Abstract
Proton pump inhibitors (PPIs) are highly effective in the treatment of upper gastrointestinal acid-related conditions and are fast becoming one of the most frequently prescribed treatments in adult -older persons. Recent data show that long-term use of PPIs in older subjects is associated with important undesirable effects, including a higher risk of osteoporotic fractures. The mechanisms of this association are unclear and the relationship between the use of PPIs and parameters of bone mass and geometry has never been fully explored.
This study investigates the relationship between the chronic use of PPIs and the parameters of bone mass (cortical and trabecular bone mineral density vBMDc and vBMDt, and bone geometry (cortical and trabecular cross sectional area-tCSA and cCSA) in older individuals. The study population consisted of 1038 subjects (452 men and 586 women) 65 years or older, selected from the InCHIANTI study, with complete information on computerized tomography performed at tibial level (pQCT) and on medications. Participants were classified as PPI users and non-users based on self-report of PPI use over the last 15 days, with PPI users (36 subjects, 14 men and 22 women) making up 3.4% of the study population (mean age 75.7±7.4 years). The relationship between use of PPIs and pQCT bone parameters were tested by multivariate linear regression analysis adjusted for age, sex and several clinical factors and/or statistically confounding variables identified by partial correlation coefficient and Spearman partial rank order correlation coefficients, as appropriate (age, sex, BMI, caloric intake, IGF-1, IL-6, calcium, estradiol, bioavailable testosterone, vitamin D, parathyroid hormone, cross-sectional muscle area, and level of physical activity). PPI users showed age- and sex-adjusted lower vBMDt than non-users (180.5±54.8 vs 207.9±59.4, p=0.001).The inverse association between PPI use and vBMDt remained almost unchanged after adjustment for multiple confounders. There was no statistically significant difference in vBMDc, - tCSA and cCSA between PPI users and non users.
In community dwelling older persons, the use of PPIs is inversely associated with vBMDt, an early marker of the osteoporotic process. These findings suggest that PPI use might increase the risk of fractures in older subjects through its detrimental effects on trabecular bone.
Keywords: Bone mineral density, trabecular bone mineral density, proton pump inhibitors, InCHIANTI Study, elderly
1.1. Introduction
Proton Pump Inhibitors (PPIs) are potent gastric acid suppressing drugs with proven efficacy both in the prevention and treatment of peptic ulcers, gastroesophageal reflux disease, and erosive esophagitis [1-2]. Since their introduction in the 1980s, PPIs have been one of the most prescribed classes of drugs worldwide due to their high effectiveness in acid-related conditions, compared to histamine receptor antagonists [3].
In the last two decades we have observed a marked increase in PPI use, especially among older persons, supported by the availability of both over-the-counter and generic formulations [4]. Despite the evident clinical benefits, recent epidemiological studies underline an often inappropriate PPI prescription for up to 50-80% of patients admitted to acute wards of geriatric and internal medicine [5-10].
There is growing evidence that high PPI exposure and/or an inappropriate treatment period are accompanied by a variety of relevant clinical adverse events. This is particularly evident in chronic PPI users and vulnerable classes of individuals such as the elderly. In this population the chronic acid suppression induced by PPIs has been associated with an increased risk of community-acquired pneumonia [11-14], C. difficile infections [15-17], and malnutrition [18] including hypomagnesemia [19]. In addition, PPIs share common metabolic pathways with several classes of medications such as non-steroidal anti-inflammatory and antiplatelet drugs and bisphosphonates whose effectiveness might be reduced by PPI use [20-21]. More recently, in older patients discharged from acute care hospitals, the use of high doses of PPIs has been associated with an increased risk of 1-year mortality [22].
The Food and Drug Administration has recently reported in a safety communication the evidence of a worrying increased risk of fractures induced by chronic PPI treatment [23]. A meta-analysis of 11 observational studies showed a mild increased risk of hip and vertebral fractures in PPI users of both sexes, compared to H2RA users [24]. Interestingly, the results were stronger in older participants and confirmed in a recent meta-analysis [24-25].
Age-related changes in bone mass, bone mineral density (BMD), bone geometry and architecture, cortical bone thickness and trabecular porosity negatively affect the bone strength, one of the most important determinants of fractures. Bone remodelling is faster and significatly more evident in trabecular bone and as far as we know, changes in trabecular bone occur early in the pathway to full blown osteoporosis [26-27]. Use of PPIs might exacerbate the age related modifications in bone density and strength.
Despite the increasing evidence of a relationship between PPI and bone fractures, few studies have explored the hypothesis that PPI use may be associated with deteriorated bone density and structural geometry.
The aim of the study is to characterize the relationship between PPI use and parameters of bone mineral density and geometry as well as markers of bone strength in older community dwelling persons.
1.2. MATERIALS AN METHODS
1.2.1. Study population
InCHIANTI is an epidemiological study of risk factors for mobility disability in the elderly, designed by the Laboratory of Clinical Epidemiology of the Italian Research Council of Aging (Florence), and conducted on a representative sample of a population living in Greve in Chianti and Bagno a Ripoli, two small towns of Tuscany, Italy. The study design and data collection have been described elsewhere [26-29]. Of the whole study population, 1260 subjects were 65 years old or older. In our analysis we excluded 46 participants affected by bone diseases such as osteoporosis or Paget’s disease: 58 participants treated with medications interfering with bone metabolism such as calcium, vitamin D, bisphosphonates, calcitonin, corticosteroids, anticonvulsive therapy and lithium, 4 participants affected by primary hyperparathyroidism and finally 114 subjects with no data available on peripheral quantitative computed tomography scans (pQCT). The final population consisted of 1038 participants (452 men and 586 women) with complete data on pQCT, medications and other variables used in the analysis presented here.
The study protocol was approved by a local ethics committee. All participants received a detailed description of the purpose and design of the study and signed an informed participation consent.
1.2.2. Measures
After obtaining written informed consent, pQTC was performed at the distal third of the tibial length. Pharmacological history was also collected and participants were asked to report any medication taken in the last two weeks. Drugs were coded according to the Anatomical Therapeutic and Chemical codes [30]. In particular, we considered PPIs users as patients taking PPI for at least 2 weeks from the time of medical history.
1.2.2.1. Tibial pQCT
pQCT was performed by the XCT 2000 device (Stratec Medizintechnik, Pforzheim, Germany). A detailed description of the tibial pQCT examination has been published elsewhere [26-27,29]. The precision error of the XCT2000 was below 1% for volumetric trabecular and cortical density and, between 1 and 3%, for composite geometry parameters [26]. We used pQCT because this technique has been shown to be effective in the assessment of osteoporosis and in predicting the risk of osteoporotic fractures [31].
The cross-sectional images obtained from the pQCT were analyzed using the BonAlyse software (BonAlyse Oy, Jyvaskyla, Finland), a software program for processing pQCT scans that automatically identifies cortical and trabecular bone tissue assessing at the same time bone mineral density and geometry. In our analysis we considered several bone parameters derived from the pQCT images. Trabecular volumetric BMD (vBMDt) (mg/cm3) was defined as the average density of the trabecular bone area detected at the 4% site of the tibial length. Cortical bone was excluded from the measurement. Cortical volumetric BMD (vBMDc) (mg/cm3), a selective measure of the apparent volumetric density of cortical bone and marker of bone material property, was measured at the 38% site of the tibial length. Total bone cross-sectional area (tCSA) (mm2), measure of bone size, was defined as the area within the circumference that delimited all cortical bone tissues with a density higher than 180 mg/cm3 and measured at the 38% site of the tibial length. Cortical bone cross-sectional area (cCSA) (mm2) was assessed as the cross-sectional area of the voxels with a density higher than 710 mg/cm3 and measured at the 38% site of the tibial length. The cortical bone area is a good measure of total cortical bone mass and a valid marker of bone resistance [26-27,29].
Calf muscle cross-sectional area (CSMA) was evaluated from a transverse scan performed at 66% of the tibia length from the distal tip of the tibia, which is the level of largest outer calf diameters, with consistency across individuals [29].
1.2.2.2. Laboratory measures
Blood samples were drawn in the morning after a 12-h overnight fast. All the routine blood tests were performed on fresh blood.
25(OH)-vitamin D, PTH, total IGF-1, total testosterone, estradiol (E2) and interleukin-6 (IL-6) assays were performed on specimens previously stored at −80°C. 25(OH)-vitamin D was measured by RIA (DiaSorin Inc., Stillwater, MN, USA), after extraction of samples with acetonitrile. Intra- and interassay CVs were 8.1 and 10.2%, respectively. Concentrations of bioavailable testosterone (Bio-T) were calculated using the Vermeulen formula [32]. Serum intact parathyroid hormone (PTH) was measured using a two-site immunoradiometric assay kit (N-tact PTHSP, DiaSorin Inc., Stillwater, MN, USA). The assay uses two affinity-purified polyclonal antibodies, the first specific for the amino-terminal 1–34 portion of the PTH molecule and the second specific for the 39–84 sequence of the hormone sensitivity assay was 1.2 ng/l. Intra- and interassay CVs were <3.0 and 5.5%, respectively. Serum levels of total IGF-1 were measured in the Laboratory of the University of Parma in duplicate by immunoradiometric assay, using commercial reagents (DSL, Webster, TX). Inter and intraassay coefficients of variation (CVs) for 3 concentrations (low, medium, high) were all less than 10%. Serum levels of interleukin-6 (IL-6) were measured in duplicate by high sensitivity enzyme-linked immunoabsorbent assays (ELISA) using commercial kits (BIOSOURCE International, Camarillo, CA). The lower detectable limit for IL-6 was 0.1 pg/ml. The interassay coefficient of variation was less than 7%. Total E2 was measured in the Laboratory of the University of Parma using an ultrasensitive radioimmunoassay (DSL-4800, Chematil, Angri, Italy), characterized by a MDC of 2.2 pg/mL and intra- and inter-assay CV of 8 and 10%, respectively. The reference range for E2 was 21.30–354.12 pg/mL. Liver function was evaluated by aspartate aminotransferase (AST) and alanine aminotransferase (ALT).
1.2.2.3. Other measures
Weight was measured to the nearest 0.1 kg using a high precision mechanical scale, with the participant wearing light clothes and without shoes. Standing height without shoes was measured to the nearest 0.1 cm. Body mass index (BMI) was calculated as [weight (kg)] / [height (m)2].
Data on dietary intake was estimated by using the food frequency questionnaire created for the European Prospective Investigation into Cancer and nutrition (EPIC) study [33] and validated in older persons [34]. Data on food consumption were transformed into daily intake of energy, macro and micronutrients, by specific software created for EPIC.
Global cognitive performance was assessed using the Mini-Mental State Examination (MMSE) performed by a trained geriatrician within a week of the blood drawn. The level of physical activity in the year prior to the interview was classified on an ordinal scale based on responses to a modified standard questionnaire [29] into: (1) hardly any physical activity; (2) mostly sitting (occasionally walks, easy gardening); (3) light exercise (no sweat) 2–4 h/week; (4) moderate exercise (sweat) 1–2 h/week (level 4); (5) moderate exercise >3 h/week; (6) intense exercise (at the limits) >3 times/week. For analytical purposes, we grouped the participants as: 1–3 inactive or having light physical activity; 4–5 having moderate physical activity; 6 having intense activity.
1.2.2.4. Statistical analysis
Variables normally distributed were reported as mean values ± standard deviations. The 25(OH)-vitamin D, PTH, Bio-T, E2, total IGF-1, IL-6, were described by reporting median values and interquartile ranges due to skewness. To approximate normal distributions, log-transformed values for 25(OH)-vitamin D, PTH, E2, Bio-T, IGF-1, and IL-6 were used in the analysis and back-transformed for data presentation. Categorical values were reported as percentages.
The relationship between PPI use (predictor) and parameters (outcomes) of bone density (BMD, vBMDc, vBMDt) and geometry (tCSA and cCSA) was tested in a age- and sex-adjusted analysis by linear or multinomial logistic regression models, as appropriate. Variables statistically correlated with bone parameters were identified by using age-adjusted partial correlation coefficients and Spearman partial rank-order correlation coefficients, as appropriate. Parsimonious models obtained by backward selection from initial fully adjusted models were used to identify independent predictors of BMD, vBMDc, vBMDt, tCSA and cCSA. The SAS 8.2 statistical package was used for all analyses (SAS Institute, Inc., Cary, NC, USA).
1.3. RESULTS
The characteristics of the participants were presented for the entire sample and participants were stratified according to PPI use. The prevalence of PPI use among the InChianti community dwelling older persons was 3.4% (22 women and 14 men). The majority of persons were on PPI (N=25; 70%) due to gastro-protection during NSAIDs or chronic aspirin treatment; the remaining 30% (N=11) were on PPI because of peptic gastro-intestinal ulcer (Table 1). Since the interaction terms sex*PPI use*trabecular bone were not statistically significant (β±SE 1.47± 20.8, p value= 0.94), nor were those between sex*PPI use*bone geometry parameters (data not shown), the data were not stratified by sex within PPI users and non-users (Table 1). The mean age of the whole population was 75.7±7.4 years. Male PPI users were older (77.7±7.5 vs 74.6±6.9) than non-users, while female PPI users were younger (74.2±4.8 vs 76.6±7.8) than non-users. After adjustment for age, PPI female users and non users did not significantly differ in estradiol (6.5 ± 4.7 vs 4.9 ± 2.2, p=0.16) and bioavailable testosterone levels (10.3 ± 5.3 vs 11.9 ± 9.8, p=0.40). Similarly, PPI male users and non users showed no difference in estradiol (13.2 ± 5.7 vs 12.9 ± 5.4, p=0.65) and bioavailable testosterone levels (68.4 ± 25.9 vs 93.7 ± 40.7, p=0.08) (data not shown).
Table 1.
Characteristics of whole study population according to PPI use.
| All (N=1038) |
PPI non users | PPI Users | P* | |
|---|---|---|---|---|
| (N=1002) | (N=36) | |||
| Age (years) | 75.7 ± 7.4 | 75.7 ± 7.5 | 75.6 ± 6.1 | 0.88 |
| Women N (%) | 564 (56.5) | 438 (43.7) | 14 (38.8) | 0.72 |
| BMI (Kg/m2) | 27.4 ± 4.05 | 27.5 ± 4.0 | 25.8 ± 4.1 | 0.01 |
| Caloric Intake (kcal/day) | 1895.3 ± 557.9 | 1894.5 ± 554.3 | 1914.9 ± 652.2 | 0.71 |
| MMSE | 24.3 ± 5.0 | 24.3 ± 5.1 | 24.0 ± 3.8 | 0.68 |
| Albumin (g/dL) | 4.2 [4.0-4.4] | 4.2 ± 0.3 | 4.2 ± 0.3 | 0.67 |
| ALT (UI/L) | 19.4 ± 12.1 | 19.4 ± 12.2 | 18.1 ± 6.4 | 0.51 |
| AST (UI/L) | 21.1 ± 7.9 | 21.0 ± 8 | 21.9 ± 6.2 | 0.52 |
| Magnesium (mg/dL) | 2.06 ± 0.53 | 2.06 ± 0.53 | 2.07 ±0.46 | 0.84 |
| Calcium intake (mg/day) | 820.27 ± 303.7 | 821.9 ± 304.6 | 774.1 ± 280.4 | 0.38 |
| Vitamin D (ng/mL) | 49.16 ± 2.6 | 49.0 ± 3.6 | 51.8 ± 3.7 | 0.54 |
| Vit B12 (pg/ml) | 372.0 [269.5-508.5] | 460 ±346.5 | 456.6±376.0 | 0.92 |
| PTH (ng/l) | 27.4 5 ± 7.3 | 27.4 ± 3.3 | 26.9 ± 2.1 | 0.83 |
| Bioavailable-T (ng/dL) | 20.4 [8.1-81.8] | 47.8 ± 49.3 | 32.9 ± 33.0 | 0.02 |
| Estradiol (pg/mL) | 8.08[4.90-12.80] | 9.50 ± 6.20 | 7.71 ±5.20 | 0.17 |
| IGF-1 (ng/mL) | 109.2[77.8-147.9] | 116.55 ± 54.4 | 94.41 ± 49.09 | 0.02 |
| IL-6 (pg /mL) | 1.47[0.86-2.31] | 2.30 ± 4.37 | 2.17 ± 2.60 | 0.88 |
| CSMA (cm2) | 60.9 ± 12.7 | 61.1 ± 12.7 | 56.7 ± 8.0 | 0.08 |
| Physical Activity level | ||||
| Sedentary/Light | 233(22.0) | 226(22.6) | 7(19.4) | |
| Moderate | 752(73.0) | 725(72.4) | 27(75.0) | 0.52 |
| High | 53(5.0) | 51(5.0) | 2(5.6) | |
Data are presented as number of cases (percentage), mean ± SD or median and interquartile ranges as appropriate.
The P values are age and sex adjusted.
Compared with non-users, PPI users had significantly lower BMI (25.8±4.1 vs 27.5±4.0, p=0.01), lower levels of bioavailable testosterone (32.9 ± 33.0 vs 47.8 ± 49.3 p=0.02) and IGF-1 (94.41 ± 49.09 vs 116.55 ± 54.4, p=0.02), while E2 levels were similar in the two groups (Table 1). After adjustment for age and sex (model 1), PPI users showed a significantly lower vBMDt than non-users (180.2 ± 54.0 vs 207.6 ± 59.4, p=0.001) (Table 2, Figure 1). No significant difference was observed in total vBMD, vBMDc, measures of bone size and markers of bone strength between PPI users and non users.
Table 2.
Parameters of bone density and geometry for the entire sample and according to PPI use.
| All (N=1038) |
PPI non-users | PPI Users | P* | |
|---|---|---|---|---|
| (N=1002) | (N=36) | |||
| BMD (mg/cm3) | 255.8 ±53.8 | 256.4± 53.6 | 239.8 ±58.9 | 0.12 |
| vBMDc (mg/cm3) | 998.2±73.6 | 998.4±73.7 | 993.5±70.7 | 0.92 |
| vBMDt (mg/cm3) | 206.6±59.4 | 207.6±59.4 | 180.2±54.0 | 0.001 |
| tCSA (mm2) | 380.9±79.9 | 381.1±80.4 | 374.50 ±6.46 | 0.78 |
| cCSA (mm2) | 293.3±64.0 | 293.5±64.1 | 287.8±62.3 | 0.98 |
PPI use.
Data are presented as means ± SD
The P values are age and sex adjusted.
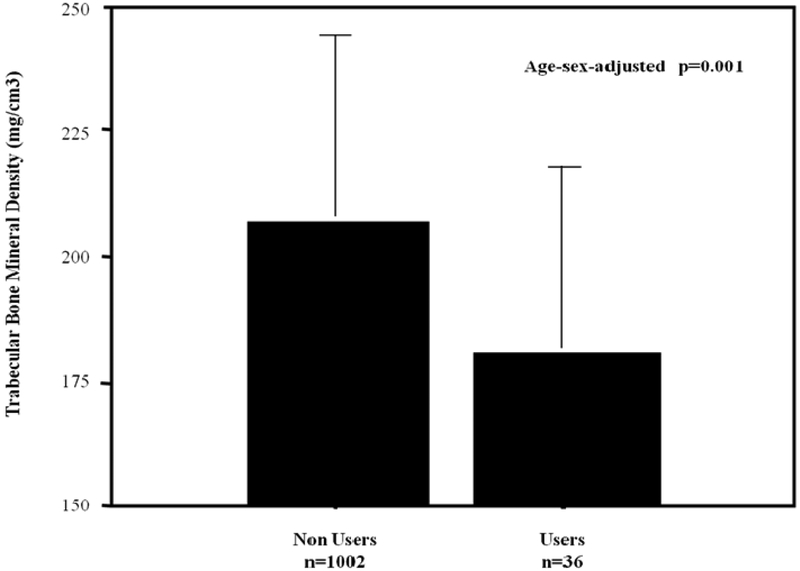
Figure 1. Relationship between proton pump inhibitors (PPI) use and volumetric bone mineral density of trabecular area (vBMDt).
The figure depicts in the vertical axis vBMDt and in the horizontal axis participants categorized according to PPI use.
The difference in vBMDt between PPI users (N=36), green column, and PPI non-users (N=1002), yellow column, was statistically significant after adjustment for age and sex (p=0.001).
After adjustment for multiple potential predictors of vBMDt (model 2) such as caloric intake, PTH, Vit D, Calcium intake, levels of E2, IL-6, IGF-1 and Bio-T, the relationship between PPI use and vBMDt remained statistically significant (Table 3). To disentangle the bias of indication we also tested the differences in pQCT measures in H2 blocker users and non users. N=12 participants were identified as H2 blocker users (1.16% of entire population). After adjustment for age and sex, we did not find any significant relationship between H2 blocker use and BMD (p=0.78), vBMDc (p=0.17), vBMDt (p=0.75), tCSA (p=0.78) and cCSA (p=0.76).
Table 3.
Multivariate analysis of the relationship between PPI use and other predictors of vBMDt (outcome).
| β±SE | p* | |||
|---|---|---|---|---|
| PPI use | −21.0 | ± | 10.7 | 0.041 |
| Sex | −24.5 | ± | 4.3 | <.0001 |
| BMI | 2.7 | ± | 0.5 | <.0001 |
| Calcium intake | −0.01 | ± | 0.006 | 0.05 |
| Physical Activity | 10.5 | ± | 4.8 | 0.02 |
Each line refers to a multivariate analysis adjusted for all the covariates presented in the table and also for IGF-1, log (vit. D), caloric intake, PTH, age, log (Estradiol), log (Bio-T), CSMA, log (IL-6).
1.4. DISCUSSION
Our data add to the literature by showing a negative association between PPI use and trabecular bone mineral density in a small sample of community dwelling older persons. These findings support the hypothesis of a possible direct effect of PPIs on bone mineral metabolism.
This is the first study testing the relationship between PPI use and parameters of bone mass and geometry in older persons. Several studies have shown a significant association between PPI use and bone fractures [24-25], although the number of prospective studies in this regard is limited. Among the four studies [35-38] investigating the relationship between PPI use and BMD, only one has been conducted in older persons [37], Our results are consistent with findings from Ozdil and colleagues [39] who demonstrated that PPI treatment is associated with lower BMD in 114 GERD patients (18-56 years). Interestingly, previous results from adult populations failed to detect any significant difference in BMD between male and female PPI users and non-users.In the 8,340 participants of the Canadian Multicenter Osteoporosis Study, Targownik and colleagues confirmed lower BMD in PPI users than non users at baseline, but this relationship was no longer significant at 5 year and 10 year follow-up periods [40].
The existing epidemiological studies did not investigate the relationship between PPI use and parameters of bone density and geometry, which play an important role in bone strength retention [41-42], Our findings excluded any significant association between use of PPIs and bone geometry. These data are not surprising because parameters of bone geometry are strongly related to bone mechanical stimulation rather than other pharmacological modulators. Conversely, we found a significant relationship between PPI use and vBMDt. This intriguing result may be due to the physiological different characteristics in density, porosity, three-dimensional structure and metabolic activity of trabecular and cortical bone areas [26], The trabecular area is more likely to be sensitive to different metabolic and pharmacological factors than the cortical, and could be assumed as the “metabolically active” part of the bone. Trabecular bone rapidly responds to mechanical stimuli, circulating growth factors and cytokines, because its primary bone cells (located in the surface) are in their closer proximity [26]. Conversely, cortical bone is mainly involved in conferring overall bone strength. By interfering with mineral metabolism PPIs confer a higher risk of fractures through detrimental effects on trabecular bone. These medications directly act on the metabolically more active bone, and may worsen bone quality and bone mineral metabolism without affecting bone geometry. Moreover, the age-related changes of bone quality increase per se the risk of fractures and morbidity [41-42]. These data may concur to explain why the negative association between PPIs and BMD is more evident in older than younger subjects.
Many hypotheses have been proposed to justify the relationship between PPI and bone fractures. However, the mechanisms underlying this association are still unclear. PPI could exert a pharmacological interference with oral bisphosphonates, particularly with alendronic acid, the most used drug for osteoporosis in older populations [21]. By excluding persons taking drugs interfering with bone metabolism, we exclude the plausibility of such a mechanism.
Another possible hypothesis underlying this intriguing relationship is the hypochlorydria and the reduction in gastric proteolysis induced by PPIs. As consequence of hypochlorydria there is decreased bioavailability or reduced absorption of important micronutrients and vitamins involved in bone metabolism such as calcium, magnesium, and vitamin B-12 [43-49]. Calcium absorption is strongly influenced by calcium solubility and gastric acidity. These are important regulators of the release of ionized calcium from calcium sales (e.g. calcium carbonate) through a competitive mechanism between H+ and Ca++ ions. PPIs increase gastric pH from 1.3 to 5.5 [50]. In vitro, calcium dissociation is 96% at a pH of 1, while it decreases to 23% at pH 6.1 [50]. Despite the potential link between PPI use and calcium, we did not find any significant difference in serum calcium levels between PPI users and non users.
Furthermore, PPI-induced chronic gastric acid suppression results in hypergastrinemia [50]. Both hypergastrinemia and reduced calcium bioavailability could negatively affect bone and mineral metabolism probably through the induction of hyperplasia and hypertrophy of the parathyroid glands resulting in an increased PTH levels [50]. The persistently elevated PTH secretion in relation to calcium serum concentration, may lead to an increased risk of fractures due to loss of bone strength and quality [41-42]. However, we failed to find any significant difference in PTH levels between PPI users and non users. Another interesting hypothesis underlying the negative relationship between use of PPIs and fractures has been linked to the known interference of this class of medications with the absorption and excretion of magnesium. Interestingly, many cases of hypomagnesemia have been observed in patients on long term PPI treatment [19,46-47]. PPI-induced hypomagnesemia could exert both direct and indirect effects on bone metabolism [49]. Despite the above mentioned evidence, our data did not show any significant difference in magnesium levels among PPI users and non-users. In elderly patients already at risk of vitamin B12 deficiency (for chronic atrophic gastritis and/or malabsorption syndromes) prolonged PPI use has been shown to worsen hypovitaminosis B12 [43-44], and hyperhomocysteinemia and interfere with collagen crosslinking, impairing bone strength [51-52]. However, similar to PTH, calcium and magnesium, vitamin B12 levels were not significantly different between PPI users and non-users in our population.
1.4.1. Study limitations and strengths
We recognize in our study several limitations. The cross-sectional nature of the study does not allow establishment of a causal relationship between PPI use and the reduction in vBMDt observed in our population. The study also considers a limited number of persons on PPIs and scant information is available about drug exposition and dose. Only 3.5% of the participants were PPI users. This data is not surprising because the InCHIANTI participants were older community dwelling persons and the baseline evaluation was performed in 1998 when the use of PPIs was less common than in the last decade. Ultimately, we cannot completely exclude bias by indication or other unmeasured confounders. However, to partially disentangle this issue we tested the difference in bone parameters between H2 blocker user and non users. After adjustment for age and sex, we did not find any significant relationship between H2 blocker use and vBMD, vBMDc, vBMDt, tCSA and cCSA.
Despite the above mentioned limitations we must consider the study’s important strengths. To our knowledge this can be considered the first study testing the relationship between PPI and vBMD in older persons providing at the same time information on drug use and measures of bone mass and geometry. We excluded participants affected by bone diseases such as osteoporosis or Paget’s disease, treated with medications interfering with bone metabolism and those subjects with primary hyperparathyroidism.
We considered in the analysis potential and important confounders including calcium intake and levels of vitamin D, sex hormones and other very well-known determinants of bone metabolism.
1.4.2. Conclusions
In community dwelling older persons, chronic use of PPIs is negatively associated with vBMDt. The detrimental effect of PPIs on vBMDt might be among the mechanisms by which PPIs increase the risk of fractures in older subjects. Further longitudinal studies are required to better understand whether and how PPI use might negatively influence the “metabolically active” part of the bone.
Highlights.
InCHIANTI study evaluates risk factors for mobility-disability in the elderly
pQCT and medications were analyzed in 1038 older men and women
Difference in bone density and geometry were analyzed in PPI users and non-users
Trabecular bone density is lower in Older PPI users than in non-users
Acknowledgements
The InCHIANTI Study was supported as a “targeted project” (ICS 110.1/RS97.71) by the Italian Ministry of Health and in part by the US National Institute on Aging (Contracts N01-AG-916413 and NO1-AG-821336), and by the Intramural Research Program of the US National Institute on Aging (Contracts 263 MD 9164 13 and 263 MD 821336) and by grant RF-2010-2312659 from the Italian Ministry of Health and Emilia Romagna Region. None of the sponsoring institutions interfered with the collection, analysis, presentation, or interpretation of the data reported here.
We thank Maurizio Conca, Pasquale Rosanova, Maria Teresa Zanelli and Pietro Schianchi for their technical support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE: All authors state that they have no conflicts of interest.
References
- [1].Pilotto A, Franceschi M, Leandro G, Scarcelli C, D'Ambrosio LP, Seripa D, et al. Clinical features of reflux esophagitis in older people: a study of 840 consecutive patients, J. Am. Geriatr. Soc 54 (2006) 1537–1542. [DOI] [PubMed] [Google Scholar]
- [2].Bhatt DL, Scheiman J, Abraham NS, Antman EM, Chan FK, Furberg CD et al. American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents, Circulation. 118 (2008) 1894–909. Erratum in: Circulation. 122 (2010) e438. [DOI] [PubMed] [Google Scholar]
- [3].Bashford JN, Norwood J, Chapman SR, Why are patients prescribed proton pump inhibitors? Retrospective analysis of link between morbidity and prescribing in the General Practice Research Database, B.M.J 317 (1998) 452–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].McCarthy DM, Adverse effects of proton pump inhibitor drugs: clues and conclusions, Curr. Opin. Gastroenterol 26 (2010) 624–631. [DOI] [PubMed] [Google Scholar]
- [5].Forgacs I, Loganayagam A, Overprescribing proton pump inhibitors, B.M.J 336 (2008) 2–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Naunton M, Peterson GM, Bleasel MD, Overuse of proton pump inhibitors, J. Clin. Pharm. Ther 25 (2000) 333–340. [DOI] [PubMed] [Google Scholar]
- [7].Mat Saad AZ, Collins N, Lobo MM, O’Connor HJ, Proton pump inhibitors: a survey of prescribing in an Irish general hospitalÌ, Int. J. Clin. Pract 59 (2005) 31–34. [DOI] [PubMed] [Google Scholar]
- [8].Walker NM, McDonald J, An evaluation of the use of proton pump inhibitors, Pharm. World. Sci 23 (2001) 116–117. [DOI] [PubMed] [Google Scholar]
- [9].Grant K, Al-Adhami N, Tordoff J, Livesey J, Barbezat G, Reith D, Continuation of proton pump inhibitors from hospital to community, Pharm. World. Sci 28 (2006) 189–193. [DOI] [PubMed] [Google Scholar]
- [10].Pasina L, Nobili A, Tettamanti M, Salerno F, Corrao S, Marengoni A et al. REPOSI Investigators. Prevalence and appropriateness of drug prescriptions for peptic ulcer and gastro-esophageal reflux disease in a cohort of hospitalized elderly, Eur. J. Intern. Med 22 (2011) 205–210. [DOI] [PubMed] [Google Scholar]
- [11].Laheij RJ, Sturkenboom MC, Hassing RJ, Dieleman J, Stricker BH, Jansen JB, Risk of community-acquired pneumonia and use of gastric acid-suppressive drugs, J.A.M.A 292 (2004)1955–1960. [DOI] [PubMed] [Google Scholar]
- [12].RodrÍguez LA, RuigÓmez A, Wallander MA, Johansson S, Acid-suppressive drugs and community-acquired pneumonia, Epidemiology. 20 (2009) 800–806. [DOI] [PubMed] [Google Scholar]
- [13].Sultan N, Nazareno J, Gregor J, Association between proton pump inhibitors and respiratory infections: a systematic review and meta-analysis of clinical trials, Can. J. Gastroenterol 22 (2008) 761–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Johnstone J, Nerenberg K, Loeb M, Meta-analysis: proton pump inhibitor use and the risk of community-acquired pneumonia, Aliment. Pharmacol. Ther 31 (2010) 1165–1177. [DOI] [PubMed] [Google Scholar]
- [15].Bavishi C, Dupont HL, Systematic review: the use of proton pump inhibitors and increased susceptibility to enteric infection, Aliment. Pharmacol. Ther 34 (2011) 1269–1281. [DOI] [PubMed] [Google Scholar]
- [16].Howell MD, Novack V, Grgurich P, Soulliard D, Novack L, Pencina M et al. Iatrogenic gastric acid suppression and the risk of nosocomial Clostridium difficile infection, Arch. Intern. Med 170 (2010) 784–790. [DOI] [PubMed] [Google Scholar]
- [17].Linsky A, Gupta K, Lawler EV, Fonda JR, Hermos JA, Proton pump inhibitors and risk for recurrent Clostridium difficile infection, Arch. Intern. Med 170 (2010) 772–778 Erratum in: Arch. Intern. Med. 170 (2010) 1100. [DOI] [PubMed] [Google Scholar]
- [18].Abraham NS, Proton pump inhibitors: potential adverse effects, Curr. Opin. Gastroenterol 28 (2012) 615–620. [DOI] [PubMed] [Google Scholar]
- [19].Regolisti G, Cabassi A, Parenti E, Maggiore U, Fiaccadori E Severe hypomagnesemia during long-term treatment with a proton pump inhibitor, Am. J. Kidney. Dis 56 (2010) 168–174. [DOI] [PubMed] [Google Scholar]
- [20].Charlot M, Grove EL, Hansen PR, Olesen JB, Ahlehoff O, Selmer C et al. Proton pump inhibitor use and risk of adverse cardiovascular events in aspirin treated patients with first time myocardial infarction: nationwide propensity score matched study, B.M.J 342 (2011) d2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Abrahamsen B, Eiken P, Eastell R, Proton pump inhibitor use and the antifracture efficacy of alendronate, Arch. Intern. Med 171 (2011) 998–1004. [DOI] [PubMed] [Google Scholar]
- [22].Maggio M, Corsonello A, Ceda GP, Cattabiani C, Lauretani F, ButtÒ V et al. Proton pump inhibitors and risk of 1-year mortality and rehospitalization in older patients discharged from acute care hospitals, J.A.M.A. Intern. Med 173 (2013) 518–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].U.S. Food and drug Administration: possible fracture risk with high dose, long-term use of proton pump inhibitors. Available from http://www.fda.gov/drugs/drugsafety/postmarketdrugsafetyinformationforpatientsand providers/ucm213206.htm. Accessed March 15, 2011.
- [24].Yu EW, Bauer SR, Bain PA, Bauer DC, Proton pump inhibitors and risk of fractures: a meta-analysis of 11 international studies, Am. J. Med 124 (2011) 519–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kwok CS, Yeong JK, Loke YK, Meta-analysis: risk of fractures with acid-suppressing medication, Bone. 48 (2011) 768–776. [DOI] [PubMed] [Google Scholar]
- [26].Russo CR, Lauretani F, Bandinelli S, Bartali B, Di Iorio A, Volpato S, Aging bone in men and women: beyond changes in bone mineral density, Osteoporos. Int 14 (2003) 531–538. [DOI] [PubMed] [Google Scholar]
- [27].Lauretani F, Bandinelli S, Griswold ME, Maggio M, Semba R, Guralnik JM, Ferrucci L. Longitudinal changes in BMD and bone geometry in a population-based study, J Bone. Miner. Res 23 (2008) 400–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ferrucci L, Bandinelli S, Benvenuti E, Di Iorio A, Macchi C, Harris TB et al. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study, J. Am. Geriatr. Soc 48 (2000) 1618–1625. [DOI] [PubMed] [Google Scholar]
- [29].Lauretani F, Bandinelli S, Russo CR, Maggio M, Di Iorio A, Cherubini A, Maggio D, Ceda GP, Valenti G, Guralnik JM, Ferrucci L, Correlates of bone quality in older persons, Bone. 39 (2006) 915–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Pahor M, Chrischilles EA, Guralnik JM, Brown SL, Wallace R, Carbonin PU, Drug data coding and analysis in epidemiologic studies, Eur. J. Clin. Epidemiol 10 (1994) 405–411. [DOI] [PubMed] [Google Scholar]
- [31].Siris ES, Miller PD, Barrett-Connor E, Faulkner KG, Wehren LE, Abbott TA, Berger ML, Santora AC, Sherwood LM, Identification and fracture outcomes of undiagnosed low bone mineral density in postmenopausal women: results from the National Osteoporosis Risk Assessment, J.A.M.A 286 (2001) 2815–2822. [DOI] [PubMed] [Google Scholar]
- [32].Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum, J. Clin. Endocrinol. Metab 84 (1999) 3666–3672. [DOI] [PubMed] [Google Scholar]
- [33].Pisani P, Faggiano F, Krogh V, Palli D, V ineis P, Berrino F, Relative validity and reproducibility of a food frequency dietary questionnaire for use in the Italian EPIC centres, Int. J. Epidemiol 26 (1997) S152–S160. [DOI] [PubMed] [Google Scholar]
- [34].Bartali B, Turrini A, Salvini S, Lauretani F, Russo CR, Corsi AM et al. Dietary intake estimated using different methods in two Italian older populations, Arch. Gerontol. Geriatr 38 (2004) 51–60. [DOI] [PubMed] [Google Scholar]
- [35].Lau YT, Ahmed NN, Fracture risk and bone mineral density reduction associated with proton pump inhibitors, Pharmacotherapy. 32 (2012) 67–79. [DOI] [PubMed] [Google Scholar]
- [36].Gray SL, aCroix LAZ, Larson J, Robbins J, Cauley JA, Manson JE et al. Proton pump inhibitor use, hip fracture, and change in bone mineral density in postmenopausal women: results from the Women’s Health Initiative, Arch. Intern. Med 170 (2010) 765–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Yu EW, Blackwell T, Ensrud KE, Hillier TA, Lane NE, Orwoll E, Acid suppressive medications and risk of bone loss and fracture in older adults, Calcif. Tissue. Int 83 (2008) 251–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Targownik LE, Lix LM, Leung S, Leslie WD, Proton-pump inhibitor use is not associated with osteoporosis or accelerated bone mineral density loss, Gastroenterology. 138 (2010) 896–904. [DOI] [PubMed] [Google Scholar]
- [39].Ozdil K, Kahraman R, Sahin A, Calhan T, Gozden EH, Akyuz U et al. Bone density in proton pump inhibitors users: a prospective study, Rheumatol. Int 2013. March 2. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- [40].Targownik LE, Leslie WD, Davison KS, Goltzman D, Jamal SA, Kreiger N et al. The relationship between proton pump inhibitor use and longitudinal change in bone mineral density: a population-based study [corrected] from the Canadian Multicentre Osteoporosis Study (CaMos), Am. J. Gastroenterol 107 (2012) 1361–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Chen H, Zhou X, Fujita H, Onozuka M, Kubo KY, Age-related changes in trabecular and cortical bone microstructure, Int. J. Endocrinol 2013 (2013) 213–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Rittweger J, Beller G, Ehrig J, Jung C, Koch U, Ramolla J et al. Bone-muscle strength indices for the human lower leg, Bone. 27 (2000) 319–326. [DOI] [PubMed] [Google Scholar]
- [43].Valuck RJ, Ruscin JM, A case-control study on adverse effects: H2 blocker or proton pump inhibitor use and risk of vitamin B12 deficiency in older adults, J. Clin. Epidemiol 57 (2004) 422–428. [DOI] [PubMed] [Google Scholar]
- [44].Marcuard SP, Albernaz L, Khazanie PG, Omeprazole therapy causes malabsorption of cyanocobalamin (vitamin B12), Ann. Intern. Med 120 (1994) 211–215. [DOI] [PubMed] [Google Scholar]
- [45].Recker RR, Calcium absorption and achlorhydria, N. Engl. J. Med 313 (1985) 70–73. [DOI] [PubMed] [Google Scholar]
- [46].Undy TC, Dissanayake A, Severe hypomagnesaemia in long-term users of proton-pump inhibitors, Clin. Endocrinol 69 (2008) 338–341. [DOI] [PubMed] [Google Scholar]
- [47].Hoorn EJ, van der Hoek J, de Man RA, Kuipers EJ, Bolwerk C, Zietse R, A case series of proton pump inhibitor-induced hypomagnesemia, Am J Kidney Dis 56 (2010) 112–116. [DOI] [PubMed] [Google Scholar]
- [48].Yang YX, Chronic proton Pump Inihibitor Therapy and Calcium Metabolism, Curr Gastroenterol. Rep 14 (2012) 473–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Faulhaber GA, Furlanetto TW, Could magnesium depletion play a role on fracture risk in PPI users?, Arch. Intern. Med 170 (2010) 1776. [DOI] [PubMed] [Google Scholar]
- [50].VerdÚ EF, Fraser R, Armstrong D, Blum AL, Effects of omeprazole and lansoprazole on 24-hour intragastric pH in Helicobacter pylori-positive volunteers, Scand. J. Gastroenterol 29 (1994) 1065–1069. [DOI] [PubMed] [Google Scholar]
- [51].Saito M, Fujii K, Marumo K, Degree of mineralization-related collagen crosslinking in the femoral neck cancellous bone in cases of hip fracture and controls, Calcif. Tissue. Int 79 (2006) 160–168. [DOI] [PubMed] [Google Scholar]
- [52].McLean RR, Jacques PF, Selhub J, Tucker KL, Samelson EJ, Broe KE, Hannan MT, et al. Homocysteine as a predictive factor for hip fracture in older persons, N. Engl. J. Med 350 (2004) 2042–2049. [DOI] [PubMed] [Google Scholar]