Abstract
A 61-year-old woman was evaluated because of 2 days of anuria, fever, anorexia, and progressive decline in mental status. On admission, she appeared confused with a GCS score of E2V2M4, and her blood ammonia level was elevated (176 μg/dL). Abdominal computed tomography showed bilateral hydronephrosis and bladder fullness with wall thickness in spite of indwelling bladder catheter. Her catheter was obstructed by blood clot. Blood cultures, blood clot culture, and one urine culture all yielded Proteus mirabilis. Obstructive urinary tract infection complicated with septic shock was diagnosed. After treatment, her ammonia level normalized.
Keywords: Obstructive urinary tract infection, Hyperammonaemia, Proteus mirabilis
Introduction
Hyperammonemia, an elevated level of ammonia in the blood, one of the causes of consciousness disturbance. When the accumulated ammonia in the blood reaches the bloodbrain barrier and crosses it, various symptoms occur including lethargy, irritability, and, in severe cases, seizures and coma. Hyperammonemia is generally caused by liver disease, often with gastrointestinal hemorrhage and/or a portosystemic shunt, and vesicorectal fistula, drugs such as 5-fluorouracil, salicylate, asparaginase, acetazolamide, diuretics, valproic acid, and barbiturate [1]. Hyperammonemia caused by non-hepatic causes are rare and may be reversible and prompt recognition and treatment can be life-saving.
We report a septic shock cause of hyperammonemic encephalopathy with a obstructive urinary tract infection (OUTI) caused by a urease-producing bacterium, Proteus mirabilis, without any of the common causes of hyperammonemia.
Case report
A 61-year-old woman was evaluated because of 2 days of anuria, fever, anorexia, and progressive decline in mental status. Her medical history included cerebral palsy, and neurogenic bladder without diabetes, and not hypertension, type 2 diabetes, depression, and immunodeficiency. She was treated with indwelling bladder catheter to relieve urinary retention for ten years. She had no medication. The patient was evaluated by her primary care provider at her house. She had a temperature of 38.7℃. Her consciousness level was Glasgow coma scale (GCS) E2V2M4. After two hours, she was admitted to the hospital because of fever with a disturbance of consciousness for further evaluation.
On examination, she appeared confused with a GCS score of 8/15 (E2V2M4). Her temperature was 37.8℃, blood pressure 151/81 mmHg, heart rate regular at 95 beats per minute, percutaneous oxygen saturation 96% (room air), and respiration rate 26 per minute. The patient mentioned a discomfort in the suprapubic region. Her breath did not smell of alcohol, acetone, and ammonia. Bulbar (palpebral) conjunctivae were not injected or congested without icterus or pallor. The respiratory examination was unremarkable and abdominal examination revealed no stigmata of chronic liver disease or focal tenderness in the suprapubic area or costovertebral angles. A large, non-tender cystic mass was palpable in the suprapubic region. The patient`s neurologic examination revealed that she was slow to answer questions and follow commands, was hard of hearing, and had no asterixis. She could not give any details of her history or symptoms and did not say that she had a headache or stiff neck. The nursing staff noted that there was no urine in her urine bag.
Laboratory analysis at the time of admission are shown in Table 1. Urinalysis showed marked pyuria, a pH of 8.0, specific gravity 1.009, protein >4+, with numerous bacteria, >100 white blood cells, 10–19 red blood cells, 1–4 ammonium magnesium phosphate crystals, and 1–4 epithelial cells per high-power field in the sediment. The white blood cell count was 25,200 /mm3, with 93% neutrophils, 15% band forms, 2% lymphocyte, 1% monocyte; hemoglobin, 11.5 g/dL; hematocrit, 34.6%; and platelet count was 132,000 /mm3. Coagulation test revealed prothrombin time INR 1.44; the serum fibrinogen 447.5 mg/dL, fibrin/fibrinogen degradation products (FDP) 37.5 μg/mL. Biochemical analysis showed aspartate aminotransferase (AST) 43 IU/L, alanine aminotransferase (ALT) 23 IU/L, lactate dehydrogenase (LDH) 275 IU/L, alkaline phosphatase (ALP) 240 IU/L, total bilirubin (TB) 1.09 mg/dL, urea nitrogen (UN) 43.2 mg/dL, creatinine 1.2 mg/dL, creatine kinase (CK) 1222 IU/L, sodium 144 mEq/L, potassium 3.1 mEq/L, and C-reactive protein (CRP) 16.97 mg/dL. Blood ammonia level was elevated (176 μg/dL).
Table 1.
Laboratory analysis on admission.
| WBC (/mm3) | 25200 | AST (IU/L) | 43 | Urinalysis | |
|---|---|---|---|---|---|
| neutrophil (%) | 93 | ALT (IU/L) | 23 | appearance | pyuria |
| band forms (%) | 15 | LDH (IU/L) | 275 | pH | 8.0 |
| lymphocyte (%) | 2 | TB (mg/dL) | 1.09 | specific gravity | 1.009 |
| monocyte (%) | 1 | UN (mg/dL) | 43.2 | protein | >4+ |
| Hb(d/dL) | 11.5 | Cr (mg/dL) | 1.20 | WBC | >100/HPF |
| Ht (%) | 34.6 | CK (IU/L) | 1222 | RBC | 10-19/HPF |
| Plt (/mm3) | 132000 | Sodium (mEq/L) | 144 | ||
| PT-INR | 1.44 | Potassium (mEq/L) | 3.1 | ||
| Fibrinogen (mg/dL) | 447.5 | CRP (mg/dL) | 16.37 | ||
| FDP (μg/mL) | 37.5 | ammonia (μg/dL) | 176 |
WBC: white blood cell, Hb: hemoglobin, PLT: platelet, PT: prothrombin time, FDP: fibrin/fibrinogen degradation products, AST: aspartate aminotransferase, ALT: alanine aminotransferase, LDH: lactate dehydrogenase, TB: total bilirubin, BUN: blood urea nitrogen, Cr: creatinine, CK: creatine kinase Na::sodium, K: potassium, CRP: C-reactive protein, HPF: high-power field.
Head computed tomography (CT) and magnetic resonance imaging (MRI) revealed no factor responsible for consciousness disturbance, and chest CT showed no pneumonitis.
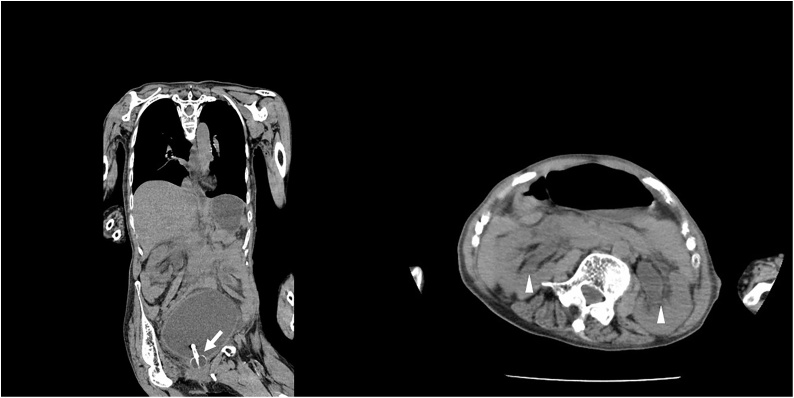
Abdominal CT and ultrasonography revealed bilateral hydronephrosis and bladder fullness with wall thickness in spite of indwelling bladder catheter, however, no sign of liver cirrhosis (Fig. 1).
Fig. 1.
Abdominal Computed Tomography showed bilateral hydronephrosis (triangle) and bladder fullness with wall thickness in spite of indwelling bladder catheter (arrow).
Sepsis was diagnosed on the basis of qSOFA criteria (GCS < 15 and respiratory rate ≥22) on admission [2] and a bladder catheter exchange was performed. Her indwelling bladder catheter on admission was obstructed by blood clots. Before antimicrobial therapy was begun, two blood samples and one urine sample had been obtained for cultures. Tazobactam / Piperacillin, 2.25 g intravenously, was administered q6h. Approximately 10 h later, the patient had a temperature of 39.8℃, her systolic blood pressure 60/30 mmHg, heart rate at 120 beats per minute, respiration rate 30 per minute, and she appeared ill. Extracellular fluid was administered for volume resuscitation. In addition, intravenous continuous infusion noradrenaline and methylprednisolone was administered. However, her mean arterial pressure (MAP) was 55 mmHg (<65), and lactate was 20 mg/dL (>2 mmol/L). Volume resuscitation, cardiovascular support, antimicrobial administration, and bladder catheterization were continued. Seven days later from admission, two blood cultures, blood clots culture, and one urine culture detected Proteus mirabilis. The P. mirabilis isolates from our patients was susceptible to Cefazolin, Cefotiam, Cefotaxime, Ceftazidime, Ceftriaxone, Cefepime, Piperacillin, Sulbactam / Ampicillin, Tazobactam / piperacillin, Gentamicin, Amikacin, Imipenem, Meropenem, Levofloxacin, and trimethoprim, but less to minocycline. The patient’s level of consciousness and general condition improved rapidly and ammonia level had normalized (46 μg/dL). After she received rehabilitation, she was discharged on the 28th hospital day.
Discussion
We report on a case of hyperammonemic encephalopathy caused by OUTI due to urease-producing bacterium, Proteus mirabilis, in a patient without a history of liver cirrhosis or portal hypertension. There were no causes for the consciousness disturbance in our case by history, physical exams, laboratory and brain imaging (CT and MRI). No evidence of underlying liver disease found. The causes of hyperammonemia without liver cirrhosis or portal hypertension included portosystemic shunt, vesicorectal fistula, drugs such as 5-fluorouracil, salicylate, asparaginase, acetazolamide, diuretics, valproic acid, and barbiturate, and shock [1]. In our case, however, the various abdominal imaging study ruled out the presence of an extra-hepatic portosystemic shunt and none of the implicated medications were involved here.
Several bacteria can produce urease and thus they are considered as ammonia producers. Arai et al. reported urease-producing bacteria detected from urine culture and showed percentage of urease-positive bacteria common found in UTI as follows; Proteus mirabilis (95%), Proteus vulgaris (97%), Morganella morganii (92%), Klebsiella pneumoniae (94%), Klebsiella oxytoca (96%), Pseudomonas aeruginosa (15%), and Escherichia coli (0%) [3]. Proteus mirabilis is a Gram-negative, facultatively anaerobic, rod-shaped bacterium. It shows swarming motility and urease activity. P. mirabilis causes 90% of all Proteus infections in humans. It is widely distributed in soil and water. It has the ability to produce high levels of urease, which hydrolyzes urea to ammonia, so makes the urine more alkaline [4], and the ammonia can enter the blood circulation, causing encephalopathy when it crosses blood-brain barrier. In our case, the various culture grew Proteus mirabilis and our treatment led to the recovery of her mental status and her blood ammonia level.
OUTI caused by urease-producing bacteria can cause hyperammonemia. Obstruction to urine flow can result from intrinsic or extrinsic mechanical blockade as well as from functional defects not associated with fixed occlusion of the drainage system. Urinary stagnation in a chronic distended bladder probably influenced ammonia diffusion through the bladder wall to the perivesical circulation. Most venous blood from the bladder drains directly into the hypogastric veins and inferior vena cava, bypassing the portal circulation and liver. As occurred in this case, the ammonia levels increased and caused encephalopathy [5]. Our case was treated with an indwelling bladder catheter to reduce urinary retention because of neurogenic bladder during home care. Two days of anuria in her medical history and bladder fullness with wall thickness in spite of indwelling bladder catheter suggested obstruction of indwelling bladder catheter.
To prevent OUTI, it is necessary to observe urine-flow and urine symptom carefully. In particular, indwelling bladder catheter should be replaced regularly by a skilled operator in a safe environment. To manage indwelling bladder catheter is an important point to prevent health care-associated infection due to indwelling bladder catheter during home care.
Urinary tract infection (UTI) with urease-producing bacteria is considered a recognized but rare cause of hyperammonemia in the adult population [5,6]. A few adult cases with Proteus UTI including OUTI with hyperammonemia have been reported before our case [7,8]. Thus, our case may be considered as the additional example for hyperammonemic OUTI reported.
In conclusion, we report a case of septic shock with hyperammonemia caused by OUTI because of indwelling bladder catheter obstruction. Management of indwelling bladder catheter is important to prevent OUTI. Additionally, blood ammonia should be measured for UTI patients with consciousness disturbance.
Conflicts of interest
We declare the authors have no conflict of interests.
Sources of funding
We declare the authors have no sources of funding for our research.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of IDcases on request
Author contribution
All authors of this case report have directly participated in treatment and writing for the case.
References
- 1.Hawkes N.D., Thomas G.A., Jurewicz A., Williams O.M., Hillier C.E., McQueen I.N. Non-hepatic hyperammonaemia: an important, potentially reversible cause of encephalopathy. Postgrad Med J. 2001;77:717–722. doi: 10.1136/pmj.77.913.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singer M., Deutschman C.S., Seymour C.W., Shankar-Hari M., Annane D., Bauer M. The third international consensus definitions for Sepsis and septic shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arai Y., Takeuchi H., Tomoyoshi T., Tatewaki K. Urease activity of bacteria in urine. Hinyokika Kiyo. 1989;35:277–281. [PubMed] [Google Scholar]
- 4.Schaffer J.N., Pearson M.M. Proteus mirabilis and urinary tract infections. Microbiol Spectr. 2015;3(5) doi: 10.1128/microbiolspec.UTI-0017-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Jonghe B., Janier V., Abderrahim N., Hillion D., Lacherade J.C. Urinary tract infection and coma. Lancet. 2002;360:996. doi: 10.1016/S0140-6736(02)11084-1. [DOI] [PubMed] [Google Scholar]
- 6.Albersen M., Joniau S., Van Poppel H., Cuyle P.J., Knockaert D.C. Urea-splitting urinary tract infection contributing to hyperammonemic encephalopathy. Nat Clin Pract Urol. 2007;4:455–458. doi: 10.1038/ncpuro0877. [DOI] [PubMed] [Google Scholar]
- 7.Soeno S., Kenzaka T., Takeda K., Tanaka Y., Kuroki S. A case of hyperammonemia due to obstructive urinary tract infection. Nihon Naika Gakkai Zasshi. 2013;102:976–978. doi: 10.2169/naika.102.976. [DOI] [PubMed] [Google Scholar]
- 8.Sato S., Yokota C., Toyoda K., Naganuma M., Minematsu K. Hyperammonemic encephalopathy caused by urinary tract infection with urinary retention. Eur J Intern Med. 2008;19:e78–79. doi: 10.1016/j.ejim.2007.10.022. [DOI] [PubMed] [Google Scholar]