM medium (StemCell Technologies) supplemented with 5% fetal bovine serum (FBS for mouse B lymphoid, StemCell Technologies), 1% gentamycin (GIBCO) with murine cytokines (100 ng/μL SCF, 100 ng/μL FLT3-L, 100 ng/μL TPO, 50 ng/μL IL-6 and 10 ng/μL IL-11 [all from Peprotech]), and then transduced at a MOI of 100 with LNGFR-lentiviral vector in the presence of Lentiboost (0.1, 0.25, and 0.5 mg/mL) or PS (4 μg/mL) for 12 hr. Transduction was stopped by adding fresh culture medium.
Flow Cytometry
Transduced CD4+ and CD8+ T cells were stained with fluorescently labeled antibodies against mouse CD4 V500 and CD8 BV421 (respectively clone RM4-5, ref. 1102765, and clone 53-6.7, ref. 1103690, from Sony Biotechnology); CD44 APC (clone IM7, ref. 559250), CD62L allophycocyanin (APC)-Cy7 (clone MEL-14, ref. 560514), and Sca-1 PE (clone D7, ref. 553108) (BD Biosciences); viability dye 7-aminoactinomycin D (7AAD); and LNGFR FITC Vio-Bright (REA648, ref. 130-110-115) (Miltenyi Biotec). Flow cytometry analysis was performed on a MACSQuant analyzer (Miltenyi Biotec).
VCN
Genomic DNA was isolated from transduced cells using a Genomic DNA Purification kit (QIAGEN). qRT-PCR was performed with TaqMan probes designed to detect a lentiviral sequence (Psi) and a sequence in the murine genome (Titin). Serial dilutions of a standard DNA plasmid containing one copy each of the Psi and Titin sequences were used to plot a standard curve. Samples and standard serial dilutions were run in duplicate.
Statistical Analysis
Statistical analyses were performed using GraphPad Prism version 6.00 for Windows (GraphPad Software, La Jolla, CA, USA; https://www.graphpad.com/) and R software. The Mann-Whitney test was used to probe quantitative data and coupled to permutation test for the VCN analysis. Contingency tables were built by taking the total number of events measured with flow cytometry. A chi-square test was used to probe the association between treatment with the enhancer and the level of transduction. To calculate the OR for transduction efficiency under the different conditions, a mixed logistic regression model was built using the lme4 package in R.
Author Contributions
Conceptualization, M.D., T.S., E.S., C.L.-P., M.C., I.A.-S.; Methodology M.D., T.S., E.S.; Investigation M.D., T.S., F.B., A. Durand; Writing original draft M.D., T.S., A. Denis; Writing review and editing M.D., T.S., E.S., M.C., I.A.-S.; Funding acquisition E.S., M.C., I.A.-S.; Supervision E.S., M.C., I.A.-S.
Conflicts of Interest
The authors declare no competing financial interests.
Acknowledgments
We thank Sirion Biotech GmbH for providing the Lentiboost reagent. We thank Gisèle Froment, Didier Nègre, and Caroline Costa from the lentiviral vector production facility/SFR BioSciences Gerland – Lyon Sud (UMS3444/US8). We also thank Olivier Pellé at the cytometry facility at SFR Necker. We thank the animal facility at the Institut Imagine. This study was funded by the French National Institute of Health and Medical Research (INSERM) and a European Research Council grant (ERC Regenerative Therapy 269037).
Footnotes
Supplemental Information includes one figure and can be found with this article online at https://doi.org/10.1016/j.omtm.2018.08.002.
Supplemental Information
References
- 1.Abate-Daga D., Davila M.L. CAR models: next-generation CAR modifications for enhanced T-cell function. Mol. Ther. Oncolytics. 2016;3:16014. doi: 10.1038/mto.2016.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morgan R.A., Boyerinas B. Genetic Modification of T Cells. Biomedicines. 2016;4:4. doi: 10.3390/biomedicines4020009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mirzaei H.R., Rodriguez A., Shepphird J., Brown C.E., Badie B. Chimeric Antigen Receptors T Cell Therapy in Solid Tumor: Challenges and Clinical Applications. Front. Immunol. 2017;8:1850. doi: 10.3389/fimmu.2017.01850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kafri T. Gene delivery by lentivirus vectors an overview. Methods Mol. Biol. 2004;246:367–390. doi: 10.1385/1-59259-650-9:367. [DOI] [PubMed] [Google Scholar]
- 5.Wong L.-F., Goodhead L., Prat C., Mitrophanous K.A., Kingsman S.M., Mazarakis N.D. Lentivirus-mediated gene transfer to the central nervous system: therapeutic and research applications. Hum. Gene Ther. 2006;17:1–9. doi: 10.1089/hum.2006.17.1. [DOI] [PubMed] [Google Scholar]
- 6.Zufferey R., Dull T., Mandel R.J., Bukovsky A., Quiroz D., Naldini L., Trono D. Self-inactivating lentivirus vector for safe and efficient in vivo gene delivery. J. Virol. 1998;72:9873–9880. doi: 10.1128/jvi.72.12.9873-9880.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dull T., Zufferey R., Kelly M., Mandel R.J., Nguyen M., Trono D., Naldini L. A third-generation lentivirus vector with a conditional packaging system. J. Virol. 1998;72:8463–8471. doi: 10.1128/jvi.72.11.8463-8471.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghosh S., Carmo M., Calero-Garcia M., Ricciardelli I., Bustamante Ogando J.C., Blundell M.P., Schambach A., Ashton-Rickardt P.G., Booth C., Ehl S. T-cell gene therapy for perforin deficiency corrects cytotoxicity defects and prevents hemophagocytic lymphohistiocytosis manifestations. J. Allergy Clin. Immunol. 2018 doi: 10.1016/j.jaci.2017.11.050. Published online January 31, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soheili T., Rivière J., Ricciardelli I., Durand A., Verhoeyen E., Derrien A.-C., Lagresle-Peyrou C., de Saint Basile G., Cosset F.L., Amrolia P. Gene-corrected human Munc13-4-deficient CD8+ T cells can efficiently restrict EBV-driven lymphoproliferation in immunodeficient mice. Blood. 2016;128:2859–2862. doi: 10.1182/blood-2016-07-729871. [DOI] [PubMed] [Google Scholar]
- 10.Passerini L., Rossi Mel E., Sartirana C., Fousteri G., Bondanza A., Naldini L., Roncarolo M.G., Bacchetta R. CD4+ T cells from IPEX patients convert into functional and stable regulatory T cells by FOXP3 gene transfer. Sci. Transl. Med. 2013;5:215ra174. doi: 10.1126/scitranslmed.3007320. [DOI] [PubMed] [Google Scholar]
- 11.Mautino M.R. Lentiviral vectors for gene therapy of HIV-1 infection. Curr. Gene Ther. 2002;2:23–43. doi: 10.2174/1566523023348165. [DOI] [PubMed] [Google Scholar]
- 12.Baumann J.G., Unutmaz D., Miller M.D., Breun S.K.J., Grill S.M., Mirro J., Littman D.R., Rein A., KewalRamani V.N. Murine T cells potently restrict human immunodeficiency virus infection. J. Virol. 2004;78:12537–12547. doi: 10.1128/JVI.78.22.12537-12547.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kerkar S.P., Sanchez-Perez L., Yang S., Borman Z.A., Muranski P., Ji Y., Chinnasamy D., Kaiser A.D., Hinrichs C.S., Klebanoff C.A. Genetic engineering of murine CD8+ and CD4+ T cells for preclinical adoptive immunotherapy studies. J. Immunother. 2011;34:343–352. doi: 10.1097/CJI.0b013e3182187600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee J., Sadelain M., Brentjens R. Retroviral transduction of murine primary T lymphocytes. Methods Mol. Biol. 2009;506:83–96. doi: 10.1007/978-1-59745-409-4_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang T., Tsang T.C., Harris D.T. Efficient transduction of murine primary T cells requires a combination of high viral titer, preferred tropism, and proper timing of transduction. J. Hematother. Stem Cell Res. 2003;12:123–130. doi: 10.1089/152581603321210208. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y., Joe G., Hexner E., Zhu J., Emerson S.G. Host-reactive CD8+ memory stem cells in graft-versus-host disease. Nat. Med. 2005;11:1299–1305. doi: 10.1038/nm1326. [DOI] [PubMed] [Google Scholar]
- 17.Cornetta K., Anderson W.F. Protamine sulfate as an effective alternative to polybrene in retroviral-mediated gene-transfer: implications for human gene therapy. J. Virol. Methods. 1989;23:187–194. doi: 10.1016/0166-0934(89)90132-8. [DOI] [PubMed] [Google Scholar]
- 18.Wurm M., Schambach A., Lindemann D., Hanenberg H., Ständker L., Forssmann W.-G., Blasczyk R., Horn P.A. The influence of semen-derived enhancer of virus infection on the efficiency of retroviral gene transfer. J. Gene Med. 2010;12:137–146. doi: 10.1002/jgm.1429. [DOI] [PubMed] [Google Scholar]
- 19.Lin P., Correa D., Lin Y., Caplan A.I. Polybrene inhibits human mesenchymal stem cell proliferation during lentiviral transduction. PLoS ONE. 2011;6:e23891. doi: 10.1371/journal.pone.0023891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Höfig I., Atkinson M.J., Mall S., Krackhardt A.M., Thirion C., Anastasov N. Poloxamer synperonic F108 improves cellular transduction with lentiviral vectors. J. Gene Med. 2012;14:549–560. doi: 10.1002/jgm.2653. [DOI] [PubMed] [Google Scholar]
- 21.Batrakova E.V., Li S., Vinogradov S.V., Alakhov V.Y., Miller D.W., Kabanov A.V. Mechanism of pluronic effect on P-glycoprotein efflux system in blood-brain barrier: contributions of energy depletion and membrane fluidization. J. Pharmacol. Exp. Ther. 2001;299:483–493. [PubMed] [Google Scholar]
- 22.Krylova O.O., Melik-Nubarov N.S., Badun G.A., Ksenofontov A.L., Menger F.M., Yaroslavov A.A. Pluronic L61 accelerates flip-flop and transbilayer doxorubicin permeation. Chemistry. 2003;9:3930–3936. doi: 10.1002/chem.200204621. [DOI] [PubMed] [Google Scholar]
- 23.Frecha C., Lévy C., Costa C., Nègre D., Amirache F., Buckland R., Russell S.J., Cosset F.L., Verhoeyen E. Measles virus glycoprotein-pseudotyped lentiviral vector-mediated gene transfer into quiescent lymphocytes requires binding to both SLAM and CD46 entry receptors. J. Virol. 2011;85:5975–5985. doi: 10.1128/JVI.00324-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frecha C., Costa C., Nègre D., Gauthier E., Russell S.J., Cosset F.-L., Verhoeyen E. Stable transduction of quiescent T cells without induction of cycle progression by a novel lentiviral vector pseudotyped with measles virus glycoproteins. Blood. 2008;112:4843–4852. doi: 10.1182/blood-2008-05-155945. [DOI] [PubMed] [Google Scholar]
- 25.Lévy C., Amirache F., Girard-Gagnepain A., Frecha C., Roman-Rodríguez F.J., Bernadin O., Costa C., Nègre D., Gutierrez-Guerrero A., Vranckx L.S. Measles virus envelope pseudotyped lentiviral vectors transduce quiescent human HSCs at an efficiency without precedent. Blood Adv. 2017;1:2088–2104. doi: 10.1182/bloodadvances.2017007773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maurice M., Verhoeyen E., Salmon P., Trono D., Russell S.J., Cosset F.-L. Efficient gene transfer into human primary blood lymphocytes by surface-engineered lentiviral vectors that display a T cell-activating polypeptide. Blood. 2002;99:2342–2350. doi: 10.1182/blood.v99.7.2342. [DOI] [PubMed] [Google Scholar]
- 27.Amirache F., Lévy C., Costa C., Mangeot P.-E., Torbett B.E., Wang C.X., Nègre D., Cosset F.L., Verhoeyen E. Mystery solved: VSV-G-LVs do not allow efficient gene transfer into unstimulated T cells, B cells, and HSCs because they lack the LDL receptor. Blood. 2014;123:1422–1424. doi: 10.1182/blood-2013-11-540641. [DOI] [PubMed] [Google Scholar]
- 28.Tervo H.-M., Goffinet C., Keppler O.T. Mouse T-cells restrict replication of human immunodeficiency virus at the level of integration. Retrovirology. 2008;5:58. doi: 10.1186/1742-4690-5-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsurutani N., Yasuda J., Yamamoto N., Choi B.-I., Kadoki M., Iwakura Y. Nuclear import of the preintegration complex is blocked upon infection by human immunodeficiency virus type 1 in mouse cells. J. Virol. 2007;81:677–688. doi: 10.1128/JVI.00870-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Costello E., Munoz M., Buetti E., Meylan P.R., Diggelmann H., Thali M. Gene transfer into stimulated and unstimulated T lymphocytes by HIV-1-derived lentiviral vectors. Gene Ther. 2000;7:596–604. doi: 10.1038/sj.gt.3301135. [DOI] [PubMed] [Google Scholar]
- 31.Pec E.A., Wout Z.G., Johnston T.P. Biological activity of urease formulated in poloxamer 407 after intraperitoneal injection in the rat. J. Pharm. Sci. 1992;81:626–630. doi: 10.1002/jps.2600810707. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.