Abstract
Most studies on the relationship between bone mineral density and atherosclerosis have used dual-energy X-ray absorptiometry, but this method is relatively insensitive to bone geometry. The aim of this study was to investigate the relationship between bone area and indices of carotid and peripheral atherosclerosis. We studied 841 persons aged 65 years or older (women = 444, mean age 73.8 years; men = 397, mean age = 75.3 years) enrolled in the InCHIANTI study and free from active malignancies, chronic use of bisphosphonates or steroids, and estrogen replacement therapy. The tibial cortical and total crosssectional area (CSA) were measured by peripheral quantitative computed tomography and their ratio was calculated (cortical/total cross-sectional area ratio, cCSA/tCSA); carotid plaques were screened by echography, and peripheral artery disease (PAD) was defined as an ankle/brachial index <0.9 or presence of intermittent claudication. No association between cCSA/tCSA and atherosclerosis was observed in men. In women, lower cCSA/tCSA was associated with both carotid plaques [odds ratio (OR) for lowest vs. best quartile = 2.09, 95 % confidence interval (CI) 1.2–3.68] and PAD (OR = 3.43, 95 % CI 1.58–8.12). After correction for potential confounders (age since menopause, body mass index, Parathyroid hormone, vitamin D, leptin, DHEA-S, testosterone, physical activity, chronic obstructive pulmonary disease, and reduced renal function), the association was not confirmed. According to partial logistic regression, the carotid plaque—cCSA/tCSA association, but not the PAD—cCSA/tCSA association, was mostly dependent on years since menopause. In women the association between osteoporosis and carotid plaques likely reflects hormonal deprivation, whereas that between osteoporosis and PAD seems multifactorial in origin.
Keywords: Bone cross-sectional area, Peripheral artery disease, Osteoporosis, Atherosclerosis, Women, Elderly
Introduction
Several epidemiologic data link a reduction of bone mineral density (BMD) with increased cardiovascular mortality in postmenopausal women [1]. At the basis of this relationship there may be an association between low BMD and atherosclerosis, which seems to be independent of age and years since menopause. It has been shown that women with osteopenia/osteoporosis are at higher risk of coronary calcifications and plaques [2, 3]. Low BMD has also been associated with atherosclerosis of carotid arteries [4–6], calcification of the abdominal aorta [3, 7], and increased arterial stiffness measured by pulse wave velocity [8]. All of these studies except one [6] estimated areal BMD (aBMD) using dual-energy X-ray absorptiometry (DXA), and all took into account age as a potential confounder.
A low BMD also seems to be related with peripheral artery disease (PAD). Farhat et al. [9] found that total-hip aBMD was associated with subclinical PAD in men but not in women. In this study, no association was found between vertebral volumetric BMD (vBMD) measured using computed tomography and PAD. Data from the Rotterdam Study, instead, showed an association between PAD and hip aBMD in women but not in men [10]. Finally, in the Rancho Bernardo study, the association between PAD and osteoporosis observed in women was not independent of age [11].
The association between BMD and atherosclerosis may be caused by shared risk factors [7, 12, 13]. However, the gender differences observed in almost all studies suggest that estrogen deprivation may play a pivotal role. Other important potential mediators of this association are vitamin D, which has both vasoprotective [14] and antireabsorptive [15] effects, and osteoprotegerin. This latter hormone prevents bone degradation and exerts an antiatherogenic effect in animal models [16], but higher levels have been found in vivo in people with severe atherosclerosis [17]. Thus, the bone—artery relationship seems to be based on complex metabolic processes that from an epidemiological point of view may be better understood by taking into account the cumulative hormone exposure and confounding factors. Indeed, some factors, such as physical activity and antioxidant intake, exert both vasoprotective and antireabsorptive effects, while others selectively affect bone or arterial structure and function. Furthermore, pharmacological interference, primarily by hormone replacement therapy (HRT) but also by several drugs affecting bone structure, deserve consideration. Furthermore, more accurate information may be obtained by using techniques more sensitive to bone composition compared to DXA. The InCHIANTI Study provides the unique opportunity of assessing the relationship between bone and arterial status because it collects a number of factors known or likely to affect one or both of them and provides information on bone composition obtained by peripheral quantitative computed tomography (pQCT).
Methods
We used data from the InCHIANTI Study, which was designed to investigate the factors contributing to the decline of mobility in older persons [18]. The participants in the study were randomly selected from the populations of two town areas in the Chianti region: Greve in Chianti and Bagno a Ripoli. The Italian National Institute of Research and Care on Aging ethical committee approved the study protocol. Participants received an extensive description of the study and signed an informed consent that included permission to conduct analyses on the biological specimens collected and stored. For those unable to fully consent because of cognitive or physical problems, surrogate consent was also obtained from a close relative. Eligible participants were interviewed at their homes by trained study researchers using a structured questionnaire aimed at investigating their heath status, their physical and cognitive performance, and other factors possibly related to loss of independence in late life. The interview was followed by a physical and instrumental examination at the study clinic, including pQCT and carotid echography.
Sample Selection
From the original data set, we selected 1,232 subjects for whom both bone and vascular data were available. We then excluded people with age <65 years (n = 274), with active malignancies, and chronically taking bisphosphonates, systemic steroids, or estrogen replacement therapy (n = 117). The final sample size was 841.
Bone Assessment
Bone mass density was estimated by pQCT using a XCT 2000 device (Stratec Medizin-technik, Pforzheim, Germany). The tibiotalar joint was identified using a pQCT longitudinal scout and used as an anatomic marker for the identification of measurements sites. Standard (2.5 mm thickness) transverse scans were obtained at different sites along the tibial length. The cross-sectional images obtained by pQCT were analyzed using BonAlyse software (Bon-Alyse Oy, Jyvaskyla, Finland), which automatically identifies cortical and trabecular bone and assesses its density. Areas with density values >710 mg/cm3 were considered as cortical bone, whereas areas with density between 180 and 710 mg/cm3 were considered as trabecular bone [19]. Total and cortical bone areas were both measured at 38 % of tibial length [20, 21].
Indicators of Vascular Disease
We evaluated the presence of atherosclerosis at two different sites: internal carotid arteries, using echographic evidence of atherosclerotic plaques at either side, and lower limb, using a composite measure including either an ankle-brachial index (ABI) <0.9 or the presence of intermittent claudication at the Rose questionnaire [22] for patients with ABI ≥0.9. The methodology for measuring ABI is reported elsewhere [23].
Analytic Approach
We grouped participants according to sex-specific quartiles of the cortical/total cross-sectional area ratio (cCSA/tCSA) measured at 38 % of tibial length. Throughout a lifetime, bone strength is maintained by remodeling, i.e., the focal replacement of old bone with new bone. In the elderly, more bone is resorbed than is replaced, leading to an increase of medullary area compared to cortical area [24]. Thus, cCSA/tCSA is a measure of bone remodeling, with lower values indicating increased bone fragility [25]; and this parameter may be more informative compared to BMD because, at least in part, it also takes into account bone geometry. Separately in men and women, groups were compared with respect to the prevalence of atherosclerosis and to the distribution of factors potentially affecting the risk of accelerated bone resorption and/or atherosclerosis. We decided to model cCSA/tCSA as a categorical variable (quartiles) instead of as a continuous variable in order to avoid assumptions about the functional form of the relationship between this variable and our outcomes.
Among lifestyle variables we took into account cumulative smoking exposure expressed as pack-years (the average number of packs of cigarettes smoked each day over the number of years a person has smoked) and low level of physical activity, defined as less than 4 h/week of light exercise. This item was evaluated in reference to both the period 20–40 years and the year preceding the assessment. We considered serum concentration of triglycerides and total, low-density lipoprotein (LDL), and high-density lipoprotein (HDL) cholesterol because of their known relationship with cardiovascular disease and their potential correlation with osteoporosis [26]. Other biochemical variables taken into account given their role in both cardiovascular and bone health were vitamin D, parathyroid hormone (PTH), dehydroepiandrosterone-sulfate (DHEA-S), and free testosterone. We analyzed adipokines such as adiponectin and leptin that may be associated with both BMD [27, 28] and cardiovascular diseases [29] as well as with markers of inflammation such as interleukin 6 (IL-6) and high-sensitivity C-reactive protein (CRP). Most of these biochemical variables showed a skewed distribution in the exploratory analyses and were log-transformed. We further took into account the nutritional intake of mono-(MUFAs) and polyunsaturated fatty acids (PUFAs) [30, 31] estimated using the Epidemiological Investigation into Cancer (EPIC) questionnaire [32, 33]. Among comorbid diseases, we considered diabetes mellitus, chronic obstructive pulmonary disease, and renal failure defined as creatinine clearance estimated using the Cockcroft and Gault formula <60 mL/min. Finally, in women time since menopause was also analyzed.
The association between cCSA/tCSA and atherosclerosis was estimated using logistic regression models adjusted for the potential confounders that showed an actual relationship with cCSA/tCSA quartiles. To provide a more thorough perspective, this analysis was also performed using trabecular and cortical BMD as dependent variables. To obtain more information on the relative weight of the individual confounders, we also evaluated partial regression models including a subset of confounders: lifestyle variables, hormone (PTH, vitamin D, adipokines) profile, inflammation markers, and comorbid diseases. All of these models were adjusted for years since menopause in women.
Results
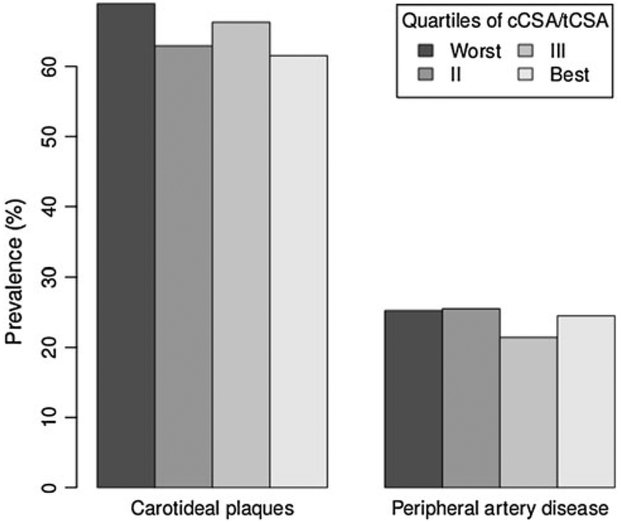
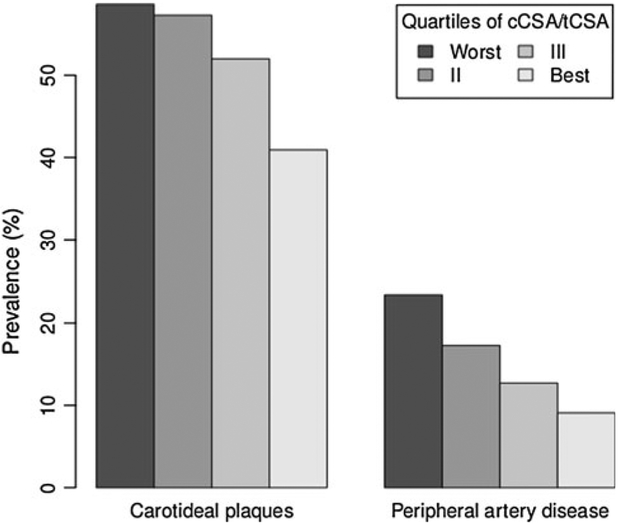
We studied 444 women and 397 men with a mean age of 73.8 and 75.3 years, respectively. Among men (Table 1), those in the worst quartile of cCSA/tCSA were older (75 vs. 73 years in the other quartiles) and had lower serum concentration of DHEA-S (log-transformed value 3.97 ± 0.94 vs. 4.3 ± 0.79 in the best quartile) and free testosterone (log-transformed value 1.1 ± 0.75 vs. 1.39 ± 0.37). In women, the differences in age across quartiles of cCSA/tCSA were more evident (Table 1), as was the expected difference in time since menopause (30 ± 10 in the worst quartile vs. 22 ± 8 in the best quartile). Body mass index (BMI) and leptin were positively associated with cCSA/tCSA. Vitamin D and PTH showed the expected trend, women with higher bone remodeling having the lower concentration of vitamin D (logtransformed value 3.41 ± 0.6 vs. 3.67 ± 0.57 for women in the best quartile) and higher concentration of PTH (logtransformed value 3.34 ± 0.49 vs. 3.03 ± 0.48). Serum concentration of free testosterone and DHEA-S were lower in women in the worst cCSA/tCSA quartile. As shown in Fig. 1, we found no association between our measure of bone remodeling and prevalence of carotid plaques or PAD. In contrast, the association was evident in women (Fig. 2): in the worst quartile of cCSA/tCSA the prevalence of carotid plaques was 58.6 % compared to 41 % in the best quartile, corresponding to an odds ratio (OR) of 2.04 [95 % confidence interval (CI) 1.17–3.58]. This association, however, was not apparent when potential confounders were taken into account (Table 2, see online appendix for the corresponding analyses using trabecular and cortical BMD as dependent variables). In this analysis, only age since menopause was associated with the outcome. In Table 3 we report the OR of having carotid plaques obtained from partial models adjusted for age alone; age plus BMI, leptin, and low physical activity; age plus PTH and vitamin D; age plus free testosterone and DHEA-S; and age plus MUFA intake. In none of these models was cCSA/tCSA associated with the odds of having carotid plaques.
Table 1.
Characteristics of the population by quartiles of cortical/total area measured at 38 % of tibial length: means (SD)
| Worst | II | III | Best | p | |
|---|---|---|---|---|---|
| Men | |||||
| Age (years) | 75 (7) | 73 (7) | 73 (6) | 73 (6) | 0.0863 |
| Smoking (pack-years) | 27 (24) | 23 (23) | 24 (26) | 25 (27) | 0.7637 |
| BMI | 27 (3) | 27 (3) | 27 (3) | 27 (3) | 0.3363 |
| Total cholesterol | 206 (38) | 211 (39) | 217 (40) | 208 (35) | 0.2374 |
| HDL cholesterol | 52 (15) | 52 (13) | 52 (14) | 51 (11) | 0.8488 |
| LDL cholesterol | 129 (36) | 134 (34) | 136 (35) | 131 (28) | 0.4678 |
| Log triglycerides | 4.71 (0.46) | 4.73 (0.46) | 4.82 (0.53) | 4.76 (0.48) | 0.3605 |
| Log PTH | 3.09 (0.46) | 3.09 (0.43) | 3.05 (0.44) | 3.01 (0.46) | 0.5387 |
| Log vitamin D | 3.9 (0.59) | 3.88 (0.58) | 4.02 (0.48) | 3.96 (0.56) | 0.3464 |
| Log adiponectin | 2.11 (0.85) | 2.28 (0.6) | 2.08 (0.69) | 2.12 (0.63) | 0.1958 |
| Log leptin | 1.49 (0.89) | 1.45 (1.07) | 1.68 (0.92) | 1.59 (0.93) | 0.3773 |
| Log high sensitivity CRP | 1.07 (1.12) | 1.1 (1.19) | 0.99 (1.04) | 0.96 (1.14) | 0.8186 |
| Log IL-6 | 0.59 (0.93) | 0.5 (0.8) | 0.45 (0.84) | 0.27 (0.9) | 0.0597 |
| Log DHEA-S | 3.97 (0.94) | 4.17 (0.75) | 4.27 (0.76) | 4.3 (0.79) | 0.019 |
| Log free testosterone | 1.1 (0.75) | 1.37 (0.42) | 1.34 (0.45) | 1.39 (0.37) | <0.001 |
| Log 17β-estradiol | 2.46 (0.45) | 2.53 (0.43) | 2.54 (0.42) | 2.55 (0.42) | 0.4614 |
| PUFA intake | 7.59 (2.18) | 7.69 (2.21) | 7.64 (2.06) | 7.61 (2.19) | 0.9894 |
| MUFA intake | 35.3 (11.13) | 35.63 (11.19) | 35.96 (10.83) | 35.91 (10.26) | 0.9734 |
| MUFA/PUFA intake ratio | 4.67 (0.65) | 4.65 (0.67) | 4.72 (0.73) | 4.76 (0.66) | 0.6176 |
| Low physical activity 20–40 years | 12 | 5 | 9 | 4 | 0.1214 |
| Low physical activity last year | 48 | 47 | 42 | 50 | 0.6855 |
| Diabetes mellitus | 14.1 | 13.3 | 16.3 | 9.2 | 0.5149 |
| COPD | 18.2 | 16.3 | 22.4 | 16.3 | 0.6507 |
| Creatinine clearance <60 mL/min | 38.1 | 26.8 | 26.8 | 23.4 | 0.1198 |
| Cerebrovascular disease | 10.1 | 10.2 | 3.1 | 10.2 | 0.1844 |
| Ischemic heart disease | 14.1 | 14.3 | 12.2 | 12.2 | 0.9538 |
| Women | |||||
| Age (years) | 80 (7) | 76 (7) | 73 (6) | 72 (6) | <0.001 |
| Smoking (pack-years) | 2 (8) | 1 (6) | 4 (8) | 2 (7) | 0.1478 |
| Years since menopause | 30 (10) | 27 (9) | 23 (8) | 22 (8) | <0.001 |
| BMI | 26 (4) | 27 (4) | 28 (5) | 28 (5) | 0.0037 |
| Total cholesterol | 227 (40) | 223 (38) | 226 (39) | 229 (39) | 0.7926 |
| HDL cholesterol | 61 (15) | 59 (17) | 61 (16) | 56 (14) | 0.0947 |
| LDL cholesterol | 141 (36) | 139 (32) | 142 (35) | 145 (35) | 0.6519 |
| Log triglycerides | 4.74 (0.41) | 4.73 (0.43) | 4.65 (0.39) | 4.78 (0.47) | 0.1625 |
| Log PTH | 3.34 (0.49) | 3.15 (0.46) | 3.19 (0.42) | 3.03 (0.48) | <0.001 |
| Log vitamin D | 3.41 (0.6) | 3.55 (0.58) | 3.58 (0.6) | 3.67 (0.57) | 0.0151 |
| Log adiponectin | 2.73 (0.71) | 2.54 (0.67) | 2.59 (0.62) | 2.49 (0.77) | 0.0793 |
| Log leptin | 2.27 (0.94) | 2.4 (0.99) | 2.67 (0.88) | 2.58 (0.97) | 0.0113 |
| Log high sensitivity CRP | 0.94 (0.97) | 0.94 (0.99) | 1.03 (1.05) | 1.14 (0.97) | 0.3683 |
| Log IL-6 | 0.34 (0.84) | 0.3 (0.73) | 0.33 (0.72) | 0.14 (0.77) | 0.1816 |
| Log DHEA-S | 3.85 (0.91) | 4.13 (0.82) | 4.27 (0.77) | 4.24 (0.9) | <0.001 |
| Log free testosterone | −1.14 (0.86) | −1.13 (1.09) | −0.8 (0.74) | −0.76 (0.82) | <0.001 |
| Log 17β-estradiol | 1.66 (0.56) | 1.73 (0.55) | 1.7 (0.59) | 1.78 (0.52) | 0.5226 |
| PUFA intake | 6.33 (2.23) | 6.7 (2.13) | 6.87 (2.16) | 6.72 (2.21) | 0.3077 |
| MUFA intake | 28.14 (10.63) | 30.36 (9.11) | 30.59 (9.77) | 31.25 (10.58) | 0.1132 |
| MUFA/PUFA intake ratio | 4.47 (0.77) | 4.6 (0.73) | 4.49 (0.69) | 4.7 (0.8) | 0.0875 |
| Menopause before 40 years | 2.8 | 1.9 | 0.9 | 1.8 | 0.7988 |
| Low physical activity 20–40 years | 14.4 | 16.4 | 23.6 | 21.8 | 0.2499 |
| Low physical activity last year | 83.8 | 77.3 | 70 | 60 | <0.001 |
| Diabetes mellitus | 6.3 | 14.5 | 9.1 | 11.8 | 0.2166 |
| COPD | 2.7 | 1.8 | 9.1 | 4.5 | 0.0458 |
| Creatinine clearance <60 mL/min | 64.4 | 58.7 | 39.6 | 37.4 | <0.001 |
| Cerebrovascular disease | 9 | 5.5 | 2.7 | 5.5 | 0.2494 |
| Ischemic heart disease | 11.7 | 11.8 | 8.2 | 6.4 | 0.4246 |
BMI body mass index, HDL high-density lypoprotein, LDL low-density lypoprotein, PTH parathormone, CRP C-reactive protein, DHEA-S dehydroepiandrosterone sulfate, PUFA polyunsaturated fatty acid, MUFA monounsaturated fatty acid, COPD chronic obstructive pulmonary disease
Fig. 1.
Prevalence of atherosclerotic disease by quartiles of cortical/total area, men
Fig. 2.
Prevalence of atherosclerotic disease by quartiles of cortical/total area, women
Table 2.
Multivariable logistic regression model estimating the risk of having carotid plaque
| OR | Lower 95 % CI |
Upper 95 % CI |
|
|---|---|---|---|
| cCSA/tCSA ratio, worst vs. best | 1.22 | 0.59 | 2.54 |
| cCSA/tCSA ratio, 2nd quartile vs. best | 1.05 | 0.53 | 2.06 |
| cCSA/tCSA ratio, 3rd quartile vs. best | 1.38 | 0.73 | 2.61 |
| Years since menopause (1-year increments) | 1.06 | 1.03 | 1.10 |
| BMI (unit increments) | 1.01 | 0.94 | 1.09 |
| Log PTH (unit) | 0.86 | 0.49 | 1.51 |
| Log vitamin D (unit) | 0.77 | 0.49 | 1.21 |
| Log leptin (unit) | 0.91 | 0.65 | 1.26 |
| Log DHEA-S (unit) | 0.97 | 0.72 | 1.29 |
| Log free testosterone (unit) | 0.79 | 0.60 | 1.04 |
| MUFA intake (unit) | 1.01 | 0.99 | 1.04 |
| Low physical activity in the last year (Y vs. N) | 0.61 | 0.36 | 1.03 |
| Diabetes mellitus | 1.12 | 0.51 | 2.48 |
| COPD | 0.52 | 0.13 | 1.82 |
| Creatinine clearance <60 mL/min | 1.03 | 0.58 | 1.82 |
cCSA/tCSA cortical/total cross-sectional area ratio, BMI body mass index, PTH parathormone, DHEA-S dehydroepiandrosterone-sulfate, MUFA monounsaturated fatty acid, COPD chronic obstructive pulmonary disease
Table 3.
Partial models estimating the risk of carotid plaques
| cCSA/tCSA ratio, worst vs. best |
cCSA/tCSA ratio, 2nd quartile vs. best |
cCSA/tCSA ratio, 3rd quartile vs. best |
|
|---|---|---|---|
| Model 1 | 1.39 (0.739–2.62) | 1.5 (0.828–2.73) | 1.48 (0.825–2.67) |
| Model 2 | 1.28 (0.656–2.52) | 1.22 (0.648–2.3) | 1.37 (0.744–2.52) |
| Model 3 | 1.44 (0.752–2.79) | 1.42 (0.776–2.62) | 1.56 (0.855–2.85) |
| Model 4 | 1.23 (0.64–2.35) | 1.38 (0.745–2.56) | 1.36 (0.75–2.46) |
| Model 5 | 1.31 (0.688–2.49) | 1.38 (0.756–2.53) | 1.5 (0.83–2.72) |
Model 1 adjusted for years since menopause, model 2 model 1 + BMI, leptin, and low physical activity, model 3 model 1 + PTH and vitamin D, model 4 model 1 + DHEA-S and free testosterone, model 5 model 1 + diabetes mellitus, chronic obstructive pulmonary disease, creatinine clearance <60 mL/min
The prevalence of PAD in the worst quartile of cCSA/tCSA was 23.4 %, compared to 9.1 % in the best quartile (OR = 3.06, 95 % CI 1.43–6.99). After full adjustment, this association was not evident (Table 4, see online appendix for the corresponding analyses using trabecular and cortical BMD as dependent variables); and once again the only statistically significant correlate of the outcome was age since menopause. The analysis of partial models (Table 5), however, showed a different role of the subsets of potential confounders taken into account. After correction for time since menopause alone, for example, the association between osteoporosis and PAD was only marginally reduced, with an OR of 2.5 (95 % CI 1.027–6.55). In the same way, the association was not weakened by the correction for vitamin D and PTH (OR = 3.22, 95 % CI 1.22–9.24). On the other hand, in the model corrected for DHEA-S and testosterone and in the model corrected for BMI, physical activity, and leptin, the association between PAD and cCSA/tCSA was not statistically significant. With respect to the latter model, the same results were obtained adjusting for physical activity in the third and fourth decades instead of physical activity in the last year. This sensitivity analysis was conducted to exclude the possibility that reverse causation accounted for the inverse association between PAD and physical activity.
Table 4.
Multivariable logistic regression model estimating the risk of peripheral artery disease
| OR | Lower 95 % CI |
Upper 95 % CI |
|
|---|---|---|---|
| cCSA/tCSA ratio, worst vs. best | 2.28 | 0.77 | 7.23 |
| cCSA/tCSA ratio, 2nd quartile vs. best | 1.76 | 0.63 | 5.24 |
| cCSA/tCSA ratio, 3rd quartile vs. best | 2.16 | 0.79 | 6.33 |
| Years since menopause (1-year increments) | 1.02 | 0.97 | 1.06 |
| BMI (unit increments) | 1.08 | 0.98 | 1.2 |
| Log PTH (unit) | 0.34 | 0.14 | 0.8 |
| Log vitamin D (unit) | 0.76 | 0.39 | 1.47 |
| Log leptin (unit) | 0.87 | 0.54 | 1.42 |
| Log DHEA-S (unit) | 0.89 | 0.6 | 1.33 |
| Log free testosterone (unit) | 0.64 | 0.45 | 0.9 |
| MUFA intake (unit) | 1.04 | 1 | 1.07 |
| Low physical activity in the last year (Y vs. N) | 1.35 | 0.61 | 3.27 |
| Diabetes mellitus | 0.71 | 0.15 | 2.42 |
| COPD | 1.3 | 0.26 | 4.9 |
| Creatinine clearance <60 mL/min | 1.71 | 0.73 | 4.15 |
cCSA/tCSA cortical/total cross-sectional area ratio, BMI body mass index, PTH parathormone, DHEA-S dehydroepiandrosterone-sulfate, MUFA monounsaturated fatty acids, COPD chronic obstructive pulmonary disease
Table 5.
Partial models estimating the risk of peripheral artery disease
| cCSA/tCSA ratio, worst vs. best |
cCSA/tCSA ratio, 2nd quartile vs. best |
cCSA/tCSA ratio, 3rd quartile vs. best |
|
|---|---|---|---|
| Model 1 | 2.5 (1.027–6.55) | 2.1 (0.873–5.46) | 1.74 (0.697–4.59) |
| Model 2 | 2.04 (0.783–5.62) | 1.77 (0.699–4.77) | 1.6 (0.627–4.3) |
| Model 3 | 3.22 (1.223–9.24) | 2.57 (1.023–7.11) | 2.29 (0.882–6.44) |
| Model 4 | 2.11 (0.856–5.61) | 1.97 (0.803–5.18) | 1.76 (0.702–4.69) |
| Model 5 | 2.36 (0.955–6.27) | 2.06 (0.839–5.4) | 1.68 (0.67–4.45) |
Model 1 adjusted for years since menopause, model 2 model 1 + BMI, leptin, and low physical activity, model 3 model 1 + PTH and vitamin D, model 4 model 1 + DHEA-S and free testosterone, model 5 model 1 + diabetes mellitus, chronic obstructive pulmonary disease, creatinine clearance <60 mL/min
Discussion
Our data confirm the repeatedly reported association between osteoporosis and atherosclerosis in women. This relationship, however, is not independent of potential confounders (especially time since menopause) when carotid plaques are the outcome of interest, while when PAD is considered as the outcome measure, the association is independent of time since menopause, vitamin D, and PTH. These findings indicate that the observed relationship between bone metabolism and atherosclerosis only in part reflects the negative effects of hormonal deprivation in postmenopausal women.
Menopause is a dramatic event for metabolism and cardiovascular function. After menopause, a noticeable worsening of the lipid profile and body composition and an increase in blood pressure are usually observed. At the same time, the reabsorptive osteoclastic activity becomes unbalanced by the counteracting osteoblastic activity [24]. These biological mechanisms likely underlie the observed relationship between postmenopausal osteoporosis and both clinical and preclinical indexes of atherosclerosis. This does not exclude that some interplay between bone and vessels occurs but identifies hormonal deprivation as the leading explanatory mechanism of this association. Nonetheless, HRT, either estrogen alone or estrogen plus progestin, could decrease the incidence of fractures but fail to improve cardiovascular outcomes. Actually, HRT was associated with increased incidence of stroke, pulmonary embolism, and deep vein thrombosis [34]. This seemingly contradictory finding should be interpreted in the light of negative effects of HRT on coagulation balance. In aging women such an effect likely outweighs the positive one on lipid profile and, thus, accounts for the observed findings. It should also be observed that in some studies the correlation between indexes of atherosclerosis and BMD in females persisted after correction for age [4, 5]. This discrepancy may be explained by differences in the methods used to assess both exposure (DXA vs. pQCT) and outcome (intima-media thickness vs. carotid plaques). Hyder et al. [6], however, found an association between lower vBMD and carotid plaques in participants of the Multi-Ethnic Study of Atherosclerosis (MESA). MESA participants were on average 10 years younger compared to our sample. Furthermore, a dilution of the association may be due to the fact that we took into account any carotid plaque, but some evidence exists that only calcified plaques are related to osteoporosis [6, 35].
While the association between carotid atherosclerosis and osteoporosis seems to be largely mediated by menopause, different mechanisms seem to underlie the association between osteoporosis and PAD. Hypovitaminosis D is frequent in patients with PAD [36], probably because of mobility restriction and reduced sunlight exposure [37]. Our data, however, indicate that the observed association between osteoporosis and PAD is also independent of vitamin D and PTH status. Instead, this association seems to be mediated by physical inactivity and leptin. With respect to leptin, our results are at least in part unexpected. Leptin is univocally considered to have a proatherogenic activity [29], but its effect on bone metabolism is not entirely clear: the current mainstream hypothesis is that it has a direct positive effect by increasing osteoblast proliferation and a centrally mediated negative action [38]. In our population the net effect of leptin seems to be in the anabolic direction; therefore, the estimated association between osteoporosis and PAD should have been stronger after taking leptin into account. However, leptin is also associated with increased fat mass and weight, and this relationship is likely to explain our findings.
This study has important strengths. First, pQCT provides a highly reliable estimation of bone structure. Second, a detailed report of actual and previous therapy was available, which allowed us to exclude women exposed to drugs known or likely to affect bone metabolism and structure. Third, the InCHIANTI database allowed us to consider a vast array of factors affecting BMD. Finally, nutrient intake was rigorously assessed by a questionnaire developed and validated in Italy.
This study also has some limitations. No measure of vertebral structure was available. The available knowledge indicates that femoral and vertebral bone are not equally associated with osteoporosis [9]. Being more metabolically active, trabecular bone may be more affected by the fall of serum estrogen levels [24]. It should also be taken into account, however, that vertebrae and tibia have different types of blood supply (two arteries for the vertebrae, a single vessel for the tibia); therefore, the tibia might suffer more than vertebral bodies from the postmenopausal fall in serum estrogen levels and the ensuing accelerated atherosclerosis.
Second, the InCHIANTI population was characterized by a healthy lifestyle and generally good health conditions. In a less healthy population the bone—artery connection might be more evident but at the expense of a greater impact of confounding factors on it. Third, we used stringent exclusion criteria, but selected and commonly used drugs, such as proton pump inhibitors and selective serotonin reuptake inhibitors, have been proposed to affect bone health through various mechanisms [39]. However, we did not introduce further exclusion criteria because the available evidence does not clarify the clinical size of these pharmacological effects.
In conclusion, this study confirms the lack of association between osteoporosis and atherosclerosis in men. In women, the association between osteoporosis and atherosclerosis of the carotid district seems to be mediated by hormonal deprivation, while the association between osteoporosis and PAD seems to be more complex and mediated by hormonal factors and low physical activity. In the context of a cross-sectional study, reverse causation remains an issue, and longitudinal studies are needed to verify the direction of this association.
Supplementary Material
Abbreviations
- BMD
Bone mineral density
- DEXA
Dual-energy X-ray absorptiometry
- PAD
Peripheral artery disease
- pQCT
Peripheral quantitative computed tomography
- ABI
Ankle-brachial index
- cCSA/tCSA
Cortical/total cross-sectional area ratio
- PTH
Parathyroid hormone
- DHEA-S
Dehydroepiandrosterone-sulfate
- IL-6
Interleukin 6
- CRP
C-reactive protein
- EPIC
EPidemiological Investigation into Cancer
- HRT
hormone replacement therapy
- PPI
Proton pump inhibitors
- SSRI
Selective serotonin re-uptake inhibitors
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00223-013-9782-y) contains supplementary material, which is available to authorized users.
The authors have stated that they have no conflict of interest.
Contributor Information
Claudio Pedone, Area of Geriatrics, Campus Biomedico University and Teaching Hospital, Via Alvaro del Portillo 200, 00128 Rome, Italy.
Simone Scarlata, Area of Geriatrics, Campus Biomedico University and Teaching Hospital, Via Alvaro del Portillo 200, 00128 Rome, Italy.
Nicola Napoli, Division of Endocrinology, Campus Bio-Medico University and Teaching Hospital, Rome, Italy.
Fulvio Lauretani, Geriatric Unit and Laboratory of Movement Analysis, Geriatric and Rehabilitation Department, University Hospital of Parma, Parma, Italy.
Stefania Bandinelli, Geriatric Rehabilitation Unit, Azienda Sanitaria Firenze, Florence, Italy.
Luigi Ferrucci, Longitudinal Study Section, Clinical Research Branch, National Institute of Aging, Baltimore, MD, USA.
Raffaele Antonelli Incalzi, Area of Geriatrics, Campus Biomedico University and Teaching Hospital, Via Alvaro del Portillo 200, 00128 Rome, Italy. San Raffaele—Cittadella della Carita Foundation, Taranto, Italy.
References
- 1.Qu X, Huang X, Jin F et al. (2013) Bone mineral density and allcause, cardiovascular and stroke mortality: a meta-analysis of prospective cohort studies. Int J Cardiol 166:385–393 [DOI] [PubMed] [Google Scholar]
- 2.Choi SH, An JH, Lim S et al. (2009) Lower bone mineral density is associated with higher coronary calcification and coronary plaque burdens by multidetector row coronary computed tomography in pre- and postmenopausal women. Clin Endocrinol (Oxf) 71:644–651 [DOI] [PubMed] [Google Scholar]
- 3.Hyder JA, Allison MA, Wong N et al. (2009) Association of coronary artery and aortic calcium with lumbar bone density: the MESA Abdominal Aortic Calcium Study. Am J Epidemiol 169:186–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sumino H, Ichikawa S, Kasama S et al. (2008) Relationship between carotid atherosclerosis and lumbar spine bone mineral density in postmenopausal women. Hypertens Res 31:1191–1197 [DOI] [PubMed] [Google Scholar]
- 5.Tamaki J, Iki M, Hirano Y et al. (2009) Low bone mass is associated with carotid atherosclerosis in postmenopausal women: the Japanese Population-based Osteoporosis (JPOS) Cohort Study. Osteoporos Int 20:53–60 [DOI] [PubMed] [Google Scholar]
- 6.Hyder JA, Allison MA, Barrett-Connor E, Detrano R et al. (2010) Bone mineral density and atherosclerosis: the Multi-Ethnic Study of Atherosclerosis, Abdominal Aortic Calcium Study. Atherosclerosis 209:283–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schulz E, Arfai K, Liu X, Sayre J, Gilsanz V (2004) Aortic calcification and the risk of osteoporosis and fractures. J Clin Endocrinol Metab 89:4246–4253 [DOI] [PubMed] [Google Scholar]
- 8.Frost ML, Grella R, Millasseau SC, Jiang BY, Hampson G, Fogelman I et al. (2008) Relationship of calcification of atherosclerotic plaque and arterial stiffness to bone mineral density and osteoprotegerin in postmenopausal women referred for osteoporosis screening. Calcif Tissue Int 83:112–120 [DOI] [PubMed] [Google Scholar]
- 9.Farhat GN, Strotmeyer ES, Newman AB et al. (2006) Volumetric and areal bone mineral density measures are associated with cardiovascular disease in older men and women: the health, aging, and body composition study. Calcif Tissue Int 79:102–111 [DOI] [PubMed] [Google Scholar]
- 10.van der Klift M, Pols HAP, Hak AE, Witteman JCM, Hofman A, de Laet CEDH (2002) Bone mineral density and the risk of peripheral arterial disease: the Rotterdam Study. Calcif Tissue Int 70:443–449 [DOI] [PubMed] [Google Scholar]
- 11.von Mühlen D, Allison M, Jassal SK, Barrett-Connor E (2009) Peripheral arterial disease and osteoporosis in older adults: the Rancho Bernardo Study. Osteoporos Int 20:2071–2078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bagger YZ, Tankó LB, Alexandersen P, Qin G, Christiansen C (2006) Radiographic measure of aorta calcification is a site-specific predictor of bone loss and fracture risk at the hip. J Intern Med 259:598–605 [DOI] [PubMed] [Google Scholar]
- 13.Bagger YZ, Rasmussen HB, Alexandersen P, Werge T, Christiansen C, Tankó LB (2007) Links between cardiovascular disease and osteoporosis in postmenopausal women: serum lipids or atherosclerosis per se? Osteoporos Int 18:505–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGreevy C, Williams D (2011) New insights about vitamin D and cardiovascular disease: a narrative review. Ann Intern Med 155:820–826 [DOI] [PubMed] [Google Scholar]
- 15.Lips P (2006) Vitamin D physiology. Prog Biophys Mol Biol 92:4–8 [DOI] [PubMed] [Google Scholar]
- 16.Morony S, Tintut Y, Zhang Z et al. (2008) Osteoprotegerin inhibits vascular calcification without affecting atherosclerosis in ldlr(−/−) mice. Circulation 117:411–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Venuraju SM, Yerramasu A, Corder R, Lahiri A (2010) Osteoprotegerin as a predictor of coronary artery disease and cardiovascular mortality and morbidity. J Am Coll Cardiol 55:2049–2061 [DOI] [PubMed] [Google Scholar]
- 18.Ferrucci L, Bandinelli S, Benvenuti E et al. (2000) Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc 48:1618–1625 [DOI] [PubMed] [Google Scholar]
- 19.Rittweger J, Beller G, Ehrig J et al. (2000) Bone—muscle strength indices for the human lower leg. Bone 27:319–326 [DOI] [PubMed] [Google Scholar]
- 20.Russo CR, Lauretani F, Bandinelli S et al. (2003) Aging bone in men and women: beyond changes in bone mineral density. Osteoporos Int 14:531–538 [DOI] [PubMed] [Google Scholar]
- 21.Russo CR, Lauretani F, Seeman E et al. (2006) Structural adaptations to bone loss in aging men and women. Bone 38:112–118 [DOI] [PubMed] [Google Scholar]
- 22.Rose G, McCartney P, Reid DD (1977) Self-administration of a questionnaire on chest pain and intermittent claudication. Br J Prev Soc Med 31:42–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Antonelli-Incalzi R, Pedone C, McDermott MM et al. (2006) Association between nutrient intake and peripheral artery disease: results from the InCHIANTI study. Atherosclerosis 186:200–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seeman E (2002) Pathogenesis of bone fragility in women and men. Lancet 359:1841–1850 [DOI] [PubMed] [Google Scholar]
- 25.Griffith JF, Genant HK (2008) Bone mass and architecture determination: state of the art. Best Res Clin Endocrinol Metab 22:737–764 [DOI] [PubMed] [Google Scholar]
- 26.Parhami F, Garfinkel A, Demer LL (2000) Role of lipids in osteoporosis. Arterioscler Thromb Vasc Biol 20:2346–2348 [DOI] [PubMed] [Google Scholar]
- 27.Napoli N, Pedone C, Pozzilli P, Lauretani F, Ferrucci L, Incalzi RA (2010) Adiponectin and bone mass density: the InCHIANTI study. Bone 47:1001–1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Biver E, Salliot C, Combescure C et al. (2011) Influence of adipokines and ghrelin on bone mineral density and fracture risk: a systematic review and meta-analysis. J Clin Endocrinol Metab 96:2703–2713 [DOI] [PubMed] [Google Scholar]
- 29.Shanker J, Rao VS, Ravindran V, Dhanalakshmi B, Hebbagodi S, Kakkar V (2012) Relationship of adiponectin and leptin to coronary artery disease, classical cardiovascular risk factors and atherothrombotic biomarkers in the IARS cohort. Thromb Haemost 108:769–780 [DOI] [PubMed] [Google Scholar]
- 30.Pedone C, Napoli N, Lauretani F, Pozzilli P, Ferrucci L, Antonelli-Incalzi R (2010) Quality of diet and potential renal acid load as risk factors for reduced bone density in elderly women. Bone 46:1063–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kontogianni MD, Melistas L, Yannakoulia M, Malagaris I, Panagiotakos DB, Yiannakouris N (2009) Association between dietary patterns and indices of bone mass in a sample of Mediterranean women. Nutrition 25:165–171 [DOI] [PubMed] [Google Scholar]
- 32.Pisani P, Faggiano F, Krogh V, Palli D, Vineis P, Berrino F (1997) Relative validity and reproducibility of a food frequency dietary questionnaire for use in the Italian EPIC centres. Int J Epidemiol 26(Suppl 1):S152–S160 [DOI] [PubMed] [Google Scholar]
- 33.Bartali B, Turrini A, Salvini S et al. (2004) Dietary intake estimated using different methods in two Italian older populations. Arch Gerontol Geriatr 38:51–60 [DOI] [PubMed] [Google Scholar]
- 34.Nelson HD, Walker M, Zakher B, Mitchell J (2012) Menopausal hormone therapy for the primary prevention of chronic conditions: a systematic review to update the U.S. Preventive Services Task Force recommendations. Ann Intern Med 157:104–113 [DOI] [PubMed] [Google Scholar]
- 35.Kim SH, Kim YM, Cho MA et al. (2008) Echogenic carotid artery plaques are associated with vertebral fractures in postmenopausal women with low bone mass. Calcif Tissue Int 82:411–417 [DOI] [PubMed] [Google Scholar]
- 36.Fahrleitner A, Dobnig H, Obernosterer A et al. (2002) Vitamin D deficiency and secondary hyperparathyroidism are common complications in patients with peripheral arterial disease. J Gen Intern Med 17:663–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holick MF (2002) Sunlight and vitamin D. J Gen Intern Med 17:733–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Motyl KJ, Rosen CJ (2012) Understanding leptin-dependent regulation of skeletal homeostasis. Biochimie 94:2089–2096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pitts CJD, Kearns AE (2011) Update on medications with adverse skeletal effects. Mayo Clin Proc 86:338–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.