Short abstract
Bulleyaconitine A, a diterpenoid alkaloid isolated from Aconitum bulleyanum plants, has been used for the treatment of chronic pain in China since 1985. Clinical studies show that the oral administration of bulleyaconitine A is effective for treating different kinds of chronic pain, including back pain, joint pain, and neuropathic pain with minimal side effect in human patients. The experimental studies have revealed that bulleyaconitine A at therapeutic doses potently inhibits the peripheral sensitization and central sensitization that underlie chronic pain and has no effect on acute pain. Bulleyaconitine A preferably blocks tetrodotoxin-sensitive voltage-gated sodium channels in dorsal root ganglion neurons by inhibition of protein kinase C, and the effect is around 600 times more potent in neuropathic animals than in naïve ones. Bulleyaconitine A at 5 nM inhibits the hypersensitivity of dorsal root ganglion neurons in neuropathic rats but has no effect on excitability of dorsal root ganglion neurons in sham group. Bulleyaconitine A inhibits long-term potentiation at C-fiber synapses in spinal dorsal horn, a synaptic model of pathological pain, preferably in neuropathic pain rats over naïve rats. The following mechanisms may underlie the selective effect of bulleyaconitine A on chronic pain. (1) In neuropathic conditions, protein kinase C and voltage-gated sodium channels in dorsal root ganglion neurons are upregulated, which enhances bulleyaconitine A’s effect. (2) Bulleyaconitine A use-dependently blocks voltage-gated sodium channels and therefore inhibits the ectopic discharges that are important for neuropathic pain. (3) Bulleyaconitine A is shown to inhibit neuropathic pain by the modulation of spinal microglia, which are involved in the chronic pain but not in acute (nociceptive) pain. Moreover, bulleyaconitine A facilitates the anesthetic effect of morphine and inhibits morphine tolerance in rats. Together, bulleyaconitine A is able to inhibit chronic pain by targeting at multiple molecules. Further clinical and experimental studies are needed for evaluating the efficacy of bulleyaconitine A in different forms of chronic pain in patients and for exploring the underlying mechanisms.
Keywords: Bulleyaconitine A, chronic pain, dorsal root ganglion, voltage-gated sodium channels, spinal dorsal horn, long-term potentiation
Introduction
Chronic pain affecting approximately one-third of the people worldwide reduces patient’s life quality and working ability, seriously.1 Pharmacotherapy is the first choice for treating the disease. While the currently used analgesics, including opioid, nonsteroidal anti-inflammatory agents, tricyclic antidepressants, and antiepileptics, are only effective in a part of patients, with many side effects, such as addiction, tolerance, nausea/vomiting, dizziness, and renal function damage,2 the pharmacological treatment of chronic pain is largely unmet.
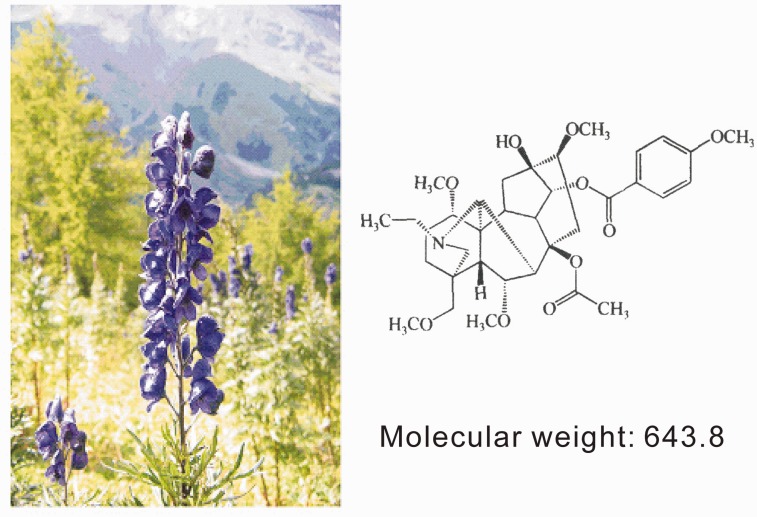
Bulleyaconitine A (BLA) is a diterpenoid alkaloid isolated from the rhizomes of Aconitum bulleyanum Diels by Kunming Institute of Botany, Chinese Academy of Sciences in 1980 (Figure 1). The plant grows in the area of Yulong Snow Mountain (altitude 4 km) in Yunnan province of China. As BLA is shown to have potent analgesic effect in animals and human patients, it is proved to treat chronic pain resulting from rheumatic and rheumatoid arthritis, strain of lumbar muscles, and scapulohumeral periarthritis by China Food and Drug Administration in 1985. The three decades of clinical application show that the oral administration of BLA is effective for treating various forms of chronic pain without addiction, tolerance, nausea/vomiting, dizziness, and renal function damage.3 To date, BLA is still the sole clinically used analgesic developed in China. To promote its clinical application and further development, the studies on mechanisms for the therapeutic effect of BLA on chronic pain are reviewed in this article.
Figure 1.
The Aconitum bulleyanum Diels growing in the area of Yulong Snow Mountain and the molecular formula of Bulleyaconitine A.
The mechanisms underlying chronic pain
To understand the pharmacological effects of BLA, the mechanisms underlying chronic pain are reviewed briefly at first.
Classification of pain
Pain can be roughly divided into two main groups: nociceptive pain and pathological pain. The nociceptive pain, also called physiological pain or acute pain, is produced by noxious stimulation of normal tissues with normal somatosensory nervous system.4 Nociceptive pain characterized by lasting short time and high threshold is an alarm signal to avoid further tissue damage. While the pathological pain is an abnormal pain resulting from injury or diseases and is subdivided into inflammatory pain and neuropathic pain. The pathological pain is manifested as allodynia (decrease in pain threshold), hyperalgesia (increase in response to noxious stimuli), and spontaneous pain. The pathological pain may persist for either short or long time. The short-lasting one is also protective, such as the local hypersensitivity produced by skin burning persisting for a few days is in favor of wound healing. Whereas, the pathological pain that persists for months, for years, or even for decades after injury or disease has been healed, for example, phantom pain and postherpetic neuralgia, only makes patients suffering without any benefit.5,6 The treatment of chronic pain remains a big challenge for pain clinicians.
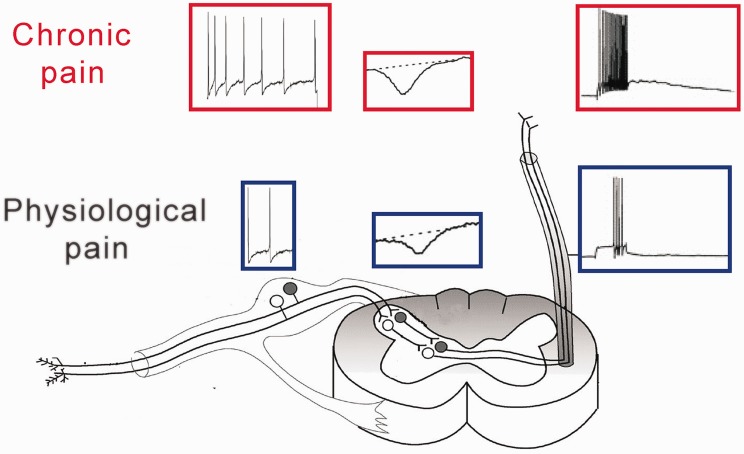
Peripheral sensitization and central sensitization underlie pathological pain
Peripheral sensitization refers to a persist hypersensitivity of nociceptors or primary afferent fibers, which increases the inputs of pain signals from peripheral nerve to central nervous system (Figure 2). Under physiological conditions, pain is conducted by afferent C-fibers and Aδ-fibers. Their cell bodies are located in dorsal root ganglion (DRG) or trigeminal ganglion. The thresholds of C- and Aδ-fibers are much higher than those of Aβ-fibers that conduct light tactile sensation, and therefore physiological pain can only be produced by noxious stimuli. In the pathological condition, however, not only C- and Aδ-fibers become hypersensitive, Aβ-fibers also conduct pain signals, leading to allodynia.7 Under physiological conditions, the primary afferent fibers produce action potentials (excitation) only when peripheral tissues or nerves are stimulated. While in pathological conditions, spontaneous action potential discharges (also named ectopic discharges) can be recorded in afferent A- and C-fibers of animals8 or human patients.9 The ectopic discharges are resulting from the abnormal expression and/or dysfunction of voltage-gated ion channels in primary afferent neurons. Among them the voltage-gated sodium (Nav) and voltage-gated calcium (Cav) channels are intensively studied. Ten subtypes of Nav (Nav1.1–1.9 and Nax) have been found in DRG neurons. The experimental studies with various animal models of neuropathic pain show that all Nav channel subtypes (apart from Nav1.3) are downregulation in injured DRG neurons (see Wang et al.10 for a review). While in uninjured DRG neurons, many of them, such as Nav1.3, Nav1.7, and Nav1.8, are upregulated.11,12 The data can explain why the uninjured afferents produce ectopic discharges following peripheral nerve injury13–15 and injury to motor fibers by transection of L5 ventral root, leaving the sensory fibers intact, produces chronic neuropathic pain.16 Studies show that tumor necrosis factor-α (TNF-α; a pro-inflammatory cytokine) overexpression is necessary and sufficient for the upregulation of Nav in uninjured DRG neurons.11,17 While anti-inflammatory cytokine interluekin-10 (IL-10) inhibits the effect of TNF-α on Nav expression.18 Cav channels are classified into three subclasses according to the amino acid sequence of α1 subunits: Cav1 (Cav1.1–1.4), Cav2 (Cav2.1–2.3), and Cav3 (Cav3.1–3.3) (see Dolphin19 for detail). The N-type calcium channels (Cav2.2) are expressed in cell body and in central terminals of DRG neurons in spinal dorsal horn.20,21 Cav2.2 channels in the central terminals, which are critical for the release of neurotransmitters, are upregulated in neuropathic pain conditions and play an important role in chronic pain (see below). A recent work22 shows that Cav2.2 channels are upregulated in the cell bodies of uninjured L4 DRG neurons but downregulated in injured L5 DRG neurons in L5-spinal nerve ligation (SNL) model. The upregulation of Cav2.2 increases the excitability of the uninjured DRG neurons. Interestingly, similar to the regulation of Nav channels the up- and downregulation of the Cav2.2 in uninjured and injured DRG neurons are also, respectively, mediated by TNF-α and IL-10.
Figure 2.
The mechanisms of pathological pain. Compared to physiological conditions (blue), the action potentials in primary afferent fibers and the synaptic potentials in spinal dorsal horn produced by the same peripheral stimulation are increased in pathological conditions (red). The former called peripheral sensitization and later central sensitization.
Central sensitization indicates the persist increase in synaptic transmission in pain pathway, that is, long-term potentiation (LTP). The pathological meaning of the LTP is to amplify pain signals (Figure 2). LTP in pain pathway is first found in spinal dorsal horn where the first order of synapses are formed in somatosensory circuits.23,24 Later, LTP is reported in anterior cingulate and in prefrontal cortex.25 Research shows that LTP is mediated by the increases in both presynaptic neurotransmitter release and postsynaptic response to the neurotransmitters (see Zhuo25 and Liu and Zhou26 for detail). It is worth to note that the upregulation of Cav2.2 in central terminals of DRG neurons in spinal dorsal horn plays an important role for chronic pain.27 Pharmacological blockage of the channel subtype attenuates neuropathic pain by the reduction of neurotransmitter release.28
The mechanisms underlying analgesic effect of BLA
In 1986, Tang et al.29 reported that subcutaneous injection of BLA inhibits inflammatory pain evoked by formalin injection, the surface (skin) pain by noxious heat, or the visceral pain by intraperitoneal injection of acetic acid, as assessed with hot plate test, radiant heat method, and writhing test. The analgesic effect of BLA is much more potent than morphine. The effect of BLA can be abolished by reserpine that exhausts catecholamine but not affected by opioid receptor blocker naloxone. Importantly, repetitive application of BLA does not induce addiction and tolerance. Recent studies show that oral administration,30 DRG local application,31 and intrathecal injection32 of BLA are effective for inhibition of neuropathic pain, bone cancer-induced pain, and chemotherapy-induced pain in rodents. Importantly, it has been reported that BLA has synergistic effect with morphine and can completely abolish the tolerance of morphine analgesic in rats.32
BLA blocks Nav channels in DRG neurons
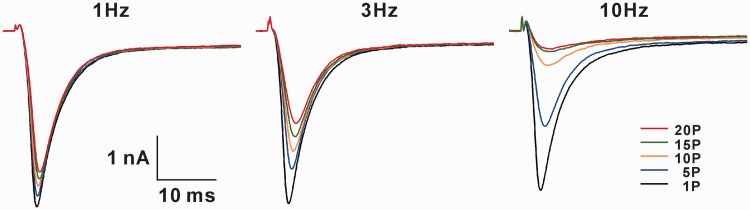
The Nav channels that allow Na+ influx play critical roles for the generation and the conduction of action potentials in all neurons. At resting state, Nav channel is closed and can be opened by depolarization. Upon opening, the channel goes rapidly to inactivated state, during which the channels are also closed but cannot be opened again.33 The drugs that act at different states of Nav channels exhibit various pharmacological effects. If a drug acts at open or inactivated channels, the blocking effect will increase gradually with repetitive opening. The phenomenon is called use-dependent or activity-dependent blockage. This is because the drug prevents channels from going back to resting state, leading to progressive decrease in the number of resting channels in cell membrane (Figure 4). If a drug acts at resting state, it will not show such feature. The use-dependent blockade enables a drug to selectively inhibit the Nav channels, which open with high frequency, and is important for the treatment of epilepsy34 and neuropathic pain.8,31,35
Figure 4.
The use-dependent blockage of BLA on uninjured DRG neurons of L5-SNL rats. In the presence of BLA at IC50 concentration (0.8 nM), the Na+ currents of same DRG neuron changes differentially to various frequency stimulation. The higher the frequency, the smaller the currents.
Wang et al. studied the effects of BLA on Nav channels in GH3 cell line expressing Nav1.1–1.3 and Nav1.636 and in HEK293t cell line expressing Nav1.7 or Nav1.837 with patch clamp technique. To test the effect of BLA on resting Nav channels, the membrane potential is hold at −140 mV (to let Nav channels at resting state), and cells are stimulated once every 30 s by a brief test pulse to open the Nav channels. With use of this protocol, they found 5 min after BLA (10 μM) application, Na+ currents is not different from predrug control. When membrane potential is hold at −70 mV for 10 s (to inactivate Nav channels), BLA (10 μM) also does not affect Na+ currents. They therefore conclude that BLA affects neither resting nor inactivated Nav channels. When the cells were repetitively stimulated at 2 Hz (1000 pulse), however, the same dose of BLA reduced the Nav currents progressively, indicating that BLA use-dependently blocks Nav channels. Although the studies reveal that BLA is able to block Nav channels but cannot explain the analgesic effect of oral application of BLA. A pharmacokinetic study in rats shows that the peak plasma concentration of BLA is 17.8 nM (11.47 ng/ml) following a single oral administration of BLA at 0.36 mg/kg,38 and oral BLA at 0.4 mg/kg is effective to inhibit neuropathic pain induced by paclitaxel in the same species.30 Apparently, 10 μM is much higher than the plasma concentration achieved by therapeutic dose. A recent work shows that DRG local application of BLA at 1–10 nM is effective to reduce allodynia produced in L5-SNL.31
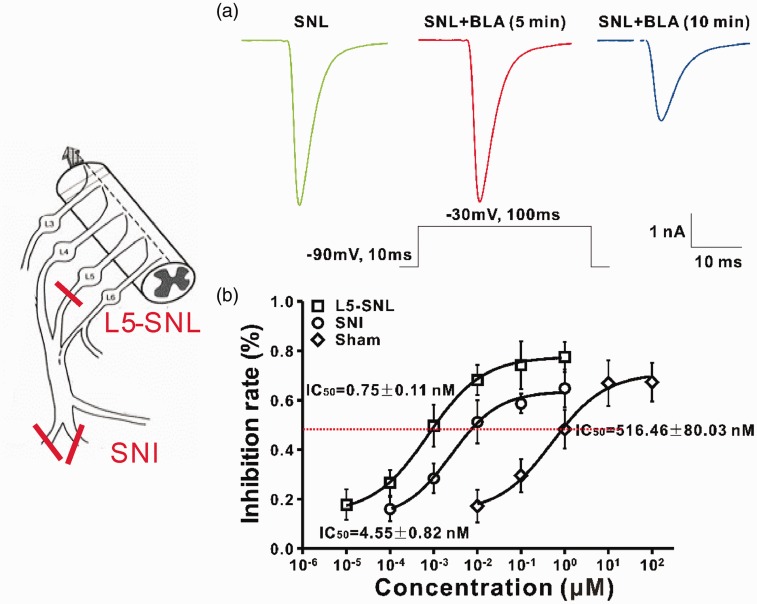
In a recent work, using both L5-SNL and spared nerve injury (SNI) models of neuropathic pain, the effects of BLA on Nav channels of DRG neurons were studied systematically.31 As Nav channels (except for Nav1.3) are downregulated in injured DRG neurons but upregulated in uninjured ones, in this work, the Na+ currents were recorded in L4–L6 DRG neurons of SNI rats (containing both injured and uninjured neurons) and in the uninjured L4 and L6 DRG neurons of L5-SNL rats before and after BLA application at different concentrations, and 50% inhibitory concentrations (IC50 values) were calculated (Figure 3). The results show that BLA is able to block resting, inactivated Nav channels and the channels opening at high frequency. The effect of BLA on Nav channels has the following features: (1) The latency is longer than 5 min, and maximal effect appears at 10 min after application (Figure 3(a)). (2) The effect of BLA cannot be reversed by washing with vehicle for 15 min. (3) BLA cannot completely abolish Na+ currents even at high concentration, and the maximal inhibitory rate is around 80%. (4) For blockage of resting Nav channels on DRG neurons BLA is 688 times more potent on the uninjured neurons of L5-SNL rats and 113 times more potent in the neurons of SNI rats, compared to those in sham rats (IC50 around 500 nM) (Figure 3(b)). (5) The effect of BLA on inactivated Nav channels is 8–12 folds more potent than on resting ones. IC50 values for resting and inactivated channels in different groups are shown in Table 1. (6) BLA preferably blocks tetrodotoxin (TTX)-S channels over TTX-R ones. IC50 values for resting and inactivated TTX-S channels are 1855 and 1843 times lower than those for TTX-R channels in the uninjured neurons of L5-SNL rats. (7) BLA use-dependently blocks Nav channels, and the effect is also most profound in L5-SNL rats, less in SNI rats and least in sham rats. Figure 4 shows the effect of BLA at IC50 concentration on the resting Nav channels of an uninjured DRG neuron.
Figure 3.
Bulleyaconitine A (BLA) preferably blocks the resting Nav channels in dorsal root ganglion neurons of neuropathic rats. The models of L5-spinal nerve ligation (SNL) and spared nerve injury (SNI) are shown on left. (a) The green line indicates the Na+ currents recorded before BLA application, red and blue ones are recorded 5 and 10 min after BLA application at IC50 concentration. (b) IC50 values of BLA on Nav channels of Sham, SNI, and SNL groups.
Table 1.
The IC50 values in different models of DRG neurons.
| Sham | SNI | SNL | |
|---|---|---|---|
| Resting state | 516 ± 80 nM | 4.55 ± 0.8 nM (113) | 0.75 ± 0.1 nM (688) |
| Inactivated state | 41.4 ± 7.2 nM | 0.56 ± 0.08 nM (74) | 0.08 ± 0.01 nM (518) |
| Times | 12 | 8 | 9 |
The times indicate fold differences in IC50 between resting and inactivated states. The digits in round brackets indicate fold differences in IC50, compared to sham groups. SNI: spared nerve injury; SNL: spinal nerve ligation.
Effects of BLA on the excitability of DRG neurons (20–35 μm in diameter) in SNI rats and on Nav1.3, Nav1.7, and Nav1.8 channel subtypes expressed in cell lines were investigated in a recent work.35 It has been shown that BLA at 5 nM is effective for inhibition of the hypersensitivity of DRG neurons produced by SNI. BLA reverses the decreased threshold and increased firing rate of action potentials of DRG neurons in SNI rats but does not affect the excitability of DRG neurons in sham rats. BLA also preferably inhibits TTX-S channels over TTX-R channels in SNI model, and effect is much more profound for inactivated channels than resting channels. Consistently, in cell line, the effects of BLA on resting Nav1.3 and Nav1.7 are, respectively, 152 and 1203 times more potent than on resting Nav1.8 (IC50 = 150 μM). BLA also preferably blocks inactivated channels over resting ones expressed in cell lines (Table 2).
Table 2.
The IC50 values of BLA for different subtypes of sodium channel.
| Nav1.8 | Nav1.7 | Nav1.3 | |
|---|---|---|---|
| Resting state | 151 ± 15 µM | 125 ± 18 nM (1203) | 995 ± 139 nM (152) |
| Inactivated state | 18 ± 3 µM | 132 ± 25 pM (135,440) | 20 ± 3.4 pM (886,670) |
| Times | 8 | 946 | 49,044 |
The times indicate fold differences in IC50 between resting and inactivated states. The digits in round brackets indicate fold differences in IC50, compared to Nav1.8.
How can BLA preferably block TTX-S Nav channels in uninjured DRG neurons of neuropathic rats? The question was also investigated in the recent work.31 The facts that the inhibitory effect of BLA on Nav channels has a long latency (>5 min) and cannot be washed out suggest that BLA may target at intracellular molecules that regulate the function of Nav channels. Indeed, intracellular application of BLA at IC50 concentration with recording electrodes reduces Na+ currents by 20% within 2 min in DRG neurons of sham, SNI, and L5-SNL rats, compared to vehicle group. The data demonstrate that the target of BLA is really within the neurons, and then, what is it? Previous works show that the activation of protein kinase C (PKC) reduces Na+ currents in hippocampal39 and cortical40 neurons but enhances Na+ currents in DRG neurons.41 Furthermore, PKC is upregulated in neuropathic pain conditions.42 Therefore, it is possible that BLA may reduce Na+ currents by inhibition of PKC in the uninjured DRG neurons. To verify the hypothesis, at first, the intracellular application of PKC inhibitors (staurosporine and Go 6983) with recording electrodes was tested to inhibit Na+ currents. Indeed, the manipulation reduces Na+ currents, and the effect is most potent on uninjured DRG neurons of L5-SNL rats, less on those of SNI rats and almost no effect on those of sham rats. In consistence with the electrophysiological results, Western blots show that PKC is upregulated in uninjured L4 DRG neurons and downregulated in injured L5 DRG neurons in L5-SNL rats. The data demonstrate that peripheral nerve injury enhances Na+ currents in uninjured DRG neurons by the upregulation of PKC. To directly test that BLA may reduce Na+ currents by inhibition of PKC, the uninjured L4 and L6 DRG neurons isolated from L5-SNL rats were incubated with PKC inhibitors for 2 h, and then Na+ currents were recorded with microelectrode containing different concentrations of BLA, and IC50 was calculated. This experiment shows that IC50 of BLA for resting Nav channels is 472 nM in L5-SNL rats and 441 nM in SNI rats, which are comparable to that obtained when BLA is extracellularly applied in sham rats (516 nM) (see Table 1). The data indicate that the preferable effect of BLA on the uninjured DRG neurons is abolished by PKC inhibition. Moreover, in the uninjured L4 DRG neurons of L5-SNL rats, BLA preferably blocks resting TTX-S channel over TTX-R one (IC50 values are 0.20 nM and 371 nM), while in the neurons pretreated with PKC inhibitors, IC50 of intracellular BLA for resting TTX-S Nav channels is increased to 309 nM. That is, the preferable effect of BLA on TTX-S channels is also abolished by PKC inhibition. Taken together, the preferable inhibitory effect of BLA on TTX-S Nav channels of the uninjured DRG neurons is mediated by inhibition of PKC that is upregulated in these neurons following peripheral nerve injury (Figure 5).
Figure 5.
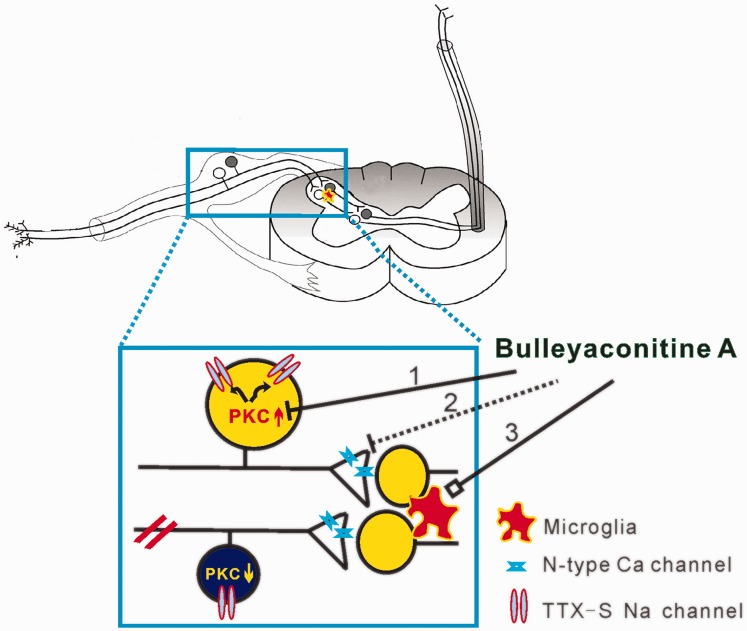
The mechanisms for BLA inhibition of chronic pain. BLA may inhibit chronic pain with following ways. (1) Nerve injury leads PKC upregulation in uninjured DRG neurons and downregulation in injured ones. The upregulated PKC promotes opening of Nav channels, especially TTX-S channels, and BLA selectively blocks the TTX-S channels in uninjured DRG neurons via inhibition of PKC. (2) BLA inhibits spinal LTP at C-fiber synapses probably by blocking N-type calcium channels in presynaptic terminals of spinal dorsal horn. (3) BLA also modulates the function of spinal microglia and thereby relieves chronic pain. TTX: tetrodotoxin; PKC: protein kinase C.
BLA inhibits the neuropathic pain and LTP at C-fiber synapses in spinal dorsal horn induced by paclitaxel
Paclitaxel, a first-line anticancer agent, is commonly used to treat solid tumors. However, similar to other chemotherapeutic agents, paclitaxel induces peripheral neuropathy in 64% of patients, manifested as paresthesias and severe pain in foot and hands.43 At present, no effective treatment is available. BLA was tested to treat this form of neuropathic pain in rats.30 Intraperitoneal injection of paclitaxel (2 mg/kg, on alternate days for four times) induces mechanical allodynia and thermal hyperalgesia in rats. The single oral administration of BLA (0.1, 0.4, and 0.8 mg/kg) attenuates the abnormal pain behaviors, dose-dependently. Chronic oral application of the drug (0.4 and 0.8 mg/kg, three times a day for seven days) during or after paclitaxel treatment produced a long-lasting inhibitory effect on thermal hyperalgesia but not on mechanical allodynia.
To investigate the central mechanisms underlying BLA’ s antineuropathic pain effect, the efficacy of synaptic transmission in spinal dorsal horn is assessed by recoding the field potentials evoked by the stimulation of afferent A-fibers and C-fibers in naïve and paclitaxel-treated rats. The field potentials evoked by C-fiber but not by A-fibers are significantly potentiated in paclitaxel-treated rats. Furthermore, the magnitudes of LTP induced by the same conditioning stimulation (40 V, 0.5 ms, 100 Hz, given in four trains of 1 s duration at 10 s intervals) in paclitaxel-treated rats are 50% higher than those in naïve rats. The data indicate that paclitaxel potentiates synaptic transmission and facilitates LTP in pain pathway. Importantly, spinal application of BLA (0.8, 8 or 80 μM) at recording segments dose-dependently inhibits LTP of C-fiber evoked field potentials, and the effect is more potent in paclitaxel-treated rats than naïve rats. BLA inhibits both early phase and late phase (>3 h) LTP.44 Accordingly, BLA may preferably inhibit the central sensitization in pathological pain.
As mentioned above, the increase in both presynaptic release and postsynaptic response underlie LTP. To determine the mechanisms underlying the inhibitory effect on the spinal LTP by BLA, miniature excitatory postsynaptic currents (mEPSCs) were recorded in spinal cord slices prepared with paclitaxel-treated rats than naïve rats. The mEPSC is produced by spontaneous presynaptic neurotransmitter release. The frequency of the mEPSC reflects the probability of presynaptic release and its amplitude the magnitude of postsynaptic response to the released neurotransmitter. It is shown that the frequency but not the amplitude of mEPSCs in paclitaxel-treated rats is higher than that in naïve rats. BLA at 0.5 μM inhibits the enhanced mEPSC frequency by paclitaxel but has no effect on its amplitude.30 As has been noted, the N-type calcium channels, which are critical for neurotransmitter release, are upregulated in spinal presynaptic terminals. Studies show that the N-type calcium channel blocker (Omega-conotoxin MVIIA) is able to inhibit paclitaxel-induced neuropathic pain.45 Our unpublished data show that BLA also blocks calcium channel in DRG neurons. It is possible that BLA may inhibit presynaptic release and attenuates neuropathic pain by blockade of N-type calcium channels in spinal dorsal horn. Further studies are needed to verify the hypothesis.
BLA is also shown to attenuate neuropathic pain by stimulating dynorphin A expression in spinal microglia, and the dynorphin A exerts its effect by the activation of κ opioid receptors.32 Further studies are needed to evaluate the effects of BLA on glial cells and the gliotransmitters.
Conclusions and prospects
The oral administration of BLA has been used for the treatment of various forms of chronic pain in China for more than 30 years. Animal studies have demonstrated that BLA at therapeutic doses preferably inhibits the peripheral and central sensitization, which underlying chronic pain, and has little effect on acute pain. BLA selectively blocks TTX-S Nav channels in DRG neurons via inhibition of PKC, and the effect is 680 folds more potent in neuropathic rats than in sham rats. BLA also preferably inhibits the spinal LTP in neuropathic rats over naïve rats. The following mechanisms may underlie the selective inhibitory effect on neuropathic pain. (1) The upregulation of PKC, Nav, and Cav channels in DRG neurons and their central terminals increases the sensibility to BLA. (2) The use-dependent blockade of BLA on Nav channels enables the drug to selectively block the ectopic discharges, which are critical for neuropathic pain. (3) Microglial activation and dysfunction are important for chronic pain but is irrelevant to nociceptive pain,46 BLA attenuates neuropathic pain by the modulation of microglial functions. Together, BLA inhibits chronic pain by acting at multitargets. It is possible that new targets of BLA will be found in the future studies. A single compound acting at multitargets may be BLA’s advantage for the treatment of chronic pain.
At present, only oral form of BLA is available in clinic. Animal studies show that local injection31 or intrathecal injection of BLA32 potently inhibits neuropathic pain. Intrathecal application of Ziconotide, a specific N-type calcium channel blocker, is proved in the United States for the treatment of severe chronic pain. The injection form of BLA is needed to treat chronic pain. Up to date, opioid is still golden standard for the treatment of server pain. However, repetitive application of opioid leads to tolerance (decrease in analgesic effect). To achieve the effective analgesic, the dosage of opioid enhances progressively, leading to aggravation of the side effects or even death. The tolerance together with the addiction of opioid leads to over 33,000 deaths per year in the United States, the situation called opioid crisis.47 Fortunately, an animal study shows that BLA has synergistic effect with morphine and can completely abolish morphine tolerance.32 Further studies are needed to verify the antitolerance effect in human patients and to elucidate the underlying mechanisms. The synergistic effect of BLA with other first-line analgesics should also be investigated in the future.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from the National Natural Science Foundation of China (U1201223, 31771166).
References
- 1.Toth C, Lander J, Wiebe S. The prevalence and impact of chronic pain with neuropathic pain symptoms in the general population. Pain Med 2009; 10: 918–929. [DOI] [PubMed] [Google Scholar]
- 2.Baron R, Binder A, Wasner G. Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol 2010; 9: 807–819. [DOI] [PubMed] [Google Scholar]
- 3.Liu YQ, Ding XN, Wang YD. The clinical studies of BLA tablets to treat common chronic pain. Chin J Pain Med 2011; 17: 314–315. [Google Scholar]
- 4.Kosek E, Cohen M, Baron R, Gebhart GF, Mico JA, Rice AS, Rief W, Sluka AK. Do we need a third mechanistic descriptor for chronic pain states? Pain 2016; 157: 1382–1386. [DOI] [PubMed] [Google Scholar]
- 5.Scholz J, Woolf CJ. Can we conquer pain? Nat Neurosci 2002; 5 Suppl: 1062–1067. [DOI] [PubMed] [Google Scholar]
- 6.Iadarola JM, Caudle RM. Good pain, bad pain. Science 1997; 278: 239–240. [DOI] [PubMed] [Google Scholar]
- 7.Devor M. Ectopic discharge in Abeta afferents as a source of neuropathic pain. Exp Brain Res 2009; 196: 115–128. [DOI] [PubMed] [Google Scholar]
- 8.Liu XG, Eschenfelder S, Blenk KH, Janig W, Habler H. Spontaneous activity of axotomized afferent neurons after L5 spinal nerve injury in rats. Pain 2000; 84: 309–318. [DOI] [PubMed] [Google Scholar]
- 9.Serra J, Bostock H, Sola R, Aleu J, Garcia E, Cokic B, Navarro X, Quiles C. Microneurographic identification of spontaneous activity in C-nociceptors in neuropathic pain states in humans and rats. Pain 2012; 153: 42–55. [DOI] [PubMed] [Google Scholar]
- 10.Wang W, Gu J, Li YQ, Tao YX. Are voltage-gated sodium channels on the dorsal root ganglion involved in the development of neuropathic pain? Mol Pain 2011; 7: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He XH, Zang Y, Chen X, Pang RP, Xu JT, Zhou X, Wei XH, Li YY, Xin WJ, Qin ZH, Liu XG. TNF-alpha contributes to up-regulation of Nav1.3 and Nav1.8 in DRG neurons following motor fiber injury. Pain 2010; 151: 266–279. [DOI] [PubMed] [Google Scholar]
- 12.Black JA, Frezel N, Dib-Hajj SD, Waxman SG. Expression of Nav1.7 in DRG neurons extends from peripheral terminals in the skin to central preterminal branches and terminals in the dorsal horn. Mol Pain 2012; 8: 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Michaelis M, Liu XG, Janig W. Axotomized and intact muscle afferents but no skin afferents develop ongoing discharges of dorsal root ganglion origin after peripheral nerve lesion. J Neurosci 2000; 20: 2742–2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu G, Ringkamp M, Hartke TV, Murinson BB, Campbell JN, Griffin JW, Meyer RA. Early onset of spontaneous activity in uninjured C-fiber nociceptors after injury to neighboring nerve fibers. J Neurosci 2001; 21: RC140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu G, Ringkamp M, Murinson BB, Pogatzki EM, Hartke TV, Weerahandi HM, Campbell JN, Griffin JW, Meyer RA. Degeneration of myelinated efferent fibers induces spontaneous activity in uninjured C-fiber afferents. J Neurosci 2002; 22: 7746–7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li L, Xian CJ, Zhong JH, Zhou XF. Effect of lumbar 5 ventral root transection on pain behaviors: a novel rat model for neuropathic pain without axotomy of primary sensory neurons. Exp Neurol 2002; 175: 23–34. [DOI] [PubMed] [Google Scholar]
- 17.Tamura R, Nemoto T, Maruta T, Onizuka S, Yanagita T, Wada A, Murakami M, Tsuneyoshi I. Up-regulation of NaV1.7 sodium channels expression by tumor necrosis factor-alpha in cultured bovine adrenal chromaffin cells and rat dorsal root ganglion neurons. Anesth Analg 2014; 118: 318–324. [DOI] [PubMed] [Google Scholar]
- 18.Shen KF, Zhu HQ, Wei XH, Wang J, Li YY, Pang RP, Liu XG. Interleukin-10 down-regulates voltage gated sodium channels in rat dorsal root ganglion neurons. Exp Neurol 2013; 247: 466–475. [DOI] [PubMed] [Google Scholar]
- 19.Dolphin AC. Calcium channel auxiliary alpha2delta and beta subunits: trafficking and one step beyond. Nat Rev Neurosci 2012; 13: 542–555. [DOI] [PubMed] [Google Scholar]
- 20.Gohil K, Bell JR, Ramachandran J, Miljanich GP. Neuroanatomical distribution of receptors for a novel voltage-sensitive calcium-channel antagonist, SNX-230 (omega-conopeptide MVIIC). Brain Res 1994; 653: 258–266. [DOI] [PubMed] [Google Scholar]
- 21.Kerr LM, Filloux F, Olivera BM, Jackson H, Wamsley JK. Autoradiographic localization of calcium channels with [125I]omega-conotoxin in rat brain. Eur J Pharmacol 1988; 146: 181–183. [DOI] [PubMed] [Google Scholar]
- 22.Yang J, Xie MX, Hu L, Wang XF, Mai JZ, Li YY, Wu N, Zhang C, Li J, Pang RP, Liu XG. Upregulation of N-type calcium channels in the soma of uninjured dorsal root ganglion neurons contributes to neuropathic pain by increasing neuronal excitability following peripheral nerve injury. Brain Behav Immun 2018; 71: 52–65. [DOI] [PubMed] [Google Scholar]
- 23.Liu XG, Sandkuhler J. Long-term potentiation of C-fiber-evoked potentials in the rat spinal dorsal horn is prevented by spinal N-methyl-D-aspartic acid receptor blockage. Neurosci Lett 1995; 191: 43–46. [DOI] [PubMed] [Google Scholar]
- 24.Randic M, Jiang MC, Cerne R. Long-term potentiation and long-term depression of primary afferent neurotransmission in the rat spinal cord. J Neurosci 1993; 13: 5228–5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhuo M. A synaptic model for pain: long-term potentiation in the anterior cingulate cortex. Mol Cells 2007; 23: 259–271. [PubMed] [Google Scholar]
- 26.Liu XG, Zhou LJ. Long-term potentiation at spinal c-fiber synapses: a target for pathological pain. Curr Pharm Des 2015; 21: 895–905. [DOI] [PubMed] [Google Scholar]
- 27.Cizkova D, Marsala J, Lukacova N, Marsala M, Jergova S, Orendacova J, Yaksh TL. Localization of N-type Ca2+ channels in the rat spinal cord following chronic constrictive nerve injury. Exp Brain Res 2002; 147: 456–463. [DOI] [PubMed] [Google Scholar]
- 28.Vink S, Alewood PF. Targeting voltage-gated calcium channels: developments in peptide and small-molecule inhibitors for the treatment of neuropathic pain. Br J Pharmacol 2012; 167: 970–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carminati GM, Nani S, Moroni E, Zampiron S. Bulleyaconitine A, a new analgesic and anti-inflammatory agent. New Drug Clin 1988; 20: 75–121. [Google Scholar]
- 30.Zhu HQ, Xu J, Shen KF, Pang RP, Wei XH, Liu XG. Bulleyaconitine A depresses neuropathic pain and potentiation at C-fiber synapses in spinal dorsal horn induced by paclitaxel in rats. Exp Neurol 2015; 273: 263–272. [DOI] [PubMed] [Google Scholar]
- 31.Xie MX, Pang RP, Yang J, Shen KF, Xu J, Zhong XX, Wang SK, Zhang XL, Liu YQ, Liu XG. Bulleyaconitine A preferably reduces tetrodotoxin-sensitive sodium current in uninjured dorsal root ganglion neurons of neuropathic rats probably via inhibition of protein kinase C. Pain 2017; 158: 2169–2180. [DOI] [PubMed] [Google Scholar]
- 32.Li TF, Fan H, Wang YX. Aconitum-derived bulleyaconitine A exhibits antihypersensitivity through direct stimulating dynorphin A expression in spinal microglia. J Pain 2016; 17: 530–548. [DOI] [PubMed] [Google Scholar]
- 33.Aldrich RW, Corey DP, Stevens CF. A reinterpretation of mammalian sodium channel gating based on single channel recording. Nature 1983; 306: 436–441. [DOI] [PubMed] [Google Scholar]
- 34.Mantegazza M, Curia G, Biagini G, Ragsdale DS, Avoli M. Voltage-gated sodium channels as therapeutic targets in epilepsy and other neurological disorders. Lancet Neurol 2010; 9: 413–424. [DOI] [PubMed] [Google Scholar]
- 35.Xie MX, Yang J, Pang RP, Zeng WA, Ouyang HD, Liu YQ, Liu XG. Bulleyaconitine A attenuates hyperexcitability of dorsal root ganglion neurons induced by spared nerve injury: The role of preferably blocking Nav1.7 and Nav1.3 channels. Mol Pain 2018; 14: 174480691877849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang CF, Gerner P, Wang SY, Wang GK. Bulleyaconitine A isolated from aconitum plant displays long-acting local anesthetic properties in vitro and in vivo. Anesthesiology 2007; 107: 82–90. DOI: 10.1097/01.anes.0000267502.18605.ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang CF, Gerner P, Schmidt B, Xu ZZ, Nau C, Wang SY, Ji RR, Wang GK. Use of bulleyaconitine A as an adjuvant for prolonged cutaneous analgesia in the rat. Anesth Analg 2008; 107: 1397–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu HR, Ji GX, Wang Q, Li XN, Gong YJ, Zhang Y, Li W, Shen T. Dose-dependent pharmacokinetics of bulleyaconitine A in rats. Pharmazie 2013; 68: 170–172. [PubMed] [Google Scholar]
- 39.Cantrell AR, Ma JY, Scheuer T, Catterall WA. Muscarinic modulation of sodium current by activation of protein kinase C in rat hippocampal neurons. Neuron 1996; 16: 1019–1026. [DOI] [PubMed] [Google Scholar]
- 40.Mittmann T, Alzheimer C. Muscarinic inhibition of persistent Nav current in rat neocortical pyramidal neurons. J Neurophysiol 1998; 79: 1579–1582. [DOI] [PubMed] [Google Scholar]
- 41.Gold MS, Levine JD, Correa AM. Modulation of TTX-R INa by PKC and PKA and their role in PGE2-induced sensitization of rat sensory neurons in vitro. J Neurosci 1998; 18: 10345–10355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chahine M, O’Leary ME. Regulation/modulation of sensory neuron sodium channels. Handb Exp Pharmacol 2014; 221: 111–135. [DOI] [PubMed] [Google Scholar]
- 43.Reyes-Gibby CC, Morrow PK, Buzdar A, Shete S. Chemotherapy-induced peripheral neuropathy as a predictor of neuropathic pain in breast cancer patients previously treated with paclitaxel. J Pain 2009; 10: 1146–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hu NW, Zhang HM, Hu XD, Li MT, Zhang T, Zhou LJ, Liu XG. Protein synthesis inhibition blocks the late-phase LTP of C-fiber evoked field potentials in rat spinal dorsal horn. J Neurophysiol 2003; 89: 2354–2359. [DOI] [PubMed] [Google Scholar]
- 45.Rigo F K, Dalmolin G D, Trevisan G, Tonello R, Silva M A, Rossato M F, Klafke J Z, Cordeiro M d N, Castro Junior C J, Montijo D, Gomez M V, Ferreira J. Effect of omega-conotoxin MVIIA and Phalpha1beta on paclitaxel-induced acute and chronic pain. Pharmacol Biochem Behav 2013; 114–115: 16–22. [DOI] [PubMed] [Google Scholar]
- 46.Gosselin RD, Suter MR, Ji RR, Decosterd I. Glial cells and chronic pain. Neuroscientist 2010; 16: 519–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Soelberg CD, Brown RE, Jr, Du Vivier D, Meyer JE, Ramachandran BK. The US opioid crisis: current federal and state legal issues. Anesth Analg 2017; 125: 1675–1681. [DOI] [PubMed] [Google Scholar]