SUMMARY
Vitamin B12 is made by only certain prokaryotes yet is required by a number of eukaryotes such as mammals, fish, birds, worms, and Protista, including algae. There is still much to learn about how this nutrient is trafficked across the domains of life. Herein, we describe ways to make a number of different corrin analogs with fluorescent groups attached to the main tetrapyrrole-derived ring. A further range of analogs were also constructed by attaching similar fluorescent groups to the ribose ring of cobalamin, thereby generating a range of complete and incomplete corrinoids to follow uptake in bacteria, worms, and plants. By using these fluorescent derivatives we were able to demonstrate that Mycobacterium tuberculosis is able to acquire both cobyric acid and cobalamin analogs, that Caenorhabditis elegans takes up only the complete corrinoid, and that seedlings of higher plants such as Lepidium sativum are also able to transport B12.
In Brief
Lawrence et al., employed chemical biology approaches to construct a range of fluorescent vitamin B12 derivatives. They demonstrated that these fluorescent variants can be used to follow intracellular B12 trafficking in bacteria, including E. coli and M. tuberculosis, the worm C. elegans, and a higher plant (Lepidium sativum).
Graphical Abstract
INTRODUCTION
The cobamides encompass a group of closely structurally related nutrients, cofactors, and coenzymes that harbor a cobalt-containing corrin ring, and are often loosely referred to as vitamin B12 (Figure 1) (Renz, 1999; Warren et al., 2002). These molecules vary in the nature of the upper ligand attached to the cobalt and the character of the lower nucleotide loop. Cobalamin, for instance, contains dimethylbenzimidazole as the base in the nucleotide loop but this is replaced with adenine in pseudocobalamin (Figure 1) (Degnan et al., 2014; Maggio-Hall and Escalante-Semerena, 2003). In the biologically active forms of cobalamin the cobalt ion is normally either adenosylated (adenosylcobalamin) or methylated (methylcobalamin) (Banerjee and Ragsdale, 2003), while vitamin B12 represents the cyanolated derivative of cobalamin that is produced during the commercial extraction and isolation of the nutrient (Martens et al., 2002). However, the term B12 is frequently used to refer to all forms of cobalamin.
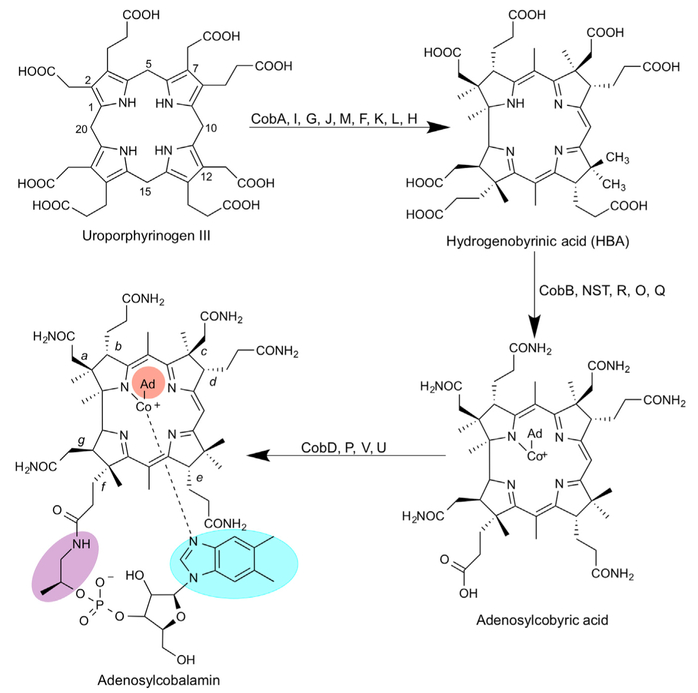
Figure 1. Biosynthesis of Adenosylcobalamin from Uroporphyrinogen III.
Initially, uroporphyrinogen III is acted upon by the enzymes in the sequence of CobA, I, G, J, M, F, K, L, and H to generate a cobalt-free corrinoid called HBA. Amidation of the a and c side chains followed by metal insertion, adenosylation, and the remaining amidations by CobB, NST, R, O, and Q gives rise to adenosylcobyric acid. The final stages of the biosynthesis see the attachment of an aminopropanol side chain and the alpha ribazole nucleotide, which contains the unusual base dimethylbenzimidazole. These reactions are mediated by the enzymes CobD, P, V, and U. The numbering of the corrin macrocyle (1–20) is shown as is the lettering associated with the side chains (a-g). Different variants of cobalamin are found through changes in the highlighted areas of the molecule. The upper ligand (orange) can be a methyl, cyano, or water group, the linking aminopropanol (purple) is an ethanolamine molecule in nor-cobalamin, and a range of different bases can replace the dimethylbenzimidazole (blue).
Cobamides are produced exclusively by certain prokaryotes (Roth et al., 1996). Apart from cobalamin and pseudocobalamin, further variants with shorter nucleotide chain lengths and/or alternative nucleotide bases are synthesized by different bacteria (Degnan et al., 2014; Krautler et al., 2003; Renz, 1999). Cobamides are made via either an anaerobic or an aerobic biochemical pathway, both involving around 30 enzyme-mediated steps (Blanche et al., 1995; Deery et al., 2012; Moore et al., 2013; Warren et al., 2002). The key steps of the aerobic pathway are shown in Figure 1, which concisely depicts contraction of the tetrapyrrole ring, insertion of the cobalt ion, and building of the nucleotide loop. Variations in the construction of the lower nucleotide loop, mediated by different substrate preferences for bases and linkers (Figure 1) during its biosynthesis, give rise to the assorted cobamides that are found in nature (Chan and Escalante-Semerena, 2011; Crofts et al., 2013; Krautler et al., 2003; Maggio-Hall and Escalante-Semerena, 2003; Zayas et al., 2007). Many prokaryotic microorganisms (Allen and Stabler, 2008; Degnan et al., 2014; Gray and Escalante-Semerena, 2009) and microbial eukaryotes (Croft et al., 2005; Helliwell et al., 2016) rely on other producers of cobamides, where both bacteria and archaea can be counted, and therefore have to recover the nutrient from the environment they inhabit. Such salvage systems often involve remodeling of the lower nucleotide loop to produce the corrin variant that suits the new host.
In this respect bacteria, as well as archaea, can be divided into a range of classes with regard to their B12 requirements. There are bacteria that make their own cobalamin, those that are auxotrophic for the nutrient, those that require cobalamin for some non-essential processes, and those that do not require the nutrient at all. In this way the nutrient plays an important role in bacterial ecology, especially in complex microbial communities (Degnan et al., 2014; Helliwell et al., 2016). However, little is known about the movement of cobamides in such communities, whether it is specifically shared or exchanged in mutualistic or symbiotic relationships.
Bacteria are also the source of cobalamin for a large number of eukaryotes that require the nutrient for either specific methylation or isomerization reactions. For instance, approximately 50% of all microalgal species require cobalamin for growth, and it has been shown that bacteria can provide this compound in course of a symbiotic exchange for photosynthate (Croft et al., 2005). Similarly, enteric bacteria are the source of cobalamin (Roth et al., 1996) in ruminants such as cattle and, as dairy products and meat represent a major form of human dietary cobalamin, and are therefore also responsible for most of the cobalamin found in humans (Brito et al., 2015; Girard et al., 2009). Other eukaryotes, including nematode worms (Yilmaz and Walhout, 2014), fish (Greibe et al., 2012), and birds (Zaman and Zak, 1989) have to acquire cobalamin as part of their diets from B12-producing bacteria. Within eukaryotic cells cobalamin is further compartmentalized, with methylcobalamin found in the cytoplasm associated with methionine synthase, and adenosylcobalamin located within the mitochondria with its cognate enzyme methylmalonyl coenzyme A (CoA) mutase (Gherasim et al., 2013; Watkins and Rosenblatt, 2013). There is the added complication of translocation through the body of multicellular organisms, requiring some form of vascular transport.
Therefore, from their prokaryotic origins, cobamides such as cobalamin undergo a complex journey through many different domains and kingdoms of life, a journey that involves scavenging, recycling, modification, and compartmentalization. In terms of understanding the role played by cobamides in ecology, health, and disease there is a need to devise sensitive methods to follow the nutrient on this biological voyage. Following the movement of cobamides has proved difficult as the nutrient is normally required in small quantities. One way of following cobamides along their transformation and transportation chain is to use fluorescent derivatives coupled to the cobalamin molecule to allow its detection via fluorescence microscopy (Lee and Grissom, 2009; Smeltzer et al., 2001). The most common of these fluorescent cobalamin derivatives involve attachment of a fluorophore to either the cobalt ion or to the ribose ring of the lower nucleotide. However, attachment to the cobalt ion involves modifications that are usually light sensitive, whereas conjugation to the ribose can be easily removed through nucleotide loop remodeling when such molecules are taken up by bacteria. Attachment of fluorophores to the side chains of the corrin macrocycle have also been reported. However, this attachment requires the hydrolysis of one of the amide side chains and the separation of a range of derivatives prior to the modification. This is low yielding process. Alternatively, a fluorophore could also be attached to the carboxylic acid side chain of cobyric acid, but this would prevent the molecule from being converted into a cobalamin analog in vivo. Herein, we describe ways to attach fluorophores directly to the C5 position of the corrin ring according to our understanding of how the corrin ring is constructed. We also report on which of these C5-modified corrin variants, together with some ribose-modified fluorophores, are taken up by a range of different organisms.
RESULTS
Synthesis of C5-allyl-HBA
The transformation of uroporphyrinogen III into a corrin involves, among other events, the addition of 8 S-adenosyl-L-methionine (SAM)-derived methyl groups, the last of which is added to the C5 position (Deery et al., 2012; Warren et al., 2002). We hypothesized that it may be possible to use SAM analogs, with extended chain lengths, to alkylate the C5 position (Figure 2). This requires the isolation of precorrin-7, the substrate for the final methyltransferase, CobL. We recently reported the construction of a recombinant Escherichia coli strain from which precorrin-7 can be purified (Deery et al., 2012). Methylation of precorrin-7 by CobL generates precorrin-8, which is isomerized by CobH to generate hydrogenobyrinic acid (HBA). We sought to modify the C5 position by employing an allyl analog of SAM (Dalhoff et al., 2006a, 2006b) with a view to making the respective C5-modified HBA derivative (C5-allyl-HBA) (Figure 2).
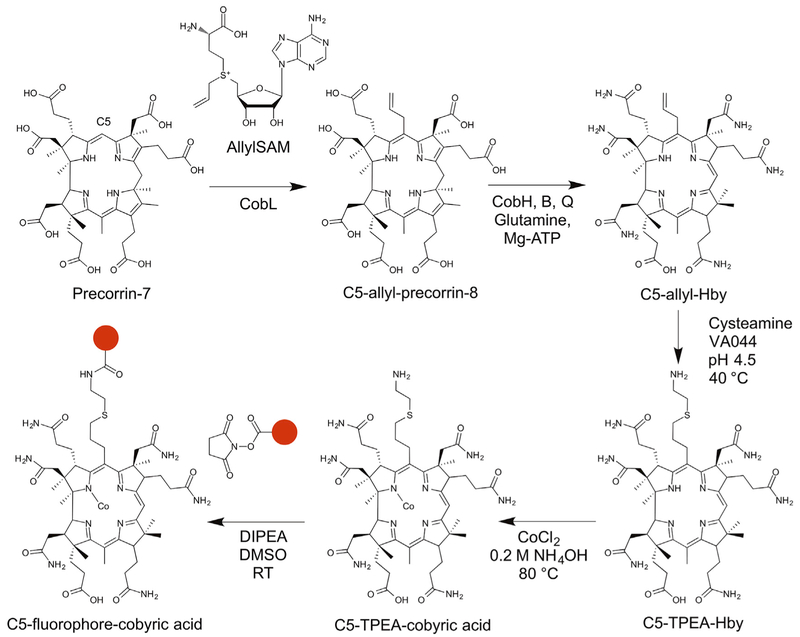
Figure 2. The ChemBio Synthesis of a C5 Analog of Cobyric Acid.
Precorrin-7 is modified with an allyl-SAM derivative by the action of the C5 methyltransferase, CobL. This results in the production of C5-allyl-precorrin-8, which is acted upon by the enzyme CobH, B, and Q to generate C5-allyl-Hby. Cysteamine is next chemically attached to the allyl group to generate C5-(TPEA)-Hby prior to the chemical insertion of cobalt to give C5-(TPEA)-cobyric acid. The coupling of a fluorophore via the free amine group on C5 generates the C5-fluorophore conjugate of cobyric acid. The fluorophore is represented by the filled red circles.
When precorrin-7 was incubated with CobL, CobH, and allyl-SAM the color of the solution changed from yellow to orange, consistent with the expected enzymatic transformation. The observed shift in the UV-visible spectrum of the starting material with a maximum absorption at 400 nm to a new species with maxima at 328, 520, and 555 nm is characteristic of the formation of an HBA-like molecule. Analysis of the reaction mixture by high-performance liquid chromatography-mass spectrometry (HPLC-MS) identified a new compound with m/z 907.4 (Figure S1). The observed increase in mass and change in UV-visible spectrum confirms the addition of an allyl group to the macrocycle and indicates that migration of the C11 methyl group to C12 has occurred.
The yield of allyl-HBA obtained from this initial reaction was less than 50%, with the other major product being allyl-precorrin-8. The presence of allyl-precorrin-8 suggested that CobH is the rate-limiting step in this reaction sequence. We considered that the lower activity of CobH could be due to spatial constraints resulting from the larger substrate and, therefore, we decided to make a number of structure-based (Shipman et al., 2001) mutations that would introduce more space into the active site, especially around the region of the protein where the C5 position of the substrate is found. One of these mutations, T85A, did indeed significantly improve the yield, such that the C5-allyl-HBA was obtained in greater than 85% yield.
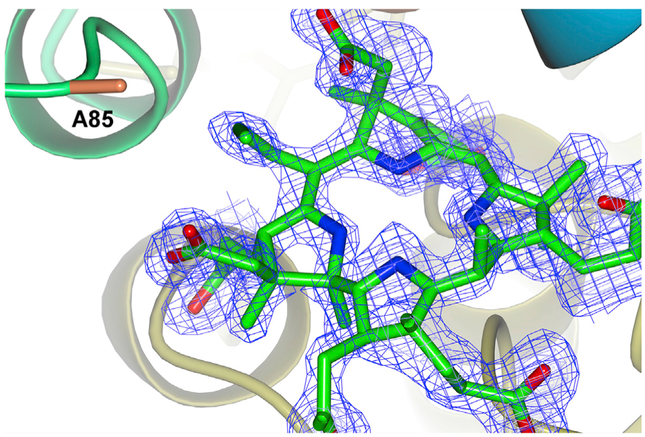
The structure of the product was further confirmed as being C5-allyl-HBA by X-ray crystallography. This was achieved by determining the structure of the CobHT85A-C5-allyl-HBA product complex at 1.6 Å resolution (Figure 3; Table S1). The extended alkyl group at the C5 position is clearly seen in the structure. Although the T85A mutation of CobH does increase the size of the active site around the C5 position, the allyl group is still constrained, suggesting that further enhancement could be afforded with more rational engineering. The PDB coordinates are deposited under the code PDB: 5N0G.
Figure 3. Product Complex of CobHT85A with C5-allyl-HBA.
The figure shows the position of the reaction product bound at the active site of the enzyme. The proximity of the C5 group to Ala85 demonstrates how the removal of the threonine side chain has provided extra space to accommodate the allyl group.
We also explored the synthesis of C5-propargyl-HBA using a propargyl SAM derivative (Dalhoff et al., 2006a, 2006b). To achieve this outcome it was necessary to prepare the respective SAM analog in situ from Se-propargyl-L-homocysteine using a modified SAM synthetase (I117A mutant of hMAT 2A) as the propargyl-SAM analog is not stable at physiological pH (Wang et al., 2013, 2014). Incubation of precorrin-7 with Se-propargyl-Hcy, Mg-ATP, hMAT 2A I117A, CobL, or CobHT85A, resulted in the same characteristic shift in the UV-visible spectrum associated with the formation of an HBA-like chromophore. Analysis of the purified product by HPLC-MS confirmed the addition of a propargyl group with an observed m/z of 905.4 (Figure S1). This approach demonstrated that a number of C5 derivatives could be made using activated SAM variants. The propargyl-compounds have the advantage of being able to be used with click chemistry approaches for further functionalization.
Conversion of the C5-allyl-HBA to Its Cobyric Acid Form
The conversion of HBA to HBA a,c-diamide (HBAD) is mediated by CobB, which amidates the acetic acid side chains attached to the C2 and C7 positions of the corrin framework (Figure 2) (Crouzet et al., 1990). Incubation of CobB with C5-allyl-HBA, in the presence of glutamine and Mg-ATP, resulted in the production of C5-allyl HBAD. The product of the reaction was found to have a shorter retention time on the HPLC column and a 2-mass unit decrease in comparison with the starting material, all of which is consistent with the amidation of the a and c side chains.
In the aerobic biosynthesis of cobalamin, HBAD undergoes cobalt chelation and adenosylation prior to the completion of the amidation reactions by CobQ (Blanche et al., 1995). However, the light-sensitive nature of the adenosylated intermediate makes this a difficult compound with which to do further modifications. We thus sought a way to bypass the metal-insertion stage of the pathway in order to complete the amidation of HBAD to generate hydrogenobyric acid (Hby), the cobalt-free analog of cobyric acid. This was achieved by using the CobQ from Allochromatium vinosum, an organism that was shown to accumulate cobalt-free corrinoids when grown in the absence of cobalt (Koppenhagen et al., 1973). The purified recombinant A. vinosum CobQ, in the presence of glutamine and Mg-ATP, did indeed catalyze the amidation of the b, d, e, and g side chains of HBAD, to generate Hby. Similarly, the enzyme also accepted the C5-allyl-HBAD as a substrate and converted it into C5-allyl Hby (Figure 2), whose structure was confirmed by HPLC-MS (m/z 901) (Figure S1) and 2D heteronuclear nuclear magnetic resonance (NMR) spectroscopy (Figure S2; Table S2).
Transformation of the C5-allyl-HBA Analogs into Cobyric Acid Analogs
The allyl group was modified to a primary amine through a thiolene reaction by reacting the C5-allyl-Hby with cysteamine in the presence of the radical initiator VA-044 (2,2′-azobis[2-(2-imidazolin-2-yl)propane]dihydrochloride) (Figure 2). The reaction product, C5-(2-thiopropane-1-aminoethane [TPEA])-Hby, was confirmed by HPLC-MS (Figure S1). Cobalt was inserted chemically using CoCl2 in dilute aqueous ammonia at elevated temperature. The reaction was quenched with KCN and the product was purified by RP18 chromatography. The structure of the C5-(TPEA)-cobyric acid was confirmed by HPLC-MS (Figure S1) and NMR (Figure S3; Table S2). Together, all of these steps demonstrate how a combination of in vitro enzyme-mediated transformations coupled with chemical approaches allow the construction of a regio-specifically derivatized form of cobyric acid (Figure 2).
C5 Fluorophore Attachment
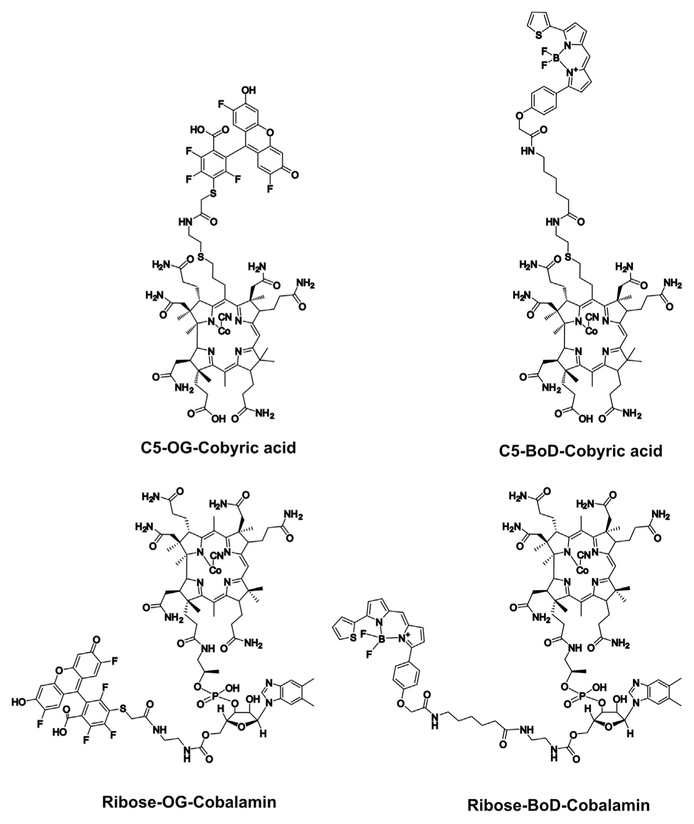
To attach the fluorophores BODIPY TR-X (BoD) and Oregon green 514 (OG) to the terminal amine of the C5 extension, the amine was reacted with the N-hydroxysuccinimide ester of the fluorophore (Figure 2). All of the products were analyzed by HPLC-MS and purified by semi-preparative HPLC. As a result it was possible to generate both BoD and OG C5-linked forms of cobyric acid (Figure 4). These compounds were further characterized in terms of their functionality and uptake in biological systems as described below.
Figure 4. Chemical Structures of the Four Fluorescent Analogs of Cobalamin.
The top two structures show the attachment of Orgeon green (GO) and BoDIPY (BoD) to the C5 position of the corrin ring, whereas the lower two structures show how these fluorophores are attached to the ribose moiety of the lower nucleotide loop.
Synthesis of Ribose-Linked Analogs
The ribose-linked analogs were synthesized by carbonyldiimidazole coupling of ethylene diamine to the C5-OH of the ribose moiety of vitamin B12, then the primary amine was linked to the N-hydroxysuccinimide ester of either OG or BoD (Figure 4). These were made following previously published methods for the construction of ribose-linked analogs of cobalamin (Horton et al., 2003; McEwan et al., 1999). The products were analyzed by HPLC-MS, and purified by semi-preparative HPLC. Making both fluorescently labeled cobyric acid and cobalamin derivatives provided the opportunity to compare their respective activities in terms of their ability to be transported into biological systems and ability to act as functionalized nutrients.
Biological Activity of the Corrin Analogs
The biological activity of the C5-cobyric acid analogs as well as the ribose-linked derivatives was investigated initially by comparing their effect in a B12-dependent microbial bioassay (Raux et al., 1996). In this case, an agar plate embedded with B12-dependent reporter strain of Salmonella enterica (AR3612; cysG, metE) was used to detect growth around an application point, where the size of any resulting growth circle can be measured against growth observed with known amounts of B12. With the microbial bioassay we looked at the efficacy of the C5-(TPEA)-cobyric acid, the BoD, and OG C5-cobyric acid, as well as the BoD and OG ribose analogs of cobalamin, to support growth of the strain. The observed growth circles can be seen in Figure 5. We were initially surprised to see that the C5-(TPEA)-cobyric acid and the two fluorescently labeled versions of cobyric acid and cobalamin all promoted growth, albeit they needed to be added at much higher concentrations than cyanocobalamin. The requirement for a higher concentration of the analog not only likely reflects poorer molecular recognition by the uptake mechanism but also recognition by methionine synthase. The cobalamin derivatives were more active than the C5 analogs, and the OG derivatives were more active than the corresponding BoD compounds. The reduced efficacy of the cobyric acid analogs in comparison with the cobalamin analogs is probably because the cobyric acid intermediates have to be converted into cobalamin forms before growth can occur.
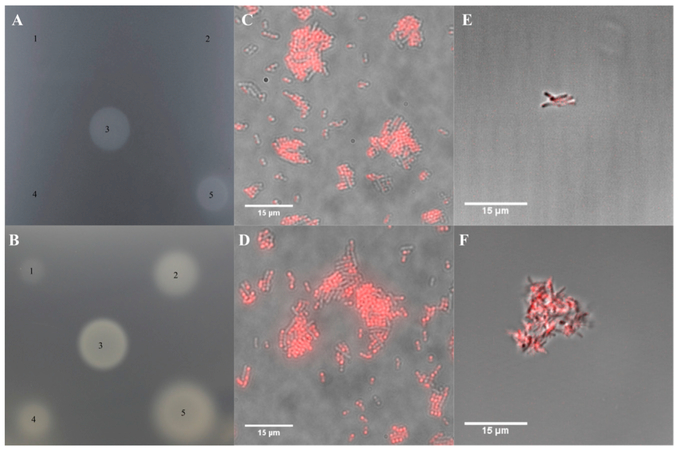
Figure 5. Effect of Cobyric Acid and Cobalamin Analogs on Bacteria.
(A) A bioassay plate containing an S. enterics strain that requires cobyric acid or later pathway intermediates for growth. Addition of a known amount of vitamin B12 (spot 3) allows growth. Spots 1, 2, and 4 represent C5-allyl-hydrogenobyrinic acid a,c-diamide (C5-allyl-HBAD), C5-allyl-Hby, and C5-(TPEA)-Hby, none of which promote growth. Spot 5 contains the C5-(TPEA)-cobyric acid analog and promotes growth.
(B) The same bioassay plate assay as in (A), with spot 3 containing a standard vitamin B12 solution. Spots 1, 2, 4, and 5 are solutions of C5-BoD-co-byricacid, ribose-BoD-cobalamin, C5-OG-cobyric acid, and ribose-OG-cobalamin, respectively. All the analogs support growth although the C5 analogs are weaker than the respective ribose-linked analogs.
(C and D) Overlay pictures of E. coli cells that have been grown in the presence of ribose-BoD-cobalamin and C5-BoD-cobyric acid, respectively. The co-localization of the red fluorescence with the cells indicates that E. coli has taken up both compounds.
(E and F) Cultures of M. tuberculosis, grown in the presence of ribose-BoD-cobalamin and C5-BoD-cobyric acid, respectively. Again, the presence of the red fluorescence with the cells indicates that M. tuberculosis is able to take up both analogs.
Uptake of Analogs into Bacteria
We next investigated the uptake of the fluorescent forms of cobyric acid and cobalamin into E. coli BL21 (DE3). The BL21 strain has a mutation in btuB, which encodes the outer membrane transport component of the cobalamin uptake system (Studier et al., 2009). To overcome this limitation, the BL21 cells were transformed with pLysS-btuB and induced overnight with isopropyl β-D-1-thiogalactopyranoside, at which point 1 μM of the OG analogs were added. The bacteria were then imaged by wide-field fluorescence microscopy and the resulting images demonstrated that, while E. coli is able to take up both OG fluorescent analogs, significantly greater fluorescence was observed with the cobalamin derivative, suggesting that it is taken up more efficiently.
For the BoD analogs, E. coli OP50 cells were transformed with a plasmid harboring both btuB and btuF (pET-BAD-btuB-btuF), where btuF encodes the periplasmic cobalamin binding protein of the B12 transporter. These were cloned in order to ensure maximal uptake of the corrin analogs, which were added to a final concentration of 1 μM at the same time as the cells were induced. Cells were incubated separately with C5-BoD-cobyric acid, ribose-BoD-cobalamin, or the unattached BoD fluorophore. As judged by the internal fluorescence of the bacteria, both the C5-BoD and ribose-BoD analogs were seen to accumulate within the cells, indicating that these compounds can be used to monitor transport into E. coli (Figure 5). No internal fluorescence was observed with the unattached BoD fluorophore. The significance of using E. coli OP50 is that this strain can also be used as a source of food for nematodes such as C. elegans.
The BoD analogs of cobalamin were also incubated with E. coli BL21 (DE3) and JM109 cells. No fluorescence was observed with the BL21 (DE3) cells, indicating that the defective btuB gene prevent uptake of the analog, even at high (1 μM) concentrations. In contrast, uptake of the C5-BoD-cobyric acid analog into JM109 cells was detectable at levels of 10 nM, while the ribose-BoD-cobalamin analogs was detectable at a concentration of 100 nM (Figure S4). These results demonstrate that the uptake of the analogs is dependent upon the presence of a functional BtuB, and that there is some variance between the analogs in regard to molecular recognition by the uptake mechanism.
There is always the possibility that the fluorescently labeled compounds undergo breakdown within the cell. To investigate the possibility of the breakdown of the analogs, we extracted both the ribose-OG-cobalamin and the C5-BoD-cobyric acid analog from E. coli that had been grown in their presence. In both cases we were able to extract the analogs intact from the cells after several days of growth. No evidence for degradation of the compounds was observed (Figure S5).
We also investigated the uptake of the BoD analogs into Mycobacterium tuberculosis. Although M. tuberculosis has a B12 pathway it is missing CobF (Gopinath et al., 2013a, 2013b; Rodionov et al., 2003) and cannot make its own cobalamin. Moreover, M. tuberculosis has a different B12 uptake mechanism, employing BacA as a transporter (Gopinath et al., 2013a, 2013b). We investigated B12 uptake into a B12-dependent strain of M. tuberculosis, H37Rv, which carries a mutation in metE (H37Rv-metE) (Warner et al., 2007). After growth in Sauton’s defined medium, supplemented with either the C5-BoD-cobyric acid or the ribose-BoD-cobalamin, the cells were found to be fluorescent after 24 and 48 hr, respectively, using fluorescence microscopy (Figure 5). It is clear, therefore, that M. tuberculosis can take up analogs of both cobyric acid and cobalamin, and that both of these can support growth.
B12 Uptake into C. elegans
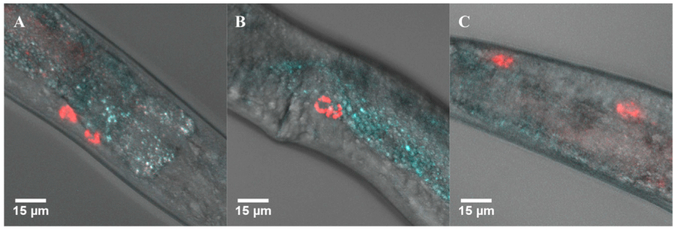
Several recent papers have demonstrated that a free-living nematode, C. elegans, represents a good eukaryotic model system to study B12 as a nutrient (Bito et al., 2013, 2017; Watson et al., 2014). Like humans, C. elegans requires B12 for the activities of both B12-dependent methionine synthase and methylmalonyl CoA mutase. Thereby, B12 deficiency causes infertility, retarded growth, and reduced lifespan (Bito et al., 2013). Bioinformatic analysis has revealed the presence in the C. elegans genome of genetic orthologs associated with human inherited B12 disorders but, interestingly, there is no evidence of the main B12 binding and trafficking proteins such as intrinsic factor or transcobalamin (Yilmaz and Walhout, 2014). To investigate B12 uptake into C. elegans, nematodes of the N2 Bristol strain were fed with E. coli OP50 that had been grown in the presence of either C5-BoD-cobyric acid, ribose-BoD-cobalamin, or unattached BoD. L4 worms that had been grown in the presence of these analogs since their embryonic stage were imaged on a confocal microscope. Only worms fed on E. coli containing the ribose-BoD-cobalamin revealed any fluorescence within the body. Interestingly, confocal microscopy revealed that, in these worms, the fluorescence was localized to discrete spots in the head, vulva, and tail regions, corresponding to the coelomocytes (Figure 6). Even within this discrete cell type, the fluorescence appears to show a degree of sub-cellular localization or compartmentalization (Figure 6). This result demonstrates that C. elegans is able to absorb and retain the BoD-labelled cobalamin (which was concentrated in some organs), whereas retention of the C5-cobyric acid analog was clearly absent.
Figure 6. Localization of Ribose-BoD-Cobalamin to Coelomocytes in C. elegans.
(A–C) C. elegans were fed with E. coli OP50 that had been grown in the presence of ribose-BoD-cobalamin and were imaged after several days. The red fluorescence associated with the fluorophore was found localized in the six coelomocytes, two of which are found at the head (A), two near the vulva (B) and two at the anus (C).
B12 Uptake into Higher Plants
It is generally accepted that land plants neither make nor require B12 and live in a B12-less world. Early reports suggesting that some plants contained B12-dependent enzyme activity were later put down to bacterial contamination. However, there does appear to be some evidence suggesting that some plants can at least take up and transport cobalamin if the nutrient is cultivated with organic fertilizers or if plants are grown in B12-enriched media (Mozafar, 1994; Sato et al., 2004).
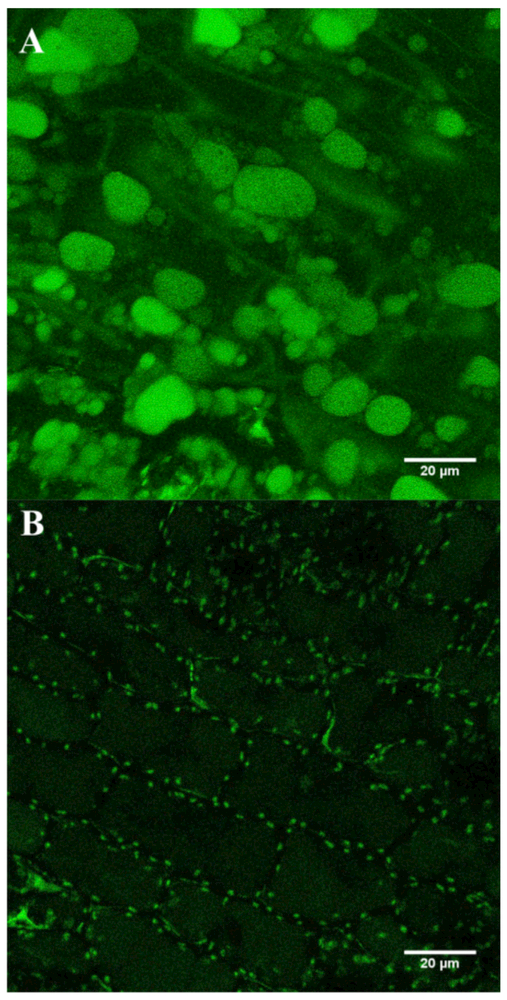
To investigate this observation further, we instigated an outreach project with a local secondary school, where pupils grew Lepidium sativum (garden cress) aseptically on an agar medium containing increasing concentrations of vitamin B12. After growth for 7 days the cotyledons from the germinating seedlings were removed, washed, and homogenized. The soluble supernatant after centrifugation was then applied to bioassay plates, which demonstrated that the seedlings were able to absorb cobalamin from the growth medium in a concentration-dependent manner (Figure S6). To confirm the ability of L. sativum to absorb cobalamin we repeated the uptake experiment using ribose-OG-cobalamin. L. sativum was therefore grown with ribose-OG-cobalamin and, after sectioning, was imaged by confocal microscopy. This approach confirmed that L. sativum absorbs ribose-OG-cobalamin, which was found to localize to the vacuoles of the cotyledons (Figure 7). These results provide definitive evidence that some plants can absorb and transport cobalamin, at least by passive diffusion. The linear shape of the concentration response and high amounts of Cbl used in the experiment (see Figure S6) indicate an unspecific uptake mechanism.
Figure 7. Localization of Ribose-OG-Cobalamin to Vacuoles in L. sativum (Garden Cress).
(A and B) Seeds were germinated in sterile media containing ribose-OG-cobalamin and were imaged after 5 days. Thin sections of the cotyledons of cress grown in the presence of the ribose-OG-cobalamin had highly fluorescent vacuoles (A), whereas control plants grown in the absence of the cobalamin derivative lacked this fluorescence (B).
DISCUSSION
In this research we have demonstrated that it is possible to functionalize the main corrin framework by substituting the methyl group of SAM with either an allyl or propargyl group. Although this approach was used to modify the C5 position, in theory it may be possible to modify any of the positions on the corrin ring that are methylated by SAM-dependent methyl transferases. Through this development we have shown that it is possible to generate either C5-allyl or C5-propargyl derivatives of HBA, and outlined how these can be transformed into cobyric acid analogs. By attaching fluorophores to the activated C5-allyl group it was possible to make C5-BoD and C5-OG cobyric acid derivatives. This has been achieved through the application of enzymatic and chemical tools to functionalize the comparatively inert corrin framework of B12. In doing so, we have been able to make analogs that can be used to investigate specific biological processes, expanding upon our initial research that allowed us to make different metal isosteres of cobalamin (Widner et al., 2016). Our chemical and biological synthesis approach allows for a regio-specific modification to the corrin molecule. Other have developed methods to generate a reactive group on the corrin molecule through non-specific acid hydrolysis of cobalamin in order to isolate variants with a free acid side chain, which was then subsequently modified (Waibel et al., 2008). This involves elaborate purification procedures and overall low yields.
The success of our approach relies on combining biosynthetic enzymes with chemical synthesis to produce new functional analogs. We wondered whether the additional chemical space occupied around the C5 position would present a steric hindrance, especially with the downstream biosynthetic enzymes. Indeed, the presence of the C5-allyl group on precorrin-8 did reduce the rate of reaction with CobH, which catalyses the methyl migration from C11 to C12 in the formation of HBA. However, with knowledge of the 3D structure of the enzyme (Shipman et al., 2001) we were able to rationally engineer more space into the active site of CobH to make it significantly more active with the C5 analogs.
The amide groups in cobalamin are added by CobB and CobQ. CobB accepts the C5-allyl-modified substrate well and transforms it into C5-HBA a,c-diamide in high yields. Normally, the next steps in the aerobic cobalamin pathway involve metal chelation and adenosylation. The amidation of the remaining side chains is only completed after the chelation and adenosylation processes have taken place. We wanted to change sequence of pathway reactions by completing the amidations prior to metal chelation, i.e., to transform HBAD into Hby. We were aware that some organisms must be able to make Hby as an intermediate, as reports from 40 years ago had highlighted that bacteria such as A. vinosum, when grown in the absence of cobalt, are able to produce cobalt-free corrinoids such as hydrogenobalamin (Koppenhagen et al., 1973). We thus cloned cobQ from A. vinosum and demonstrated that the encoded enzyme is indeed able to convert HBA-diamide into Hby and similarly convert the C5 analogs into the C5-Hby equivalents. With all these enzyme tools in hand we were able to produce the C5-allyl Hby on the tens of mg scale. After coupling of the fluorophore to the C5-allyl position cobalt was then inserted to generate the C5-cobyric acid analogs containing either an OG or BoD fluorophore. These cobyric acid analogs were then compared with cobalamin analogs where the fluorophores were attached to the ribose position.
The functional analysis of the various corrin and cobalamin analogs was surprising. Addition of the C5-(TPEA)-cobyric acid to a microbial B12 bioassay plate promoted growth around the application point, indicating that the C5 material must be taken up by the S. enterica reporter strain, which then presumably converted it into a functional cobalamin analog. The other analogs such as the C5-OG and C5-BoD variants also promoted growth, albeit less well. The high levels of the analogs required to promote growth reflect several constraints on their molecular recognition in terms of their uptake and incorporation into methionine synthase. For instance, it is clear from our results that the ribose-OG-cobalamin is taken up more efficiently than the C5-linked cobyric acid analog, while, conversely, the C5-BoD-cobyric acid analog is taken up more easily that the ribose-linked cobalamin equivalent. What is most surprising of all is that all these analogs appear to fit into the active site of methionine synthase. The crystal structure of the E. coli methionine synthase suggests that there is a certain amount of room to accommodate some extra bulk on the ribose group (Drennan et al., 1994). However, within the corrin binding site there is less room for the further chemical space required for the C5 attachment. Nonetheless, as mentioned above, the same is true with CobH, the enzyme required to convert the modified C5-allyl-precorrin-8 intermediate into C5-allyl-HBA, where the enzyme still catalyses the reaction, albeit at a reduced rate in comparison with the natural substrate. So, with methionine synthase the extra group on the corrin ring may force some conformational change within the protein so that the extra bulk is accommodated. There is always the possibility that the fluorescent analogs are broken down in the cell to release the fluorophore from the corrin. However, extraction of C5-BoD-cobyric acid from E. coli that had been grown with the compound resulted only in the identification of intact C5-BoD-cobyric acid. Overall, this tells us that the S. enterica and E. coli uptake mechanisms will accept corrin and B12 analogs with significant extra chemical bulk and that these compounds are relatively active inside the cell.
We investigated whether these fluorescent corrin analogs were also taken up by M. tuberculosis. Interestingly, both the C5-BoD-cobyric acid and the ribose-BoD-cobalamin analogs were taken up by cultured M. tuberculosis cells, with the C5-BoD analog appearing to be taken up more quickly than the ribose analog. Significantly, both analogs appear to support growth of this B12-dependent tuberculosis (TB) strain indicating that the molecules are able to get inside the cells. The B12 uptake mechanism in M. tuberculosis, which is different to that found in most other bacteria (Gopinath et al., 2013a, 2013b), is clearly able to take up incomplete corrinoids. As humans are not able to take up cobyric acid, the use of cobyric acid conjugates could prove a useful method for specifically labeling and targeting TB infections.
There is still much to learn about how B12 makes its journey from bacteria to specific compartments in eukaryotic cells. Several recent reports have highlighted the fact that C. elegans may prove to be a good model to learn more about the movement, distribution, and storage of B12 (Bito et al., 2013, 2017; Watson et al., 2014; Yilmaz and Walhout, 2014). Indeed, we were able to show that a fluorescent B12 derivative is absorbed from bacteria by C. elegans and is observed to accumulate within coelomocytes. Interestingly, only the ribose-linked cobalamin analog was absorbed indicating that the worm is able to differentiate between complete and incomplete corrinoids. It is not clear whether this is due to the B12 analog being recognized as foreign material or whether the cells represent a storage area for excess cobalamin. Nonetheless, the results demonstrate that this is a powerful model to learn about how B12 is absorbed and, as this system is different to mammalian systems, there is the possibility of exploiting this difference to try and treat worm-based parasite such as hook worms.
It is well documented that vegetarians are more prone to B12 deficiency as plants lack B12 (Stabler and Allen, 2004). However, it has been suggested that some plants are able to absorb the nutrient if added exogenously to their growth media (Mozafar, 1994; Sato et al., 2004). Through an outreach project we demonstrated that that garden cress, L. sativum, can indeed take up cobalamin. The amount of B12 absorbed by garden cress is dependent upon the amount present in the growth medium, and displayed a linear rather than a hyperbolic relationship. This would suggest an unspecific form of uptake. Nonetheless, we were able to confirm B12 uptake using our OG analog of cobalamin, showing that the nutrient ends up in the vacuole in the leaf. The uptake of B12 into plants is irrelevant in terms of plant metabolism, since plants do not possess any B12-dependent enzymes, but the finding that some plants are able to accumulate B12 is important, as such nutrient-enriched plants would be important in helping overcome dietary limitations in countries such as India, which have a high proportion of vegetarians.
STAR★METHODS
CONTACT FOR REAGENT AND RESOURCES SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Professor Martin J. Warren (m.j.warren@kent.ac.uk)
EXPERIMENTAL MODEL AND SUBJECT DETAILS
All bacterial strains used in this study are listed in the Key Resources Table together with their source and genotypes. For E. coli, unless stated otherwise, the strains were grown in LB media supplemented with the appropriate antibiotics. All Mycobacterium tuberculosis cultures were grown in cobalamin deficient Sauton’s defined medium (0.5 g KH2PO4, 0.5 g MgSO4·7H2O, 2 g citric acid, 0/05 g ferric ammonium citrate, 60 mLs glycerol, 4 g asparagine and 500 μL TWEEN 80 in 1 L pH 7.0. Worm studies were carried out with Caenorhabditis elegans var. Bristol (strain N2) and maintained on nematode growth medium (NGM) agar.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Bacterial and Virus Strains | ||
|
E. coli: JM109 endA1 recA1 gyrA96, thi hsdR17 (rκ-mκ+) relA1 supE44 Δ(lac-proAB) [F’, traD36 proAB lacIqZΔM15] |
Promega | JM109 |
|
E. coli: BL21(DE3) pLysS F−
ompT hsdSB (rB−, mB−) gal dcm (DE3) pLysS (CmR) |
Novagen | BL21(DE3) pLysS |
|
E. coli: BL21(DE3) F−
ompT hsdSB
(rB−, mB−) gal dcm (DE3) |
Novagen | BL21(DE3) |
|
E. coli: Rosetta (DE3) F−
ompT hsdSB(rB−, mB−) gal dcm (DE3) pRARE (CamR) |
Novagen | Rosetta (DE3) |
| E. coli: OP50 Uracil auxotroph | Gift from J. Tullet | N/A |
| E. coli: OP50 (pET-BAD-btuBF) | This paper | N/A |
| E. coli: OP50 (pET 3a) | This paper | N/A |
| M. tuberculosis: H37Rv ATCC® 27294 | Gift from H. Boshoff | N/A |
| M. tuberculosis: H37RvJO | Gift from H. Boshoff | N/A |
| M. tuberculosis: ΔbacA H37RvJO with hyg-marked ΔbacA deletion |
Warner et al., 2007; Gopinath et al., 2013a, 2013b) |
N/A |
| M. tuberculosis: ΔmetE H37RvJO with hyg-marked ΔmetE deletion. |
Warner et al., 2007; Gopinath et al., 2013a, 2013b) |
N/A |
|
M. tuberculosis: ΔmetH H37RvJO with ΔmetH deletion. |
Warner et al., 2007; Gopinath et al., 2013a, 2013b) |
N/A |
| S. enterica: AR3612; cysG, metE | (Raux et al., 1996) | N/A |
| Chemicals, Peptides, and Recombinant Proteins | ||
| S-(5’-Adenosyl)-L-homocysteine | Sigma | Cat# A9384 |
| Allyl-bromide | Sigma | Cat# A29585 |
| Silver perchlorate | Sigma | Cat# 674583 |
| Adenosine 5′-triphosphate disodium salt hydrate | Sigma | Cat# A2383 |
| L-Glutamine | Sigma | Cat# G3126 |
| Lichroprep RP18 | VWR | Cat# 1.09303.0500 |
| Diethylaminoethyl–Sephacel® aqueous ethanol suspension | Sigma | Cat# I6505 |
| Cysteamine hydrochloride | Sigma | Cat# M6500 |
| VA-044 2,2’-Azobis[2-(2-imidazolin-2-yl)propane] Dihydrochloride, Water Soluble Azo Initiator |
Alpha Laboratories | Cat# 017–19362 |
| BODIPY® TR-X NHS Ester (Succinimidyl Ester) | ThermoFisher | Cat# D-6116 |
| Oregon Green® 514 Carboxylic Acid, Succinimidyl Ester |
ThermoFisher | Cat# O-6139 |
| Porpargyl bromide solution | Sigma | Cat# 81831 |
| Seleno-L-methionine | Sigma | Cat# S3132 |
| CobL | (Deery et al., 2012) | N/A |
| CobH | (Deery et al., 2012) | N/A |
| CobB | (Deery et al., 2012) | N/A |
| CobQAlv | This paper | N/A |
| BtuF | (Deery et al., 2012) | N/A |
| CobH (T85A) | This paper | N/A |
| hMAT2A (I117A) | Gift from U. Oppermann (Oxford) | N/A |
| SAHH | Gift from J. Micklefield (Manchester) | N/A |
| Molecular dimensions Structure Screen 1 | Molecular dimensions | Cat# MD1–01 |
| Deposited Data | ||
| Structure of R. capsulatus CobH (T85A) in complex with C5-allyl-HBA. | This paper | PDB: 5NOG |
| Experimental Models: Organisms/Strains | ||
| N2 C. elegans | Gift from J. Tullet (Kent) | N/A |
| A. thaliana | Gift from A. Smith (Cambridge) | N/A |
| L. sativum | Meadow Grange Nursery, Blean. | N/A |
| Oligonucleotides | ||
| Allochromatium vinosum CobQ Forward: CTACATATGACCGATTCAGCCCCCAC | This paper | N/A |
| Allochromatium vinosum CobQ Reverse: CATACTAGTTCAGCGTGCCAGTTCGAG | This paper | N/A |
| R. capsulatus CobH (T85A) Forward: | This paper | N/A |
| R. capsulatus CobH (T85A) Reverse: | This paper | N/A |
| Recombinant DNA | ||
| pET14b | Novagen | Cat# 69660 |
| pET3a | Novagen | Cat# 69418-3 |
| pLysS | Novagen | Cat# 69659-3 |
| pETcoco-2 | Novagen | Cat# 71148-3 |
| pET3a-cobAIGJFMKLCEHis | (Deery et al., 2012) | N/A |
| pET14b-cobL | (Deery et al., 2012) | N/A |
| pET14b-cobH | (Deery et al., 2012) | N/A |
| pET14b-cobB | (Deery et al., 2012) | N/A |
| pET14b-cobQ | This paper | N/A |
| pET14b-btuF | (Deery et al., 2012) | N/A |
| pLysS-btuB | (Deery et al., 2012) | N/A |
| pET14b-cobH(T85A) | This paper | N/A |
| pET14b-hMat2A(I117A) | This paper | N/A |
| pET28-SAHH | Gift from J. Micklefield (Manchester) | N/A |
| Software and Algorithms | ||
| Coot | (Emsley et al., 2010) | https://www2.mrc-lmb.cam.ac.uk/personal/pemsley/coot/ |
| XIA2 version 0.3.8.0 | (Winter, 2010) | https://xia2.github.io |
| CCP4i | (Potterton et al., 2003) | http://www.ccp4.ac.uk/ccp4i_main.php |
| CCPN analysis | (Vranken et al., 2005) | http://www.ccpn.ac.uk/v2-software/software/analysis |
METHODS DETAILS
Chemicals and Reagents
Oregon Green® 514 (OG) and BODIPY® TR-X (BoD) succinimidyl ester were both purchased from Thermo Fisher Scientific, Inc., as was the P-Per for plant extraction. Allyl-Bromide, Cysteamine hydrochloride, Seleno-L-methionine, Dowex 50WX4 hydrogen form, Diethylaminoethyl-Sephacel, and N, N-DiisopropylethylamineReagentPlus® were bought from Sigma Aldrich, Ltd. Other chemicals and equipment include LICHROPREP RP-18 from VWR, VA-044 2,2’-Azobis[2-(2-imidazolin-2-yl)propane] dihydrochloride from alpha laboratories Ltd. and crystal screens from Molecular Dimensions Ltd.
Protein Production and Purification
All cobalamin biosynthetic genes were amplified by PCR using genomic DNA from either Rhodobacter capsulatus SB1003 (cobL, H and B), or Allochromatium vinosum ATCC 17899 (cobQ). The cob genes were amplified individually with primers then cloned into modified pET14b plasmids. These were then transformed into BL21 star (DE3) pLysS. hMATIIA I117A was transformed into Rosetta 2 (DE3) pLysS. The recombinant strains were grown in LB at 37°C and protein overexpression was induced with 0.4 mM of isopropyl-1-thio-β-D-galactopyranoside. The cells were resuspended in buffer A (20 mM Tris pH 8, 500 mM NaCl, 10 mM Imidazole) and sonicated. The protein was purified using Chelating Sepharose™ charged with NiSO4. Unbound proteins were washed off with buffer A, buffer A containing 30 mM imidazole and buffer A containing 80 mM Imidazole. Proteins were eluted with buffer A containing 400 mM imidazole and passed through a PD10 column equilibrated with buffer B (20 mM Tris, pH 8.0, containing 100 mM NaCl) for CobL and H, or buffer C (20 mM Tris, pH 8.0, containing 500 mM NaCl) for CobB and Q.
Production and Purification of Precorrin-7
E. coli BL21 star (DE3) pLysS was transformed with pET3a/cobA-I-G-J-M-F-K-LC-E and grown in 2TY at 28°C for 24 hours (Deery et al., 2012). The cells were resuspended in 20 mM Tris, pH 8.0, and sonicated. Proteins were precipitated with acetonitrile and following clarification by centrifugation precorrin-7 was purified over DEAE-Sepherose. The column was washed with 20 mM Tris, pH 8.0, containing 100 mM NaCl, before elution with 500 mM NaCl. The pH of the eluent was lowered to pH 4.0 with TFA and the precorrin-7 was further purified over RP18. The column was washed with a stepwise gradient of methanol in 0.1% (v/v) TFA and elution was achieved with 50% methanol.
Synthesis of SAM Analogues
Allyl-SAM was prepared from homocysteine and allylbromide as described previously (Wang et al., 2011). Allyl bromide (44 μL, 0.214 mmol) and AgClO4 (6 mg, 0.025 mmol) were added to S-adenosyl-L-homocysteine (5 mg, 0.013 mmol) dissolved in a mixture of formic acid and acetic acid (1:1, 0.6 mL) at 0°C. The mixture was allowed to warm up to room temperature and stirred continuously for 2.5 h. A further portion of allyl bromide (44 μL, 0.214 mmol) and AgClO4 (6 mg, 0.025 mmol) were added and the reaction mixture was stirred for another 2.5 h. following centrifugation to remove the precipitate, H2O (3 mL) was added and the residual allyl bromide was extracted with diethyl ether (5 mL × 3). The aqueous phase was then concentrated under vacuum.
Synthesis of Se-propargyl-L-selenohomocysteine
Se-propargyl-L-selenohomocysteine was synthesized from L-selenomethionine as described previously (Singh et al., 2014). L-selenomethionine (100 mg) was dissolved in 20 mL liquid ammonia at −78°C and stirred. Small fragments of sodium metal (34 mg) were added until a deep blue colour persisted for at least 1 min. Progargyl bromide (46.5 μL) was then added followed by ammonium chloride (20 mg). The reaction removed from the cooling bath and the flask was left open to the atmosphere for the ammonia to evaporate. The resulting residue was dissolved in H2O (5 mL) and the pH was adjusted to pH 5 - 7 by the addition of HCl (1 M). The solvent was removed under reduced pressure.
Synthesis C5-allyl-HBA
Precorrin-7 (60 μM) was incubated with CobL(6 μM), CobH (30 μM) and allyl-SAM (170 μM) in buffer Bat 28°C for 16 hours in the dark. The reaction mixture was then heated to 65°C and the precipitate was removed by centrifugation. The resulting supernatant was applied to a DEAE-Sepharose column equilibrated in 20 mM Tris, pH 8.0. The column was washed with 20 mM Tris, pH 8.0, and the product was eluted with 20 mM Tris, pH 8.0, containing 500 mM NaCl.
Synthesis of C5-propargyl-HBA
C5-propargyl-HBA was prepared from precorrin-7 and Se-propargyl-L-selenohomocysteine. Precorrin-7 (60 μM), Se-propargyl-L-selenohomocysteine (1.5 mM), ATP (2 mM), MgCl2 (5 mM), hMAT2A ∣117A (60 μM), CobL (6 μM), CobH (30 μM), Se-propargyl-Hcy (1.5 mM) and SAHH (50 μM) were incubated in 20 mM Tris, pH 8.0, containing 100 mM NaCl at 28°C in the dark for 16 h. The reaction mixture was heated to 80°C for 15 minutes and the precipitate was removed by centrifugation. The supernatant was applied to DEAE column pre-equilibrated in 20 mM Tris, pH 8.0. The column was washed with 20 mM Tris, pH 8.0, containing 100 mM NaCl, and eluted with 20 mM Tris, pH 8.0, containing 500 mM NaCl.
Synthesis of C5-allyl-Hby
C5-allyl-HBA (30 μM), CobB (2 μM), CobQ (5 mM), ATP (8 mM), L-Glutamine (8 mM), and MgCl2 (20 mM) were incubated in buffer C at 28°C for 16 hours in the dark. The reaction mixture was heated to 80°C, acidified to pH 4 with trifluoroacetic acid (TFA) and the precipitate removed by centrifugation. The supernatant was applied to an RP18 column equilibrated in 0.1% TFA, washed with 0.1% TFA followed by 20% methanol before elution in 50% methanol. The product was then dried in a vacuum centrifuge.
C5 Allyl Group Extension
The C5-allyl group was modified through a thiol-ene coupling reaction with cysteamine. A solution of C5-allyl-Hby (30 μM) was incubated at 55°C in acetate buffer (pH 4.0, 0.25 M) containing cysteamine (100 mM) and VA-044 (20 mM) in the dark for 15 mins. The product was purified over RP18 and analyzed by HPLC-MS.
Cobalt Insertion
Cobalt insertion was achieved through incubation of the desired metal-free corrinoid (20 μM) with CoCl2 (10 mM) in ammonium hydroxide (0.2 M) at 80°C under an N2 atmosphere. The reaction was followed UV-visible spectroscopy until deemed complete (~ 60 mins) after which it was quenched with KCN and the product purified over RP18 and eluted in methanol.
Synthesis of Fluorescent C5-Cobyric Acid Derivatives
CN2-C5-TPEA-cobyric acid was dissolved in DMSO and either the succinimidyl ester of OG or BoD along with 2 equivalents of DIPEA were added. The reaction was stirred at room temperature for 16 hours in the dark. The solvent was removed in vacuo and the compound was purified by semi-preparative HPLC.
Ribose Linked Fluorescent Vitamin B12 Derivatives
The ribose-5’-hydroxyl group of vitamin B12 was activated with carbonyldiimidazole and coupled with 1,2-diaminoethane as described previously (McEwan et al., 1999). The derivative was added to either the succinimidyl ester of OG or BoD with 2 equivalents of DIPEA in DMSO. The reaction was stirred for 16 hours in the dark at room temperature. The product was precipitated with acetone and collected by filtration. Fluorescent derivatives were dissolved in 50% acetonitrile and purified by semi-preparative HPLC.
Crystal Structure Determination of CobHT85A with Bound C5-allyl-HBA
Recombinantly produced CobHT85A was purified on a nickel affinity column. After affinity purification CobHT85A was subject to gel filtration on a G200 Superdex column (GE Healthcare). Protein fractions were collected and concentrated to 7 mg mL−1. Allyl-HBA was added to the protein in a 1:1 ratio prior to crystallisation at 19°C using the hanging drop vapour diffusion method. Initial crystals were obtained in 0.2 M calcium acetate hydrate, 0.1 M sodium cacodylate pH 6.5,18 % (w/v) PEG 8000 (Molecular Dimensions, MD01 condition 20). Crystal optimisation required the addition of 20% methanol to the well condition and increasing the PEG 8000 to 20%. This resulted in multiple square shaped crystals which formed within three days. A single crystal was picked, transferred to a drop of in 0.2 M calcium acetate hydrate, 0.1 M sodium cacodylate pH 6.5, 20 % (w/v) PEG 8000 containing 20% glycerol as a cryoprotectant and then flash frozen in liquid nitrogen. The crystal diffracted to 1.6 Å and was found to belong to space group C 1 2 1 which is the same as the published structure of R. capsulatus CobH co-crystallised with HBA (PDB 4AU1) (Deery et al., 2012). Data for the crystal was collected using beamline IO4–1 at the Diamond Light Source (UK). The data was auto-integrated and scaled using the Xia2 package using XDS (X-ray data software) and XSCALE (3dii) at the beamline. An initial model was generated by refinement of the scaled and merged data against PDB:4AU1 in Refmac 5. The model was then refined through cycles of manual model building in Coot and refinement with Refmac 5. The final model was validated in Coot and with wwPDB. The PDB coordinates were deposited with the code 5N0G.
Bioassay Plates
A quantitative bioassay using S. typhimurium AR3612 was performed as described previously (Rauxet al., 1996). The bioassay plates containing the indicator strain AR3612 were prepared from lawns of the corresponding bacteria. The bacteria were scraped from an overnight minimal medium (M9) plate containing methionine (50 mg/L) and cysteine (50 mg/L) and washed with 0.9 % (w/v) NaCl to remove traces of methionine. The cells were mixed with 300 mL of minimal medium (M9) containing cysteine (50 mg/L) agar at 47°C, and poured into plates.
Imaging in Escherichia coli
TheOP50 E. coli was transformed with pET-BAD-btuSFand grown in a 4 mL LB culture after inoculation with 16 μL of starter culture. This was grown with 1 μM of C5-BoD-cobyric acid or ribose-BoD-cobalamin overnight with induction with 0.02% arabinose. Cultures (1 mL) were spun down and washed three times with fresh LB to remove external fluorophore. The final pellet was resuspended in 1 mL of fresh LB, 5 μL of this culture was dried on to a 1 % (w/v) LB-agarose pad and imaged on an Olympus IX81 widefield microscope with PlanApo 150 × OTIRFM-SP 1.49 numerical aperture lens mounted on ASI stage (Applied Scientific), and illuminated using LED light sources (Cairn Research Ltd) with appropriate filters (YFP and mCherry, Chroma). The samples were visualised using a Princeton ProEM 1024 back-thinned EMCCD camera (Princeton Instruments) on Metamorph software (Molecular Devices). Each 3D-maximum projection of volume data was calculated from 13 z-plane images, each 0.2 μm apart.
Imaging in Mycobacterium tuberculosis
Relevant strains of M. tuberculosis were grown in 150 μL cultures in a 96 well plate containing various concentrations of the BODIPY-labelled analogues of cobalamin and cobyric acid. The cells were grown for up to 48 h. After growth, the cells were spun down and washed three times to remove the fluorophore. Cells were pipetted directly onto a slide and imaged live on a Leica SP5 microscope using 594 nm excitation and imaging between 600-700 nm. The images were processed using Leica Lite confocal software and FIJI (Schindelin et al., 2012).
Extraction of Cobalamin from Lepidium sativum Using P-Per
Lepidium sativum seeds were sterilised by washing with 70 % ethanol three times then rinsed five times with sterile water. The seeds were placed on Murashige-Skoog agar containing different concentrations of cobalamin, and grown for one week in sunlight at room temperature. The cotyledons of the cress were collected and washed 5 times with water. The residual water was removed by pipette, after centrifugation. The P-Per was prepared as instructed in the enclosed manual (Thermo Fisher): 283 μL reagent A, 2.9 μL of reagent B, and 214 μL of reagent C. A little sand was added to the cotyledons along with 100 μL of the P-Per. The cotyledons were ground using a hand-held pellet pestle for 2 minutes. The suspension was vortexed and then centrifuged for 3 minutes at 15,000 rpm rotation. The lower aqueous phase was collected and applied to the bioassay plate as described above.
Imaging in L. sativum
L. sativum seeds were sterilised by washing with 70 % ethanol three times, then rinsed five times with sterile water. The seeds were placed on the Murashige-Skoog agar with 0.5 μM ribose-OG cobalamin, and grown at room temperature in the dark for five days. Whole cotyledons or sectioned cress were placed directly on the glass slide with water. The samples were imaged with a Leica TCS SP2 laser scanning confocal microscope (Leica Microsystems, Germany) with AOBS (Acoust-Optical Beam Splitter) detected using PMTs (photomultiplier tubes). Both the 40 × and 63 × HCX PL APO oil lenses, numerical aperture 1.25 and 1.4 respectively, were used. Samples were excited at 514 nm from an Argon–Krypton-mixed gas laser and images were acquired in the green/yellow region of the light spectrum (525–590 nm). The software used to image was LCS (Leica Confocal Software) and the images were processed using Leica Lite confocal software and FIJI (Schindelin et al., 2012).
Imaging in Caenorhabditis elegans
C. elegans were maintained on normal E. coli OP50 at 20°C unless otherwise stated. A food source carrying the fluorescent analogues was made by transforming the OP50 with pET-BAD-btuSF and were grown in a 4 mL LB culture after inoculation with 16 μL of starter culture. This was incubated with 1 μM of C5-BoD-cobyric acid or ribose-BoD-cobalamin overnight with induction with 0.02% arabinose. 1 mL of culture was spun down and washed three times with fresh LB to remove external fluorophore. The final pellet was resuspended in 1 mL of fresh LB and 200 μL of the OP50 culture was pipetted on to the centre of NGM agar plates. These plates were left to dry in a sterile culture hood for 4 hours and then stored at 4°C until used. Three forth larval stage (L4) C. elegans were transferred to the seeded plates and left to grow at 20°C for four days before imaging. Three third larval stage (L3) C. elegans were transferred to the seeded plates and allowed to lay eggs. These progeny were then imaged at the L4 stage.
For each slide, 3-5 L4 stage C. elegans were mounted in M9 + 0.2 % levamisole on a 2 % agarose pad and imaged within 30 min at the University of Bristol on a Leica SP8X AOBS confocal laser scanning microscope attached to a Leica DMi8 inverted epifluorescence microscope with ‘Adaptive Focus Control’. The sample was excited with a white light laser at 594 nm and detected between 599-712 nm using a hybrid gated detector for the fluorophore, and excited with a 405 nm laser, with images acquired between 410-505 nm for the gut granule autofluorescence. All images were taken using a 20 × numerical aperture 0.75 dry lens. Again the images were processed using Leica Lite confocal software and FIJI (Schindelin et al., 2012).
HPLC MS Analysis
Samples were separated on an Agilent 1000 series HPLC coupled to a micrOTOF-Q II (Bruker) mass spectrometer using an Ace 5 AQ column (2.1 × 150 mm; Advanced Chromatography Technologies) maintained at 30°C and with a flow rate of 0.2 mL/min. The mobile phase consisted of 0.1% TFA (v/v) in water (solvent A) and 100% acetonitrile (solvent B). Three different HPLC gradients were employed. Gradient 1 (methionine and SAM and analogues): Linear gradient from 0-30% solvent B over 30 mins. Gradient 2 (pathway intermediates): 5-20% B over 6 mins, 20-30% B over 19 mins followed by 30-90% B over 5 mins. Gradient 3 (fluorescent cobalamin analogues): Linear gradient from 0-100% solvent B over 45 mins. Semi-preparative HPLC was performed on an Agilent 1000 series HPLC using a CS SIL C18 column (250 mm × 10 mm; 5 μm; Charlton Scientific) at a flow rate of 5 mL/min running the gradients described above.
NMR Analysis
NMR experiments were performed using a 600 MHz (1H) Bruker Avance III spectrometer with a 5 mm QCI-F cryoprobe. Assignments were completed using DQF-COSY, TOCSY (20 ms and 80 ms mixing times), NOESY (500 ms mixing time), 13C-HSQC-TOCSY, 13C,1H-HSQC and 13C,1H-HMBC experiments with WaterGATE or excitation sculpting water suppression on 0.1-1 mM samples dissolved in deuterium oxide. All 13C data was obtained using natural isotopic abundance. Data were processed and analysed using Bruker Topspin version 3.2 and CCPN analysis. 1H Chemical shift referencing was based on the position of the water resonance, 13C referencing used 1H/13C gyromagnetic ratios to define indirect carrier position (Wishart and Case, 2001) and all data were obtained at 25°C.
QUANTIFICATION AND STATISTICAL ANALYSIS
Bioassays (Figures 5 and S6) were performed three times (n=3) and the values for the amount of B12 present in the samples was generated from a standard curve. For B12 uptake into plants the averages of the separate measurements were then calculated and plotted using Microsoft Excel. The structure of CobH was refined with internal statistical analysis as reported in the deposition in the PDB
No additional statistical tests were performed.
DATA AND SOFTWARE AVAILABILITY
All software used in this study is reported in Method Details and indicated in the Key Resources Table. The coordinates for the CobH T85A crystal structure were deposited in the Protein Data Bank PDB:5N0G (https://www.rcsb.org/structure/5n0g).
Supplementary Material
Highlights.
SAM analogs have been used to make variants of vitamin B12
Analogs of B12 with fluorescent groups attached to the corrin ring are described
The uptake of B12 analogs into E. coli and M. tuberculosis is shown
Vitamin B12 analogs are shown to accumulate in worms and higher plants
SIGNIFICANCE.
Vitamin B12, cobalamin, is an essential dietary component that is synthesized solely by certain prokaryotes. Although some eukaryotes, including mammals, require the nutrient, kingdoms such as Plantae and Fungi have evolved to live in a B12-less environment, which has implications for people on a vegan diet. To follow more closely the journey undertaken by cobalamin, from its prokaryotic inception, through sharing in microbial communities and its uptake by animals, we devised ways to construct a range of fluorescent analogs of cobalamin. By manipulation of the B12 biosynthetic pathway we were able to modify the C5 position of the corrin framework so as to incorporate an allyl substituent, where the vinyl group could be used to attach fluorophores to give tagged cobyric acid analogs. Fluorescent cobalamin variants were also constructed through attachment of fluorophores to the ribose group in the lower nucleotide loop. Together, these fluorescent analogs were used to show that M. tuberculosis is able to absorb both complete and incomplete corrinoids, demonstrating that cobalamin can be used to carry cargo into the cell. C. elegans was also found to absorb cobalamin, demonstrating that this approach can be used to follow intracellular trafficking in a model organism. Moreover, it also represents a target for parasites such as hook worms. Finally, and very surprisingly, certain plants were observed to be able to absorb B12 from their growth medium, accumulating the molecule in the leaf vacuole. This latter finding may be important as a way to address the global challenge of providing a nutrient-complete vegetarian diet, a valuable development as the world becomes increasingly meat-free due to population expansion.
ACKNOWLEDGMENTS
This work was supported, in part, by the Intramural Research Program of NIAID and in part by grants from the Biotechnology and Biological Sciences Research Council (BBSRC; BB/L010208/1, BB/K009249/1, and BB/I012079, and the Wellcone Trust 097627/Z/11/Z). We thank Prof Jason Micklefield (Manchester) and Prof Udo Oppermann (Oxford) for the gift of SAHH and hMATIIA. Our research into B12 uptake into plants was done as part of an outreach program to engage pupils in real scientific research, inspired by the Authentic Biology project (www.authentic-biology.org). The detection and measurement of B12 in the cotyledons of garden cress was performed with the help of the biology teachers and year 11 and 12 pupils at Sir Roger Manwood School in Sandwich, Kent. Teachers: Dr Jackie Wilson and Vicki Beale. Pupils: Megan Battenfield, Megan Betts, Alex Gallagher, Inga Loo, Shiree Kinder, George Morris, Emma Rudge, James Russell, Rebekah Smith, Maddy Stock, Pema Tamang, Elizabeth Westbrook, Himmy Wu, and Katie Fidock.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information includes six figures and two tables and can be found with this article online at https://doi.org/10.1016/j.chembiol.2018.04.012.
DECLARATION OF INTERESTS
The authors declare no competing interests.
REFERENCES
- Allen RH, and Stabler SP (2008). Identification and quantitation of cobalamin and cobalamin analogues in human feces. Am. J. Clin. Nutr 87, 1324–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee R, and Ragsdale SW (2003). The many faces of vitamin B12: catalysis by cobalamin-dependent enzymes. Annu. Rev. Biochem 72, 209–247. [DOI] [PubMed] [Google Scholar]
- Bito T, Matsunaga Y, Yabuta Y, Kawano T, and Watanabe F (2013). Vitamin B12 deficiency in Caenorhabditis elegans results in loss of fertility, extended life cycle, and reduced lifespan. FEBS Open Bio 3, 112–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bito T, Misaki T, Yabuta Y, Ishikawa T, Kawano T, and Watanabe F (2017). Vitamin B12 deficiency results in severe oxidative stress, leading to memory retention impairment in Caenorhabditis elegans. Redox Biol. 11, 21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanche F, Cameron B, Crouzet J, Debussche L, Thibaut D, Vuilhorgne M, Leeper FJ, and Battersby AR (1995). Vitamin B12 - how the problem of its biosynthesis was solved. Angew. Chem. Int. Ed 34, 383–411. [Google Scholar]
- Brito A, Chiquette J, Stabler SP, Allen RH, and Girard CL (2015). Supplementing lactating dairy cows with a vitamin B12 precursor, 5,6-dimethylbenzimidazole, increases the apparent ruminal synthesis of vitamin B12. Animal 9, 67–75. [DOI] [PubMed] [Google Scholar]
- Chan CH, and Escalante-Semerena JC (2011). ArsAB, a novel enzyme from Sporomusa ovata activates phenolic bases for adenosylcobamide biosynthesis. Mol. Microbiol 81, 952–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croft MT, Lawrence AD, Raux-Deery E, Warren MJ, and Smith AG (2005). Algae acquire vitamin B12 through a symbiotic relationship with bacteria. Nature 438, 90–93. [DOI] [PubMed] [Google Scholar]
- Crofts TS, Seth EC, Hazra AB, and Taga ME (2013). Cobamide structure depends on both lower ligand availability and CobT substrate specificity. Chem. Biol 20,1265–1274. [DOI] [PubMed] [Google Scholar]
- Crouzet J, Cauchois L, Blanche F, Debussche L, Thibaut D, Rouyez MC, Rigault S, Mayaux JF, and Cameron B (1990). Nucleotide sequence of a Pseudomonas denitrificans 5.4 kilobase DNA fragment containing 5 cob genes and identification of structural genes encoding S-adenosyl-L-methionine - uroporphyrinogen III methyltransferase and cobyrinic acid a,c-diamide synthase. J. Bacteriol. 172, 5968–5979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalhoff C, Lukinavicius G, Klimasauskas S, and Weinhold E (2006a). Direct transfer of extended groups from synthetic cofactors by DNA methyltransferases. Nat. Chem. Biol 2, 31–32. [DOI] [PubMed] [Google Scholar]
- Dalhoff C, Lukinavicius G, Klimasauskas S, and Weinhold E (2006b). Synthesis of S-adenosyl-L-methionine analogs and their use for sequence-specific transalkylation of DNA by methyltransferases. Nat. Protoc 1, 1879–1886. [DOI] [PubMed] [Google Scholar]
- Deery E, Schroeder S, Lawrence AD, Taylor SL, Seyedarabi A, Waterman J, Wilson KS, Brown D, Geeves MA, Howard MJ, et al. (2012). An enzyme-trap approach allows isolation of intermediates in cobalamin biosynthesis. Nat. Chem. Biol 8, 933–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degnan PH, Taga ME, and Goodman AL (2014). Vitamin B12 as a modulator of gut microbial ecology. Cell Metab 20, 769–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drennan CL, Huang S, Drummond JT, Matthews RG, and Ludwig ML (1994). How a protein binds B12 - a 3.0-Angstrom X-ray structure of B12-binding domains of methionine synthase. Science 266, 1669–1674. [DOI] [PubMed] [Google Scholar]
- Emsley P, Lohkamp B, Scott WG, and Cowtan K (2010). Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr 66, 486–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gherasim C, Lofgren M, and Banerjee R (2013). Navigating the B12 road: assimilation, delivery, and disorders of cobalamin. J. Biol. Chem 288, 13186–13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard CL, Berthiaume R, Stabler SP, and Allen RH (2009). Identification of cobalamin and cobalamin analogues along the gastrointestinal tract of dairy cows. Arch. Anim. Nutr 63, 379–388. [DOI] [PubMed] [Google Scholar]
- Gopinath K, Moosa A, Mizrahi V, and Warner DF (2013a). Vitamin B12 metabolism in Mycobacterium tuberculosis. Future Microbiol 8, 1405–1418 [DOI] [PubMed] [Google Scholar]
- Gopinath K, Venclovas C, loerger TR, Sacchettini JC, McKinney JD, Mizrahi V, and Warner DF (2013b). A vitamin B12 transporter in Mycobacterium tuberculosis. Open Biol. 3, 120175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray MJ, and Escalante-Semerena JC (2009). In vivo analysis of cobinamide salvaging in Rhodobacter sphaeroides strain 2.4.1. J. Bacteriol 191, 3842–3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greibe E, Fedosov S, and Nexo E (2012).The cobalamin-binding protein in zebrafish is an intermediate between the three cobalamin-binding proteins in human. PLoS One 7, e35660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helliwell KE, Lawrence AD, Holzer A, Kudahl UJ, Sasso S, Krautler B, Scanlan DJ, Warren MJ, and Smith AG (2016). Cyanobacteria and eukaryotic algae use different chemical variants of vitamin B12. Curr. Biol 26, 999–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton RA, Bagnato JD, and Grissom CB (2003). Structural determination of 5’-OH alpha-ribofuranoside modified cobalamins via 13C and DEPT NMR. J. Org. Chem 68, 7108–7111. [DOI] [PubMed] [Google Scholar]
- Koppenhagen VB, Wagner F, and Pfiffner JJ (1973). Alpha-(5,6 dimethylbenzimidazolyl)rhodibamide and rhodibinamide, the rhodium analogues of vitamin B12 and cobinamide. J. Biol. Chem 248, 7999–8002. [PubMed] [Google Scholar]
- Krautler B, Fieber W, Ostermann S, Fasching M, Ongania KH, Gruber K, Kratky C, and Mikl C (2003). The cofactor of tetrachloroethene reductive dehalogenase of Dehalospirillum multivorans is norpseudo B12, a new type of a natural corrinoid. Helv. Chim. Acta 86, 3698–3716. [Google Scholar]
- Lee M, and Grissom CB (2009). Design, synthesis, and characterization of fluorescent cobalamin analogues with high quantum efficiencies. Org. Lett 11, 2499–2502. [DOI] [PubMed] [Google Scholar]
- Maggio-Hall LA, and Escalante-Semerena JC (2003). Alpha-5,6-dimethyl-benzimidazole adenine dinucleotide (alpha-DAD), a putative new intermediate of coenzyme B12 biosynthesis in Salmonella typhimurium. Microbiology 149, 983–990. [DOI] [PubMed] [Google Scholar]
- Martens JH, Barg H, Warren MJ, and Jahn D (2002). Microbial production of vitamin B12. Appl. Microbiol. Biotechnol 58, 275–285. [DOI] [PubMed] [Google Scholar]
- McEwan JF, Veitch HS, and Russell-Jones GJ (1999). Synthesis and biological activity of ribose-5’-carbamate derivatives of vitamin B12. Bioconjug. Chem 10, 1131–1136. [DOI] [PubMed] [Google Scholar]
- Moore SJ, Lawrence AD, Biedendieck R, Deery E, Frank S, Howard MJ, Rigby SE, and Warren MJ (2013). Elucidation of the anaerobic pathway for the corrin component of cobalamin (vitamin B12). Proc. Natl. Acad. Sci. USA 110, 14906–14911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozafar A (1994). Enrichment of some B-vitamins in plants with application of organic fertilizers. Plant Soil 167, 305–311. [Google Scholar]
- Potterton E, Briggs P, Turkenburg M, and Dodson E (2003). A graphical user interface to the CCP4 program suite. Acta Crystallogr. D Biol. Crystallogr D59, 1131–1137. [DOI] [PubMed] [Google Scholar]
- Raux E, Lanois A, Levillayer F, Warren MJ, Brody E, Rambach A, and Thermes C (1996). Salmonella typhimurium cobalamin (vitamin B12) biosynthetic genes: functional studies in S. typhimurium and Escherichia coli. J. Bacteriol 178, 753–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renz P (1999). Biosynthesis of the 5,6-dimethylbenzidazole moiety of cobalamin and of the other bases found in natural corrinoids In Chemistry and Biochemistry of B12, Banerjee R, ed. (John Wiley and sons), pp. 557–575. [Google Scholar]
- Rodionov DA, Vitreschak AG, Mironov AA, and Gelfand MS (2003). Comparative genomics of the vitamin B12 metabolism and regulation in prokaryotes. J. Biol. Chem 278, 41148–41159. [DOI] [PubMed] [Google Scholar]
- Roth JR, Lawrence JG, and Bobik TA (1996). Cobalamin (coenzyme B12): synthesis and biological significance. Annu. Rev. Microbiol 50,137–181. [DOI] [PubMed] [Google Scholar]
- Sato K, Kudo Y, and Muramatsu K (2004). Incorporation of a high level of vitamin B12 into a vegetable, kaiware daikon (Japanese radish sprout), by the absorption from its seeds. Biochim. Biophys. Acta 1672, 135–137. [DOI] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, et al. (2012). FIJI: an open-source platform for biological-image analysis. Nat. Methods 9,676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipman LW, Li D, Roessner CA, Scott AI, and Sacchettini JC (2001). Crystal stucture of precorrin-8x methyl mutase. Structure 9, 587–596. [DOI] [PubMed] [Google Scholar]
- Singh S, Zhang J, Huber TD, Sunkara M, Hurley K, Goff RD, Wang G, Zhang W, Liu C, Rohr J, et al. (2014). Facile chemoenzymatic strategies for the synthesis and utilization of S-adenosyl-(L)-methionine analogues. Angew. Chem. Int. Ed 53, 3965–3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeltzer CC, Cannon MJ, Pinson PR, Munger JD Jr., West FG, and Grissom CB (2001). Synthesis and characterization of fluorescent cobalamin (CobalaFluor) derivatives for imaging. Org. Lett 3, 799–801. [DOI] [PubMed] [Google Scholar]
- Stabler SP, and Allen RH (2004). Vitamin B12 deficiency as a worldwide problem. Annu. Rev. Nutr. 24, 299–326. [DOI] [PubMed] [Google Scholar]
- Studier FW, Daegelen P, Lenski RE, Maslov S, and Kim JF (2009). Understanding the differences between genome sequences of Escherichia coli B strains REL606 and BL21(DE3) and comparison of the E. coli B and K12 genomes. J. Mol. Biol 394, 653–680. [DOI] [PubMed] [Google Scholar]
- Vranken WF, Boucher W, Stevens TJ, Fogh RH, Pajon A, Llinas M, Ulrich EL, Markley JL, Ionides J, and Laue ED (2005). The CCPN data model for NMR spectroscopy: development of a software pipeline. Proteins 59, 687–696. [DOI] [PubMed] [Google Scholar]
- Waibel R, Treichler H, Schaefer NG, van Staveren DR, Mundwiler S, Kunze S, Kuenzi M, Alberto R, Nuesch J, Knuth A, et al. (2008). New derivatives of vitamin B12 show preferential targeting of tumors. Cancer Res 68, 2904–2911. [DOI] [PubMed] [Google Scholar]
- Wang R, Islam K, Liu Y, Zheng W, Tang H, Lailler N, Blum G, Deng H, and Luo M (2013). Profiling genome-wide chromatin methylation with engineered posttranslation apparatus within living cells. J. Am. Chem. Soc 735, 1048–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Zheng W, and Luo M (2014). A sensitive mass spectrum assay to characterize engineered methionine adenosyltransferases with S-alkyl methionine analogues as substrates. Anal Biochem. 450, 11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Zheng W, Yu H, Deng H, and Luo M (2011). Labeling substrates of protein arginine methyltransferase with engineered enzymes and matched S-adenosyl-L-methionine analogues. J. Am. Chem. Soc. 733, 7648–7651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner DF, Savvi S, Mizrahi V, and Dawes SS (2007). A riboswitch regulates expression of the coenzyme B12-independent methionine synthase in Mycobacterium tuberculosis: implications for differential methionine synthase function in strains H37Rv and CDC1551. J. Bacteriol 189, 3655–3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren MJ, Raux E, Schubert HL, and Escalante-Semerena JC (2002). The biosynthesis of adenosylcobalamin (vitamin B12). Nat. Prod. Rep 19, 390–412. [DOI] [PubMed] [Google Scholar]
- Watkins D, and Rosenblatt DS (2013). Lessons in biology from patients with inborn errors of vitamin B12 metabolism. Biochimie 95, 1019–1022. [DOI] [PubMed] [Google Scholar]
- Watson E, MacNeil LT, Ritter AD, Yilmaz LS, Rosebrock AP, Caudy AA, and Walhout AJ (2014). Interspecies systems biology uncovers metabolites affecting C. elegans gene expression and life history traits. Cell 156, 759–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widner FJ, Lawrence AD, Deery E, Heldt D, Frank S, Gruber K, Wurst K, Warren MJ, and Krautler B (2016). Total synthesis, structure, and biological activity of adenosylrhodibalamin, the non-natural rhodium homologue of coenzyme B12. Angew. Chem. Int. Ed 55, 11281–11286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter G (2010). XIA2: an expert system for macromolecular crystallography data reduction. J. Appl. Crystallogr 43, 186–190. [Google Scholar]
- Wishart DS, and Case DA (2001). Use of chemical shifts in macromolecular structure determination. Methods Enzymol 338, 3–34. [DOI] [PubMed] [Google Scholar]
- Yilmaz LS, and Walhout AJ (2014). Worms, bacteria, and micronutrients: an elegant model of our diet. Trends Genet. 30, 496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaman K, and Zak Z (1989). Purification and partial characterization of a cobalamin-binding protein from chicken egg yolk. Biochim. Biophys. Acta 998, 102–104. [DOI] [PubMed] [Google Scholar]
- Zayas CL, Claas K, and Escalante-Semerena JC (2007). The CbiB protein of Salmonella enterica is an integral membrane protein involved in the last step of the de novo corrin ring biosynthetic pathway. J. Bacteriol 189, 7697–7708. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.