Abstract
The mechanisms underlying the evolution of morphological novelties have remained enigmatic but co-option of existing gene regulatory networks (GRNs), recruitment of genes and the evolution of orphan genes have all been suggested to contribute. Here, we study a morphological novelty of beetle pupae called gin-trap. By combining the classical candidate gene approach with unbiased screening in the beetle Tribolium castaneum, we find that 70% of the tested components of the wing network were required for gin-trap development. However, many downstream and even upstream components were not included in the co-opted network. Only one gene was recruited from another biological context, but it was essential for the anteroposterior symmetry of the gin-traps, which represents a gin-trap-unique morphological innovation. Our data highlight the importance of co-option and modification of GRNs. The recruitment of single genes may not be frequent in the evolution of morphological novelties, but may be essential for subsequent diversification of the novelties. Finally, after having screened about 28% of annotated genes in the Tribolium genome to identify the genes required for gin-trap development, we found none of them are orphan genes, suggesting that orphan genes may have played only a minor, if any, role in the evolution of gin-traps.
Keywords: wing, gin-trap, morphological novelty, recruitment, co-option, orphan genes
1. Background
The emergence of morphological novelties has been key for the evolution of animals, but the underlying genetic mechanisms have remained poorly studied. It has been suggested that entire gene regulatory networks (GRNs) are co-opted and modified in order to generate new structures. An alternative is the de novo assembly of GRNs by the recruitment of genes from other contexts. Finally, there is evidence that some novelties emerged through the function of taxonomically restricted genes (or orphan genes). While there are examples for each of these processes, empirical data are badly needed to determine the relative importance of these processes [1–8].
Insects are the most species-rich animal taxon on the planet, representing more than half of all living animals [9]. More than 80% of extant insect species belong to the holometabola, which show an overwhelming morphological diversity [9]. This suggests that metamorphosis was a key innovation in promoting insect diversity [10]. Holometabolous insects produce distinct larval and adult morphologies that allow the stages to explore different food sources and to adapt to different ecological habitats. Hence, metamorphosis provides an excellent opportunity to study the evolution of morphological novelties. During that process, a large part of larval cells is integrated into the adult animal while another part undergoes apoptosis. In addition, imaginal cells proliferate and differentiate to form parts of the adult structures. The relative contribution of larval versus imaginal cells varies from species to species and from organ to organ within one species [11]. The epidermis of Drosophila melanogaster and other dipterans represents an extreme case where all larval epidermal cells are replaced by imaginal cells [11,12]. Coleopterans are more typical for insects in that larval cells contribute significantly to the adult epidermis [11,13].
Innovation and diversification during metamorphosis have been studied in a number of insect taxa. For instance, the genetic basis of wing pigmentation has been scrutinized in butterflies and flies [14–17] and morphological evolution was investigated with respect to fore- and hindwings in beetles [18–20], beetle horns [21,22] and genital lobes in drosophilids [23]. However, studies in drosophilids reveal mechanisms acting during the rather derived mode of metamorphosis based on imaginal discs. Most studies in other insects, on the other hand, have been based on a candidate gene approach because efficient large-scale screening tools are lacking in those non-model species. Hence, this approach leads to a bias towards the identification of conserved gene functions, while the contribution of unexpected or even taxonomically restricted genes (orphan genes) [3,6,24] may go unnoticed.
The red flour beetle Tribolium castaneum is an excellent model system for studying morphological evolution during metamorphosis. First, beetles show a typical mode of insect metamorphosis based on a large contribution of larval cells to the adult epidermis [11]. Second, RNA interference (RNAi) is very efficient and systemic [25–27], and a number of transgenic and genome editing tools have become available [28–32]. Importantly, in the ongoing genome-wide iBeetle screen, randomly selected genes have been studied for both embryonic and metamorphic phenotypes, which are documented in the iBeetle-Base [33,34]. Hence, T. castaneum has become a model system where unbiased large-scale phenotypic screening is feasible. Owing to the systemic nature of RNAi, genes acting during metamorphosis can be tested by injection of double-stranded RNA (dsRNA) into late larval stages, avoiding potential lethality due to earlier functions [27,34].
In this work, we focused on a structure called gin-trap, which is an epidermal outgrowth consisting of an anterior and a posterior part armoured with a denticular sclerotization, respectively [35,36]. Gin-traps are located at the dorso-lateral side of the first to seventh abdominal segments in Tribolium (figure 1a). Interestingly, they are found exclusively on pupae of Coleoptera of the closely related families Tenebrionidae and Colydiidae [35]. Functionally equivalent but morphologically different gin-traps (dorsal gin-trap) are found on the dorsal part of abdominal segments of Coleoptera and Lepidoptera [35,37]. Gin-traps are defensive organs, which grasp the appendages of predators in response to mechanical stimulation of the otherwise helpless pupa [35,38,39]. The fact that lateral gin-traps are found only on a Holometabola-specific life stage (the pupa) of very few taxonomically related taxa strongly suggests that gin-traps evolved after the radiation of holometabolous insects. Hence, they represent an excellent case study for the evolution of a morphological novelty. On the basis of the expression and function of the wing selector gene vestigial in gin-trap development and a homeotic transformation from gin-trap to wing-like structures after Hox gene RNAi in the tenebrionid beetle Tenebrio molitor, it has been suggested that gin-traps are wing serial homologues [40]. However, it has remained unclear how many genes are used by both the wing and gin-trap GRNs and how many are recruited only to the gin-trap GRN. Further, the importance of orphan genes in the evolution of this morphological novelty remains unknown.
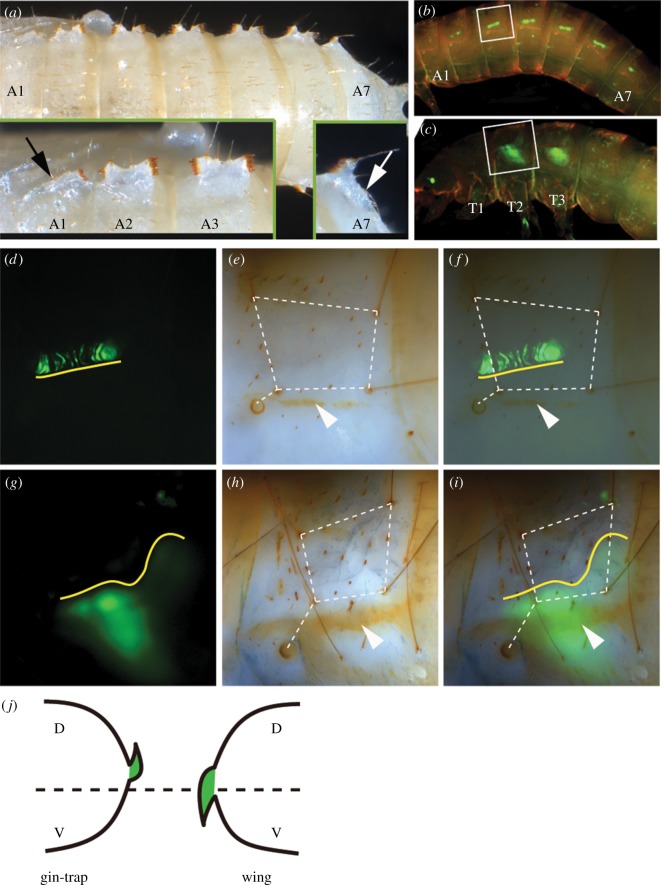
Figure 1.
Morphology and segmental origin of gin-traps in Tribolium. (a) Dorsal view on a pupa with gin-traps on A1–A7. Note the symmetry in A2–A6 but asymmetry in A1 and A7 (insets). (b) The transgenic line GöGal41152 marks cells within the gin-traps from prepupal stages onwards. (c) The pu11 line marks wing primordia. (d–f) Location of the gin-trap primordium (d) relative to cuticular markers (e). (g–i) Location of the wing primordium relative to the corresponding cuticular markers. (j) Gin-traps and wings grow towards the dorsal (D) and ventral (V) sides, respectively. Hence, the region of outgrowth (yellow line in d–i) is quite similar for both structures.
In order to address these questions, we first systematically score orthologues of the Drosophila wing GRN for a role in gin-trap formation in Tribolium in a classical candidate gene approach. Then, we identify novel genes involved in gin-trap and wing formation in an unbiased way by mining the results of the iBeetle screen. Our data reveal that gin-trap development is based on the co-option of about 70% of the tested wing GRN components, where mainly downstream genes were removed. Unexpectedly, central upstream components of the wing GRN like engrailed and Dpp signalling were lost as well. We estimate that recruitment of genes from other contexts accounts for about 10% of the gin-trap GRN while, surprisingly, we do not find involvement of orphan genes after having screened 28% of annotated genes of the Tribolium genome.
2. Methods
(a). Animals
Wild-type San Bernardino strain (SB) and enhancer trap lines (pu11 and GöGal41152) were used and reared on whole-wheat flour supplemented with 5% yeast powder at 32°C for all experiments.
(b). Inverse PCR
Genomic DNA was isolated from four adults by standard phenol–chloroform extraction and then separately digested by the restriction enzymes Bsp143I and HhaI. After self-ligation at room temperature for 1 h, inverse PCR was performed with the primer sets in electronic supplementary material, table S3 and the amplified fragment was sent for sequencing (LGC Genomics).
(c). Cloning and sequencing of Tribolium genes
Homologues of Ultrabithorax (Ubx), bursicon (burs) and partner of bursicon (pburs) were isolated from pupal cDNAs of Tribolium by PCR (see electronic supplementary material, table S1, for primer sequences), cloned into pJET1.2/blunt vector and their sequence was confirmed by sequencing (LGC Genomics).
(d). dsRNA synthesis
dsRNA was produced from cloned genes for: Tc-EGFR, Tc-Ser, Tc-Dl, Tc-wg, Tc-en, Tc-hh, Tc-ci, Tc-dpp, Tc-omb, Tc-iro and Tc-ASH. Vectors with the following cDNAs were kindly provided by Yoshinori Tomoyasu: Tc-nub, Tc-srf, Tc-dad, Tc-apA/B, Tc-dsh and Tc-sal [19,20,41]. Templates were generated by PCR adding terminal T7 promoter sequences (see electronic supplementary material, table S1, for sequences) and dsRNA was synthesized by in vitro transcription (Megascript T7; Ambion). The dsRNA products were denatured in a 94°C heating block for 5 min and then reannealed by slowly cooling down to room temperature. Specificity of the products was confirmed via agarose gel electrophoresis. dsRNAs targeting iBeetle candidate genes were ordered from Eupheria Biotech GmbH (Dresden) (see electronic supplementary material, table S2, for iBeetle numbers).
(e). Tribolium injection
Injection were performed in penultimate or last larval stage (L6 or L7). dsRNAs were titrated from 100 to 1 ng µl−1 according to different genes and approximately 0.5–0.7 µl of dsRNA solution was injected into at least 10 larvae for each set of injections, similar to the procedure in the iBeetle screen [34]. After injection, the larvae were kept on flour at 32°C until pupation. For novel genes identified from the iBeetle database, non-overlapping fragments (1 µg µl−1) were injected to control for off-target effects (see electronic supplementary material, table S3).
(f). Image processing and documentation
Images were captured by using a Zeiss Axioplan microscope (dorsal and ventral of pupa and close-up of gin-trap), a Leica M205 FA microscope (enhancer trap lines) and a Zeiss LSM 510 laser scanning microscope (enhancer trap lines). Adjustments for brightness and contrast were done with Adobe Photoshop and figures were assembled in Adobe Illustrator.
3. Results
(a). The elytra and gin-trap primordia originate from conserved developmental field
In order to visualize the developing gin-traps prior to their emergence in the pupa, we searched for enhancer trap lines generated in a Gal4 enhancer trap screen (G.B., 2016, unpublished results). We characterized one line, GöGal41152, which marked cells within the gin-traps but not their epidermis (see electronic supplementary material, figure S1). We asked whether wings and gin-traps develop at the same location in the respective segments by comparing the signal of GöGal41152 with the enhancer trap line pu11, which marks wing anlagen [20] (figure 1c). Both trachea and setae were used as segmental cuticular landmarks to compare the location of gin-trap and wing primordia. The wing primordium was larger than the signal marking the gin-traps and it extended more to the ventral side relative to the cuticular landmarks (compare figure 1d–f with 1g–i). However, the gin-trap primordium is bent to the dorsal side within the prepupa, while the wing primordium grows towards the ventral side (figure 1j). Taking this into account, it appears that both primordia originate from very similar regions in the respective segments (yellow line in figure 1), which is in line with the previous suggestion of serial homology of wings and gin-traps [40].
(b). Gin-traps recruited only parts of the wing gene regulatory network
In order to test to what degree the development of gin-traps is based on the wing GRN, we knocked down known homologues of wing patterning genes in both wild-type and the GöGal41152 line. We included genes known from Drosophila wing development, for which either a wing RNAi phenotype or wing-specific expression had been described in Tribolium [19,20,41]. In Drosophila, the wing imaginal disc is subdivided into anteroposterior (AP) and dorsoventral (DV) compartments [42]. Previous studies showed that the wing gene network is largely conserved between Tribolium and Drosophila except for divergent expression patterns of dpp and its target genes optomotor-blind (omb), spalt (sal) and daughter against dpp (dad), which are expressed at the distal tip of the AP boundary in Tribolium rather than along the entire AP border [19,20].
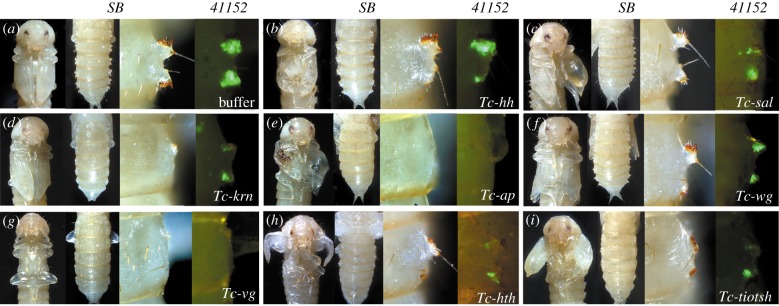
First, we tested components of the AP patterning system. Double knockdown of Tc-engrailed (Tc-en) and its paralogue Tc-invected (Tc-inv) [43] resulted in altered orientation and an irregular surface of pupal elytra, and about 50% of pupae and adult animals showed blistered elytra in the distal part (electronic supplementary material, figures S3a and S4d; wing blisters arise by detachment of dorsal and ventral wing epithelia, indicating loss of their usually tight adhesion). However, defects in gin-trap formation were not detected. Depletion of Tc-hedgehog (Tc-hh) resulted in deformation of elytra, walking legs and antennae, and the posterior part of the gin-traps was strongly reduced (figure 2b). Analysis of other members of the Hh pathway (cubitus interruptus (ci), smoothened (smo)) confirmed this result (electronic supplementary material, figure S2). This asymmetric posterior requirement in gin-traps contrasts the Hh function at the boundary between anterior and posterior compartments of the wing disc. Unexpectedly, Tc-dpp, which is the central AP morphogen of the Drosophila wing, and its target genes Tc-omb and Tc-dad were not involved in gin-trap formation in Tribolium (electronic supplementary material, figure S3b–d). However, Tc-sal affected both anterior and posterior parts of the gin-traps (figure 2c). Depletion of Tc-keren (Tc-krn), encoding the only activating EGFR ligand in Tribolium, resulted in the reduction in both parts of the gin-traps similar to knock-down phenotypes of Tc-EGFR (figure 2d; electronic supplementary material, figure S2). In addition, Tc-krn RNAi affected the formation of veins in the elytra (electronic supplementary material, figure S4b).
Figure 2.
Function of candidate wing genes in wing and gin-trap development. Panels show (from left to right) ventral view of a pupa showing the wings, dorsal view showing the gin-trap, close-up of one gin-trap and the signal of the GöGal41152 transgenic line. (a) Negative control. (b–i) RNAi phenotypes. In the GöGal41152 line, no RNAi larvae hatched after Tc-hth RNAi. Hence, the signal in the prepupa is shown (h).
Next, we tested components of wing DV patterning. Tc-apterous (Tc-ap) RNAi caused the complete absence of gin-traps, while its phenotype in wings was moderate compared with the results based on RNAi at the penultimate stage. The shape of the elytra was deformed and their dorsal surface abnormal, sometimes showing necrosis (figure 2e). Depletion of the Tc-Notch receptor led to death at the prepupal stage, but the size of the gin-trap signal was decreased in prepupa of the line GöGal41152 (electronic supplementary material, figure S2). To further test the involvement of the Notch pathway, we knocked down the ligand Tc-Serrate (Tc-Ser). Gin-traps were moderately smaller and their orientation was irregular, while elytra became smaller and blistered (electronic supplementary material, figure S2). RNAi targeting the Wnt ligand Tc-wingless (Tc-wg) affected predominantly the posterior part of the gin-traps, with minor alterations to the anterior parts (figure 2f). This was phenocopied by knocking down Tc-dishevelled (Tc-dsh), another component of the Wnt signalling pathway (electronic supplementary material, figure S2). Knocking down Tc-vestigial (Tc-vg) induced the complete deletion of gin-traps and wings (figure 2g), confirming the vg RNAi phenotype found in T. molitor [40].
In Drosophila, homothorax (hth) and teashirt (tsh) are expressed in the proximal part of the wing disc to specify the notum [44–46]. RNAi of Tc-hth and Tc-tiotsh (the single orthologue of the Drosophila tsh-related genes [47]) led to moderate phenotypes of the gin-traps and deformation of elytra (figure 2h,i).
The terminal wing specification genes Dll, ASH, srf and iro, which specify the margin, sensory, intervein and vein differentiation of wings, respectively, were not required for gin-trap formation (electronic supplementary material, figures S3e–h and S4c,e). Likewise, the wing pouch marker gene nubbin (nub) was not involved (electronic supplementary material, figure S3i).
(c). Unbiased RNAi screen reveals novel gin-trap and wing patterning genes
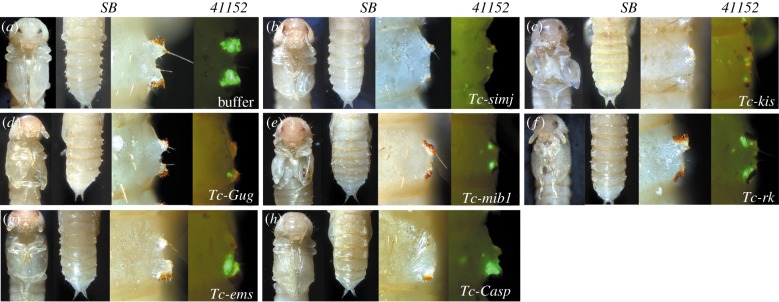
Next, we wanted to assess the portion of genes that were recruited from other biological contexts to the gin-trap GRN and search for the potential involvement of orphan genes (i.e. beetle-specific genes). Therefore, we searched for gin-trap phenotypes annotated with a penetrance of more than 50% at the iBeetle-Base (http://ibeetle-base.uni-goettingen.de) [33,34]. The iBeetle project is a first-pass screen, where false-positive and off-target phenotypes need to be considered. Hence, for genes screened for a role in gin-trap formation, we repeated the injection of the iBeetle dsRNA fragment in the same and in a different genetic background (SB) and analysed phenotypes induced by non-overlapping dsRNA fragments [34,48]. Ten phenotypes were confirmed (see electronic supplementary material, table S2). Several of these genes were known to be involved in Drosophila wing patterning like Tc-vg, the EGFR ligand Tc-krn as well as the Notch pathway component Tc-mind bomb1 (Tc-mib1) (figure 3e), confirming the involvement of these pathways. The size of the gin-traps was moderately decreased in RNAi targeting Tc-Grunge (Tc-Gug; in Drosophila also called atrophin) (figure 3d), a nuclear repressor protein, which in Drosophila regulates EGFR, Hh signalling and tsh in wings and other tissues [49–51]. Intriguingly, we found defects only in the anterior part of the gin-traps after RNAi targeting the Hox gene Tc-abdA (see electronic supplementary material, figure S5). In Tc-rickets (Tc-rk) RNAi pupae, RNAi gin-traps were slightly smaller in size and showed irregular orientation, while the elytra showed a wrinkled surface as previously described (figure 3f) [52]. rk is a G-protein-coupled receptor involved in the bursicon signalling pathway, which is involved in moulting-related behaviours, neuropeptide-induced tanning and Tribolium wing and gin-trap development [52–54]. We confirmed the involvement of this pathway in gin-trap formation by testing the hormones Tc-bursicon (Tc-burs) and Tc-pbursicon (Tc-pburs) (electronic supplementary material, figure S2g–i).
Figure 3.
Genes identified by unbiased screening. Panels as in figure 2. (a) Negative control. (b–h) RNAi phenotype of Tc-simj, Tc-kis, Tc-Gug, Tc-mib1, Tc-rk and Tc-ems. Tc-casp is the only gene with a gin-trap phenotype that does not show a wing phenotype as well (h). No orphan genes were involved in gin-trap formation.
Three genes, Tc-simjang (Tc-simj), Tc-kismet (Tc-kis) and Tc-empty-spiracles (Tc-ems), had not been connected to Drosophila wing development before but showed phenotypes in both gin-traps and wings of Tribolium (figure 3b,c,g). Both simj and kis are involved in chromatin gene regulation [55,56]. The respective RNAi pupae showed reduced gin-traps and denticles (figure 3b,c). Tc-ems is a transcription factor and was required mainly for the anterior part of the gin-traps (figure 3g). Knockdown of these genes affected the wing pattern to different degrees. Size, orientation or shape of the pupal wing was abnormal when Tc-simj and Tc-ems were knocked down (figure 3b,g), while the elytra were deformed and blistered in Tc-kis RNAi pupae (figure 3c).
One gene affected gin-traps but not wings: caspar (casp) is a repressor of the immune deficiency pathway (but not Toll signalling) [57]. In Tribolium, it was essential for the formation of the anterior part of the gin-traps (figure 3h).
Eight of the above-mentioned genes/pathways, including Tc-hth, Tc-Dll, Wnt signalling (Tc-wg, Tc-dsh), Dpp signalling (Tc-dpp), Hh signalling (Tc-smo, Tc-ptc), Notch signalling (Tc-ser, Tc-mib1), EGFR signalling (Tc-krn) and bursicon signalling pathways (Tc-burs, Tc-pburs), were also involved in leg metamorphosis (electronic supplementary material, figures S2 and S6). The phenotypes we found were identical to the description of previous studies in Tribolium [52,58,59].
4. Discussion
(a). A highly reduced wing gene regulatory network is co-opted in the gin-traps
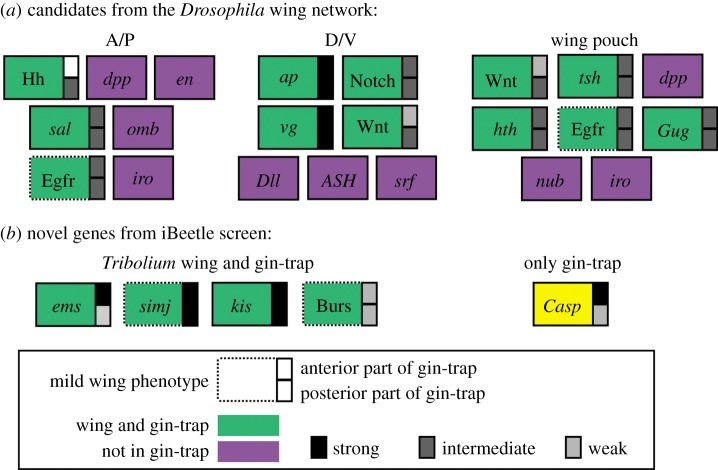
Co-option of existing GRNs, recruitment of genes to a novel context and emergence of orphan genes have all been suggested to contribute to the evolution of morphological novelties [2–8]. The relative importance of the contribution of each of these processes remained obscure as unbiased phenotypic screening tools were missing in insects outside Drosophila. The ongoing genome-wide RNAi screen iBeetle offers the unique possibility to determine the gene sets required for the development of novel structures. We found that 14 out of 15 genes required for gin-trap formation acted in the wing GRN as well and we confirmed serially homologous position of both structures and transformation of gin-traps into wings [40]. On the basis of these findings, we prefer the hypothesis that co-option and subsequent modification of the wing GRN led to the emergence of gin-traps rather than the hypothesis of a de novo recruitment of genes into a novel GRN. Specifically, our results show that about 70% (14 out of 20; each signalling pathway counted as one component, figure 4; electronic supplementary material, table S4) of samples of the tested wing GRN was co-opted. This figure might be an overestimation because it is based on the number of involved components, but not on their regulatory interactions. If the interactions of conserved components are quite different, an alternative interpretation would be independent recruitment of that component or gain of a novel function. Tc-ems could be such a case because it has only mild defects in the wings but severe reduction in the anterior part of the gin-trap (figure 3g).
Figure 4.
Components of the wing and gin-trap GRNs. (a) Many genes known from Drosophila wing development have a function in gin-trap development as well (green). Many downstream components but also the upstream components dpp and en did not show gin-trap phenotypes (purple). Components are arranged according to their approximate position in the network (upstream top row versus downstream bottom row) and their involvement in AP, DV and wing pouch formation. Based on [19]. (b) The unbiased screen revealed novel genes required for wing and gin-trap development (green) and one gene, which was clearly recruited from another biological context (yellow). Hh and Wnt signalling together with Tc-ems and Tc-casp showed asymmetric requirement for the anterior versus posterior part of the gin-traps despite the fact that the gin-traps have mirror image symmetry. Note that a role of bursicon in Tribolium wing and gin-trap formation has been described before by Bai & Palli [52].
Further, we find that an unexpectedly large portion of the tested wing GRN components were not co-opted (30%). These were mainly downstream genes in line with the morphological differences of wings and gin-traps. However, we also found that some components acting upstream in the Drosophila wing network were lost as well (i.e. engrailed and the Dpp pathway). This suggests a large degree of flexibility in the co-option of modules within a network (figure 4) [4,7]. What could be the evolutionary scenario of GRN co-option? It could be that an ancestral wing serial homologue GRN acted at the tergal edges [41] and independently recruited genes to build the wing GRN and later in evolution was modified to form the gin-trap GRN. Alternatively, the gin-trap GRN could be based on co-option and subsequent modification of the wing GRN.
It has been suggested that orphan genes or taxonomically restricted genes (i.e. genes that evolved only in a certain lineage) may be essential to the evolution of morphological diversity [2,5,60–62]. In contrast with this prediction, we did not find any such gene in the gin-trap GRN, arguing against a central role. However, we cannot exclude the possibility that orphan genes will be found to be involved in gin-trap formation in the gene set that remains to be screened.
Our unbiased screening revealed only Tc-caspar (Tc-casp) as a component required for gin-trap but not wing development. casp is a negative regulator of the immune deficiency pathway in Drosophila and several anopheline species [57,63]. Hence, this is a prime example of recruitment of a gene from a completely different context. This work is based on the first part of the iBeetle screen where 4480 randomly selected genes were scored for phenotypes during metamorphosis (28% of the gene set) [34]. A similar portion of novel genes acting in wing and/or gin-trap GRNs is predicted to be present in the remainder of the genome. Under this assumption, the portion of genes newly recruited in the gin-trap GRN would be around 10% (see electronic supplementary material, table S2, for calculation).
Surprisingly, we identified three genes involved in wing formation in Tribolium, which had not been connected to this process in Drosophila. This suggests that either the Drosophila GRN has not been comprehensively studied or that significant differences exist to the Tribolium GRN.
(b). Gene regulatory network reduction reflects morphological differences
Compared with the wing with its asymmetry along AP, DV and PD axes and its complex vein pattern, the gin-traps are simple epidermal outgrowths decorated with spines and setae. The large degree of reduction in downstream components of the co-opted wing GRN reflects this simplification. Along the proximo-distal axis, the body wall patterning genes (hth, tsh, EGFR, sal) but not the distal wing patterning genes (dpp, nub, omb, dad) were required. This indicates that mainly the proximal part of the wing GRN was co-opted for the gin-traps (figure 4). It is surprising that nub did not show a phenotype. nub is a transcription factor that is expressed in the wing primordium (wing pouch) and is very specifically required for wing formation [41,64]. While we find it not to be required for gin-trap formation, a Tc-nub enhancer trap line (Tc-nub1 L) marks both wing and gin-trap primordia [65]. Hence, it may represent a gene that is still regulated by the co-opted GRN but has lost an apparent functional role in gin-trap epidermis development. Unexpectedly, we did not find loss of upstream DV patterning genes, despite the fact that gin-traps are symmetric along this axis. However, the gin-traps appear to be built by the dorsal but not the ventral component of the wing anlagen, which might explain the loss of DV polarity [65].
(c). Recruitment and gain of function required for the evolution of a morphological innovation
Intriguingly, the gin-traps show mirror image symmetry along the AP axis, while the walking legs, the wings and the underlying segments all have a clear AP asymmetry. Hence, the gin-trap GRN had to evolve a mechanism to realize this innovation. Our results suggest that several modifications of the co-opted wing GRN contributed. First, it formed without the functional input of engrailed, which is a key factor of posterior identity of segments, legs and wings. Indeed, gin-trap anlagen do not comprise engrailed-positive cells [65]. Hence, the development of symmetry did not have to overcome this very fundamental posterior identity. Second, the segment polarity genes Tc-hh and Tc-wg specify the posterior part of the gin-traps. Hh signalling, which emerges from engrailed-positive cells in embryos, legs and wings, was required exclusively for the posterior part of the gin-trap (figure 2b). Segmental wg expression is located anterior to engrailed/hh-positive cells and was required predominantly for the posterior part of the gin-traps (figure 2f). Hence, two segment polarity genes active in the posterior half of each segment specify the posterior part of the gin-trap. Third, a mirror-image copy of the posterior part of the gin-trap needs to be specified independently of the highly conserved segmental AP asymmetry. Intriguingly, the only newly recruited gene that we found in our search, Tc-casp, affected predominantly the anterior part of the gin-traps (figure 3h). Hence, while recruitment was rare overall, it appeared to have been essential for formation of an innovation regarding gin-trap morphology. Tc-ems is the second component, which was required mainly for the anterior part. Interestingly, the Tc-ems wing phenotype was rather mild (figure 3g), while the phenotype in the anterior gin-trap was among the strongest that we observed. Apparently, Tc-ems has gained a novel upstream function in the anterior gin-trap GRN. Intriguingly, the Tc-abdA RNAi phenotype showed a loss of the anterior part only. Hence, it appears that the two symmetric parts of the gin-trap are under different control by abdominal Hox genes. In summary, specification of an innovation (mirror image symmetry) involved recruitment of one and gain of upstream functions of another component and required the asymmetric involvement of abdominal Hox genes.
(d). Gin-trap evolution and serial homology
On the basis of vg expression in gin-trap anlagen, absence of both structures in RNAi and homeotic transformations, it was suggested that gin-traps are wing serial homologues [40]. Further, it was suggested that both structures evolved from a more ancestral network active at the tergal edges, the wing serial homologues GRN [41]. In line with previous work, we find transformation in Ubx/abdA double RNAi. But we also note a complex regulation of gin-trap development where Ubx and abdA cooperate to repress the wing development, while at the same time they seem to be required for the different parts of the gin-traps (electronic supplementary material, figures S5 and S7). These data, together with the location and the extensive overlap of components involved in wing and gin-trap GRNs, are in line with the view of serial homology. However, we find a large degree of loss of tested network components (about 30%) and identify an innovation, which depends on a recruited gene and the gain of function of at least one other component. Together with the lack of ventral and engrailed-positive tissues in the gin-traps [65], this amounts to a significant degree of divergence of both developmental origin and GRN. Hence, we wonder how far ‘serial homology’ fully reflects this situation or whether the concept of ‘homocracy’ (i.e. being regulated by the same genes) may be more appropriate [66].
Supplementary Material
Acknowledgements
We thank Yoshinori Tomoyasu, David Linz, Ernst A. Wimmer and Nico Posnien for discussions; Elke Küster for providing enhancer trap lines; Claudia Hinners for technical support; Yoshinori Tomoyasu for sharing clones. We would like to thank the two anonymous reviewers for their insightful comments and suggestions to improve the quality of the paper.
Ethics
Animals were kept in the best possible conditions on the basis of the biology of this species. The study was conducted in accordance with ARRIVE guidelines. We also adhered to the legal requirements of Germany and the University of Göttingen. Experimental animals were preserved at −20°C in a freezer and stored.
Data accessibility
DNA sequences: Genbank accession numbers MH664125-MH664127.
Authors' contributions
Y.H. conceived the initial idea for the study, performed the experiments, analysed the data and wrote the draft of the manuscript. J.S., C.S.-E., T.R., N.S. and U.M. identified the phenotypes in the screen. G.B. conceived the study, designed the study, coordinated the study and helped draft the manuscript. All authors gave final approval for publication.
Competing interests
The authors declare that they have no competing interests.
Funding
G.B.: Deutsche Forschungsgemeinschaft (DFG) (FOR1234 iBeetle; BU1443/7-1). Y.H.: Chinese Government Graduate Student Overseas Study Program set by the China Scholarship Council (no. 201206990023), Göttingen University School of Science (GAUSS), Göttingen International and Universitätsbund Göttingen.
References
- 1.Jasper WC, Linksvayer TA, Atallah J, Friedman D, Chiu JC, Johnson BR. 2015. Large-scale coding sequence change underlies the evolution of postdevelopmental novelty in honey bees. Mol. Biol. Evol. 32, 334–346. ( 10.1093/molbev/msu292) [DOI] [PubMed] [Google Scholar]
- 2.Kaessmann H. 2010. Origins, evolution, and phenotypic impact of new genes. Genome Res. 20, 1313–1326. ( 10.1101/gr.101386.109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khalturin K, Hemmrich G, Fraune S, Augustin R, Bosch TCG. 2009. More than just orphans: are taxonomically-restricted genes important in evolution? Trends Genet. 25, 404–413. ( 10.1016/j.tig.2009.07.006) [DOI] [PubMed] [Google Scholar]
- 4.Moczek AP. 2009. On the origins of novelty and diversity in development and evolution: a case study on beetle horns. Cold Spring Harb. Symp. Quant. Biol. 74, 289–296. ( 10.1101/sqb.2009.74.010) [DOI] [PubMed] [Google Scholar]
- 5.Santos ME, Le Bouquin A, Crumière AJJ, Khila A. 2017. Taxon-restricted genes at the origin of a novel trait allowing access to a new environment. Science 358, 386–390. ( 10.1126/science.aan2748) [DOI] [PubMed] [Google Scholar]
- 6.Tautz D, Domazet-Lošo T. 2011. The evolutionary origin of orphan genes. Nat. Rev. Genet. 12, 692–702. ( 10.1038/nrg3053) [DOI] [PubMed] [Google Scholar]
- 7.True JR, Carroll SB. 2002. Gene co-option in physiological and morphological evolution. Annu. Rev. Cell Dev. Biol. 18, 53–80. ( 10.1146/annurev.cellbio.18.020402.140619) [DOI] [PubMed] [Google Scholar]
- 8.Wagner GP, Lynch VJ. 2010. Evolutionary novelties. Curr. Biol. 20, R48–R52. ( 10.1016/j.cub.2009.11.010) [DOI] [PubMed] [Google Scholar]
- 9.Grimaldi D, Engel MS. 2005. Evolution of the insects. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 10.Rainford JL, Hofreiter M, Nicholson DB, Mayhew PJ. 2014. Phylogenetic distribution of extant richness suggests metamorphosis is a key innovation driving diversification in insects. PLoS ONE 9, e109085 ( 10.1371/journal.pone.0109085) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Snodgrass R. 1954. Insect metamorphosis: Smithsonian miscellaneous collections , vol. 122, No. 9, Smithsonian Miscellaneous Collections. Washington, DC: Literary Licensing. [Google Scholar]
- 12.Fristrom D, Fristrom J. 1993. The metamorphic development of the adult epidermis. The development of Drosophila melanogaster, vol. 2 (eds Martinez-Arias Alfonso, Bates Michael), pp. 843–897. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press. [Google Scholar]
- 13.Truman JW, Riddiford LM. 2002. Endocrine insights into the evolution of metamorphosis in insects. Annu. Rev. Entomol. 47, 467–500. ( 10.1146/annurev.ento.47.091201.145230) [DOI] [PubMed] [Google Scholar]
- 14.Arnoult L, Su KFY, Manoel D, Minervino C, Magriña J, Gompel N, Prud'homme B. 2013. Emergence and diversification of fly pigmentation through evolution of a gene regulatory module. Science 339, 1423–1426. ( 10.1126/science.1233749) [DOI] [PubMed] [Google Scholar]
- 15.Gompel N, Prud'homme B, Wittkopp PJ, Kassner VA, Carroll SB. 2005. Chance caught on the wing: cis-regulatory evolution and the origin of pigment patterns in Drosophila. Nature 433, 481–487. ( 10.1038/nature03235) [DOI] [PubMed] [Google Scholar]
- 16.Keys DN, Lewis DL, Selegue JE, Pearson BJ, Goodrich LV, Johnson RL, Gates J, Scott MP, Carroll SB. 1999. Recruitment of a hedgehog regulatory circuit in butterfly eyespot evolution. Science 283, 532–534. ( 10.1126/science.283.5401.532) [DOI] [PubMed] [Google Scholar]
- 17.Reed RD, et al. 2011. Optix drives the repeated convergent evolution of butterfly wing pattern mimicry. Science 333, 1137–1141. ( 10.1126/science.1208227) [DOI] [PubMed] [Google Scholar]
- 18.Medved V, Marden JH, Fescemyer HW, Der JP, Liu J, Mahfooz N, Popadić A. 2015. Origin and diversification of wings: insights from a neopteran insect. Proc. Natl. Acad. Sci. USA 112, 15 946–15 951. ( 10.1073/pnas.1509517112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tomoyasu Y, Arakane Y, Kramer KJ, Denell RE. 2009. Repeated co-options of exoskeleton formation during wing-to-elytron evolution in beetles. Curr. Biol. 19, 2057–2065. ( 10.1016/j.cub.2009.11.014) [DOI] [PubMed] [Google Scholar]
- 20.Tomoyasu Y, Wheeler SR, Denell RE. 2005. Ultrabithorax is required for membranous wing identity in the beetle Tribolium castaneum. Nature 433, 643–647. ( 10.1038/nature03272) [DOI] [PubMed] [Google Scholar]
- 21.Moczek AP, Rose DJ. 2009. Differential recruitment of limb patterning genes during development and diversification of beetle horns. Proc. Natl Acad. Sci. USA 106, 8992–8997. ( 10.1073/pnas.0809668106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wasik BR, Rose DJ, Moczek AP. 2010. Beetle horns are regulated by the Hox gene, Sex combs reduced, in a species- and sex-specific manner. Evol. Dev. 12, 353–362. ( 10.1111/j.1525-142X.2010.00422.x) [DOI] [PubMed] [Google Scholar]
- 23.Glassford WJ, Johnson WC, Dall NR, Smith SJ, Liu Y, Boll W, Noll M, Rebeiz M. 2015. Co-option of an ancestral hox-regulated network underlies a recently evolved morphological novelty. Dev. Cell 34, 520–531. ( 10.1016/j.devcel.2015.08.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Domazet-Loso T, Tautz D. 2003. An evolutionary analysis of orphan genes in Drosophila. Genome Res. 13, 2213–2219. ( 10.1101/gr.1311003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown SJ, Mahaffey JP, Lorenzen MD, Denell RE, Mahaffey JW. 1999. Using RNAi to investigate orthologous homeotic gene function during development of distantly related insects. Evol. Dev. 1, 11–15. ( 10.1046/j.1525-142x.1999.99013.x) [DOI] [PubMed] [Google Scholar]
- 26.Bucher G, Scholten J, Klingler M. 2002. Parental RNAi in Tribolium (Coleoptera). Curr. Biol. 12, R85–R86. ( 10.1016/S0960-9822(02)00666-8) [DOI] [PubMed] [Google Scholar]
- 27.Tomoyasu Y, Denell RE. 2004. Larval RNAi in Tribolium (Coleoptera) for analyzing adult development. Dev. Genes Evol. 214, 575–578. ( 10.1007/s00427-004-0434-0) [DOI] [PubMed] [Google Scholar]
- 28.Berghammer AJ, Klingler M, Wimmer EA. 1999. A universal marker for transgenic insects. Nature 402, 370–371. ( 10.1038/46463) [DOI] [PubMed] [Google Scholar]
- 29.Gilles AF, Schinko JB, Averof M. 2015. Efficient CRISPR-mediated gene targeting and transgene replacement in the beetle Tribolium castaneum. Dev. Camb. Engl. 142, 2832–2839. ( 10.1242/dev.125054) [DOI] [PubMed] [Google Scholar]
- 30.Lorenzen MD, Kimzey T, Shippy TD, Brown SJ, Denell RE, Beeman RW. 2007. piggyBac-based insertional mutagenesis in Tribolium castaneum using donor/helper hybrids. Insect. Mol. Biol. 16, 265–275. ( 10.1111/j.1365-2583.2007.00727.x) [DOI] [PubMed] [Google Scholar]
- 31.Schinko JB, Weber M, Viktorinova I, Kiupakis A, Averof M, Klingler M, Wimmer EA, Bucher G. 2010. Functionality of the GAL4/UAS system in Tribolium requires the use of endogenous core promoters. BMC Dev. Biol. 10, 53 ( 10.1186/1471-213X-10-53) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trauner J, Schinko J, Lorenzen MD, Shippy TD, Wimmer EA, Beeman RW, Klingler M, Bucher G, Brown SJ. 2009. Large-scale insertional mutagenesis of a coleopteran stored grain pest, the red flour beetle Tribolium castaneum, identifies embryonic lethal mutations and enhancer traps. BMC Biol. 7, 73 ( 10.1186/1741-7007-7-73) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dönitz J, Schmitt-Engel C, Grossmann D, Gerischer L, Tech M, Schoppmeier M, Klingler M, Bucher G. 2015. iBeetle-Base: a database for RNAi phenotypes in the red flour beetle Tribolium castaneum. Nucleic Acids Res. 43, D720–D725. ( 10.1093/nar/gku1054) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmitt-Engel C, et al. 2015. The iBeetle large-scale RNAi screen reveals gene functions for insect development and physiology. Nat. Commun. 6, 7822 ( 10.1038/ncomms8822) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hinton HE. 1946. The ‘gin-traps’ of some beetle pupae; a protective device which appears to be unknown. Trans. R. Entomol. Soc. Lond. 97, 473–496. ( 10.1111/j.1365-2311.1946.tb00273.x) [DOI] [Google Scholar]
- 36.Wilson MCL. 1971. The morphology and mechanism of the pupal gin-traps of Tenebrio molitor L. (Coleoptera, Tenebrionidae). J. Stored Prod. Res. 7, 21–30. ( 10.1016/0022-474X(71)90034-8) [DOI] [Google Scholar]
- 37.Hinton HE. 1955. Protective devices of endopterygote pupae. Trans. Soc. Brit. Entomol. 12, 49–92. [Google Scholar]
- 38.Eisner T, Eisner M. 1992. Operation and defensive role of ‘gin traps’ in a coccinellid pupa (Cycloneda Sanguinea). Psyche J. Entomol. 99, 265–273. ( 10.1155/1992/54859) [DOI] [Google Scholar]
- 39.Ichikawa T, Kurauchi T. 2009. Larval cannibalism and pupal defense against cannibalism in two species of tenebrionid beetles. Zool. Sci. 26, 525–529. ( 10.2108/zsj.26.525) [DOI] [PubMed] [Google Scholar]
- 40.Ohde T, Yaginuma T, Niimi T. 2013. Insect morphological diversification through the modification of wing serial homologs. Science 340, 495–498. ( 10.1126/science.1234219) [DOI] [PubMed] [Google Scholar]
- 41.Clark-Hachtel CM, Linz DM, Tomoyasu Y. 2013. Insights into insect wing origin provided by functional analysis of vestigial in the red flour beetle, Tribolium castaneum. Proc. Natl. Acad. Sci. USA 110, 16 951–16 956. ( 10.1073/pnas.1304332110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morata G. 2001. How Drosophila appendages develop. Nat. Rev. Mol. Cell Biol. 2, 89–97. ( 10.1038/35052047) [DOI] [PubMed] [Google Scholar]
- 43.Peel AD, Telford MJ, Akam M. 2006. The evolution of hexapod engrailed-family genes: evidence for conservation and concerted evolution. Proc. R. Soc. B 273, 1733–1742. ( 10.1098/rspb.2006.3497) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Azpiazu N, Morata G. 2000. Function and regulation of homothorax in the wing imaginal disc of Drosophila. Development 127, 2685–2693. [DOI] [PubMed] [Google Scholar]
- 45.Casares F, Mann RS. 2000. A dual role for homothorax in inhibiting wing blade development and specifying proximal wing identities in Drosophila. Development 127, 1499–1508. [DOI] [PubMed] [Google Scholar]
- 46.Wang S-H, Simcox A, Campbell G. 2000. Dual role for Drosophila epidermal growth factor receptor signaling in early wing disc development. Genes Dev. 14, 2271–2276. ( 10.1101/gad.827000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shippy TD, Tomoyasu Y, Nie W, Brown SJ, Denell RE. 2008. Do teashirt family genes specify trunk identity? Insights from the single tiptop/teashirt homolog of Tribolium castaneum. Dev. Genes Evol. 218, 141–152. ( 10.1007/s00427-008-0212-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kitzmann P, Schwirz J, Schmitt-Engel C, Bucher G. 2013. RNAi phenotypes are influenced by the genetic background of the injected strain. BMC Genomics 14, 5 ( 10.1186/1471-2164-14-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Charroux B, Freeman M, Kerridge S, Baonza A. 2006. Atrophin contributes to the negative regulation of epidermal growth factor receptor signaling in Drosophila. Dev. Biol. 291, 278–290. ( 10.1016/j.ydbio.2005.12.012) [DOI] [PubMed] [Google Scholar]
- 50.Erkner A, et al. 2002. Grunge, related to human atrophin-like proteins, has multiple functions in Drosophila development. Development 129, 1119–1129. [DOI] [PubMed] [Google Scholar]
- 51.Zhang Z, Feng J, Pan C, Lv X, Wu W, Zhou Z, Liu F, Zhang L, Zhao Y. 2013. Atrophin–Rpd3 complex represses Hedgehog signaling by acting as a corepressor of CiR. J. Cell Biol. 203, 575–583. ( 10.1083/jcb.201306012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bai H, Palli SR. 2010. Functional characterization of bursicon receptor and genome-wide analysis for identification of genes affected by bursicon receptor RNAi. Dev. Biol. 344, 248–258. ( 10.1016/j.ydbio.2010.05.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baker JD, Truman JW. 2002. Mutations in the Drosophila glycoprotein hormone receptor, rickets, eliminate neuropeptide-induced tanning and selectively block a stereotyped behavioral program. J. Exp. Biol. 205, 2555–2565. [DOI] [PubMed] [Google Scholar]
- 54.Luo C-W, Dewey EM, Sudo S, Ewer J, Hsu SY, Honegger H-W, Hsueh AJW. 2005. Bursicon, the insect cuticle-hardening hormone, is a heterodimeric cystine knot protein that activates G protein-coupled receptor LGR2. Proc. Natl Acad. Sci. USA 102, 2820–2825. ( 10.1073/pnas.0409916102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Daubresse G, Deuring R, Moore L, Papoulas O, Zakrajsek I, Waldrip WR, Scott MP, Kennison JA, Tamkun JW. 1999. The Drosophila kismet gene is related to chromatin-remodeling factors and is required for both segmentation and segment identity. Development 126, 1175–1187. [DOI] [PubMed] [Google Scholar]
- 56.Terriente-Félix A, Molnar C, Gómez-Skarmeta JL, de Celis JF. 2011. A conserved function of the chromatin ATPase Kismet in the regulation of hedgehog expression. Dev. Biol. 350, 382–392. ( 10.1016/j.ydbio.2010.12.003) [DOI] [PubMed] [Google Scholar]
- 57.Kim M, Lee JH, Lee SY, Kim E, Chung J. 2006. Caspar, a suppressor of antibacterial immunity in Drosophila. Proc. Natl. Acad. Sci. USA 103, 16 358–16 363. ( 10.1073/pnas.0603238103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Angelini DR, Smith FW, Jockusch EL. 2012. Extent with modification: leg patterning in the beetle Tribolium castaneum and the evolution of serial homologs. G3 Bethesda 2, 235–248. ( 10.1534/g3.111.001537) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shah MV, Namigai EK, Suzuki Y. 2011. The role of canonical Wnt signaling in leg regeneration and metamorphosis in the red flour beetle Tribolium castaneum. Mech. Dev. 128, 342–358. ( 10.1016/j.mod.2011.07.001) [DOI] [PubMed] [Google Scholar]
- 60.Dai H, Chen Y, Chen S, Mao Q, Kennedy D, Landback P, Eyre-Walker A, Du W, Long M. 2008. The evolution of courtship behaviors through the origination of a new gene in Drosophila. Proc. Natl. Acad. Sci. USA 105, 7478–7483. ( 10.1073/pnas.0800693105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Harpur BA, Kent CF, Molodtsova D, Lebon JM.D., Alqarni AS, Owayss AA, Zayed A. 2014. Population genomics of the honey bee reveals strong signatures of positive selection on worker traits. Proc. Natl. Acad. Sci. USA 111, 2614–2619. ( 10.1073/pnas.1315506111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Khalturin K, Anton-Erxleben F, Sassmann S, Wittlieb J, Hemmrich G, Bosch TC.G. 2008. A novel gene family controls species-specific morphological traits in hydra. PLoS Biol. 6, e278 ( 10.1371/journal.pbio.0060278) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Garver LS, Dong Y, Dimopoulos G. 2009. Caspar controls resistance to Plasmodium falciparum in diverse anopheline species. PLoS Pathog. 5, e1000335 ( 10.1371/journal.ppat.1000335) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ng M, Diaz-Benjumea FJ, Cohen SM. 1995. Nubbin encodes a POU-domain protein required for proximal-distal patterning in the Drosophila wing. Development 121, 589–599. [DOI] [PubMed] [Google Scholar]
- 65.Linz DM, Tomoyasu Y. 2018. Dual evolutionary origin of insect wings supported by an investigation of the abdominal wing serial homologs in Tribolium. Proc. Natl. Acad. Sci. USA 2108, 201711128 ( 10.1073/pnas.1711128115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nielsen C, Martinez P. 2003. Patterns of gene expression: homology or homocracy? Dev. Genes Evol. 213, 149–154. ( 10.1007/s00427-003-0301-4) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
DNA sequences: Genbank accession numbers MH664125-MH664127.