Abstract
Cooperation and division of labour are fundamental in the ‘major transitions’ in evolution. While the factors regulating cell differentiation in multi-cellular organisms are quite well understood, we are just beginning to unveil the mechanisms underlying individual specialization in cooperative groups of animals. Clonal ants allow the study of which factors influence task allocation without confounding variation in genotype and morphology. Here, we subjected larvae and freshly hatched workers of the clonal ant Platythyrea punctata to different rearing conditions and investigated how these manipulations affected division of labour among pairs of oppositely treated, same-aged clonemates. High rearing temperature, physical stress, injury and malnutrition increased the propensity of individuals to become subordinate foragers rather than dominant reproductives. This is reflected in changed gene regulation: early stages of division of labour were associated with different expression of genes involved in nutrient signalling pathways, metabolism and the phenotypic response to environmental stimuli. Many of these genes appear to be capable of responding to a broad range of stressors. They might link environmental stimuli to behavioural and phenotypic changes and could therefore be more broadly involved in caste differentiation in social insects. Our experiments also shed light on the causes of behavioural variation among genetically identical individuals.
Keywords: division of labour, social dominance, environmental stress, clonality, Formicidae
1. Introduction
The ‘major transitions’ in evolution are characterized by cooperation and division of labour among the components of higher level evolutionary units [1–3], e.g. individual cells in multi-cellular organisms, individual zooids in colonial animals and individual insects in insect societies. While the drivers of cellular differentiation in metazoan embryogenesis have been tracked down, e.g. to maternal cytoplasmic determinants or inductive signalling [4], it is less well understood what proximately triggers the functional specialization of the components of higher level transitions. For example, which environmental or social factors influence division of labour among individuals in cooperative groups, and which genes control the early stages of this differentiation?
Division of labour is the fundament of the evolutionary and ecological success of social insects (ants, termites, and social bees and wasps). Insect societies consist of reproductives (queens and, in termites, kings) and non-reproductive workers, which again may specialize in different non-reproductive tasks, such as foraging, nest defence or brood care [5,6]. Social feedback [7] and variation in morphology, age, genotype and personal experience have been shown to affect the propensity of individuals to engage in particular tasks [8]. Nevertheless, similarly efficient division of labour arises in the absence of such variation among morphologically identical full sisters of similar age and experience. In these cases, task allocation has been suggested to be controlled by individual-specific ‘personalities’ (i.e. consistent individual differences in behaviour) or ‘response thresholds’ (i.e. likelihood to respond to stimuli) [9–12], but the causes of such idiosyncrasies remain largely unexplored [12,13]. Clonal ants, in which all nestmates are genetically and morphologically identical, offer a powerful system to study which factors influence task allocation without confounding variation in genotype [14–16].
Most colonies of Platythyrea punctata (Smith F. 1858) lack morphological queens (figure 1a). Instead, a single, socially dominant worker produces new workers from unfertilized eggs by thelytokous parthenogenesis [17] (here, we use ‘worker’ as a morphological rather than functional term irrespective of reproductive status). Because of a greatly reduced recombination rate, all offspring are genetically identical and colonies have a clonal structure [18,19]. The experimental isolation of two young clonemates in a new nest box quickly leads to stable division of labour between a dominant reproductive and a subordinate forager (A. Bernadou, J. Heinze 2014, unpublished observations). This resembles the emergence of division of labour in foundress associations of several wasps [20,21] and ants [22] and allows investigation of the early stages in the development of individual behavioural profiles in insect societies [20].
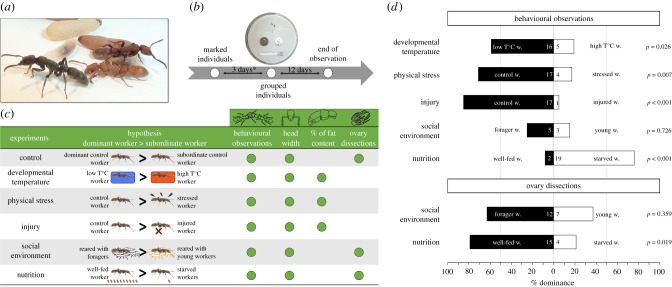
Figure 1.
Workers of the ant P. punctata and experimental treatments. (a) Workers, pupae and larvae of the thelytokous ant P. punctata. Workers change their cuticle colour with age from a yellowish to blackish coloration (worker body length approx. 8 mm). (b) Experimental workflow used in this study. Individuals were marked with a colour code on the day of their eclosion. Three days later, two similarly or differently treated clonemates were placed into a Petri dish with a plaster floor and a cavity serving as nest (temperature, stress, injury). Asterisk indicates for the nutrition and social environment experiment, 3-day-old clonemates were first exposed to different conditions for 10 or 18 days (see main text) before grouping. (c) Experimental manipulations performed, hypotheses tested and the different variables measured for each treatment. (d) Effect of experimental treatments on the likelihood of workers to become dominant or subordinate in dyadic encounters with a same-aged, but differently treated clonemate.
In mammals and fishes, behavioural differences among clonal or otherwise genetically similar individuals have been suggested to arise from influences during development [23–25]. We therefore manipulated larval rearing conditions and ‘early experience’ of clonally identical, same-aged workers of P. punctata to determine how environmental and social factors during early life affect social status and division of labour (figure 1). We hypothesized that unfavourable environmental conditions during development, physical stress, injury, presence of aggressive young nestmates during eclosion and poor nutritional status might decrease the likelihood of an individual assuming reproductive tasks when starting a new colony together with a similarly aged, but oppositely treated clonemate (figure 1c). We complemented our behavioural studies by analysing gene expression changes in the heads of young workers after they had been placed together and started to develop different behavioural profiles.
Our results show that environmental conditions and early experience affect dominance status and division of labour. Matching this, several genes that were differentially expressed have previously been shown in other species to cause phenotypic changes in response to environmental stressors [26,27]. These findings not only give insights into the emergence of division of labour and caste differentiation in social insects in general, but also into factors that might underlie behavioural differentiation among clonally identical animals.
2. Methods
(a). Stock colony origin and maintenance
In April and May 2012, we collected colonies of P. punctata in El Yunque National Forest, Puerto Rico. Thereafter, colonies were kept under near-natural conditions (12 h 26°C/12 h 22°C, 70% humidity) in plastic boxes with a plaster floor in climate chambers. Colonies were fed three times per week with diluted honey, cockroaches and Drosophila ad libitum. The plaster floor was regularly moistened. Behavioural and genomic experiments were done in 2015 and 2016. Prolonged laboratory rearing does not have any visible effect on division of labour. While field colonies occasionally consist of different clonal lineages owing to the adoption of alien workers or the fusion of colonies [28], genetic heterogeneity is quickly lost in the laboratory, as non-reproductive workers have a lifespan of only 200 days [14]. Clonality was confirmed by genotyping a subset of 63 freshly eclosed workers from six colonies at five microsatellite loci (electronic supplementary material, table S1). We used stock colonies containing several dozen workers and brood at all stages.
(b). Basic experimental set-up
To set up the experimental colonies, stock colonies were checked daily over several weeks for newly hatched workers. These were marked individually with Edding® 751 paint markers on the day of eclosion and returned to their colonies to recover. After 3–18 days, depending on treatment, two similarly treated clonemates (control experiments, emerging from the same clonal stock colony) or two differently treated (or to be treated) clonemates (experimental manipulations, emerging from the same or differently treated parts of the same clonal stock colony, see below), which had eclosed within the same 24 h were placed into a Petri dish (figure 1b; Ø 13.5 cm, height 3 cm) with a plaster floor and a cavity covered by a microscope slide and red plastic film serving as nest. We monitored these dyads for aggression (antennal boxing and biting [16,17]) twice daily for 10 min over 12 consecutive days and during the last 6 days of observation also noted the occurrence of self- and allo-grooming, inactivity and time spent outside the nest (20 min day−1 nest−1, 4 h observations per nest in total). To ensure data consistency, all observers received the same instructions and training for the recording of behaviour. The social status of the two clonemates was determined by using the number of days an individual was dominant, i.e. performing most of the aggressive interactions, over the 12 days observation (for details, see the electronic supplementary material).
(c). Hierarchy formation and division of labour among clonemates
We first confirmed that pairs of young, unmanipulated clonemates establish social and reproductive rank orders when placed together (control experiment, n = 25). Subsequently, we determined how different environmental and social conditions affect division of labour among same-aged clonemates. We subjected them to different rearing temperatures during larval development, physical stress, small injuries, different social environment and food availability (figure 1c). In all experiments, we originally set up greater than or equal to 25 dyads, but because a few workers died during the observation period, sample sizes are typically lower.
The effect of temperature during ontogeny was investigated by splitting clonal colonies into two equal fragments and keeping them under constant 22°C and 27°C, respectively, for 22 months (n = 27; mean size of colony fragments ± 95% confidence interval (CI95): 74.7 ± 12.6 and 72.7 ± 11.3 workers for 22°C and 27°C colonies, respectively). Three-day-old workers from low- and high-temperature fragments were paired in a new nest and observed for 12 days following the basic experimental procedure. ‘Stress’, mimicking an attack by another ant, was induced by gently squeezing one of the two paired clonemates with forceps for 1 min twice per day, 15–60 min before each observation (n = 24) throughout the 12 day observation period. Workers were injured by clipping the distal part of the middle leg of one of the two clonemates immediately before putting them together (n = 20). Injuries, such as the loss of antennae or legs, are common in insects in nature. Nutritional status was manipulated by isolating 3-day-old workers in small plastic tubes and providing them with three Drosophila per day for 10 days (30 in total) or three Drosophila only on day 5 (n = 25). After 10 days of this feeding treatment, a well-fed and a starved worker were paired and observed for 12 days (see Basic experimental set-up). Finally, we examined the effect of social environment by keeping a 3-day-old worker for 18 days in a nest containing either six old foragers or six aggressive young workers [16] (n = 20). The 3-day-old focal worker and the six (young or old) workers were simultaneously moved to a new nest. After 18 days, a focal worker raised with young workers and a focal worker raised with old foragers were placed together and observed for 12 days (see Basic experimental set-up).
(d). Body size and nutritional status
After the 12 days observation period, workers were placed individually in Eppendorf cups, killed by freezing, and stored at –20°C, except for those workers that were kept until they started laying eggs (see below and Results). We measured maximum cephalic width on mounted and dried workers under a digital microscope. In addition, as a proxy of nutritional status, we determined abdominal fat content using a petroleum ether extraction protocol (electronic supplementary material) [16].
(e). Ovary dissections
In the experiment with different social environment, social status could not reliably be determined from aggressive interactions. We therefore determined reproductive rank orders by ovary dissection. We also dissected the ovaries of workers from the control and the food-manipulation experiment (cf. Results). In these cases, individuals were kept alive until they started laying eggs. We measured the length of the largest oocyte under a microscope (Zeiss Primo Star). Images were analysed using the free software ImageJ 1.48v.
(f). RNA-seq experiments
To investigate, which early transcriptomic changes are associated with the emergence of division of labour, we set up five experimental dyads in Petri dishes containing two unmanipulated 3-day-old workers each, studied hierarchy formation for 12 days, and thereafter analysed gene expression in the heads of the five dominant and the five subordinate individuals by RNA-seq (electronic supplementary material, tables S2–S4). Differential gene expression analysis was done with DEseq2, using a generalized linear model of the form design = ∼ colony + social status. Further details about sequencing, de novo transcriptome assembly, gene expression analysis and gene ontology enrichment analysis are given in the electronic supplementary material.
(g). Statistical analyses
The analyses were performed using the software R v. 3.2.1 [29]. Data in the text are given as mean ± CI95 or as median and range according to the analysis used. For more detailed methods, see the electronic supplementary material.
3. Results
(a). Hierarchy formation and division of labour among control clonemates
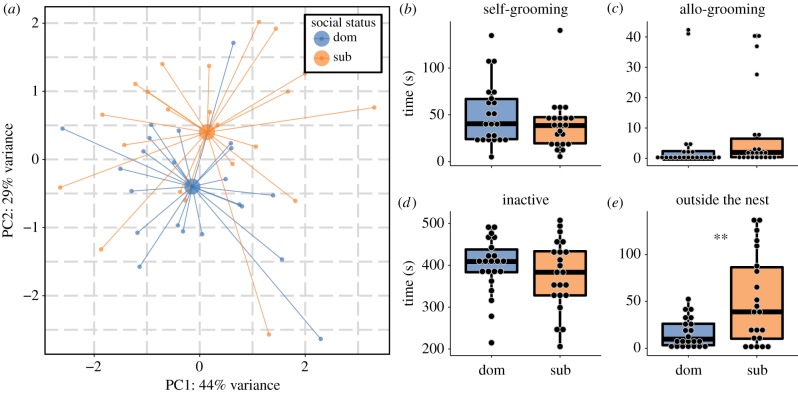
When placed together in pairs, unmanipulated, equally young clonemates of P. punctata rapidly established social and reproductive ranks order by antennal boxing (for all pairs of young workers except one, difference in aggressiveness: binomial test, p < 0.05, n = 22). Social status could not be explained by differences in body size (electronic supplementary material, table S5). The mean durations of the behaviour of individuals were subjected to a principal component analysis (PCA) (figure 2a; electronic supplementary material, table S6). The first two principal components, accounting for 73% of the variance, were retained (eigenvalues > 1). The first axis was positively correlated with self- and allo-grooming and negatively with inactivity; the second axis was positively associated with time spent outside the nest (figure 2a; electronic supplementary material, table S6).
Figure 2.
PCA and boxplots based on the behaviour of dominant and subordinate workers of the ant P. punctata. (a) PCA based on the behaviour of pairs of young workers of identical genotype and age with similar history placed together. The bigger dots in the plot indicate the centroid of each group (n = 22 for each group). (b–e) Four behaviours were recorded for the dominant and subordinate individuals: time spent on self- and allo-grooming inside the nest, time spent inactively inside the nest and time spent outside the nest (**p < 0.01, n = 22 for each group). (Online version in colour.)
The dominance hierarchy led to significant task partitioning between the two clonemates (figure 2a; PERMANOVA: F1,42 = 2.19, p = 0.01) (electronic supplementary material, video S1). The subordinate worker spent significantly more time outside the nest (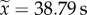 , range: 0.0–137.5 s, n = 22) than the dominant (
, range: 0.0–137.5 s, n = 22) than the dominant (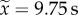 , range: 0.0–52.42 s, n = 22) (figure 2e; paired Wilcoxon rank-sum test: V = 32, p = 0.003). Dominant workers had also a tendency to be more inactive inside the nest than subordinate workers (mean time inactive ± CI95: 399.12 ± 30.11 and 377.52 ± 36.57 s for dominant and subordinate workers, respectively; n = 22) (figure 2d; paired t-test: t = 1.91, p = 0.06).
, range: 0.0–52.42 s, n = 22) (figure 2e; paired Wilcoxon rank-sum test: V = 32, p = 0.003). Dominant workers had also a tendency to be more inactive inside the nest than subordinate workers (mean time inactive ± CI95: 399.12 ± 30.11 and 377.52 ± 36.57 s for dominant and subordinate workers, respectively; n = 22) (figure 2d; paired t-test: t = 1.91, p = 0.06).
To confirm that the behavioural dominance observed during the first 12 days leads to reproductive division of labour, 17 colonies were kept for an additional three to six weeks to allow workers to lay eggs. The six other colonies were frozen after the 12 days and the workers were genotyped (electronic supplementary material, table S1). The worker categorized as dominant after the 12 day observation period had better developed ovaries than the subordinate (oocyte length, 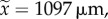 range: 346–1472 µm and
range: 346–1472 µm and 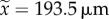 , range: 0.0–1060 µm for dominant and subordinate workers, respectively, n = 14, as several workers had died; paired Wilcoxon rank-sum test: V = 105, p < 0.001).
, range: 0.0–1060 µm for dominant and subordinate workers, respectively, n = 14, as several workers had died; paired Wilcoxon rank-sum test: V = 105, p < 0.001).
(b). Division of labour and experimental manipulations
In subsequent experiments, we explored what might cause the specialization into reproductive and non-reproductive individuals (figure 1c). As in the control experiment, pairs of differently treated clonemates engaged rapidly in antennal boxing (total 4715 cases among 116 pairs, observation time 464 h) and occasional biting (22 cases). From the distribution of antennal boxing and/or ovarian status, we could determine the social and reproductive rank relationships between clonemates in 98 of these pairs (for all pairs: binomial test, p < 0.05). Across all treatments, workers showed a similar division of labour as in the control colonies (results; electronic supplementary material, figure S1 and table S6).
(c). Effect of treatment on social status
We next investigated in which way treatment affected division of labour (figure 1d). Rearing temperature during larval development strongly influenced the propensity of workers to become dominant. The clonemate experiencing low rearing temperatures (22°C versus 27°C) became dominant in 16 of 21 dyads in which rank relations could be determined (binomial test, 16 versus 5: p = 0.026; generalized linear mixed model (GLMM), z = −3.211, p = 0.001).
Stressing workers by gently squeezing them twice per day with forceps negatively affected their status. Rank relationships could be determined in 21 of 24 dyads, in 17 of which the non-stressed worker became dominant (binomial test, 17 versus 4: p = 0.007; GLMM, z = 3.682, p < 0.001).
Similarly, an injury strongly decreased the likelihood of workers to become dominant. A significant difference in aggressive behaviour was found in 18 of 20 pairs of young workers. The unharmed ant became dominant in 17 of 18 dyads (binomial test, 17 versus 1: p < 0.001; GLMM, z = 3.894, p < 0.001).
Among the workers that had experienced different social environments, we could determine rank relationships from aggression in only eight of 20 colonies. In five of these, the worker reared with foragers was significantly more aggressive than the worker reared with nurses (binomial test, 5 versus 3: p = 0.726; GLMM, z = 0.989, p = 0.323). To obtain additional information about division of labour, we waited until workers had laid eggs. Workers reared with foragers had better developed ovaries than their clonemates reared with young workers in 12 of 19 colonies (binomial test, 12 versus 7: p = 0.359; GLMM, z = 1.603, p = 0.109; one pair was discarded because ovary dissection failed) (mean oocyte length ± CI95: 840 ± 200 and 290 ± 150 µm for workers reared with foragers and young individuals, respectively, n = 12).
Finally, in the nutrition experiment, antennal boxing differed significantly between clonemates in 21 of 25 pairs. In contrast with the other treatments, workers with poor nutritional status engaged more in antennal boxing than well-fed workers (binomial test, 19 versus 2: p < 0.001; GLMM, z = −4.283, p < 0.001). We therefore kept these colonies until eggs had been laid. Well-fed clonemates had better developed ovaries than starved workers in 15 of the 19 dyads (binomial test, 15 versus 4: p = 0.019; GLMM, z = 3.322, p < 0.001; mean oocyte length ± CI95: 920 ± 120 versus 280 ± 117 µm, n = 15). In six colonies, one or both workers died and no eggs were laid.
(d). Effect of experimental treatment on body size and fat content
To determine whether manipulation might have influenced social status indirectly via other traits, we investigated head width and fat content of clonemates. Head width did not differ significantly between dominant and subordinate clonemates in the injury, stress, nutrition and social environment treatment (electronic supplementary material, table S5). By contrast, temperature during larval development can affect adult body size [30]. Indeed, workers reared at lower temperature were on average 3% larger than workers reared at higher temperature, i.e. lower rearing temperature might have indirectly affected social status by increasing body size.
Fat content did not differ between dominant and subordinate workers in the temperature and social environment experiments, but unstressed, dominant workers had a higher fat content than stressed subordinates (electronic supplementary material, table S5), indicating that physical stress prevented workers from building up fat stores. Fat content could not be determined when workers were dissected.
(e). RNA-seq experiments
Our behavioural results suggested that molecular mechanisms underlying hierarchy formation and division of labour comprise changes in pathways pertaining to nutrition, stress and social behaviour. To test this assumption, we compared gene expression in heads of individuals from five unmanipulated clonal pairs after 12 days of hierarchy formation.
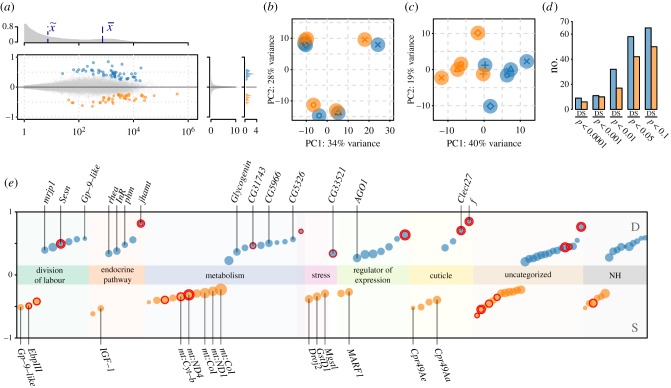
The transcriptomic study generated between 26 and 39 million reads per sample that were jointly assembled into 162 673 de novo transcripts. Gene-level expression analysis on 75 977 assembled transcript clusters (genes) showed mild divergence in expression profiles between dominant and subordinate individuals (figure 3a). PCA revealed a strong colony effect on gene expression patterns (figure 3b). After correcting for this effect, samples were separated according to hierarchy by PC1 (figure 3c). One hundred genes showed differential expression (false discovery rate (FDR) < 0.05) in the dominant versus subordinate comparison with log2 fold changesDom/Sub ranging from −0.64 to 0.85 (figure 3a,d; electronic supplementary material, table S2). Of these, 58 genes were upregulated in dominants compared to subordinates (figure 3d,e).
Figure 3.
Comparison of head gene expression in 14-day-old dominant and subordinate workers of P. punctata. (a) Overview of average expression levels (x-axis) and logarithmic expression fold changes (log2, dominant versus subordinate, y-axis) for each gene. Coloured dots show genes with significant overexpression at FDR < 0.05 in dominant (blue) and subordinate workers (orange). The top density plot shows the distribution of gene expression levels (mean expression 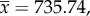 median expression
median expression 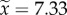 ), the density plots to the right show the distribution of expression differences. (b,c) PCA results without (b) and with correcting (c) for a colony effect. The plot shows PCs 1 and 2 for the variance stabilizing transformed expression levels of the top 500 genes with the highest variance in the dataset. Dominant (blue) and subordinate (orange) individuals from the same colony are plotted with the same symbol. (d) Overview of the number of genes called to be differentially expressed at different FDR cut-offs. D, dominant (blue); S, subordinate (orange). (e) Detailed summary of the 100 genes differentially expressed (FDR < 0.05) between heads of subordinate and dominant workers. Genes were sorted in four a priori and two a posteriori defined functional groups (see main text). Genes that could not be grouped into either of these categories (uncategorized) and genes without homology (NH) to known proteins from D. melanogaster, A. mellifera or H. saltator are shown separately. Dot sizes are proportional to the base mean (mean of normalized counts of all samples). Genes overexpressed in dominants or subordinates are depicted in blue and orange, respectively. Expression fold change is shown on the y-axis. FDR values below 1 × 10−3 and 1 × 10−5 are indicated by one or two red circles, respectively. Labelled genes are discussed in the results and discussion in more detail.
), the density plots to the right show the distribution of expression differences. (b,c) PCA results without (b) and with correcting (c) for a colony effect. The plot shows PCs 1 and 2 for the variance stabilizing transformed expression levels of the top 500 genes with the highest variance in the dataset. Dominant (blue) and subordinate (orange) individuals from the same colony are plotted with the same symbol. (d) Overview of the number of genes called to be differentially expressed at different FDR cut-offs. D, dominant (blue); S, subordinate (orange). (e) Detailed summary of the 100 genes differentially expressed (FDR < 0.05) between heads of subordinate and dominant workers. Genes were sorted in four a priori and two a posteriori defined functional groups (see main text). Genes that could not be grouped into either of these categories (uncategorized) and genes without homology (NH) to known proteins from D. melanogaster, A. mellifera or H. saltator are shown separately. Dot sizes are proportional to the base mean (mean of normalized counts of all samples). Genes overexpressed in dominants or subordinates are depicted in blue and orange, respectively. Expression fold change is shown on the y-axis. FDR values below 1 × 10−3 and 1 × 10−5 are indicated by one or two red circles, respectively. Labelled genes are discussed in the results and discussion in more detail.
Homology to proteins from Drosophila melanogaster, Apis mellifera and the ant Harpegnathos saltator was inferred for 84 of the 100 differentially expressed genes (DEGs) and used to assign putative functional annotations and gene names (electronic supplementary material, table S2). Based on these, DEGs were manually grouped in four a priori defined categories (division of labour, endocrine pathway, metabolism and stress) and two categories emerging from manual annotation (regulator of expression, cuticle). The four a priori categories were chosen based on the behavioural experiments (metabolism, stress [26,27]) or previous knowledge about social insect caste differentiation (division of labour, endocrine pathway [26,27]). We could assign 57 DEGs to one of these six categories, 43 genes remained uncategorized (16 without inferred homologues) (figure 3e). We emphasize that this grouping is not intended to imply statistically significant over-representation of DEGs in either of these categories but for jointly discussing sets of DEGs in a functional context.
Full information on DEGs can be found in figure 3 and electronic supplementary material, tables S2–S4. Here, we highlight only several particularly interesting results. Of the 20 genes in the largest category (metabolism), two homologues to lipid metabolic genes (CG5966, CG5326) and two homologues to carbohydrate metabolic genes (glycogenin, CG31743) were upregulated in dominants. By contrast, several mitochondrial respiratory chain genes (mt:Cyt-b, mt:ND4, mt:ND1, mt:Col) were upregulated in subordinates, suggesting an increased demand on energy generation in these individuals. In the category endocrine pathway, homologues of the insulin receptor InR, the InR-binding protein rhea, the juvenile hormone (JH) methyl transferase jhamt and the ecdysone metabolic gene phantom [31] were upregulated in dominants. In subordinates, a homologue of the insulin receptor substrate IGF-1 was upregulated, matching previous studies that JH and insulin-like signalling (ILS) affect division of labour in social insects [26,27,32,33].
Similarly, nine additional DEGs found in P. punctata had previously been implied to be associated with division of labour in other social insects (category division of labour). Homologues of the odorant-binding protein Gp-9, sestrin and major royal jelly protein 1 (MRJP1) were upregulated in dominants while ejaculatory-bulb-specific protein 3 (EbpIII) was upregulated in subordinates [26,34–36]. We also detected differential expression in two small-ncRNA-binding transcriptional regulators (AGO1 and CG17018, a homologue to Meiosis Arrest Female 1 (MARF1)), which were grouped in the category regulator of expression together with transcription factors and transcription factor interacting proteins.
The enrichment analyses suggest that transcripts involved in oxidation–reduction processes and transmembrane proteins were over-represented in our DEG set (electronic supplementary material, results).
4. Discussion
Clonemates of the ant P. punctata in dyadic encounters readily form a rank order, which is tightly associated with division of labour between a dominant reproductive and a subordinate forager. This highlights that genetically identical individuals may differ strikingly in behaviour and adds to the increasing interest in behavioural individuality in clones [23–25,37]. Recent studies suggested that ‘factors unfolding or emerging during development contribute to individual differences in structural brain plasticity and behaviour’ [24,25]. By manipulating the rearing conditions of clonemates during development or shortly after eclosion, we could identify environmental or social factors that affected an individual's probability of taking over a particular task.
Rearing temperature, stress, injury and nutritional status influenced whether a clonemate became a dominant reproductive or a subordinate forager in dyadic encounters, while previous social environment played only a limited role. The transcriptomes of workers shortly after hierarchy establishment corroborate these results: several genes that were differently expressed between subordinates and dominants link environmental stimuli with physiological responses and/or belong to key pathways that affect caste differentiation in other social insects. In the following, we discuss our results in more detail.
Lower rearing temperature may have increased the likelihood of becoming dominant indirectly via an effect on body size. In many ectotherms, slower growth rate at lower temperature leads to larger body size [30]. We could not identify an advantage of larger body size in contests among workers from control colonies, but body size differences were considerably larger in the temperature experiment than in control colonies. Larger body size has been shown to give an advantage in dominance contests in several social and solitary insects [38–41]. Alternatively, temperature during larval development may have affected division of labour indirectly via other physiological parameters. For example, honeybee workers exposed to higher temperatures during pupal development started to forage earlier and recruited more nestmates [42,43], and pre-imaginal temperature affected the temperature preferences for brood location of brood-tending workers in the ant Camponotus rufipes [44].
Regularly opening the nest and squeezing one individual with forceps apparently disturbed both nestmates, as indicated by both dominant and subordinate workers having a lower fat content than in other experiments. Despite the short duration of the manipulation, squeezed workers obviously suffered more than their clonemates, documented by a more significant reduction in fat content and a lower propensity to become dominant. Squeezing with forceps resembles dominance grabbing and pulling, thus directly or indirectly (via ‘loser effects’ [45]) reducing the fighting ability of stressed workers. Alternatively, squeezing by itself may have negatively influenced the worker's ‘emotional state’, as suggested to explain why vigorously shaking honeybee workers causes them to classify neutral stimuli as predicting punishment [46].
Small injuries and the associated immune challenge may direct resources from reproduction and dominance to the activation of the immune system [47,48] and may also affect aggressiveness [49]. In ants, injuries lead to temporarily decreased fecundity [50] and precocious foraging [51]. The trade-off between establishing dominance and immune defence may explain why almost none of the injured P. punctata workers became dominant.
Workers that had experienced poor nutritional conditions were less likely to become reproductive than their well-fed clonemates. Nutritional status, in particular fat content, has been suggested to be a mechanism for division of labour in social insects [52,53], in that low lipid stores predispose individuals to forage. Interestingly, from the direction of antennal boxing alone, we would have assumed that poorly fed P. punctata are dominant over well-fed clonemates, contrasting the different times the two clonemates spent outside the nest and also opposite to previous findings on hierarchy formation in ants. The discrepancy is probably resolved by the fact that food begging may also involve rapid antennation in many insects, including species related to P. punctata [54,55].
Surprisingly, social environment during early adulthood had no or only a small effect on later division of labour. We had expected that the clonemate kept with young workers would rarely become dominant because young workers rapidly form social hierarchies, into which the young clonemate would have had to integrate. By contrast, old foragers only slowly begin to lay eggs when all younger individuals are removed [16]. The absence of a significant effect of social environment on division of labour in our dyads might indicate that a dominant individual had already been established in several forager-only colonies. The low aggressiveness in this experiment might be a consequence of the long duration of the exposure to different social environments: one individual might have secured its dominant status already before the start of the experiment and later signalled its status via pheromones rather than defending it aggressively [56].
The results of the transcriptomic analysis complement the insights from our behavioural experiments according to which differences in nutritional and immune state and individual stress level govern division of labour in P. punctata. Intriguingly, these differences appear to translate into social status and division of labour by recruiting genomic pathways known to be involved in caste differentiation in social insect larvae. The period following hierarchy formation in adult P. punctata might not only conceptually be analogous to the caste differentiation period in eusocial insect development. Rather, both mechanisms appear to rely on similar molecular toolkits to establish division of labour in the colony. By altering rearing conditions, we probably induced changes in these molecular pathways, eventually affecting the outcome of hierarchy formation.
Several of the genes differentially expressed between dominant reproductives and subordinate foragers, e.g. IGF-1, InR, rhea and sestrin, are part of or interact with the target of rapamycin (TOR) and ILS pathways. These pathways are known to respond to oxidative and social stress [57], nutritional [58] and immune status [48] and in turn affect morphology, reproduction and lifespan [59,60]. The over-representation of transcripts involved in oxidation–reduction processes could be linked to oxidative stress and therefore imply the possibility that dominant and subordinate workers will experience different lifespans. TOR and ILS are key for the induction of queen development in honeybee larvae and hierarchy formation in the ant Diacamma sp. by regulating juvenile hormone levels [61,62]. The differential expression of a central JH-metabolic enzyme (jhamt) suggests the involvement of JH also in hierarchy formation and/or division of labour in P. punctata. In the wasp Synoeca surinama, adult JH titres are associated with the development of functional ovaries but not with the expression of dominance [63]. Similarly, JH-application induces early wing loss in female sexuals of the ant H. saltator, but treated individuals fail to lay eggs earlier than control individuals [64].
While mostly recognized as a moulting hormone, ecdysteroids also play a role in the adult brain [65]. The upregulation of a phantom-homologue—an ecdysteroid metabolic enzyme—in dominant P. punctata might point towards a role for ecdysteroids in regulating neuronal changes associated with dominance. Similar to ecdysteroids, MRJP1 is involved in both larval development and the regulation of adult behaviour and learning [66,67]. In the honeybee, MRJP1 is essential for the development of queen- and worker-destined larvae by exerting growth-factor-like functions on Egfr signalling pathways [68]. Furthermore, MRJP1 is more strongly expressed in the brains of adult honeybee nurses than those of foragers [69,70]. This resembles the higher expression in dominants of P. punctata.
Several found DEGs have not yet been associated with caste differentiation or task allocation in social Hymenoptera. The differential expression of odorant-binding proteins in P. punctata suggests a so far unappreciated role in division of labour [71]. Genes coding for cuticular proteins (Cpr49Ae, Cpr49Aa) and other cuticle-associated proteins (e.g. forked or Clect27) were also differentially expressed, indicating that task allocation may involve specialization of the cuticle towards a reproductive or a foraging role.
One particularly curious finding is the different expression of two genes coding for small-ncRNA-binding proteins, AGO1 and MARF1. These proteins are recognized for their general role in transcriptional silencing and epigenetic regulation [72–74]. Their differential expression between subordinate and dominant individuals might encourage future studies on non-coding transcriptional regulators in hierarchy formation and caste differentiation of social insects.
5. Conclusion
What do these results mean for task allocation? The importance of genotype, experience and age for the specialization in particular tasks is well known, but efficient division of labour can also develop in single-age cohorts composed of genetically very similar individuals. Our study shows that, temperature gradients in the nest, physical stress, injuries and food availability during larval development or shortly after eclosion may affect behaviour and thus underlie different ‘response thresholds’ or ‘personalities’. The differential expression analysis during the early phase of division of labour in P. punctata indicates the involvement of genes from several molecular pathways, known to link environmental stimuli to behavioural and phenotypic changes and to be involved in caste differentiation in social insect larvae [26,27]. Interestingly, many of these genes appear to be confer responses to a broad range of environmental stressors [26,27]. Together with the data from our experimental manipulations, these results suggest that different environmental cues can affect division of labour via a small set of molecular pathways.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank Bartosz Walter, Marion Fuessl, Tina Wanke, Katrin Kellner and Jon N. Seal for their help in collecting the ants, Bartosz Walter and Bert Rivera Marchand for their help with obtaining permits. We thank Helena Lowack and Andreas Trindl for RNA extractions and for help in the wet laboratory, Agnes Paech for help with morphological measurements.
Ethics
Collecting colonies in Puerto Rico was permitted by USDA Forest Service and Departamento de Recursos Naturales y Ambientales, 2012-IC-036.
Data accessibility
Data available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.9f33sq1 [75].
Authors' contributions
A.B. and J.H. designed the study. A.B., J.P. and E.H. performed the experiments. A.B., L.S., K.M. and J.H. analysed and interpreted the data. A.B., L.S. and K.M. prepared the figures and tables. A.B., L.S. and J.H. discussed the results; A.B. and J.H. wrote the paper with support of L.S and K.M. All authors gave final approval for publication.
Competing interests
We have no competing interests.
Funding
The study was supported by DFG grants no. He1623/33, KO 1895/20-1 and He1623/37 (FOR 2218).
References
- 1.Buss LW. 1988. The evolution of individuality. Princeton, NJ: Princeton University Press. [Google Scholar]
- 2.Smith JM, Szathmary E. 1998. The major transitions in evolution. Oxford, UK: Oxford University Press. [Google Scholar]
- 3.Michod RE. 2000. Darwinian dynamics: evolutionary transitions in fitness and individuality. Princeton, NJ: Princeton University Press. [Google Scholar]
- 4.Gilbert SF, Barresi MJ. 2016. Developmental biology, 11th edn Sunderland, MA: Sinauer Associates. [Google Scholar]
- 5.Hölldobler B, Wilson EO. 2009. The superorganism: the beauty, elegance, and strangeness of insect societies. New York, NY: W.W. Norton & Company. [Google Scholar]
- 6.Bourke AFG. 2011. Principles of social evolution. Oxford, UK: Oxford University Press. [Google Scholar]
- 7.Gordon DM. 1996. The organization of work in social insect colonies. Nature 380, 121–124. ( 10.1038/380121a0) [DOI] [Google Scholar]
- 8.Ravary F, Lecoutey E, Kaminski G, Châline N, Jaisson P. 2007. Individual experience alone can generate lasting division of labor in ants. Curr. Biol. 17, 1308–1312. ( 10.1016/j.cub.2007.06.047) [DOI] [PubMed] [Google Scholar]
- 9.Théraulaz G, Bonabeau E, Deneubourg JL. 1998. Response threshold reinforcement and division of labour in insect societies. Proc. R. Soc. Lond. B 265, 327–332. ( 10.1098/rspb.1998.0299) [DOI] [Google Scholar]
- 10.Dall SRX, Bell AM, Bolnick DI, Ratnieks FLW. 2012. An evolutionary ecology of individual differences. Ecol. Lett. 15, 1189–1198. ( 10.1111/j.1461-0248.2012.01846.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jandt JM, Bengston S, Pinter-Wollman N, Pruitt JN, Raine NE, Dornhaus A, Sih A. 2014. Behavioural syndromes and social insects: personality at multiple levels. Biol. Rev. 89, 48–67. ( 10.1111/brv.12042) [DOI] [PubMed] [Google Scholar]
- 12.Jeanson R, Weidenmüller A. 2014. Interindividual variability in social insects: proximate causes and ultimate consequences. Biol. Rev. 89, 671–687. ( 10.1111/brv.12074) [DOI] [PubMed] [Google Scholar]
- 13.Toth AL, Rehan SM. 2017. Molecular evolution of insect sociality: an eco-evo-devo perspective. Annu. Rev. Entomol. 62, 419–442. ( 10.1146/annurev-ento-031616-035601) [DOI] [PubMed] [Google Scholar]
- 14.Hartmann A, Heinze J. 2003. Lay eggs, live longer: division of labor and life span in a clonal ant species. Evolution 57, 2424–2429. ( 10.1554/03-138) [DOI] [PubMed] [Google Scholar]
- 15.Oxley P, Ji L, Fetter-Pruneda I, McKenzie SK, Li C, Hu H, Zhang G, Kronauer DJ. 2014. The genome of the clonal raider ant (Cerapachys biroi), a new model system for social evolution and behavior. Curr. Biol. 24, 451–458. ( 10.1016/j.cub.2014.01.018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bernadou A, Busch J, Heinze J. 2015. Diversity in identity: behavioral flexibility, dominance, and age polyethism in a clonal ant. Behav. Ecol. Sociobiol. 69, 1365–1375. ( 10.1007/s00265-015-1950-9) [DOI] [Google Scholar]
- 17.Heinze J, Hölldobler B. 1995. Thelytokous parthenogenesis and dominance hierarchies in the ponerine ant, Platythyrea punctata. Naturwissenschaften 82, 40–41. ( 10.1007/BF01167871) [DOI] [Google Scholar]
- 18.Schilder K, Heinze J, Gross R, Hölldobler B. 1999. Microsatellites reveal clonal structure of populations of the thelytokous ant Platythyrea punctata (F. Smith) (Hymenoptera; Formicidae). Mol. Ecol. 8, 1497–1507. ( 10.1046/j.1365-294x.1999.00727.x) [DOI] [PubMed] [Google Scholar]
- 19.Kellner K, Heinze J. 2011. Mechanism of facultative parthenogenesis in the ant Platythyrea punctata. Evol. Ecol. 25, 77–89. ( 10.1007/s10682-010-9382-5) [DOI] [Google Scholar]
- 20.West MJ. 1967. Foundress associations in polistine wasps: dominance hierarchies and the evolution of social behaviour. Science 157, 1584–1585. ( 10.1126/science.157.3796.1584) [DOI] [PubMed] [Google Scholar]
- 21.Brahma A, Mandal S, Gadagkar R. 2018. Emergence of cooperation and division of labor in the primitively eusocial wasp Ropalidia marginata . Proc. Natl Acad. Sci. USA 115, 756–761. ( 10.1073/pnas.1714006115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kolmer K, Heinze J. 2000. Rank orders and division of labour among unrelated cofounding ant queens. Proc. R. Soc. Lond. B 267, 1729–1734. ( 10.1098/rspb.2000.1202) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Archer GS, Friend TH, Piedrahita J, Nevill CH, Walker S. 2003. Behavioral variation among cloned pigs. Appl. Anim. Behav. Sci. 81, 321–331. ( 10.1016/S0168-1591(02)00272-1) [DOI] [Google Scholar]
- 24.Freund J, Brandmaier AM, Lewejohann L, Kirste I, Kritzler M, Krüger A, Sachser N, Lindenberger U, Kempermann G. 2013. Emergence of individuality in genetically identical mice. Science 340, 756–759. ( 10.1126/science.1235294) [DOI] [PubMed] [Google Scholar]
- 25.Bierbach D, Laskowski KL, Wolf M. 2017. Individual differences in behaviour of clonal fish arise despite near-identical rearing conditions. Nat. Commun. 8, 15361 ( 10.1038/ncomms15361) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Corona M, Libbrecht R, Wheeler DE. 2016. Molecular mechanisms of phenotypic plasticity in social insects. Curr. Opin. Insect. Sci. 13, 55–60. ( 10.1016/j.cois.2015.12.003) [DOI] [PubMed] [Google Scholar]
- 27.Weitekamp CA, Libbrecht R, Keller L. 2017. Genetics and evolution of social behavior in insects. Annu. Rev. Genet. 51, 219–239. ( 10.1146/annurev-genet-120116-024515) [DOI] [PubMed] [Google Scholar]
- 28.Kellner K, Barth B, Heinze J. 2000. Colony fusion causes within-colony variation in a parthenogenetic ant. Behav. Ecol. Sociobiol. 64, 737–746. ( 10.1007/s00265-009-0891-6) [DOI] [Google Scholar]
- 29.R Development Core Team. 2014. R: a language and environment for statistical computing. See http://www.R-project.org/.
- 30.Atkinson D, Sibly RM. 1997. Why are organisms usually bigger in colder environments? Making sense of a life history puzzle. Trends Ecol. Evol. 12, 235–239. ( 10.1016/S0169-5347(97)01058-6) [DOI] [PubMed] [Google Scholar]
- 31.Warren JT, et al. 2004. Phantom encodes the 25-hydroxylase of Drosophila melanogaster and Bombyx mori: a P450 enzyme critical in ecdysone biosynthesis. Insect Biochem. Mol. Biol. 34, 991–1010. ( 10.1016/j.ibmb.2004.06.009) [DOI] [PubMed] [Google Scholar]
- 32.Corona M, Velarde RA, Remolina S, Moran-Lauter A, Wang Y, Hughes KA, Robinson GE. 2007. Vitellogenin, juvenile hormone, insulin signaling, and queen honey bee longevity. Proc. Natl Acad. Sci. USA 104, 7128–7133. ( 10.1073/pnas.0701909104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manfredini F, Brown MJ, Toth AL.. 2018. Candidate genes for cooperation and aggression in the social wasp Polistes dominula. J. Comp. Physiol. A 204, 449–463. ( 10.1007/s00359-018-1252-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steller MM, Kambhampati S, Caragea D. 2010. Comparative analysis of expressed sequence tags from three castes and two life stages of the termite Reticulitermes flavipes. BMC Genomics 11, 463 ( 10.1186/1471-2164-11-463) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Antikainen H, Driscoll M, Haspel G, Dobrowolski R. 2017. TOR-mediated regulation of metabolism in aging. Aging Cell 16, 1219–1233. ( 10.1111/acel.12689) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walsh JT, Warner MR, Kase A, Cushing BJ, Linksvayer TA. 2018. Ant nurse workers exhibit behavioural and transcriptomic signatures of specialization on larval stage. Anim. Behav. 141, 161–169. ( 10.1016/j.anbehav.2018.05.015) [DOI] [Google Scholar]
- 37.Schuett W, Dall SR, Baeumer J, Kloesener MH, Nakagawa S, Beinlich F, Eggers T. 2011. Personality variation in a clonal insect: the pea aphid, Acyrthosiphon pisum. Dev. Psychobiol. 53, 631–640. ( 10.1002/dev.20538) [DOI] [PubMed] [Google Scholar]
- 38.O'Neill KM. 1983. The significance of body size in territorial interactions of male beewolves (Hymenoptera: Sphecidae. Philanthus). Anim. Behav. 31, 404–411. ( 10.1016/S0003-3472(83)80059-1) [DOI] [Google Scholar]
- 39.Alcock J. 1996. The relation between male body size, fighting, and mating success in Dawson's burrowing bee, Amegilla dawsoni (Apidae, Apinae. Anthophorini). J. Zool. 239, 663–674. ( 10.1111/j.1469-7998.1996.tb05469.x) [DOI] [Google Scholar]
- 40.Turillazzi S, Pardi L. 1977. Body size and hierarchy in polygynic nests of Polistes gallicus (L) (Hymenoptera Vespidae). Monit. Zool. Ital. 11, 101–112. [Google Scholar]
- 41.Heinze J, Oberstadt B. 1999. Worker age, size and social status in queenless colonies of the ant Leptothorax gredleri. Anim. Behav. 58, 751–759. ( 10.1006/anbe.1999.1188) [DOI] [PubMed] [Google Scholar]
- 42.Tautz J, Maier S, Groh C, Rossler W, Brockmann A. 2003. Behavioral performance in adult honey bees is influenced by the temperature experienced during their pupal development. Proc. Natl Acad. Sci. USA 100, 7343–7347. ( 10.1073/pnas.1232346100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Becher MA, Scharpenberg H, Moritz RFA. 2009. Pupal developmental temperature and behavioral specialization of honeybee workers (Apis mellifera L). J. Comp. Physiol. A 195, 673–679. ( 10.1007/s00359-009-0442-7) [DOI] [PubMed] [Google Scholar]
- 44.Weidenmüller A, Mayr C, Kleineidam CJ, Roces F. 2009. Preimaginal and adult experience modulates the thermal response behavior of ants. Curr. Biol. 19, 1897–1902. ( 10.1016/j.cub.2009.08.059) [DOI] [PubMed] [Google Scholar]
- 45.Hsu Y, Wolf LL. 2001. The winner and loser effect: what fighting behaviours are influenced? Anim. Behav. 61, 777–786. ( 10.1006/anbe.2000.1650) [DOI] [Google Scholar]
- 46.Bateson M, Desire S, Gartside SE, Wright GA. 2011. Agitated honeybees exhibit pessimistic cognitive biases. Curr. Biol. 21, 1070–1073. ( 10.1016/j.cub.2011.05.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moret Y, Schmid-Hempel P. 2000. Survival for immunity: the price of immune system activation for bumblebee workers. Science 290, 1166–1168. ( 10.1126/science.290.5494.1166) [DOI] [PubMed] [Google Scholar]
- 48.Schwenke RA, Lazzaro BP, Wolfner MF. 2016. Reproduction-immunity trade-offs in insects. Annu. Rev. Entomol. 61, 239–256. ( 10.1146/annurev-ento-010715-023924) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Adamo SA, Gomez-Juliano A, LeDue EE, Little SN, Sullivan K. 2015. Effect of immune challenge on aggressive behaviour: how to fight two battles at once. Anim. Behav. 105, 153–161. ( 10.1016/j.anbehav.2015.04.018) [DOI] [Google Scholar]
- 50.von Wyschetzki K, Lowack H, Heinze J. 2016. Transcriptomic response to injury sheds light on the physiological costs of reproduction in ant queens. Mol. Ecol. 25, 1972–1985. ( 10.1111/mec.13588) [DOI] [PubMed] [Google Scholar]
- 51.Moroń D, Witek M, Woyciechowski M. 2008. Division of labour among workers with different life expectancy in the ant Myrmica scabrinodis. Anim. Behav. 75, 345–350. ( 10.1016/j.anbehav.2007.06.005) [DOI] [Google Scholar]
- 52.Toth AL, Kantarovich S, Meisel AF, Robinson GE. 2005. Nutritional status influences socially regulated foraging ontogeny in honey bees. J. Exp. Biol. 208, 4641–4649. ( 10.1242/jeb.01956) [DOI] [PubMed] [Google Scholar]
- 53.Smith CR, Suarez AV, Tsutsui ND, Wittman SE, Edmonds B, Freauff A, Tillberg CV. 2011. Nutritional asymmetries are related to division of labor in a queenless ant. PLoS ONE 6, e24011 ( 10.1371/journal.pone.0024011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hashimoto Y, Yamauchi K, Hasegawa E. 1995. Unique habits of stomodeal trophallaxis in the ponerine ant Hypoponera sp. Insectes Soc. 42, 137–144. ( 10.1007/BF01242450) [DOI] [Google Scholar]
- 55.Liebig J, Heinze J, Hölldobler B. 1997. Trophallaxis and aggression in the ponerine ant, Ponera coarctata: implications for the evolution of liquid food exchange in the hymenoptera. Ethology 103, 707–722. ( 10.1111/j.1439-0310.1997.tb00180.x) [DOI] [Google Scholar]
- 56.Hartmann A, d'Ettorre P, Jones GR, Heinze J. 2005. Fertility signaling: the proximate mechanism of worker policing in a clonal ant. Naturwissenschaften 92, 282–286. ( 10.1007/s00114-005-0625-1) [DOI] [PubMed] [Google Scholar]
- 57.Giannakou ME, Goss M, Jacobson J, Vinti G, Leevers SJ, Partridge L. 2007. Dynamics of the action of dFOXO on adult mortality in Drosophila. Aging Cell 6, 429–438. ( 10.1111/j.1474-9726.2007.00290.x) [DOI] [PubMed] [Google Scholar]
- 58.Stanley PD, Ng'oma E, O'Day S, King EG. 2017. Genetic dissection of nutrition-induced plasticity in insulin/insulin-like growth factor signaling and median lifespan in a Drosophila multiparent population. Genetics 206, 587–602. ( 10.1534/genetics.116.197780) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brogiolo W, Stocker H, Ikeya T, Rintelen F, Fernandez R, Hafen E. 2001. An evolutionarily conserved function of the Drosophila insulin receptor and insulin-like peptides in growth control. Curr. Biol. 11, 213–221. ( 10.1016/S0960-9822(01)00068-9) [DOI] [PubMed] [Google Scholar]
- 60.Badisco L, Van Wielendaele P, Broeck JV. 2013. Eat to reproduce: a key role for the insulin signaling pathway in adult insects. Front. Physiol. 4, 202 ( 10.3389/fphys.2013.00202) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mutti NS, Dolezal AG, Wolschin F, Mutti JS, Gill KS, Amdam GV. 2011. IRS and TOR nutrient-signaling pathways act via juvenile hormone to influence honey bee caste fate. J. Exp. Biol. 214, 3977–3984. ( 10.1242/jeb.061499) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Okada Y, Watanabe T, Tin MMY, Tsuji K, Mikheyev AS. 2017. Social dominance alters nutrition-related gene expression immediately: transcriptomic evidence from a monomorphic queenless ant. Mol. Ecol. 26, 2922–2938. ( 10.1111/mec.13989) [DOI] [PubMed] [Google Scholar]
- 63.Kelstrup HC, Hartfelder K, Nascimento FS, Riddiford LM. 2014. The role of juvenile hormone in dominance behavior, reproduction and cuticular pheromone signaling in the caste-flexible epiponine wasp, Synoeca surinama. Front. Zool. 11, 78 ( 10.1186/s12983-014-0078-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Penick CA, Liebig J, Brent CS. 2011. Reproduction, dominance, and caste: endocrine profiles of queens and workers of the ant Harpegnathos saltator. J. Comp. Physiol. A 197, 1063–1071. ( 10.1007/s00359-011-0667-0) [DOI] [PubMed] [Google Scholar]
- 65.Ishimoto H, Kitamoto T. 2010. The steroid molting hormone ecdysone regulates sleep in adult Drosophila melanogaster. Genetics 185, 269–281. ( 10.1534/genetics.110.114587) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Drapeau MD, Albert S, Kucharski R, Prusko C, Maleszka R. 2006. Evolution of the yellow/major royal jelly protein family and the emergence of social behavior in honey bees. Genome Res. 16, 1385–1394. ( 10.1101/gr.5012006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Buttstedt A, Moritz RF, Erler S. 2013. More than royal food: major royal jelly protein genes in sexuals and workers of the honeybee Apis mellifera. Front. Zool. 10, 72 ( 10.1186/1742-9994-10-72) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kamakura M. 2011. Royalactin induces queen differentiation in honeybees. Nature 473, 478–483. ( 10.1038/nature10093) [DOI] [PubMed] [Google Scholar]
- 69.Peixoto LG, Calábria LK, Garcia L, Capparelli FE, Goulart LR, de Sousa MV, Espindola FS. 2009. Identification of major royal jelly proteins in the brain of the honeybee Apis mellifera. J. Insect. Physiol. 55, 671–677. ( 10.1016/j.jinsphys.2009.05.005) [DOI] [PubMed] [Google Scholar]
- 70.Hojo M, Kagami T, Sasaki T, Nakamura J, Sasaki M. 2010. Reduced expression of major royal jelly protein 1 gene in the mushroom bodies of worker honeybees with reduced learning ability. Apidologie 41, 194–202. ( 10.1051/apido/2009075) [DOI] [Google Scholar]
- 71.Pelosi P, Zhou JJ, Ban LP, Calvello M. 2006. Soluble proteins in insect chemical communication. Cell. Mol. Life Sci. 63, 1658–1676. ( 10.1007/s00018-005-5607-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hutvagner G, Simard MJ. 2008. Argonaute proteins: key players in RNA silencing. Nat. Rev. Mol. Cell. Biol. 9, 22–32. ( 10.1038/nrm2321) [DOI] [PubMed] [Google Scholar]
- 73.Wu L, Zhou H, Zhang Q, Zhang J, Ni F, Liu C, Qi Y. 2010. DNA methylation mediated by a microRNA pathway. Mol. Cell. 38, 465–475. ( 10.1016/j.molcel.2010.03.008) [DOI] [PubMed] [Google Scholar]
- 74.Su YQ, Sugiura K, Sun F, Pendola JK, Cox GA, Handel MA, Schimenti JC, Eppig JJ. 2012. MARF1 Regulates essential oogenic processes in mice. Science 335, 1496–1499. ( 10.1126/science.1214680) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bernadou A, Schrader L, Pable J, Hoffacker E, Meusemann K, Heinze J. 2018. Data from: Stress and early experience underlie dominance status and division of labour in a clonal insect Dryad Digital Repository. ( 10.5061/dryad.9f33sq1) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Bernadou A, Schrader L, Pable J, Hoffacker E, Meusemann K, Heinze J. 2018. Data from: Stress and early experience underlie dominance status and division of labour in a clonal insect Dryad Digital Repository. ( 10.5061/dryad.9f33sq1) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.9f33sq1 [75].