Abstract
Purpose:
To evaluate cervical mucus secretory leukocyte protease inhibitor (SLPI) concentrations in patients with high-risk human papillomavirus (hrHPV) 16 or 18 positive and low-grade squamous intraepithelial lesions (LGSIL) or high-grade squamous intraepithelial lesions (HGSIL).
Method:
Patients with HPV 16 or 18 positive from 30 to 45 years of age whose cervical cancer screening results reported cytologically LGSIL or HGSIL were included in the study. In the control group, we included participants in the same age with cytology negative and HPV-negative healthy women. All cytological LGSIL or HGSIL results were histopathologically confirmed with colposcopic biopsy specimens. Finally, the study consisted of a total of 3 groups each containing 25 participants as follows: (1) Pap smear and HPV-negative control group, (2) HPV 16 or HPV 18 and LGSIL-positive participants, and (3) HPV 16 or 18 and HGSIL-positive participants. Cervical mucus SLPI levels were analyzed using the enzyme-linked immunosorbent assay method.
Results:
The mean cervical mucus SLPI levels were 32.94 ng/mL (range: 23-41.29 ng/mL) in the hrHPV + LGSIL group, 29.40 ng/mL (range: 21.03-38.95 ng/mL) in the hrHPV + HGSIL, and 18.75 ng/mL (range: 13.58-29.24 ng/mL) in the healthy control group. Cervical mucus SLPI levels were found to be significantly higher in the hrHPV + LGSIL and hrHPV + HGSIL groups compared to the control group (P < .001).
Conclusions:
The data from the present study indicate that SLPI seems to be one of the important immunomodulatory proteins that provide local immune response in cervical mucosa.
Keywords: cervical immune response, human papillomavirus, high-risk HPV, LGSIL, HGSIL, secretory leukocyte protease inhibitor (SLPI)
Introduction
Cervical cancer (CC) is the second most common malignancy in women worldwide, and nearly 85% of the cases occur in developing countries. Initial reports have revealed that the infection of human papillomavirus (HPV) is a fundamental issue in CC, and nearly all cases with CC also have an HPV infection.1 Globally, an estimated 291 million women are HPV-positive; of that number, 105 million have been exposed to high-risk HPV (hrHPV) 16 or HPV 18 infection.2 Persistent HPV infection, mostly hrHPV, is a precondition for the occurrence of pre-CC lesions and CC.3 The most significant factor in the progress of HPV infection is the immune response.4 Persistent HPV infections are essential for the development of cervical intraepithelial neoplasia (CIN) and its subsequent advancement to cancer, and this can be justified as an effect of host immune response.5
Secretory leukocyte protease inhibitor (SLPI) is a low-molecular-weight protein in the mucosal surfaces of lung alveoli and the intestinal and genital reproductive systems.6 Inhibition of antimicrobial, anti-inflammatory, and antiprotease effects is the most significant role of SLPI.7 Several serine proteases, such as trypsin, cathepsin G, and neutrophil elastase, are responsible for the antiprotease activity of SLPI. It is acknowledged that SLPI is overexpressed in inflammation triggered by lipopolysaccharides and pro-inflammatory cytokines.8
The SLPI is an antimicrobial peptide secreted by keratinocytes, the target cells of HPV infection, which contributes to cervical mucosal immunity. Therefore, in the present study, we aimed to evaluate local cervical immune response in patients with hrHPV 16 or 18 low-grade squamous intraepithelial lesions (LGSIL) and those with high-grade squamous intraepithelial lesions (HGSIL) using cervical mucus SLPI concentrations.
Materials and Methods
Study Design and Study Population
This study was conducted at the Kayseri Education and Research Hospital, Kayseri, Turkey, and approved by the Erciyes University Ethics Committee (Approval no: 2016/555). All steps were carried out in accordance with the Helsinki Declaration. All participants gave their informed consent prior to taking part in the study.
As a routine practice of this hospital, CC was screened in women aged >30 years as a cotesting with cervical cytology and HPV testing. Patients with HPV 16 or 18 from 30 to 45 years of age whose CC screening results reported cytologically LGSIL or HGSIL were included in the study. In the control group, we included participants in the same age with cytology negative and HPV-negative healthy women. After this initial analysis and classification, colposcopy was preferred in patients with cytologically LGSIL or HGSIL positive and HPV 16 or 18 positive results according to American Society for Colposcopy and Cervical Pathology guidelines. In the present study, evaluating SLPI levels in patients with other high-risk HPV serotype patients could be a suitable approach, but we excluded these patients because we prefer to evaluate the most frequently isolated and most carcinogenic types (HPV 16 and 18).
Since SLPI levels may demonstrate differences during menstrual cycles, samples of cervical mucus were obtained from all study population before the colposcopic examination and in follicular phase when menstrual bleeding does not occur. In the control group, samples were obtained during first regular clinic visits to assess the results of cotest screening. In study population group, all cytological LGSIL or HGSIL results were histopathologically confirmed with colposcopic biopsy specimens. Finally, the study consisted of a total of 3 groups each containing 25 participants as follows: (1) Pap smear and HPV-negative control group, (2) HPV 16 or HPV 18 and LGSIL-positive participants, and (3) HPV 16 or 18 and HGSIL-positive participants.
Patients exhibiting pregnancy; types 1 and 2 diabetes; chronic liver, heart, and kidney diseases; malignancy; history of chemotherapy or radiotherapy; nonsteroidal drug use within the previous 1 month, and HIV seropositivity were not included in the study. In addition, the use of oral contraceptives that could decrease local cervical immune response, alcohol use and/or smoking, presence of active cervicovaginal infection (fungal vulvovaginitis, trichomoniasis, and bacterial vaginosis), and areas of cervical ulceration and erosion which might affect the SLPI levels were exclusion criteria.
Cervical Mucus Sampling and Data Collection
During vaginal examination with a speculum, a cotton-tipped sterile swab was inserted through the external cervical orifice and left there for 10 seconds to obtain samples of cervical mucus. Since the sample is obtained by cotton-tipped swab, it is possible that the differences in SLPI amount are due to different content of mucus in the swab and not due to different level of the SLPI molecule. To minimize this difference, all samples of cervical mucus were obtained by the same researcher, similar cotton-tipped swab, same second left cervix to obtain samples, and as possible same mucus amount to avoid interobserver variability. Cervical mucus samples on the cotton tip of the swab were dipped into 1 mL phosphate-buffered saline solution, stirred, and dispersed. The dissociated samples were centrifuged at 15 000 rpm at 4°C for 10 minutes, and the supernatant was stored at −80°C until the day of analysis. The SLPI concentrations were analyzed after 4 months first sample was obtained, using the enzyme-linked immunosorbent assay method in The Clinic of Biochemistry of Kayseri Training and Research Hospital.
Enzyme-Linked Immunosorbent Assay Technique
This assay employs the quantitative sandwich enzyme immunoassay technique. A monoclonal antibody specific for human SLPI has been precoated onto a microplate. Standards and samples are pipetted into the wells, and any SLPI present is bound by the immobilized antibody. After washing away any unbound substances, an enzyme-linked polyclonal antibody specific for human SLPI is added to the wells. Following a wash to remove any unbound antibody enzyme reagent, a substrate solution is added to the wells and color develops in proportion to the amount of SLPI bound in the initial step. The color development is stopped, and the intensity of the color is measured.
Human Papillomavirus DNA Analysis
DNA analysis of HPV was performed at Kayseri Education and Research Hospital Pathology clinic. Fourteen hrHPV types including HPV types 16, 18, 31, 33, 39, 45, 51, 52, 56, 58, 59, 66, and 68 were investigated by transcription-mediated amplification and hybridization protection method (Optima-Panther system). Human papillomavirus (+) cases were reevaluated with the genotyping study (Aptima HPV16,18/45 Genotype Assay-Panther System) for detection of HPV16,18/45, and the results were reported.
Statistical Analysis
The Shapiro-Wilk test was used to test the normality assumption of the data. Levene test was used to test the variance homogeneity assumption. Values are expressed as mean (standard deviation[SD]), median (25th-75th percentile), or n (%). Parametric comparisons were made using a t test or a z test, and nonparametric comparisons were made using the Mann-Whitney U test. All comparisons were made with a PASW Statistics version 18 program (SPSS Inc, Chicago, Illinois; http://www.spss.com). A P value of <.05 was considered statistically significant.
Results
A total of 75 participants were enrolled in the study: 25 in the hrHPV + LGSIL group; 25 in the hrHPV + HGSIL; and 25 in the healthy control group. The mean age was 39.44 (2.82) years in the hrHPV + LGSIL group, 38.04 (2.70) years in the hrHPV + HGSIL group, and 39.43 (4.69) years in the control group (P = .170). Gravidity, body mass index, and race were similar for both the groups (P = .941, P = .756, P = 1, respectively). A comparison of the participants’ characteristics is shown in Table 1.
Table 1.
Comparison of Patient’s Characteristics Among the Groups.a
| Characteristics | HrHPV + LGSIL Group (n = 25) | HrHPV + HGSIL Group (n = 25) | Control Group (n = 25) | P Value |
|---|---|---|---|---|
| Age (year) | 39.44 (2.82) | 38.04 (2.70) | 39.43 (4.69) | .170 |
| Gravidity | 2 (1-3) | 2 (1.50-2.50) | 2 (1.50-3) | .941 |
| BMI (kg/m2) | 25 (24.50-25.70) | 25 (24.65-25.25) | 24.90 (24.10-25.60) | .756 |
| Race (white), n (%) | 25 (100%) | 25 (100%) | 25 (100%) | - |
Abbreviations: BMI, body mass index; HGSIL, high-grade squamous intraepithelial lesion; HPV, high-risk human papillomavirus; LGSIL, low-grade squamous intraepithelial lesion.
a The Shapiro-Wilk test was used to test the normality assumption of the data. Levene test was used to test the variance homogeneity assumption. Values are expressed as mean (standard deviation), median (25th-75th percentile) or n (%). Parametric comparisons were made using a t test or a z test, and nonparametric comparisons were made using the Mann-Whitney U test. All comparisons were made with the PASW Statistics 18 program, and a P value of <.05 was considered statistically significant.
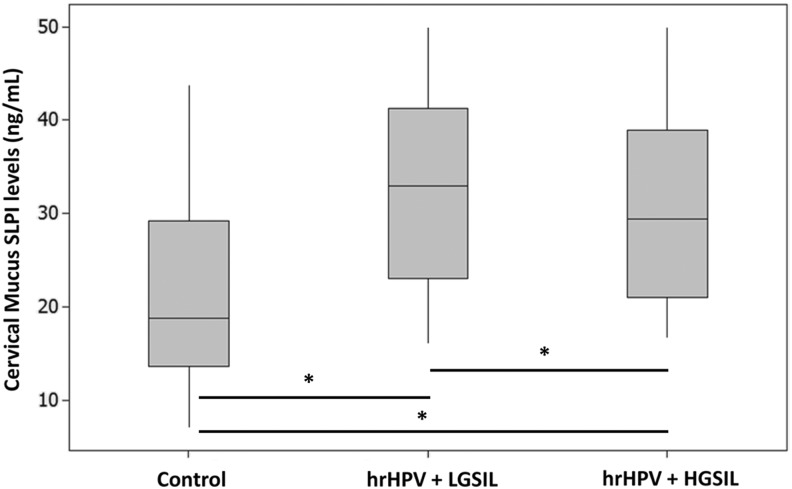
A comparison of the cervical mucus SLPI levels is shown in Figure 1. The mean serum cervical mucus SLPI levels were 32.94 ng/mL (range: 23-41.29 ng/mL) in the hrHPV + LGSIL group, 29.40 ng/mL (range: 21.03-38.95 ng/mL) in the hrHPV HGSIL, and 18.75 ng/mL (range: 13.58-29.24 ng/mL) in the healthy control group. Cervical mucus SLPI levels were found to be significantly higher in the hrHPV + LGSIL and hrHPV + HGSIL groups, compared to the control group (P < .001).
Figure 1.
Comparison of cervical mucus secretory leukocyte protease inhibitor (SLPI) levels among the groups.
Discussion
In the present investigation, the effect of cervical immune response on hrHPV infection was examined. The main finding of the study was that cervical mucus SLPI concentrations were significantly increased in patients with hrHPV-positive LGSIL and HGSIL, compared to the control group. The SLPI seems to be an important immunomodulatory protein that provides local immune response in the cervical mucosa.
Although many studies have evaluated the level of SLPI, the different methods used in these studies, which were performed on diverse tissue types, make the immune effect of SLPI an increasingly complex phenomenon to understand. This condition has been demonstrated by Wen et al who reported that the immune effect of SLPI changes depending on the localization of the tumor, its stage of differentiation, infectious agents present, and prevailing endocrine effects.9 Although SLPI expression has been investigated in many tumoral tissues, its role in tumorigenesis is debatable, and the up- or downregulation of its expression appears to be related to cancer type.10,11 Bouchard et al stated that SLPI is underexpressed in bladder, nasopharyngeal, and breast carcinomas, while it is overexpressed in papillary thyroid, pancreatic, cervical, endometrial, and ovarian cancers.11 In a study that evaluated genital cervical mucosal immunity, Mhatre et al reported that cervicovaginal lavage SLPI levels were significantly lower in patients with hrHPV-positive CIN 1 and CIN 3 compared to the healthy controls.12 In another study that evaluated cervical mucosal immune response in primary HPV infection, Gardella et al reported that cervicovaginal levels of myeloperoxidase and lactoferrin, which provide immunity against viral infection, were significantly higher in HPV-positive women compared to HPV-negative women.13
We may be able to explain our results with a local immune response in the cervical mucosa.14 The 2 most important HPV proteins in the pathogenesis of malignant disease are early antigen 6 (E6) and early antigen 7 (E7). It is well documented that, with the binding of P53 by E6 antigen and of the retinoblastoma (Rb) gene by E7 antigen, defective DNA escapes from the G1 growth arrest of the DNA cell cycle. In the absence of genetic control mechanisms, intercellular cytokine is the most important defense mechanism mediating suppression of malignant transformation.
The host launch immune response becomes the primary shield throughout the initial periods of an HPV disease. Dendritic cells, Langerhans cells, T-cells (natural killer cells), and keratinocytes are key cells required in endorsing a suitable response against HPV infection. Being the chief focus of HPV infection, keratinocytes perform an essential part throughout the induction of HPV and afterward conjoin to endorse an efficient immune reaction. Keratinocytes are part of the immune resistance and perform as antigen displaying cells, which are able to provoke Th1 and Th2 type cytokine expression.15,16 When the release of SLPI from keratinocytes is considered, SLPI appears to be one of the significant immunomodulator proteins to specify regional immune reaction in cervical mucosa.
The strength of our study consists in analyzing the local immune response of the genital tract through the cervix. Additionally, we excluded some environmental and social factors, including smoking, oral contraceptive use, presence of vaginal infection, and HIV seropositivity, which could be responsible for reduction in the immune defense. The main limitation of the study is absence of other observations such as decrease in/clearance of hrHPV, regression of LSIL/HSIL, presence of inflammatory cells in the cervix, and correlation of these conditions with cervical mucus SLPI levels, as well as, the small sample size.
Evaluating cases 1 year later might be significant in identifying the type of infection and its possible development based on mucosal immunity. Cervical mucus SLPI levels may be one of the determining factors for HPV infection and the progression of cervical precancerous lesions.
Conclusions
The data from the present study indicate that cervical mucus SLPI concentrations were increased in patients with hrHPV-positive LGSIL and HGSIL. The SLPI appears to be an important immunomodulatory protein that provides local immune response in the cervical mucosa. However, large-scale prospective studies are required to understand the clinical importance of this finding regarding the long-term consequences and underlying mechanisms of elevated SLPI levels.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Yusuf Madendag  http://orcid.org/0000-0002-7622-2991
http://orcid.org/0000-0002-7622-2991
References
- 1. Luo H, Belinson JL, Du H, et al. Evaluation of viral load as a triage strategy with primary high-risk human papillomavirus cervical cancer screening. J Low Genit Tract Dis. 2017;21(1):12–16. [DOI] [PubMed] [Google Scholar]
- 2. Burchell AN, Winer RL, de Sanjosé S, Franco EL. Chapter 6: Epidemiology and transmission dynamics of genital HPV infection. Vaccine. 2006;24(suppl 3):S3/52–61. [DOI] [PubMed] [Google Scholar]
- 3. Xiao SS, Fan JL, He SL, et al. Analysis of human papillomavirus infection in 16,320 patients from a gynecology clinic in Central South China. J Low Genit Tract Dis. 2016;20(4):327–331. [DOI] [PubMed] [Google Scholar]
- 4. Einstein MH, Schiller JT, Viscidi RP, et al. Clinician’s guide to human papillomavirus immunology: knowns and unknowns. Lancet Infect Dis. 2009;9(6):347–356. [DOI] [PubMed] [Google Scholar]
- 5. Cromme FV, Meijer CJ, Snijders PJ, et al. Analysis of MHC class I and II expression in relation to presence of HPV genotypes in premalignant and malignant cervical lesions. Br J Cancer. 1993;67(6):1372–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wilkinson TS, Roghanian A, Simpson AJ, Sallenave JM. WAP domain proteins as modulators of mucosal immunity. Biochem Soc Trans. 2011;39(5):1409–1415. [DOI] [PubMed] [Google Scholar]
- 7. Moreau T, Baranger K, Dadé S, Dallet-Choisy S, Guyot N, Zani ML. Multifaceted roles of human elafin and secretory leukocyte proteinase inhibitor (SLPI), two serine protease inhibitors of the chelonianin family. Biochimie. 2008;90(2):284–295. [DOI] [PubMed] [Google Scholar]
- 8. Choi BD, Jeong SJ, Wang G, et al. Temporal induction of secretory leukocyte protease inhibitor (SLPI) in odontoblasts by lipopolysaccharide and wound infection. J Endod. 2009;35(7):997–1002. [DOI] [PubMed] [Google Scholar]
- 9. Wen J, Nikitakis NG, Chaisuparat R, et al. Secretory leukocyte protease inhibitor (SLPI) expression and tumor invasion in oral squamous cell carcinoma. Am J Pathol. 2011;178(6):2866–2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Devoogdt N, Revets H, Ghassabeh GH, De Baetselier P. Secretory leukocyte protease inhibitor in cancer development. Ann N Y Acad Sci. 2004;1028:380–389. [DOI] [PubMed] [Google Scholar]
- 11. Bouchard D, Morisset D, Bourbonnais Y, Tremblay GM. Proteins with whey-acidic-protein motifs and cancer. Lancet Oncol. 2006;7(2):167–174. [DOI] [PubMed] [Google Scholar]
- 12. Mhatre M, McAndrew T, Carpenter C, Burk RD, Einstein MH, Herold BC. Cervical intraepithelial neoplasia is associated with genital tract mucosal inflammation. Sex Transm Dis. 2012;39(8):591–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gardella B, Iacobone AD, Musacchi V, et al. The mucosal innate immune response in primary human papillomavirus infection: a pilot study. J Low Genit Tract Dis. 2016;20(4):338–342. [DOI] [PubMed] [Google Scholar]
- 14. Amador-Molina A, Hernández-Valencia JF, Lamoyi E, Contreras-Paredes A, Lizano M. Role of innate immunity against human papillomavirus (HPV) infections and effect of adjuvants in promoting specific immune response. Viruses. 2013;5(11):2624–2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nestle FO, Di Meglio P, Qin JZ, Nickoloff BJ. Skin immune sentinels in health and disease. Nat Rev Immunol. 2009;9(10):679–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Black AP, Ardern-Jones MR, Kasprowicz V, et al. Human keratinocyte induction of rapid effector function in antigen-specific memory CD4+ and CD8+ T cells. Eur J Immunol. 2007;37(6):1485–1493. [DOI] [PubMed] [Google Scholar]