Abstract
Majority of malignant pancreatic neoplasms are epithelial in origin and mostly arise from exocrine gland. Ductal adenocarcinoma compromises the major histological type of such tumors. Primary non-epithelial tumors of exocrine pancreatic gland are extremely rare and incorporate lymphoma and sarcoma. Primary pancreatic lymphoma compromises less than 0.5% of pancreatic malignancies. Primary pancreatic lymphoma can be difficult to differentiate from pancreatic adenocarcinoma and other neoplasms on imaging, and a correct diagnosis is crucial for appropriate patient management.
Keywords: Primary pancreatic lymphoma, Malignant tumors of the pancreas, Primary anaplastic lymphoma, Pancreas
1. Introduction
Lesions involving the pancreas can broadly be subdivided, radiographically, into cystic versus solid, and non-neoplastic versus neoplastic lesions. Solid appearing neoplastic lesions usually include pancreatic adenocarcinoma as the most common, followed by less common solid pancreatic neuroendocrine tumors, solid pseudopapillary tumors, pancreatoblastoma, and pancreatic metastasis. Primary pancreatic lymphoma (PPL) is an extremely rare solid neoplasm. It constitutes of less than 2% of extranodal lymphomas and 0.5% of all pancreatic masses [1]. Radiographic studies, such as computed tomography (CT), various forms of ultrasonography (US), and magnetic resonance imaging (MRI), may demonstrate key features to help the radiologist offer confident reports to guide the workup and management of patients with pancreatic masses, as current treatment strategies and prognosis are substantially different for neoplastic and nonneoplastic lesions and for type of neoplastic lesion. Therefore, we report a case of PPL, focusing on current literature regarding key imaging features and clinical presentation, and why the ability to differentiate at the stage of diagnostic imaging can influence further management.
2. Case presentation
A 36-year-old female with history of tobacco and alcohol abuse presented with 4 weeks of progressive constant circumferential pain across the upper abdomen and lumbar region accompanied by nausea, vomiting, diarrhea and unintentional 11-pound weight loss.
2.1. Investigations
A contrast enhanced CT (CECT) of the abdomen and pelvis was performed with series obtained in the late arterial and delayed phases, revealing a large hypodense lesion centered within the junction of the neck/body of the pancreas with mild dilation of the pancreatic duct (Fig. 1). Adjacent to the lesion was a bulky conglomerate of mesenteric lymph nodes extending below the level of the renal veins. It was concluded that a primary malignancy with central necrosis was the primary concern. However, with the clinical history, additional considerations were given to inflammatory or infectious causes. The patient was clinically treated as having pancreatitis and provided with parental fluids, bowel rest, and pain control with improvement of symptoms and tolerance of oral intake after four days of inpatient management. Therefore, the patient was discharged home with arrangement made for an outpatient endoscopic ultrasound (EUS) and potential biopsy.
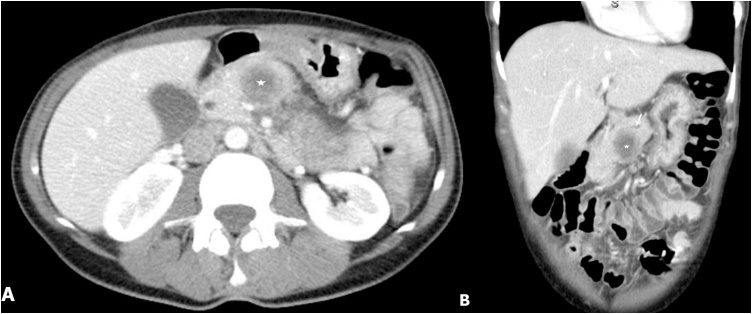
Fig. 1.
(A) Contrast enhanced axial, and (B) Coronal CT scan demonstrates a hypodense mass within the proximal pancreatic body (star). Pancreatic duct is minimally dilated despite the large size of mass (arrow).
Within two days of discharge, the patient was unable to tolerate oral intake and had a return of symptoms. A repeat CECT with late arterial and delayed phases revealed interval enlargement of the hypoenhancing mass and adjacent bulky mesenteric adenopathy. The patient was transferred to a tertiary center and admitted for further workup.
Upon admission to the referring hospital, an abdominal US demonstrated a complex avascular cystic lesion surrounded with thick soft tissue rim (Fig. 2). There was mild dilatation of the extrahepatic biliary ducts without a definitive cause. This was followed with further workup of magnetic resonance cholangiopancreatography (MRCP). MR identified a T2W intermediate lesion corresponding to the cyst seen on US (Fig. 3). There was associated mild dilatation and irregularity of the upstream main pancreatic duct. There were multiple enlarged peripancreatic, periportal, and mesenteric lymph nodes with a conglomerate surrounding the superior mesenteric artery (SMA) (Fig. 3). DWI/ADC demonstrated heterogeneous areas of diffusion restriction in the primary mass. However, the mesenteric lymphadenopathy demonstrated definitive and homogenous diffusion restriction (Fig. 4).
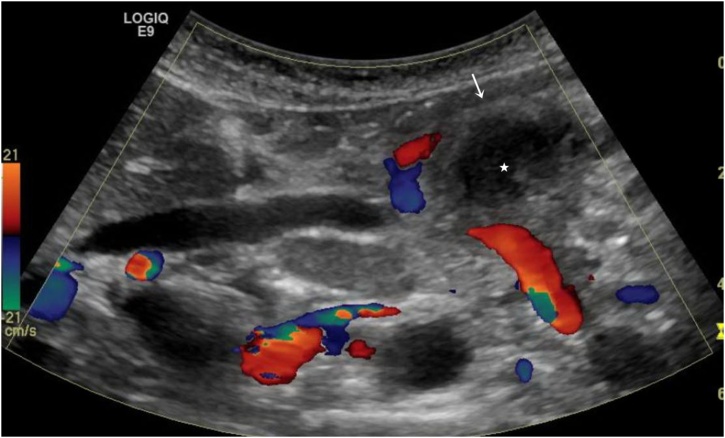
Fig. 2.
Targeted US shows a complex avascular cystic lesion (star) surrounded by thick soft tissue rim (arrow).
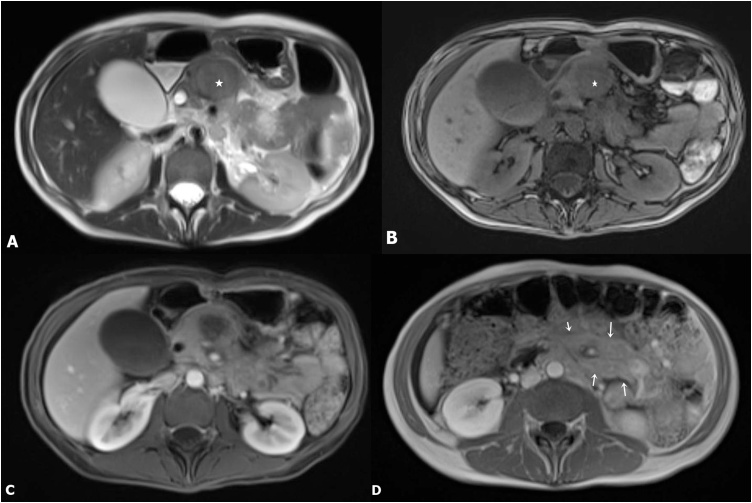
Fig. 3.
(A–B) Axial T1W MR Images shows corresponding hypointense lesion (star) in the pancreas. Lesion appears heterogeneous on T2W images and demonstrates intermediate signal intensity; (C) Axial post contrast fat suppressed T1W image shows mild enhancement; (D) Extensive mesenteric lymphadenopathy surrounding SMA (arrows).
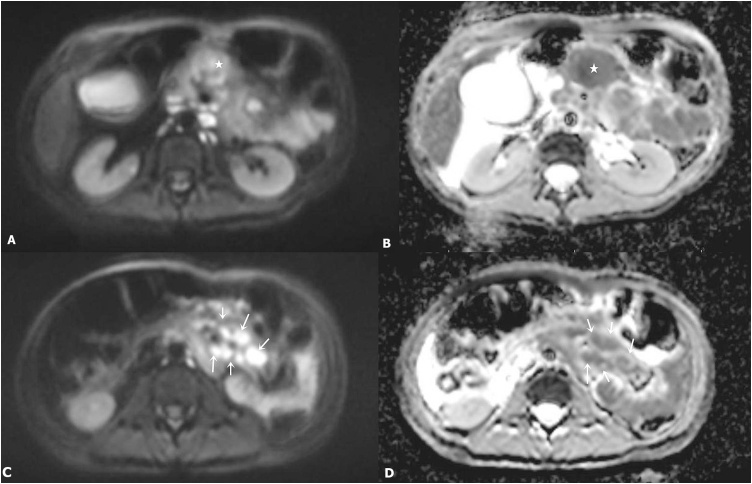
Fig. 4.
(A–B) Axial DWI and corresponding ADC images demonstrate heterogeneous areas of diffusion restriction within the primary mass (star); (C–D) The mesenteric lymphadenopathy demonstrates definitive diffusion restriction (arrows).
2.2. Differential diagnosis
After review of the radiologic studies, and consideration given to the patient’s relatively young age, the leading diagnosis for the pancreatic mass and pattern of adjacent lymphadenopathy was a primary B-Cell pancreatic lymphoma.
2.3. Outcome and follow-up
A follow-up EUS guided pancreatic biopsy yielded pleomorphic malignant neoplasm (Fig. 5) strongly positive for ALK and CD30 on immunohistochemical stains (Fig. 6). CD20 was essentially negative, showing very rare cells with nonspecific granular staining, negative for CD3, and negative for cytokeratin AE1/AE3. Genetic analysis demonstrated fusion of the NPM/ALK gene, suggestive of a chromosome (2;5)(p23; q35) translocation. The overall combined findings were most consistent with anaplastic large cell lymphoma.
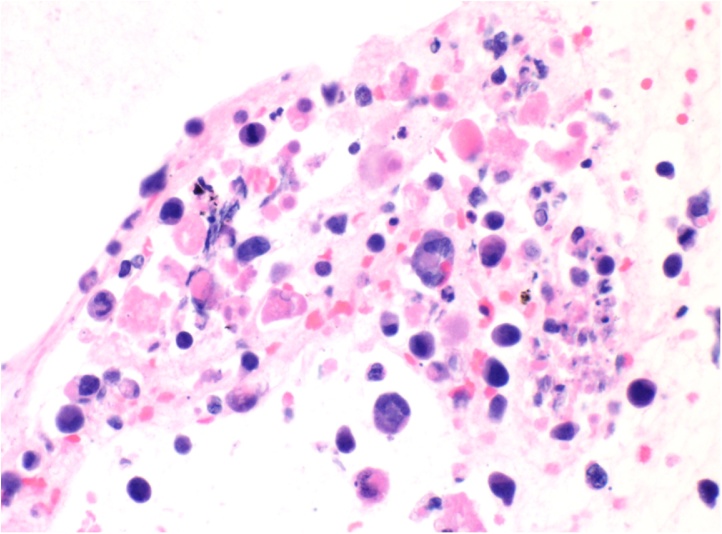
Fig. 5.
The cell block from the pancreatic neck mass fine needle aspiration shows a neoplastic cell population with increased nuclear to cytoplasmic ratio and marked pleomorphism. Scattered, enlarged, horseshoe-shaped cells (hallmark cells) are present (arrow) and are commonly found in anaplastic large cell lymphomas (400×).
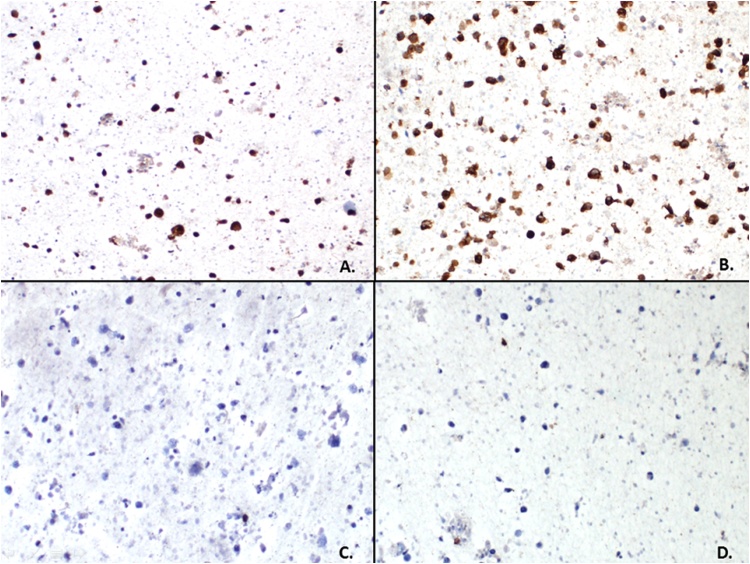
Fig. 6.
Immunoperoxidase studies on the cell block material demonstrates the pleomorphic neoplastic cells are strongly positive for ALK (A, 100×) and CD30 (B, 100×) with no expression of cytokeratin AE1/AE3 (C, 100×) and CD3 (D, 100×) which is consistent with an anaplastic large cell lymphoma. Additional molecular cytogenetic studies revealed a t(2;5) NPM/ALK fusion gene, found in 30–50% of anaplastic large cell lymphomas.
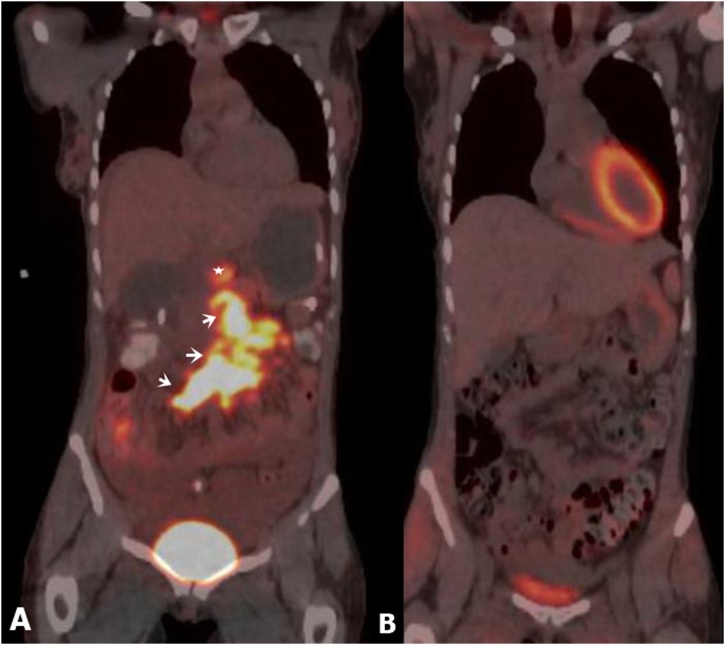
Further staging workup was performed, including CT of the chest as well as a positron emission tomography (PET). PET revealed increased FDG uptake corresponding with the pancreatic lymphoma and adjacent lymphadenopathy (Fig. 7). The patient was initiated on chemotherapy that included etoposide, prednisolone, oncovin, cyclophosphamide, and doxorubicin (EPOCH) with repeat PET/CT one month later demonstrating complete functional response.
Fig. 7.
(A) Pretreatment Coronal fused PET image demonstrates uptake in the region of pancreatic mass (star) and mesenteric adenopathy (adenopathy); (B) PET following once cycle of EPOCH demonstrating complete functional response.
3. Discussion
As mentioned previously, PPL is an extremely rare presentation of extranodal lymphoma and a high level of clinical suspicion must be maintained in order to differentiate from its more commonly encountered counterpart, adenocarcinoma. Current treatment strategies vary, and prognosis are substantially different with primary pancreatic lymphoma having a more favorable prognosis and response to radiation and/or chemotherapy. Therefore, when faced with a solid pancreatic mass, knowing when to direct the next steps in management towards a potential cure without surgical resection [2].
Imaging can suggest the diagnosis of primary pancreatic lymphoma and institute further evaluation with endoscopic ultrasound guided biopsy to ascertain a cytohistological diagnosis for appropriate treatment planning. Using comprehensive treatment approaches, cure rates may reach up to 30% [3].
CT is the most common imaging modality in which the first suggestion of pancreatic lymphoma will usually be encountered. With CT, most lesions will appear homogeneously hypodense with poor enhancement with contrast administration [3]. The pancreas may appear diffusely enlarged from an infiltrative appearance and usually does not result in obstruction of the pancreatic duct, even with ductal invasion. In contrast, pancreatic adenocarcinoma will commonly dilate the more distal pancreatic duct when more proximal ductal invasion has occurred [3]. Another helpful clue is if bulky lymphadenopathy is seen below the level of the renal veins, lymphoma is more likely the culprit rather than adenocarcinoma [3,4]. With MRI, pancreatic lymphoma generally demonstrates low signal characteristics within the pancreas on T1-weighted images with subtle enhancement following gadolinium-containing contrast. On T2-weighted images, pancreatic lymphoma may appear relatively heterogeneous in signal characteristics relative to surrounding pancreatic parenchyma [3]. Imaging features on DWI/ADC have not been well described in the literature [5]. PET imaging can be useful to assess treatment response.
Diagnosis of primary pancreatic lymphoma can be made, if following criteria are met: (1) Lack of coexisting superficial and mediastinal lymphadenopathy on clinical examination and chest radiography, (2) a normal white blood count in peripheral blood, (3) Primary mass in the pancreas with confined peripancreatic lymphadenopathy, and (4) Lack of hepatic or splenic involvement [6]. These criteria were met in our case.
In addition to radiographic features, clinical factors may help influence the diagnosis of pancreatic lymphoma over adenocarcinoma. Primary pancreatic lymphoma is considered more common between 35 and 75 years of age with a strong male predominance [7]. Adenocarcinoma is considered rare before the age of 45. A review of early case reports demonstrates that the most common presenting complaint includes abdominal pain. Other presentations may include weight loss, abdominal mass, acute pancreatitis, and icterus [3,8].
Once primary pancreatic lymphoma is suggested radiographically and followed by a tissue sampling, cytohistology is considered a routine part of diagnosis and management. Flow cytology aids in the detection of cell clonality and differentiation from chronic pancreatitis. With pancreatic lymphoma, expression of B-cell type cluster of differentiation (CD) receptors is usually demonstrated such as CD20. It is even rarer to have T-cell type CD receptors expressed, such as CD3. Most cases of B-cell pancreatic lymphoma are intermediate or high-grade non-hodgkin lymphoma with diffuse large cell lymphoma (DLBCL) being the most predominant [7]. Anaplastic large cell lymphomas (ALCL) are less commonly encountered. With ALCL, the most common genetic variation is translocation of t (2; 5) (p23; q35) between anaplastic lymphoma kinase (ALK) gene on chromosome 2 and nucelophosmin (NPM) gene on chromosome 5 [9]. As with the case presented, this results in the production of a chimeric NPM-ALK protein which can be detected immunohistochemically using monoclonal or polyclonal antibodies by polymerase chain reaction (PCR) or fluorescence in situ hybridization (FISH).
In regards to treatment, currently, anthracycline-based chemotherapy is considered the standard of care, and six to eight cycles of R-CHOP or EPOCH are usually performed for patients of all ages [10]. Behrns et al. [6] described a median survival of 13–22 months if treated with radio or chemotherapy alone, whereas it was ≤26 months with combined radiochemotherapy. Additionally, Tuchek et al. found total pancreatectomy (Whipple procedure) is not generally recommended for the diagnosis and/or treatment of PPL, as it has no impact on survival or associated morbidity [11]. However, more recent case reports are raising the possibility that surgery may have a therapeutic role in association with chemotherapy [12].
To conclude, PPL is a rare subset of solid pancreatic neoplasms that can mimic pancreatic adenocarcinoma; however, at the stage of diagnostic imaging, the radiologist can provide a critical role by maintaining a high level of suspicion and paying close attention to imaging features as described. Regardless, clinical and radiological findings are not pathognomonic and tissue sampling and cytohistological pathology is needed for diagnosis. As such, patients with PPL currently require chemotherapy-based treatment with or without radiation and surgical intervention and have a much better prognosis than those with adenocarcinoma.
4. Conclusion
-
•
Primary pancreatic lymphoma, an extranodal presentation of malignant lymphoma, is a rare subset of solid pancreatic neoplasms, which can closely mimic the more commonly encountered adenocarcinoma.
-
•
Imaging characteristics can provide helpful clues that suggest PPL rather than adenocarcinoma. While not pathognomonic, these include hypodense mass typically within the head of the pancreas with an enlarged infiltrative appearance of the pancreas without dilatation of the pancreatic duct congruent with the size of the mass, and peripancreatic lymphadenopathy extending below the renal veins.
-
•
Currently staging and treatment strategies differ between PPL and pancreatic adenocarcinoma. The former is worked up radiographically and by tissue sampling with treatment primarily involving chemotherapy and/or radiation. The later requiring surgical intervention such as a Whipple’s procedure.
-
•
Immunohistochemistry from tissue sampling can provide additional information regarding the subset of lymphoma, with diffuse large B-cell being the most common.
-
•
Patients with PPL have a much better prognosis than those with pancreatic adenocarcinoma.
Disclosure
None.
Conflict of interest
None.
Contributor Information
Bradley Aaron Cagle, Email: bcagle@wakehealth.edu.
Brenda L. Holbert, Email: bholbert@wakehealth.edu.
Stephanie Wolanin, Email: ssoiseth@wakehealth.edu.
Rafel Tappouni, Email: rtappoun@wakehealth.edu.
Neeraj Lalwani, Email: nlalwani@wakehealth.edu.
References
- 1.Saif M.W. Primary pancreatic lymphomas. JOP. 2006;7(3):262–273. [PubMed] [Google Scholar]
- 2.Sugishita H., Watanabe Y., Yamamoto Y., Yoshida M., Sato K., Horiuchi A., Kawachi K. Primary pancreatic lymphoma: the role of surgical treatment. Case Rep. Gastroenterol. 2010;4(1):104–110. doi: 10.1159/000283405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Merkle E.M., Bender G.N., Brambs H.J. Imaging findings in pancreatic lymphoma: differential aspects. AJR Am. J. Roentgenol. 2000;174(3):671–675. doi: 10.2214/ajr.174.3.1740671. [DOI] [PubMed] [Google Scholar]
- 4.Lalwani N., Mannelli L., Ganeshan D.M., Shanbhogue A.K., Dighe M.K., Tiwari H.A., Maximin S., Monti S., Ragucci M., Prasad S.R. Uncommon pancreatic tumors and pseudotumors. Abdom. Imaging. 2015;40(1):167–180. doi: 10.1007/s00261-014-0189-7. [DOI] [PubMed] [Google Scholar]
- 5.Fujinaga Y., Lall C., Patel A., Matsushita T., Sanyal R., Kadoya M. MR features of primary and secondary malignant lymphoma of the pancreas: a pictorial review. Insights Imaging. 2013;4(3):321–329. doi: 10.1007/s13244-013-0242-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Behrns K.E., Sarr M.G., Strickler J.G. Pancreatic lymphoma: is it a surgical disease? Pancreas. 1994;9(5):662–667. [PubMed] [Google Scholar]
- 7.Nayer H., Weir E.G., Sheth S., Ali S.Z. Primary pancreatic lymphomas: a cytopathologic analysis of a rare malignancy. Cancer. 2004;102(5):315–321. doi: 10.1002/cncr.20488. [DOI] [PubMed] [Google Scholar]
- 8.Arcari A., Anselmi E., Bernuzzi P., Berta R., Lazzaro A., Moroni C.F., Trabacchi E., Vallisa D., Vercelli A., Cavanna L. Primary pancreatic lymphoma. A report of five cases. Haematologica. 2005;90(1):ECR09. [PubMed] [Google Scholar]
- 9.Tsuyama N., Sakamoto K., Sakata S., Dobashi A., Takeuchi K. Anaplastic large cell lymphoma: pathology, genetics, and clinical aspects. J. Clin. Exp. Hematop. 2017;57(3):120–142. doi: 10.3960/jslrt.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zelenetz A.D., Advani R.H., Buadi F., Cabanillas F., Caligiuri M.A., Czuczman M.S., Damon L.E., Fayad L., Flinn I.W., Forero A., Glenn M.J., Gockerman J.P., Gordon L.I., Harris N.L., Hoppe R.T., Kaminski M.S., Lacasce A.S., Nademanee A., Porcu P., Press O., Prosnitz L., Smith M.R., Sotomayor E.M., Vose J.M., Yahalom J. N. National Comprehensive Cancer, non-Hodgkin's lymphoma. Clinical practice guidelines in oncology. J. Compr. Canc. Netw. 2006;4(3):258–310. doi: 10.6004/jnccn.2006.0025. [DOI] [PubMed] [Google Scholar]
- 11.Tuchek J.M., De Jong S.A., Pickleman J. Diagnosis, surgical intervention, and prognosis of primary pancreatic lymphoma. Am. Surg. 1993;59(8):513–518. [PubMed] [Google Scholar]
- 12.Battula N., Srinivasan P., Prachalias A., Rela M., Heaton N. Primary pancreatic lymphoma: diagnostic and therapeutic dilemma. Pancreas. 2006;33(2):192–194. doi: 10.1097/01.mpa.0000227910.63579.15. [DOI] [PubMed] [Google Scholar]