Abstract
Accumulating evidence suggests that microRNAs (miRs) exert vital functions in the development and progression of multiple types of human cancer. However, the role of miR-6852 in gastric cancer (GC) remains unclear. In the present study, miR-6852 expression was significantly downregulated in GC tissues compared with adjacent normal tissues determined by reverse transcription-quantitative polymerase chain reaction (RT-qPCR) analysis. Furthermore, miR-6852 expression levels in patients with GC were reversely correlated with tumor metastasis and TNM stage. Through Cell Counting kit-8 and Transwell assays, it was demonstrated that overexpression of miR-6852 significantly inhibited the proliferation, migration and invasion of GC cells. With regards to the mechanism involved, luciferase reporter assays suggested that miR-6852 directly target forkhead box J1 (FOXJ1) in GC cells. Furthermore, overexpression of miR-6852 markedly inhibited the mRNA and protein expression levels of FOXJ1 in GC cells determined by RT-qPCR and western blot analysis. Additionally, FOXJ1 was overexpressed in GC tissues and cell lines, and its expression was negatively correlated with that of miR-6852 in GC tissues. Rescue assays indicated that overexpression of FOXJ1 significantly reversed the effects of miR-6852 transfection on GC cell proliferation, migration and invasion. Taken together, the present findings demonstrated that miR-6852 exerted a tumor suppressive role through targeting FOXJ1 in GC. These results implied that miR-6852 may be a novel therapeutic target of GC treatment.
Keywords: microRNA-6852, gastric cancer, proliferation, migration, invasion, FOXJ1
Introduction
Gastric cancer (GC) remains the second most common malignant cancer and gives rise to a number of cancer-associated fatalities worldwide every year (1). GC has become a major threat to human life. Notably, the majority of patients with GC are diagnosed at an advanced stage, which makes GC treatment difficult due to extensive tumor invasion and lymphatic metastasis (2,3). The pathogenesis of GC is correlated with a complexity of factors, including oncogene activation and tumor-suppressor inactivation (4). Therefore, in order to provide improved intervention of GC, a greater understanding of GC pathogenesis and the identification of novel biomarkers and therapeutic targets is urgently required.
MicroRNAs (miRs) belong to a class of short non-coding RNAs of ~22 nucleotides in length that have the ability to regulate gene expression by directing target mRNAs for degradation (5,6). In past decades, a large number of studies have indicated that miRs serve as key regulators in various physiological processes, including cell proliferation, metabolism, apoptosis, invasion and migration (5,7,8). Aberrant expression of miRs has been linked to the development and progression of various types of human cancer (9), including GC (10). Increasing evidence has demonstrated that miRs are effective biomarkers for the diagnosis and prognosis of human cancer (11). Also several reports imply miRs may be promising therapeutic targets for cancer treatment (12).
A recent study demonstrated that miR-6852 overexpression induces necrosis in cervical cancer cells (13). However, the role of miR-6852 in GC cells remains largely unknown. The aim of the present study was to assess the role of miR-6852 in GC cells in order to clarify if the miR may be a promising therapeutic target for treating GC.
Materials and methods
Patient samples
A total of 56 fresh GC tissue samples and adjacent normal tissues (male, 30; female, 26; median age, 54±11 years) were collected at Huazhong University of Science and Technology (Wuhan, China) between June 2014 and December 2016. Tissues were immediately snap frozen in liquid nitrogen and stored at −80°C until total RNA was extracted. Patients were diagnosed with GC prior to this study. Patients who received radiation- or chemotherapy prior to surgery were excluded. Written informed consent was obtained from each patient who participated in the present research. Furthermore, the present study was approved by the Ethics Committee of Huazhong University of Science and Technology.
Cell culture
Human GC cell lines (MGC-803, MKN-1, SGC-7901, BGC-823 and AGS) and the normal gastric epithelium GES-1 cell line were acquired from the Shanghai Institute of Biochemistry and Cell Biology (Shanghai, China). All cell lines were cultured in Dulbecco's modified Eagle's medium (DMEM, Invitrogen, Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS, Invitrogen, Thermo Fisher Scientific, Inc.), 100 U/ml of penicillin and 100 mg/ml of streptomycin (Invitrogen, Thermo Fisher Scientific, Inc.) at 37°C in an atmosphere containing 5% CO2.
Reverse transcription-polymerase chain reaction (RT-qPCR)
Total RNAs were extracted from GC tissues or cultured cell lines using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. Total RNA was reverse transcribed into cDNA using the PrimeScript RT reagent kit with gDNA Eraser (Takara Biotechnology Co., Ltd., Dalian, China), and 1 µg total RNA was reverse transcribed for each sample. qPCR was performed with a Taqman MicroRNA Assay kit (Applied Biosystems; Thermo Fisher Scientific, Inc.) according to the manufacturer's instructions. Thermocycling conditions were as follows: Denaturation at 95°C for 10 min, followed by 40 cycles of denaturation at 95°C for 15 sec and elongation at 60°C for 1 min. Results were normalized to U6 or GAPDH expression. Expression fold change was determined by the 2−ΔΔCq method (14). Primer sequences were as follows: miR-6852 forward, 5′-AACGAGACGACGACAGAC-3′ and reverse, 5′-CCCTGGGGTTCTGAGGACATG-3′; U6 forward, 5′-AACGAGACGACGACAGAC-3′ and reverse, 5′-GCAAATTCGTGAAGCGTTCCATA-3′; forkhead box J1 (FOXJ1) forward, 5′-CAGAATCGCTGCCTCCTCTC-3′ and reverse, 5′-CAGGGTCCTTTAGCCGGTTT-3′; GAPDH forward, 5′-ATGTTGCAACCGGGAAGGAA-3′ and reverse, 5′-AGGAAAAGCATCACCCGGAG-3′.
Cell Counting Kit (CCK)-8 proliferation assays
Transfected cells were collected at 24 h post-transfection and seeded into 96-well plates at a density of 3×103 cells per well. Following incubation for 0, 24, 48 and 72 h at 37°C, the CCK-8 assay was performed according to the manufacturer's instructions. In brief, 10 µl of CCK-8 reagent (Beyotime Institute of Biotechnology, Shanghai, China) was added to each well. The cells were incubated at 37°C in an atmosphere containing 5% CO2 for 2 h. Absorbance was determined at a wavelength of 450 nm using an ELx808 absorbance reader (BioTek Instruments, Inc., Winooski, VT, USA). Each assay was performed in triplicate and repeated three times.
Migration and invasion assays
Migration was measured using 6.5-mm Transwell inserts with 8.0 µm pore polycarbonate membranes (Costar; Corning Incorporated, Corning, NY, USA). Cell invasion assays were performed with 6.5-mm Transwell inserts with 8.0 µm pore polycarbonate membranes (pre-coated with Matrigel for invasion; Costar; Corning Incorporated). Briefly, 2×105 transfected and non-transfected cells were suspended and seeded into the upper chambers of the inserts in 200 µl serum-free DMEM, while the 600 µl complete DMEM containing 10% FBS was added to the lower chambers. A total of 24 h following incubation at 37°C, cells on the upper surface of the membrane were removed but cells in the lower membrane were fixed with 100% methanol at room temperature for 20 min and stained with 0.1% crystal violet at room temperature for 20 min. Cells were observed using an optical microscope (magnification, ×100; Olympus Corporation, Tokyo, Japan) and counted in five random fields of view from each well. The mean number of migrated or invaded cells was calculated.
Luciferase assay
The mutated FOXJ1 3′-untranslated region (UTR) sequence (in which all predicted sites were mutated) was synthetized by Sangon Biotech Co., Ltd. (Shanghai, China). The wild-type 3′-UTR sequence of FOXJ1 or the mutated 3′-UTR sequence of FOXJ1 was incorporated into the pGL3 control vector (Promega Corporation, Madison, WI, USA), to obtain the wild-type FOXJ1-3′UTR or mutant FOXJ1-3′UTR, respectively. GC cells were seeded into 24-well plates the day prior to transfection and then cotransfected with wild-type or mutant 3′-UTR FOXJ1, along with miR-6852 mimics or controls using Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's instructions. At 48 h after co-transfection, the luciferase activity for the wild-type or mutant FOXJ1 3′-UTR was measured using a dual luciferase reporter assay (Promega Corporation). Luciferase activity was normalized to Renilla.
Statistical analysis
All statistical analyses were performed using SPSS 20.0 (IBM, SPSS, Inc., Chicago, IL, USA) and GraphPad Prism (version 6; GraphPad Software, Inc., La Jolla, CA, USA). The Student's t-test and one-way analysis of variance followed by Tukey's post hoc test were used to analyze two or multiple groups for statistical significance, respectively. Pearson correlation coefficient analysis was used to determine the correlations. The χ2 test was used to assess the association between miR-6852 expression and clinicopathological features in patients with GC. P<0.05 was considered to indicate a statistically significant difference.
Results
miR-6852 is underexpressed in GC tissues
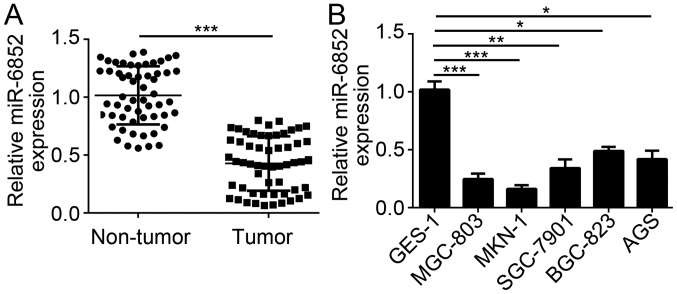
To explore the potential function of miR-6852 in GC cells, the expression pattern of miR-6852 was assessed by RT-qPCR. Results indicated that miR-6852 expression was significantly downregulated in GC tissues compared with adjacent normal tissues (Fig. 1A). Consistently, RT-qPCR results also indicated that the expression of miR-6852 was downregulated in GC tissues compared with the normal gastric epithelium GES-1 cells (Fig. 1B). Furthermore, the correlation between miR-6852 expression and clinicopathological features in patients with GC was determined. Notably, lower expression of miR-6852 in patients with GC was associated with increased tumor metastasis, higher TNM stage and poorer tumor differentiation; however, there was no correlation between miR-6852 expression level and patient age (Table I). These results demonstrated that miR-6852 was downregulated in GC tissues and implied that miR-6852 may serve as a tumor suppressor in GC.
Figure 1.
miR-6852 was underexpressed in GC tissues. (A) Expression levels of miR-6852 in 56 pairs of GC tissues and adjacent normal tissues were determined using reverse transcription-quantitative polymerase chain reaction. (B) Relative expression of miR-6852 in GC cell lines and normal cell line GES-1 was indicated. All data were presented as the mean ± standard deviation of three independent experiments. *P<0.05, **P<0.01 and ***P<0.001 vs. control group. GC, gastric cancer; miR-6852, microRNA-6852.
Table I.
Correlation between microRNA-6852 expression and clinicopathological features in patients with gastric cancer.
| Feature | n=56 | miR-6852 low (n=31) | miR-6852 high (n=25) | P-value |
|---|---|---|---|---|
| Age (years) | 0.559 | |||
| <60 | 17 | 8 | 9 | |
| ≥60 | 39 | 23 | 16 | |
| Differentiation | 0.418 | |||
| Well/moderate | 23 | 11 | 12 | |
| Poor | 33 | 20 | 13 | |
| Metastasis | 0.007 | |||
| Absent | 26 | 9 | 17 | |
| Present | 30 | 22 | 8 | |
| TNM stage | ||||
| I–II | 32 | 13 | 19 | 0.015 |
| III–IV | 24 | 18 | 6 | |
miR-6852 overexpression suppresses GC cell proliferation, migration and invasion
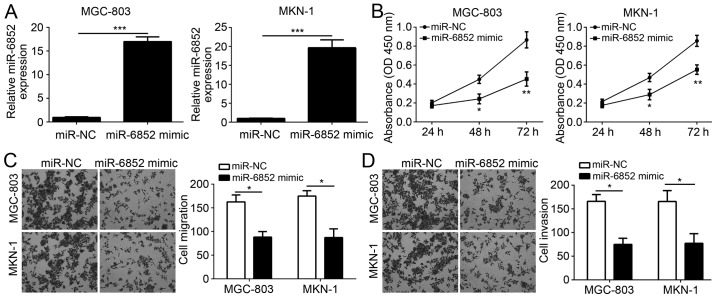
To investigate the role of miR-6852, miR-6852 was overexpressed in GC cell lines MGC-803 and MKN-1. RT-qPCR analysis indicated that miR-6852 was effectively overexpressed in MGC-803 and MKN-1 cells following miR-6852 transfection (Fig. 2A). Subsequently, CCK-8 assays were performed to assess cellular proliferation. Notably, overexpression of miR-6852 significantly inhibited the proliferation of MGC-803 and MKN-1 cells (Fig. 2B). Furthermore, the identified correlation between miR-6852 expression and tumor metastasis was validated using Transwell assays. Results indicated that overexpression of miR-6852 significantly decreased the numbers of migrated or invaded MGC-803 and MKN-1 cells (Fig. 2C and D). Taken together, these data demonstrated that miR-6852 suppressed the proliferation, migration and invasion of GC cells.
Figure 2.
miR-6852 overexpression suppresses gastric cancer cell proliferation, migration and invasion. (A) Reverse transcription-quantitative polymerase chain reaction analysis of miR-6852 expression in MGC-803 and MKN-1 cells. (B) Cell Counting Kit-8 assays were used for analysis of cellular proliferation in MGC-803 and MKN-1 cells. (C and D) Transwell assays were used for detection of cell migration and invasion in MGC-803 and MKN-1 cells (magnification, ×100). All data were presented as the mean ± standard deviation of three independent experiments. *P<0.05, **P<0.01 and ***P<0.001 vs. control group. OD, optical density; miR-6852, microRNA-6852.
FOXJ1 is a target of miR-6852
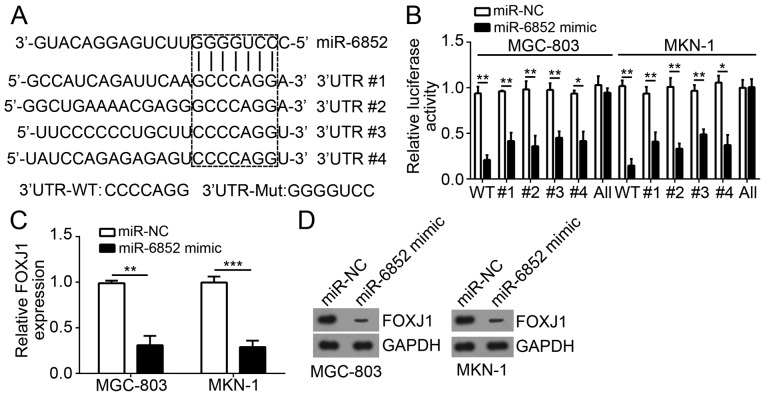
miRs have been demonstrated to regulate gene expression via targeting the 3′-UTR region of mRNAs. To determine the molecular mechanism of miR-6852 function, the target genes of miR-6852 in GC cells were investigated. Bioinformatics analysis determined that FOXJ1 was a potential target of miR-6852. As indicated in Fig. 3A, there were four potential binding sites of miR-6852 in the 3′-UTR region of FOXJ1 mRNA. Subsequently, a luciferase reporter plasmid containing wild-type or mutant 3′-UTR binding site of FOXJ1 mRNA was constructed (Fig. 3A). Luciferase reporter assays indicated that overexpression of miR-6852 significantly inhibited the luciferase activity in MGC-803 and MKN-1 cells transfected with wild-type FOXJ1 3′-UTR but not with mutant FOXJ1 3′-UTR (all four sites mutated; Fig. 3B). Notably, mutation of either predicted recognition site in FOXJ1 3′-UTR could not affect the inhibitory effect of miR-6852 on luciferase activity (Fig. 3B), suggesting the four sites could be recognized by miR-6852. Furthermore, overexpression of miR-6852 significantly inhibited the mRNA expression levels of FOXJ1 in MGC-803 and MKN-1 cells (Fig. 3C). Similarly, the protein expression levels of FOXJ1 were also decreased in MGC-803 and MKN-1 cells transfected with miR-6852 mimics (Fig. 3D). The above findings demonstrated that FOXJ1 was a direct target of miR-6852 in GC cells.
Figure 3.
FOXJ1 was a target of miR-6852. (A) Predicted binding sites of miR-6852 in the 3′-UTR region of FOXJ1 mRNA were determined. (B) Luciferase reporter assays were used for analysis of the interaction between miR-6852 and FOXJ1 3′-UTR. (C) Reverse transcription-quantitative polymerase chain reaction analysis was used to determine FOXJ1 mRNA expression levels in MGC-803 and MKN-1 cells transduced with miR-6852 mimics or control. (D) Protein expression levels of FOXJ1 in MGC-803 and MKN-1 cells transduced with miR-6852 mimics or control were measured using western blot analysis. GAPDH was used as a loading control. All data were presented as the mean ± standard deviation of three independent experiments. *P<0.05, **P<0.01 and ***P<0.001 vs. control group. miR-6852, microRNA-6852; FOXJ1, forkhead box J1; UTR, untranslated region.
FOXJ1 expression is upregulated and reversely correlated with miR-6852 expression levels in GC tissues
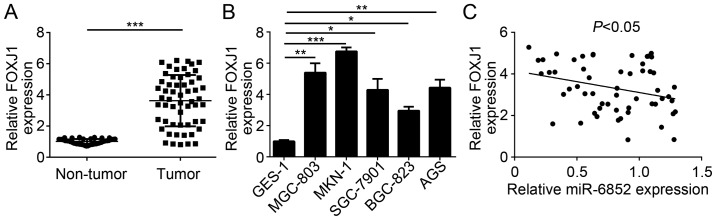
To further confirm the previous findings, the expression patterns of FOXJ1 in GC cells were determined. RT-qPCR analysis revealed that FOXJ1 was significantly upregulated in GC tissues compared with adjacent normal tissues (Fig. 4A). Consistently, the expression of FOXJ1 was significantly upregulated in GC cell lines compared with GES-1 cells (Fig. 4B). Furthermore, a reverse correlation between the expression of miR-6852 and FOXJ1 expression levels was indicated in patients with GC (Fig. 4C). These data indicated that FOXJ1 was targeted by miR-6852 and may be involved in GC progression.
Figure 4.
FOXJ1 expression was upregulated and reversely correlated with miR-6852 expression levels in GC tissues. (A) The expression of FOXJ1 in GC tissues and adjacent normal tissues was indicated. (B) RT-qPCR analysis was used to determine the expression patterns of FOXJ1 in GC cell lines. (C) RT-qPCR analysis indicated that there was an inverse correlation between miR-6852 and FOXJ1 expression levels in GC tissues. All data were presented as the mean ± standard deviation of three independent experiments. *P<0.05, **P<0.01 and ***P<0.001 vs. control group. GC, gastric cancer; FOXJ1, forkhead box J1; RT-qPCR, reverse transcription-quantitative polymerase chain reaction; miR-6852, microRNA-6852.
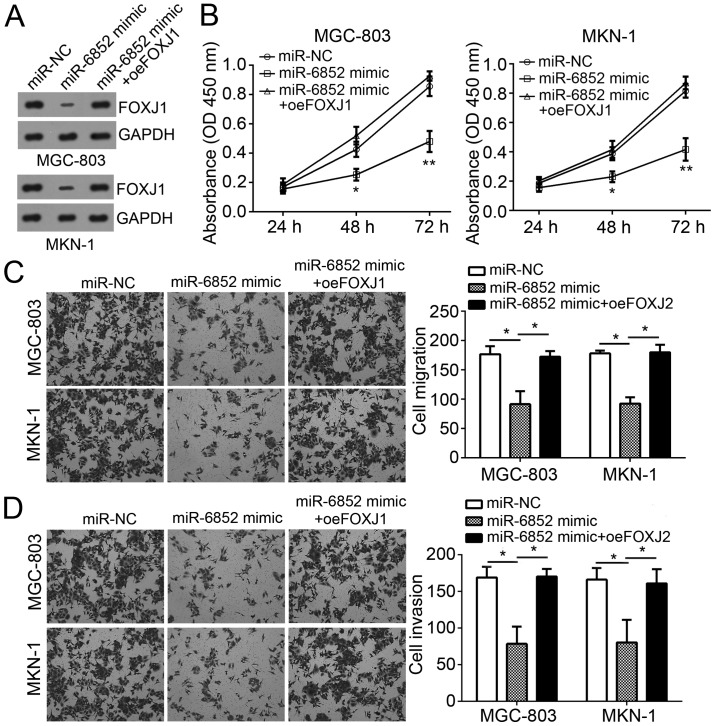
Restoration of FOXJ1 expression reverses the effects of miR-6852 transfection
To determine the role of FOXJ1 in miR-6852-regulated GC progression, FOXJ1 expression was restored in MGC-803 and MKN-1 cells transfected with miR-6852 mimics. Western blot analysis indicated that FOXJ1 was markedly upregulated in GC cells (Fig. 5A). CCK-8 assay analysis revealed that miR-6852 significantly inhibited cellular proliferation, whereas overexpression of FOXJ1 abolished this effect in MGC-803 and MKN-1 cells (Fig. 5B). In addition, Transwell assay results also demonstrated that overexpression of FOXJ1 abrogated the suppressive effects of miR-6852 on the migration and invasion of MGC-803 and MKN-1 cells (Fig. 5C and D). Taken together, these results demonstrated that miR-6852 suppressed the proliferation, migration and invasion of GC cells by targeting FOXJ1.
Figure 5.
Restoration of FOXJ1 expression reversed the effects of miR-6852 transfection. (A) Western blot analysis was used to determine FOXJ1 expression in MGC-803 and MKN-1 cells. (B) Cell Counting Kit-8 assays were used for analysis of cell proliferation. (C and D) Transwell assays were utilized for detection of cell migration and invasion (magnification, ×100). All data were presented as the mean ± standard deviation of three independent experiments. *P<0.05, **P<0.01 and ***P<0.001 vs. control group. FOXJ1, forkhead box J1; miR-6852, microRNA-6852.
Discussion
GC is the second leading cause of cancer-associated mortality around the world (15). Patients with GC typically have a very low 5-year overall survival, which is primarily due to systemic tumor metastasis (16). Thus, it is crucial to determine the molecular mechanism underlying the development and progression of GC. In the present study, the function of miR-6852 in GC was explored and the results revealed that miR-6852 was significantly downregulated in GC tissues and cell lines. Furthermore, miR-6852 expression was significantly correlated with tumor differentiation, metastasis and TNM stage, which suggested miR-6852 may be involved in GC progression. Results from functional experiments indicated that miR-6852 overexpression inhibited the proliferation, migration and invasion of GC cells. These data demonstrated that miR-6852 serves as a tumor suppressor in GC and may be a promising therapeutic target for GC treatment.
miRs have been acknowledged as important players in tumorigenesis and can regulate tumor cell proliferation, survival and metastasis (17–19). A large number of studies indicate that miRs serve as oncogenes or tumor suppressors in almost all types of human cancer, including breast cancer (20), non-small cell lung cancer (21), prostate cancer (22), osteosarcoma (23), glioma (24) and GC (25). For example, Ding et al (26) reported that miR-367 regulates cell proliferation and metastasis by targeting metastasis-associated protein 3 in clear-cell renal cell carcinoma. He et al (27) indicated that miR-186 regulates the invasion and metastasis of bladder cancer via vascular endothelial growth factor C. Notably, a recent study demonstrated that miR-6852 overexpression induces necrosis in cervical cancer cells (13). However, the role of miR-6852 in other tumors remains elusive. In the present study, miR-6852 was downregulated in GC tissues and negatively correlated with GC severity. Furthermore, it was demonstrated that miR-6852 suppressed GC cell proliferation, migration and invasion according to CCK-8 and Transwell assay results.
FOXJ1 belongs to the forkhead box gene family, which is a large group of transcription factors, and widely participates in various biological processes, including development and tumorigenesis (28,29). Several reports have demonstrated that FOXJ1 regulates human cancer development and progression (30,31). For instance, Liu et al (32) reported that FOXJ1 is upregulated and promotes colorectal cancer progression via activating b-catenin signaling. Another study also indicated that FOXJ1 was downregulated in GC and serves as a prognostic marker for patients with GC (33). Consistent with a previous report, the present study indicated that FOXJ1 was a target of miR-6852 and its expression was upregulated in GC tissues. Furthermore, it was revealed that FOXJ1 expression was inversely correlated with that of miR-6852 in GC tissues. Additionally, through a series of functional experiments, it was indicated that restoration of FOXJ1 reversed the effects of miR-6852 transfection on GC cell proliferation, migration and invasion.
In conclusion, the present findings demonstrated that miR-6852 expression was downregulated in GC tissues and correlated with tumor severity. Furthermore, the results suggested that miR-6852 may be a tumor suppressor in GC through targeting FOXJ1. These findings indicate that miR-6852 may be a potential therapeutic target for GC treatment.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
JL, HY and JZ initiated and designed the present work and analyzed and interpreted the results. QW, YD, WZ and FL performed experiments. JL wrote the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
For the use of human samples, the protocol for the present study was approved by the Institutional Ethics Committee of Tongji Medical College, Huazhong University of Science and Technology (Wuhan, China) and all enrolled patients signed a written informed consent document.
Patient consent for publication
No applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Wu HH, Lin WC, Tsai KW. Advances in molecular biomarkers for gastric cancer: miRNAs as emerging novel cancer markers. Expert Rev Mol Med. 2014;16:e1. doi: 10.1017/erm.2013.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dassen AE, Lemmens VE, van de Poll-Franse LV, Creemers GJ, Brenninkmeijer SJ, Lips DJ, Wurff Vd AA, Bosscha K, Coebergh JW. Trends in incidence, treatment and survival of gastric adenocarcinoma between 1990 and 2007: A population-based study in the Netherlands. Eur J Cancer. 2010;46:1101–1110. doi: 10.1016/j.ejca.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 4.Yang H, Wang L, Tang X, Bai W. miR-203a suppresses cell proliferation by targeting E2F transcription factor 3 in human gastric cancer. Oncol Lett. 2017;14:7687–7690. doi: 10.3892/ol.2017.7199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 6.He L, Hannon GJ. MicroRNAs: Small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5:522–531. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- 7.Wienholds E, Plasterk RH. MicroRNA function in animal development. FEBS Lett. 2005;579:5911–5922. doi: 10.1016/j.febslet.2005.07.070. [DOI] [PubMed] [Google Scholar]
- 8.Agrawal L, Sahu S, Ghosh S, Shiga T, Fujita D, Bandyopadhyay A. Inventing atomic resolution scanning dielectric microscopy to see a single protein complex operation live at resonance in a neuron without touching or adulterating the cell. J Integr Neurosci. 2016;15:435–462. doi: 10.1142/S0219635216500333. [DOI] [PubMed] [Google Scholar]
- 9.Zhang S, Ge W, Zou G, Yu L, Zhu Y, Li Q, Zhang Y, Wang Z, Xu T. MiR-382 targets GOLM1 to inhibit metastasis of hepatocellular carcinoma and its down-regulation predicts a poor survival. Am J Cancer Res. 2018;8:120–131. [PMC free article] [PubMed] [Google Scholar]
- 10.Kang W, Huang T, Zhou Y, Zhang J, Lung RWM, Tong JHM, Chan AWH, Zhang B, Wong CC, Wu F, et al. miR-375 is involved in Hippo pathway by targeting YAP1/TEAD4-CTGF axis in gastric carcinogenesis. Cell Death Dis. 2018;9:92. doi: 10.1038/s41419-017-0134-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilmott JS, Zhang XD, Hersey P, Scolyer RA. The emerging important role of microRNAs in the pathogenesis, diagnosis and treatment of human cancers. Pathology. 2011;43:657–671. doi: 10.1097/PAT.0b013e32834a7358. [DOI] [PubMed] [Google Scholar]
- 12.Kashyap D, Tuli HS, Garg VK, Goel N, Bishayee A. Oncogenic and tumor-suppressive roles of MicroRNAs with special reference to apoptosis: Molecular mechanisms and therapeutic potential. Mol Diagn Ther. 2018;22:179–201. doi: 10.1007/s40291-018-0316-1. [DOI] [PubMed] [Google Scholar]
- 13.Poudyal D, Herman A, Adelsberger JW, Yang J, Hu X, Chen Q, Bosche M, Sherman BT, Imamichi T. A novel microRNA, hsa-miR-6852 differentially regulated by Interleukin-27 induces necrosis in cervical cancer cells by downregulating the FoxM1 expression. Sci Rep. 2018;8:900. doi: 10.1038/s41598-018-19259-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 15.Yasui W, Oue N, Aung PP, Matsumura S, Shutoh M, Nakayama H. Molecular-pathological prognostic factors of gastric cancer: A review. Gastric Cancer. 2005;8:86–94. doi: 10.1007/s10120-005-0320-0. [DOI] [PubMed] [Google Scholar]
- 16.Carcas LP. Gastric cancer review. J Carcinog. 2014;13:14. doi: 10.4103/1477-3163.146506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shimagaki T, Yoshizumi T, Harimoto N, Yoshio S, Naito Y, Yamamoto Y, Ochiya T, Yoshida Y, Kanto T, Maehara Y. MicroRNA-125b expression and intrahepatic metastasis are predictors for early recurrence after hepatocellular carcinoma resection. Hepatol Res. 2018;48:313–321. doi: 10.1111/hepr.12990. [DOI] [PubMed] [Google Scholar]
- 18.Hu X, Wang Y, Liang H, Fan Q, Zhu R, Cui J, Zhang W, Zen K, Zhang CY, Hou D, et al. miR-23a/b promote tumor growth and suppress apoptosis by targeting PDCD4 in gastric cancer. Cell Death Dis. 2017;8:e3059. doi: 10.1038/cddis.2017.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao N, Lin T, Zhao C, Zhao S, Zhou S, Li Y. MicroRNA-588 is upregulated in human prostate cancer with prognostic and functional implications. J Cell Biochem. 2017 Oct 5; doi: 10.1002/jcb.26417. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 20.Shivapurkar N, Vietsch EE, Carney E, Isaacs C, Wellstein A. Circulating microRNAs in patients with hormone receptor-positive, metastatic breast cancer treated with dovitinib. Clin Transl Med. 2017;6:37. doi: 10.1186/s40169-017-0169-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu C, Xie Z, Peng Q. MiRNA-107 enhances chemosensitivity to paclitaxel by targeting antiapoptotic factor Bcl-w in non small cell lung cancer. Am J Cancer Res. 2017;7:1863–1873. [PMC free article] [PubMed] [Google Scholar]
- 22.Egan SM, Karasik E, Ellis L, Gollnick SO. miR-30e* is overexpressed in prostate cancer and promotes NF-κB-mediated proliferation and tumor growth. Oncotarget. 2017;8:67626–67638. doi: 10.18632/oncotarget.18795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li J, Wu QM, Wang XQ, Zhang CQ. Long noncoding RNA miR210HG sponges miR-503 to facilitate osteosarcoma cell invasion and metastasis. DNA Cell Biol. 2017;36:1117–1125. doi: 10.1089/dna.2017.3888. [DOI] [PubMed] [Google Scholar]
- 24.Zheng Y, Lu X, Xu L, Chen Z, Li Q, Yuan J. MicroRNA-675 promotes glioma cell proliferation and motility by negatively regulating retinoblastoma 1. Hum Pathol. 2017;69:63–71. doi: 10.1016/j.humpath.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 25.Wang X, Liu B, Wen F, Song Y. MicroRNA-454 inhibits the malignant biological behaviours of gastric cancer cells by directly targeting mitogen-activated protein kinase 1. Oncol Rep. 2018;39:1494–1504. doi: 10.3892/or.2017.6171. [DOI] [PubMed] [Google Scholar]
- 26.Ding D, Zhang Y, Wen L, Fu J, Bai X, Fan Y, Lin Y, Dai H, Li Q, Zhang Y, An R. MiR-367 regulates cell proliferation and metastasis by targeting metastasis-associated protein 3 (MTA3) in clear-cell renal cell carcinoma. Oncotarget. 2017;8:63084–63095. doi: 10.18632/oncotarget.18647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He X, Ping J, Wen D. MicroRNA-186 regulates the invasion and metastasis of bladder cancer via vascular endothelial growth factor C. Exp Ther Med. 2017;14:3253–3258. doi: 10.3892/etm.2017.4908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Overdier DG, Ye H, Peterson RS, Clevidence DE, Costa RH. The winged helix transcriptional activator HFH-3 is expressed in the distal tubules of embryonic and adult mouse kidney. J Biol Chem. 1997;272:13725–13730. doi: 10.1074/jbc.272.21.13725. [DOI] [PubMed] [Google Scholar]
- 29.Abedalthagafi MS, Wu MP, Merrill PH, Du Z, Woo T, Sheu SH, Hurwitz S, Ligon KL, Santagata S. Decreased FOXJ1 expression and its ciliogenesis programme in aggressive ependymoma and choroid plexus tumours. J Pathol. 2016;238:584–597. doi: 10.1002/path.4682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xian S, Shang D, Kong G, Tian Y. FOXJ1 promotes bladder cancer cell growth and regulates Warburg effect. Biochem Biophys Res Commun. 2018;495:988–994. doi: 10.1016/j.bbrc.2017.11.063. [DOI] [PubMed] [Google Scholar]
- 31.Lan Y, Hu X, Jiang K, Yuan W, Zheng F, Chen H. Significance of the detection of TIM-3 and FOXJ1 in prostate cancer. J BUON. 2017;22:1017–1021. [PubMed] [Google Scholar]
- 32.Liu K, Fan J, Wu J. Forkhead box protein J1 (FOXJ1) is overexpressed in colorectal cancer and promotes nuclear translocation of β-catenin in SW620 cells. Med Sci Monit. 2017;23:856–866. doi: 10.12659/MSM.902906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang J, Cai X, Xia L, Zhou J, Xin J, Liu M, Shang X, Liu J, Li X, Chen Z, et al. Decreased expression of FOXJ1 is a potential prognostic predictor for progression and poor survival of gastric cancer. Ann Surg Oncol. 2015;22:685–692. doi: 10.1245/s10434-014-3742-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.