Abstract
Forest ecosystems are an integral component of the global carbon cycle as they take up and release large amounts of C over short time periods (C flux) or accumulate it over longer time periods (C stock). However, there remains uncertainty about whether and in which direction C fluxes and in particular C stocks may differ between forests of high versus low species richness. Based on a comprehensive dataset derived from field-based measurements, we tested the effect of species richness (3–20 tree species) and stand age (22–116 years) on six compartments of above- and below-ground C stocks and four components of C fluxes in subtropical forests in southeast China. Across forest stands, total C stock was 149 ± 12 Mg ha−1 with richness explaining 28.5% and age explaining 29.4% of variation in this measure. Species-rich stands had higher C stocks and fluxes than stands with low richness; and, in addition, old stands had higher C stocks than young ones. Overall, for each additional tree species, the total C stock increased by 6.4%. Our results provide comprehensive evidence for diversity-mediated above- and below-ground C sequestration in species-rich subtropical forests in southeast China. Therefore, afforestation policies in this region and elsewhere should consider a change from the current focus on monocultures to multi-species plantations to increase C fixation and thus slow increasing atmospheric CO2 concentrations and global warming.
Keywords: BEF-China, carbon storage, carbon flux, ecosystem functioning, evergreen broad-leaved forest, forest biodiversity
1. Introduction
As an integral component of the global carbon cycle, forests store about 45% of terrestrial carbon (C) and account for a net sink of 1.1 Pg C yr−1 [1,2]. Regulating atmospheric C concentration by preserving and enhancing forest C stocks has been recognized as a major political target to mitigate global climate change [3]. In particular, reliable benchmark estimates of forest carbon stocks and knowledge how forest C density can be promoted are required for the successful implementation of forest management strategies [4]. In this context, tree diversity, typically measured as tree species richness per area, is regarded as an important component influencing forest productivity and carbon storage at global and local scales [5,6]. However, our knowledge about the significance of tree species richness for forest C cycling is still limited and largely concentrated on above-ground assessments [5,7].
Global syntheses have confirmed that plant species richness in general has positive effects on plant productivity and other components of the C cycle [8,9]. Previous research conducted in tree plantations [10,11] and forests [5,7,12,13] suggests that the results from grassland ecosystems might also apply to forest ecosystems. Several mechanisms of how species richness may affect ecosystem productivity, and thereby the size of ecosystem C stocks and the balance between C gains and losses have been identified. Besides selection effects, niche complementarity and biotic and abiotic facilitation are major mechanisms underlying biodiversity–ecosystem functioning (BEF) relationships [14]. Selection effects result from the increasing chance that well-performing and dominant species driving ecosystem functions are present in diverse communities [15]. According to the niche complementarity hypothesis [16], species richness promotes resource use and nutrient retention and thus allows larger C stocks per area [17]. It has also been suggested that variability of C stocks and fluxes should decrease with species richness, resulting in a more predictable balance between C gains and losses over longer time periods [18,19]. Facilitation occurs when a species enhances the performance of another species in a plant community [20]. Biotic facilitation comprises, for example, enemy and pathogen dilution effects when trees grow better in species-rich forests due to lower encounter and transmission rates of herbivores [21] and plant pathogens [22]. By contrast, abiotic facilitation refers to an improved abiotic environment for plant growth, which can be related, for example, to better nutrient availability or microclimatic conditions [20].
Tree species richness has been shown to positively affect above-ground stand productivity [23], above-ground tree C storage [24], leaf litter production [25], litter decomposition [26] or soil C storage [13]. Whereas stand productivity, including leaf- and root-litter production, and tree C storage can be directly influenced by tree species richness, the size of other C stocks such as those in soil and litter are rather controlled by the balance between litter input and decomposition dynamics. There is evidence that richness has the potential to positively influence C fluxes in and out of forest ecosystems, but it is not clear if these positive effects on fluxes cancel out and leave multiple components of C stocks unchanged in their sum [27] or increase them [24]. For example, the balance could be shifted if species richness has a stronger influence on decomposition dynamics and consequently carbon loss than on C capture.
To understand how tree species richness influences total C storage, it is crucial to consider as many of the major components of the C cycle as possible. Furthermore, because many previous studies focused on intensively managed forests [10,11], with richness levels rarely exceeding four tree species, earlier results might only be transferable to a limited extent to more species-rich forests [28,29]. That biodiversity effects on above-ground biomass are prevalent even at high richness levels has been recently shown by several studies [12,30]. In a subtropical tree-biodiversity experiment above-ground wood productivity was 122% higher in the most species-rich communities composed of 24 tree species than in the average monoculture [31]. In that analysis, stand productivity increased linearly with the logarithm of species richness, indicating a flattening of the strength of richness effects, possibly due to increasing functional redundancy among species [32]. Weak or no BEF relationships have also been observed in some studies in old-growth tropical forests, which suggested that these relationships can also depend on spatial scale [33–35].
Besides species richness, stand age is often considered an even more important factor driving C-cycling processes in forest ecosystems. During secondary forest succession, C accumulates in above- and below-ground C stocks while stand productivity often declines because of increased mortality and declining tree densities [36]. Because age and richness may be correlated, effects of the two may be confounded, such that richness effects are in part explained by age effects and the other way round [32]. Furthermore, age and richness may interactively affect ecosystem functioning [37]. For example, assuming low functional redundancy between species in early successional forests, an increase in richness might have stronger effects on productivity than in late successional forests with high functional redundancy. Only by resolving the relative effects of tree species richness and stand age on C-cycling processes, reasonable predictions about the role of species richness during forest succession are possible.
Subtropical broad-leaved forests in East Asia are a hotspot of tree species richness but still underrepresented in C-cycle research. Owing to large afforestation programmes, forest cover in this region is increasing, thus representing an important C sink in China [38]. However, most of current afforestation focuses on monoculture plantations [39], and it remains unknown if this can compensate for previous C losses due to earlier deforestation of species-rich forest. Using a comparative study approach, we estimated the effects of species richness and stand age on multiple components of the C cycle (six components of C stocks and four components of C fluxes) and the resulting total forest C stock in a subtropical forest in southeast China. Our study included 27 forest plots established along crossed gradients in richness and age to estimate multiple components of C stocks and fluxes. We address the following overall research question: how are total and component carbon stocks and fluxes controlled by tree species richness and stand age? Specifically, we test the following hypotheses. Hypothesis H1 refers to effects of species richness: richness promotes component C stocks (H1a) and C fluxes (H1b), with a positive effect on total forest C storage (H1c). We expect that the positive effect of richness on C gain (tree above-ground C increment) outweighs richness effects on C loss (respiration, decomposition), resulting in an overall positive richness effect on forest C storage. Hypothesis H2 refers to effects of stand age: component C stocks (H2a) and C fluxes (H2b) are larger in old than in young stands, with a positive effect of stand age on total forest C storage (H2c). To conclude, we compare results of our study with C stocks of conventional single-species plantations in subtropical China to estimate potential C gains and financial assets under a multi-species planting scheme.
2. Material and methods
(a). Study site
The study was conducted within the Gutianshan National Nature Reserve (GNNR) in Zhejiang Province, southeast China (29°14' N, 118°07' E) GNNR covers an area of about 81 km2. The region is characterized by a warm-temperate climate with distinct seasonality [40]. A large portion of the GNNR is covered by mixed evergreen broad-leaved subtropical forest of advanced and young successional stages harbouring a total of 1426 plant species belonging to 648 genera and 149 families. The most prevalent soils of GNNR are sandy-loamy and silty-loamy acidic Cambisols developed on granite or on saprolite. More site information is provided by Bruelheide et al. [40].
In 2008, 27 plots of 30 × 30 m distributed within an area of 33 km2 were established to assess variables related to ecosystem C-cycling along crossed gradients of species richness (3–20 tree species per plot) and stand age (22–116 years). This plot size was chosen as representative for this forest ecosystem with a canopy height of less than 30 m; that is, we considered C-cycling variables as scale-invariant above this plot size. Larger plots would have increased the precision of measurements (and thus reduced the error variation) but would not have allowed us to measure as many as 27 plots across the same total area. More importantly, because of the species–area relationship, richness is not scale-invariant and increases continually with plot size, so that correlations with ecosystem variables also change. We found these correlations to be strongest for plot sizes of 30 × 30 and 40 × 40 m using data from a nearby 24 ha permanent forest plot [41], consistent with the assumption that this was a relevant ecosystem scale. For each plot, richness was determined as the number of species with at least one tree individual with diameter at breast height greater than 10 cm, whereas stand age was determined by tree ring analysis based on the age of the fifth-largest tree in each plot [40]. The different stand ages were due to different times since the last agricultural use or tree harvesting before the Nature Reserve was established [40]. Despite the current protection status, we observed that in two of the 27 selected plots, some trees were harvested, leading to the exclusion of these two plots from some analyses (table 1).
Table 1.
Definitions of variables of C stocks and fluxes. Particularly time-consuming measurements (Root_C and Rs) were assessed on a subset of plots; in two plots, Tree_AGC, AGC_F and derived variables could not be assessed because farmers harvested some trees.
| abbreviation | definition | measuring period | no. repeated measures | no. plots | |
|---|---|---|---|---|---|
| C stocks (Mg C ha−1) | Tree_AGC Herb_AGC Litter_C DW_C Root_C SOC |
above-ground tree C above-ground herb C litter-layer C deadwood C root C soil C |
2008 2008 2009–2010 2009 2008–2009 2008 |
1 1 4 1 1 1 |
25 27 27 27 15 27 |
| AGC_total GC_total BGC_total |
total above-ground C (Tree_AGC + Herb_AGC) total ground-layer C (Litter_C + DW_C) total below-ground C (Coarseroot Ca + SOC) |
2008 2009–2010 2008 |
1 1 1 |
25 27 25 |
|
| Total_C | total C stock | 2008–2010 | 1 | 25 | |
| C fluxes (Mg C ha−1 yr−1) | AGC_F Litterfall_F CWD_F Rs |
above-ground tree C increment total litterfall flux coarse woody debris flux soil respiration |
2008–2010 2009–2014 2009–2015 2009–2012 |
2 6 2 4 |
25 27 27 16 |
aHere, the BGC total only included coarse-root C, not fine roots and herb roots, to maximize the number of plots for the below-ground calculation.
(b). Quantification of C stocks and fluxes
To estimate C stocks (expressed in Mg C ha−1), we included six components and grouped them into three layers (table 1). These components were above-ground C in trees (Tree_AGC), above-ground C in herbs (Herb_AGC), forest-floor litter-layer C (Litter_C), deadwood C (DW_C), root C (Root_C) and soil C (SOC). The layers were above-ground total C (AGC_total = Tree_AGC + Herb_AGC), ground total C (GC_total = Litter_C + DW_C) and below-ground total C (BGC_total = Root_C + SOC). Finally, the six component stocks were summed up to an estimate of the total C stock (Total_C). C fluxes (expressed in Mg C ha−1 yr−1) were calculated from repeated measurements of tree sizes, soil respiration, litter production and coarse deadwood decomposition. The four components were above-ground C increment in trees (AGC_F), litterfall C flux (Litterfall_F), coarse woody debris C flux (CWD_F) and soil respiration (Rs) (table 1).
Detailed information of the field measurement and estimation method for each component of C stocks and fluxes is provided in the electronic supplementary material (Material and methods).
(c). Measurements of environmental covariates
According to previous studies and the local situation, we considered elevation, slope, aspect and soil pH as covariates in our study, because they potentially have strong impacts on C cycling processes. Plot elevation ranged from 251 to 903 m.a.s.l. Because aspect is a circular variable, we used cosine and sine of aspect as northness and eastness, respectively. Soil pH was determined potentiometrically in a 1 : 2.5 soil–water suspension for the top 5 cm of the mineral soil. We then used a principal component analysis (PCA) to extract orthogonal axes of these five environmental covariates. At last, the first axis with highest loading from soil pH and elevation was used to characterize environmental variation among plots for subsequent analyses.
(d). Statistical analyses
We used several approaches to explain variation in the dependent variables, C stocks and fluxes, as a function of the explanatory variables, species richness, stand age and environmental variation. All these analyses were based on regression. We first applied hierarchical partitioning to separate the amount of variation explained by the different explanatory variables when they were all considered together in multiple regression (R package ‘hier.part’ [42]). Second, we used linear regression to determine how the two main explanatory variables, richness and age, affected the dependent variables individually. Third, we used multiple regression with richness and age to inspect the richness effects on C stocks and fluxes at different ages and to inspect the age effects at different richness levels. We did not include the environmental variation in the multiple regression analyses presented in the main text because its influence was generally small and plots were not deliberately selected to represent environmental variation (but see electronic supplementary material, table S2 for an analysis including environmental variation). To display richness effects, we plotted—along with the regression lines from the linear regression—the partial regression lines from the multiple regression for the three ages 37, 64 and 95 years (figures 3 and 4). These are the mean ages for eight plots classified as young forests, nine plots classified as medium forests and 10 plots classified as old forests, respectively. A similar approach was used to display age effects (see electronic supplementary material, figure S1). Fourth, we tested for interactions between richness and age in multiple regression analyses but because these were never significant we did not include them in the final models. All values of C stocks and fluxes in all analyses except the benchmarking (figure 1) were log-transformed to meet the normality assumption. At last, we used structural equation models as implemented in the R package ‘lavaan’ [43] to explore hypothesized causal relationships between variables and derive path-analytic diagrams (see electronic supplementary material, figure S3). For these analyses, we included the first principal component (PC1) from the environmental PCA as covariate. All analyses were conducted with the R statistical software [44].
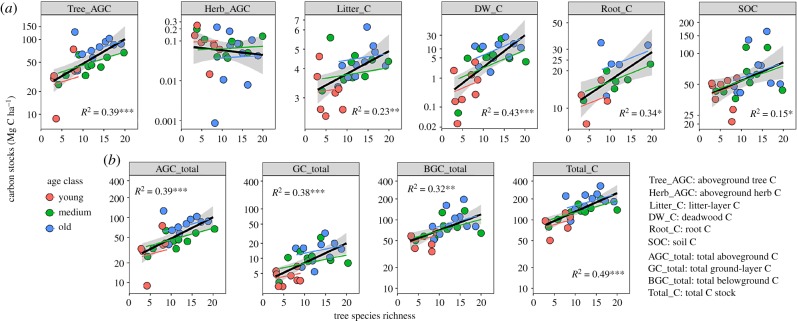
Figure 3.
Relationships between C stocks and tree species richness for (a) six components and (b) three layers and total C. Each dot represents a plot. Significant R2-values from linear regressions are shown (***p < 0.001, **p < 0.01, *p < 0.05). Partial regression lines for three levels of stand ages (young, medium, old) are shown in different colours. Logarithmic scales are used for all dependent variables (y-axes). (Online version in colour.)
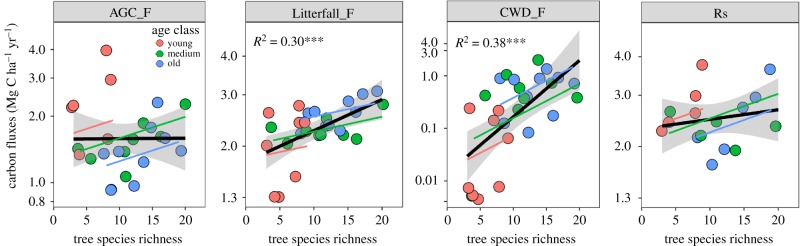
Figure 4.
Relationships between the four C fluxes and tree species richness. Each dot represents a plot. Significant R2-values from linear regressions are shown (***p < 0.001, **p < 0.01). Partial regression lines for three levels of stand ages (young, medium, old) are shown in different colours. Logarithmic scales are used for all dependent variables (y-axes). (Online version in colour.)
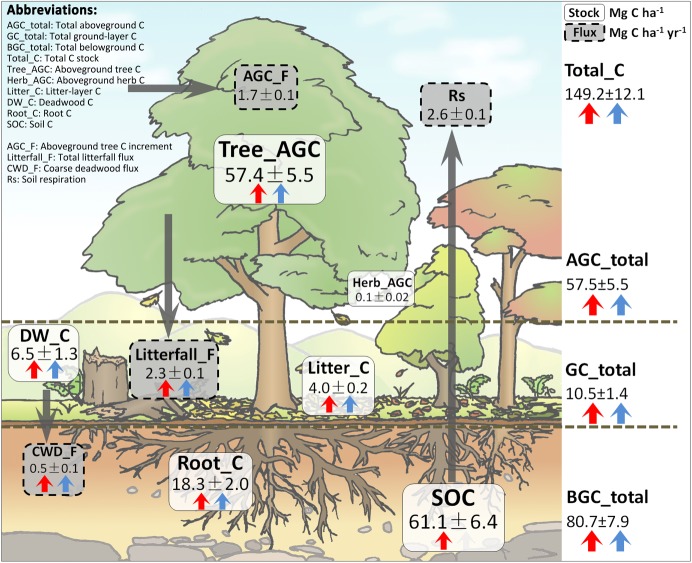
Figure 1.
Benchmarking map of carbon stocks (solid white boxes) and fluxes (dashed grey boxes) averaged across all 27 forest stands. Numbers represent means and standard errors. Different colours of arrows indicate effects of tree species richness (red) and stand age (blue). Directions of arrows show positive (upward) and negative (downward) relationships of richness and age with respective C stocks and fluxes. No arrows are shown when effects were not significant.
3. Results
(a). Benchmarking of C stocks
Overall, the mean Total_C across all 27 plots was 149.2 Mg C ha−1, with AGC_total, GC_total and BGC_total contributing 57.5, 10.5 and 80.7 Mg C ha−1, respectively (figure 1). On average, the largest amount of C was stored in the soil (SOC, 61.1 Mg C ha−1), followed by Tree_AGC (57.4 Mg C ha−1, figure 1). The other C stocks ranking after these two were Root_C (18.3 Mg C ha−1) > DW_C (6.5 Mg C ha−1) > Litter_C (4.0 Mg C ha−1) > Herb_AGC (0.1 Mg C ha−1). More C was generally stored below than above ground (figure 1).
(b). Hierarchical partitioning of variation in C stocks and fluxes due to species richness, stand age and environmental variation
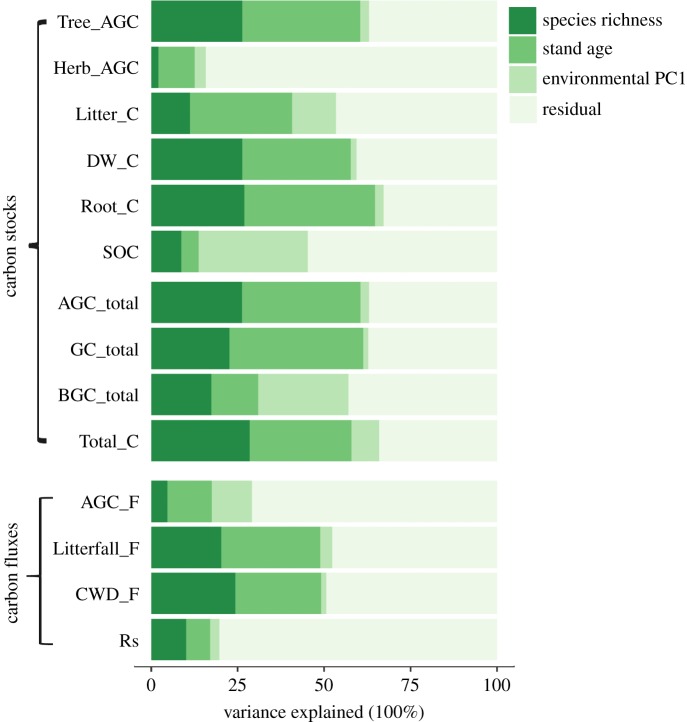
The amount of variation in each C component, C layer, total C and C flux explained by species richness, stand age and environmental variation is shown in figure 2 and electronic supplementary material, table S1. For C components, richness played a strong role in explaining variation in Tree_AGC, DW_C and Root_C, but played a lesser role in Herb_AGC and SOC. In comparison with richness, age accounted for a slightly higher proportion of variation in these components. For Total_C, richness explained about the same proportion (28.5%) of variation as age did (29.4%). Overall, richness and age together explained around 50% of the variation in all carbon stocks except Herb_AGC, SOC and BGC_total. The variation attributed to the environment was generally low except for SOC and BGC_total (ranging from 1.7 to 31.6%). With regard to carbon fluxes, the effects of richness and age were both large and similar for Litter_F (20.2% versus 28.7%) and CWD_F (24.3% versus 24.8%). However, richness and age could only explain a small proportion in the variation of the productivity-related component fluxes AGC_F and Rs. The environment explained from 1.4 to 11.6% of the variation in component C fluxes.
Figure 2.
Hierarchical partitioning of the variation explained for each component and layer of carbon stocks and fluxes by species richness, stand age and environmental variation. The term for environmental variation was obtained as principal component axis (environmental PC1) of an ordination that incorporated elevation, slope, eastness of aspect, northness of aspect and soil pH at the 27 plots. (Online version in colour.)
(c). Effects of species richness and stand age on C stocks (H1a, c and 2a, c)
All components of C stocks except Herb_AGC increased significantly with increasing species richness (H1a, figure 3a). Likewise, C stocks were larger in older than in younger stands except for Herb_AGC and SOC (H2a, electronic supplementary material, figure S1a), which showed marginally negative and marginally positive trends with age, respectively. Estimated AGC_total, GC_total, BGC_total and, in particular, Total_C were larger in more species-rich and older compared with less species-rich and younger stands (figure 3b; electronic supplementary material, figure S1b; H1c and H2c). Partial regressions predicted that the relationship between richness and C stocks was positive for given age levels (figure 3b), suggesting an independent positive effect of richness increasing C stocks in young, medium and old forests.
(d). Effects of species richness and stand age on C fluxes (H1b and 2b)
Two of the C fluxes, Litterfall_F and CWD_F, increased significantly with increasing species richness (H1b) and these effects were consistently positive across stand ages (figure 4). Although AGC_F and Rs did not significantly change with richness in simple linear regressions, the partial regressions predicted increasing C flux within given age levels with increasing richness (figure 4). This result was due to the negative effect of age on AGC_F and Rs (electronic supplementary material, figure S1c), which counterbalanced the positive effect of richness in the simple linear regression. The other two components of C fluxes, Litterfall_F and CWD_F, were larger in older than in younger stands.
(e). Structural equation modelling
With the structural equation models using the overall correlation matrix between variables (electronic supplementary material, figure S2), we first found that AGC_F was indeed influenced by all three explanatory variables, but with a negative effect of age balancing the positive effect of richness (electronic supplementary material, figure S3a). AGC_F then increased Tree_AGC, but richness still had an additional strongly positive effect on Tree_AGC, indicating that the total richness effect was only partly mediated by the indirect effect via AGC_F (electronic supplementary material, figure S3b). Richness also increased Root_C, which was correlated with Tree_AGC. In contrast with these direct effects, the significant effects of richness on litterfall and deadwood variables found in the regression analyses mentioned above appeared to be indirect, i.e. working via the increased Tree_AGC which in turn promoted litterfall and deadwood variables (electronic supplementary material, figure S3c,d). For SOC and Rs, we could not find good explanations in the form of path analyses, indicating that these were affected by other unmeasured variables. In the case of SOC, the influence of environmental variation was particularly strong (figure 2).
4. Discussion
Total ecosystem C in the subtropical forest studied here was 149 Mg C ha−1 across all forest stands. Similar or higher C stocks have been reported from other subtropical forests in China [45,46]. Across all forest stands, we found that most C was stored in tree standing biomass with tree above-ground and root C contributing 57 and 18 Mg C ha−1, respectively. Soil as the second largest C stock after tree biomass accounted for 41% of total ecosystem C stock, which was similar to other studies [47]. Whereas previous studies have focused on above-ground tree biomass or soil C storage, our study demonstrates that roots, ground litter (4 Mg C ha−1) and deadwood (6.5 Mg C ha−1) represent major components of C stocks and thus should not be neglected in forest inventories [48].
(a). Effects of species richness on C storage
Our research underlines the importance of tree species richness as a driver of the C cycle in the studied subtropical forest and provides comprehensive evidence for diversity-mediated C sequestration above and below ground. Using a study design with plots selected to represent a range of species richness levels and stand ages, we found that higher richness was associated not only with larger C stocks (H1a) and C fluxes (H1b) in these stands but also with larger total C storage (H1c). To a certain extent, these richness effects were correlated with effects of age, because these two explanatory variables were not fully orthogonal. Thus, partial regression coefficients, i.e. effects of richness for plots of given age, were usually somewhat smaller than linear regression coefficients, except for two components of C fluxes (AGC_F and Rs), where the opposite was the case (figures 3 and 4). Beyond this, the richness effects on C stocks and fluxes did not change with age, that is none of the interactions between these two explanatory variables were significant, indicating that richness and age had additive effects on C stocks and fluxes. Previous studies using sample-survey designs, that is with most plots having intermediate richness, also found positive relationships with measures of above-ground forest productivity [5,23].
As summarized by Poorter et al. [12], tree species richness could promote higher stand productivity and thus higher accumulated tree C stocks by niche complementarity or sampling-/selection-/insurance-type effects. For our study site, there is evidence that higher richness allows both a higher stem density and larger tree sizes [49], and thus higher tree above-ground C stocks per unit area, probably as a result of reduced interspecific competition due to complementary resource use between species [47]. Complementary resource use between tree species may be related to below-ground root complementarity, as observed in a manipulative biodiversity experiment near the field site of the present study [50], or to above-ground crown complementarity allowing higher canopy packing and greater light interception in more diverse forest stands [51].
We showed that higher richness promotes C stocks of deadwood and the litter layer, which is probably a result of a generally higher tree biomass translating into more litter and deadwood. Among the components of C stocks that we studied, only the herb layer (Herb_AGC) was negatively related to tree species richness (figure 3a), which was likely caused by increased competition from trees [52].
Although AFG_F and soil respiration increased with richness for plots of given age (figure 4), the linear regressions of these two dependent variables against richness were not significant because these components of C fluxes were smaller in older than in younger stands (electronic supplementary material, figure S1c) and there were more species-rich old than young stands among the 27 study plots. Overall, as shown by the results of path analyses, the positive effect of richness and the negative effect of age on AFG_F were nearly counterbalanced (see electronic supplementary material, figure S3a). This suggests that increased richness may slow down or even prevent declines of stand AFG_F in older forest. Autotrophic respiration by roots can be a major contributor to soil respiration, thus allowing richness to affect soil respiration by increased root biomass and productivity [53]. The significantly higher C fluxes via litter production and deadwood decomposition in more species-rich plots were probably the result of diversity-promoted C stocks in living and dead biomass as indicated by path analyses (see electronic supplementary material, figure S3c,d).
Although we could not relate tree species richness to whole-ecosystem productivity because we did not account for below-ground productivity (root growth, root exudates), our data indicate that richness promoted a positive balance between C gains and losses over time. It has previously been argued that richness might primarily affect C fluxes, whereas the total C stock would be less responsive [27]. In this perspective, increased C uptake would be compensated by higher respiratory C loss, thereby leaving ecosystem C storage unchanged. By contrast, our results indicate that richness effects on C stocks can be stronger than richness effects on C fluxes. Thus, our results suggest that for the studied subtropical forests, richness does not simply speed up the C cycle but also allows the forests to retain a larger amount of C in C storage.
(b). Effects of stand age on C storage
Accumulations of C stocks with forest age are well studied [54], even though rarely corrected for correlated effects of increasing species richness with increasing age. In our study, we analysed both overall (linear regressions) and richness-corrected (partial regression coefficients) effects of forest age on C stocks (H2a), C fluxes (H2b) and the total C accumulation (H2c) and found that in part the effects of stand age were correlated with effects of richness, again because these two explanatory variables were not fully orthogonal (see electronic supplementary material, figure S1). Our plots represented a chronosequence from 22- to 116-year-old stands. Obviously, forests could potentially get much older, but no older forests could be found in the study region due to past agricultural land use. Over the observed forest-age gradient, C stocks in live trees (Tree AGC and Root_C) were 122% larger in the oldest stands than in the youngest ones, and soil C was 72% larger, strongly suggesting that these C stocks increase as a forest ages. Large C accumulation in regenerating forests have also been found in other studies [45,54], underlining the large C storage potential of subtropical forests in China. For example, total ecosystem C increased from 101.4 Mg C ha−1 in a young (18 years) to 260.2 Mg C ha−1 in a 60-year-old subtropical forest [46].
Tang et al. [55] found that young forests allocate assimilated C predominantly to tree biomass, whereas in old forests, soil is the most important C stock. This is in contrast to our study, where the larger total C stocks in older stands were underpinned by stronger age-effects on component stocks in tree biomass than in soil, although all components except Herb_AGC were larger in older than in younger stands (see electronic supplementary material, figure S1). It is conceivable that with further increases in age a similar change as the one described by Tang et al. [55] could occur in our forests. This would be expected due to the observed negative effect of age on above-ground primary productivity (AGC_F) combined with positive effects on ground litter (Litterfall_F) and deadwood decomposition (CWD_F).
5. Conclusion
Previous biodiversity–productivity studies in forest ecosystems reported positive effects of tree species richness on above-ground components of primary productivity, suggesting that more C might be stored in species-rich forests. We found more C below than above ground in our subtropical forests, raising the question whether richness effects might be changed if total rather than only above-ground C storage is considered. Our findings demonstrate that indeed total above- and below-ground C storage in subtropical forest increases with species richness if major components of C fluxes and C stocks are considered. At the regional scale, subtropical forests in southeast China are a major potential C sink because of the implementation of large afforestation and plantation programmes in the last 36 years. However, these plantations were mainly established with single species and even though they will continue to accumulate C if their continued growth can be maintained into the future, much larger amounts of C could have been stored according to our study if plantation programmes would have adopted multi-species planting schemes.
Assuming a total amount of 30.3 Tg C stored per year by the planted monoculture forests in China [56], tentatively valued at 0.4 billion dollar, and extrapolating our results to other types of forests, an additional 6.4% C ha−1 could have been stored per year for every additional species. As a consequence, amounts in the order of 0.3 billion dollars per year (0.4 × 0.0649) might have been gained by using 10-species mixtures instead of monocultures for the 0.89 × 106 ha of planted forests in China. This assumes that the relationship between monocultures and 10-species mixtures has a similar slope to the one we found here between three- and 20-species mixtures. At the same time, this indicates the potential gains that could still be realized by switching to species-rich plantation schemes now. Such plantation schemes may be costly to carry out but could have the additional advantage that species-rich forests can be expected to be more stable and resistant to global change and to extreme events, and they contribute to biodiversity maintenance. Therefore, biodiversity should be considered as an integral component in forest-carbon management strategies.
Supplementary Material
Acknowledgements
We acknowledge the support of the BEF-China research group, especially many students and local helpers involved in setting up the plots and collecting the numerous data. We also thank the local support from the administration Bureau of the Gutianshan National Nature Reserve. We thank Norma Fowler, Lourens Poorter and an anonymous reviewer for their extensive and very helpful comments about earlier versions of this paper.
Ethics
This research was performed in accordance with the laws, guidelines and ethical standards of China, Switzerland and the European Union, where the research was performed.
Data accessibility
The data supporting the findings of this study are deposited in the Dryad Digital Repository at: http://dx.doi.org/10.5061/dryad.t9t0sc4 [57].
Authors' contributions
X.L., S.T., B.S. and K.M. designed the study; X.L., S.T., J-S.H., H.B., Z.T., A.E., M.S.-L., K.A.P., B.Y., P.K., T.S., Y.H., C.W., M.S., K.N.L. and P.A.N. conducted the measurements and contributed data, X.L., S.T. and B.S. conducted the analyses and wrote the manuscript, with help from all other co-authors.
Competing interests
We have no competing interests.
Funding
This study was financially supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB31000000), the National Science and Technology Ministry Major Project (2017YFA0605103), the National Key Project for Basic Research Program of China (no. 2014CB954100), the German Research Foundation (DFG FOR 891), the Swiss National Science Foundation (SNSF no. 130720, 147092) and the University of Zurich Research Priority Program on Global Change and Biodiversity (URPP GCB).
References
- 1.Bonan GB. 2008. Forests and climate change: forcings, feedbacks, and the climate benefits of forests. Science 320, 1444–1449. ( 10.1126/science.1155121) [DOI] [PubMed] [Google Scholar]
- 2.Pan Y, et al. 2011. A large and persistent carbon sink in the world's forests. Science 333, 988–993. ( 10.1126/science.1201609) [DOI] [PubMed] [Google Scholar]
- 3.Grassi G, House J, Dentener F, Federici S, Elzen MD, Penman J. 2017. The key role of forests in meeting climate targets requires science for credible mitigation. Nat. Clim. Change 7, 220–226. ( 10.1038/nclimate3227) [DOI] [Google Scholar]
- 4.Pichancourt J-B, Firn J, Chadès I, Martin TG. 2014. Growing biodiverse carbon-rich forests. Glob. Change Biol. 20, 382–393. ( 10.1111/gcb.12345) [DOI] [PubMed] [Google Scholar]
- 5.Liang J, et al. 2016. Positive biodiversity-productivity relationship predominant in global forests. Science 354, 6309 ( 10.1126/science.aaf8957) [DOI] [PubMed] [Google Scholar]
- 6.van der Sande MT, et al. 2017. Biodiversity in species, traits, and structure determines carbon stocks and uptake in tropical forests. Biotropica 49, 593–603. ( 10.1111/btp.12453) [DOI] [Google Scholar]
- 7.Paquette A, Messier C. 2011. The effect of biodiversity on tree productivity: from temperate to boreal forests. Glob. Ecol. Biogeogr. 20, 170–180. ( 10.1111/j.1466-8238.2010.00592.x) [DOI] [Google Scholar]
- 8.Grace JB, et al. 2016. Integrative modelling reveals mechanisms linking productivity and plant species richness. Nature 529, 390 ( 10.1038/nature16524) [DOI] [PubMed] [Google Scholar]
- 9.Hooper DU, et al. 2012. A global synthesis reveals biodiversity loss as a major driver of ecosystem change. Nature 486, 105–108. ( 10.1038/nature11118) [DOI] [PubMed] [Google Scholar]
- 10.Piotto D. 2008. A meta-analysis comparing tree growth in monocultures and mixed plantations. Forest Ecol. Manag. 255, 781–786. ( 10.1016/j.foreco.2007.09.065) [DOI] [Google Scholar]
- 11.Erskine PD, Lamb D, Bristow M. 2006. Tree species diversity and ecosystem function: can tropical multi-species plantations generate greater productivity? Forest Ecol. Manag. 233, 205–210. ( 10.1016/j.foreco.2006.05.013) [DOI] [Google Scholar]
- 12.Poorter L, et al. 2015. Diversity enhances carbon storage in tropical forests. Glob. Ecol. Biogeogr. 24, 1314–1328. ( 10.1111/geb.12364) [DOI] [Google Scholar]
- 13.Gamfeldt L, et al. 2013. Higher levels of multiple ecosystem services are found in forests with more tree species. Nat. Commun. 4, 1340 ( 10.1038/ncomms2328) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hooper DU, et al. 2005. Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecol. Monogr. 75, 3–35. ( 10.1890/04-0922) [DOI] [Google Scholar]
- 15.Loreau M. 2000. Biodiversity and ecosystem functioning: recent theoretical advances. Oikos 91, 3–17. ( 10.1034/j.1600-0706.2000.910101.x) [DOI] [Google Scholar]
- 16.Tilman D, Lehman CL, Thomson KT. 1997. Plant diversity and ecosystem productivity: theoretical considerations. Proc. Natl Acad. Sci. USA 94, 1857–1861. ( 10.1073/pnas.94.5.1857) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams LJ, Paquette A, Cavender-Bares J, Messier C, Reich PB. 2017. Spatial complementarity in tree crowns explains overyielding in species mixtures. Nat. Ecol. Evol. 1, 63 ( 10.1038/s41559-016-0063) [DOI] [PubMed] [Google Scholar]
- 18.Musavi T, et al. 2017. Stand age and species richness dampen interannual variation of ecosystem-level photosynthetic capacity. Nat. Ecol. Evol. 1, 48 ( 10.1038/s41559-016-0048) [DOI] [PubMed] [Google Scholar]
- 19.Sakschewski B, von Bloh W, Boit A, Poorter L, Peña-Claros M, Heinke J, Joshi J, Thonicke K. 2016. Resilience of Amazon forests emerges from plant trait diversity. Nat. Clim. Change 6, 1032 ( 10.1038/nclimate3109) [DOI] [Google Scholar]
- 20.Wright AJ, Wardle DA, Callaway R, Gaxiola A. 2017. The overlooked role of facilitation in biodiversity experiments. Trends Ecol. Evol. 32, 383–390. ( 10.1016/j.tree.2017.02.011) [DOI] [PubMed] [Google Scholar]
- 21.Jactel H, Brockerhoff EG. 2007. Tree diversity reduces herbivory by forest insects. Ecol. Lett. 10, 835–848. ( 10.1111/j.1461-0248.2007.01073.x) [DOI] [PubMed] [Google Scholar]
- 22.Hantsch L, Bien S, Radatz S, Braun U, Auge H, Bruelheide H. 2014. Tree diversity and the role of non-host neighbour tree species in reducing fungal pathogen infestation. J. Ecol. 102, 1673–1687. ( 10.1111/1365-2745.12317) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vilà M, Vayreda J, Comas L, Ibáñez JJ, Mata T, Obón B. 2007. Species richness and wood production: a positive association in Mediterranean forests. Ecol. Lett. 10, 241–250. ( 10.1111/j.1461-0248.2007.01016.x) [DOI] [PubMed] [Google Scholar]
- 24.Ruiz-Benito P, Gómez-Aparicio L, Paquette A, Messier C, Kattge J, Zavala MA. 2014. Diversity increases carbon storage and tree productivity in Spanish forests. Glob. Ecol. Biogeogr. 23, 311–322. ( 10.1111/geb.12126) [DOI] [Google Scholar]
- 25.Huang Y, Ma Y, Zhao K, Niklaus PA, Schmid B, He J-S. 2017. Positive effects of tree species diversity on litterfall quantity and quality along a secondary successional chronosequence in a subtropical forest. J. Plant Ecol. 10, 28–35. ( 10.1093/jpe/rtw115) [DOI] [Google Scholar]
- 26.Gessner MO, Swan CM, Dang CK, McKie BG, Bardgett RD, Wall DH, Hättenschwiler S. 2010. Diversity meets decomposition. Trends Ecol. Evol. 25, 372–380. ( 10.1016/j.tree.2010.01.010) [DOI] [PubMed] [Google Scholar]
- 27.Körner C. 2017. A matter of tree longevity. Science 355, 130–131. ( 10.1126/science.aaal2449) [DOI] [PubMed] [Google Scholar]
- 28.Ruiz-Jaen MC, Potvin C. 2011. Can we predict carbon stocks in tropical ecosystems from tree diversity? Comparing species and functional diversity in a plantation and a natural forest. New Phytol. 189, 978–987. ( 10.1111/j.1469-8137.2010.03501.x) [DOI] [PubMed] [Google Scholar]
- 29.Wardle DA. 2016. Do experiments exploring plant diversity–ecosystem functioning relationships inform how biodiversity loss impacts natural ecosystems? J. Veg. Sci. 27, 646–653. ( 10.1111/jvs.12399) [DOI] [Google Scholar]
- 30.Poorter L, et al. 2017. Biodiversity and climate determine the functioning of Neotropical forests. Glob. Ecol. Biogeogr. 26, 1423–1434. ( 10.1111/geb.12668) [DOI] [Google Scholar]
- 31.Fichtner A, Härdtle W, Bruelheide H, Kunz M, Li Y, von Oheimb G. 2018. Neighbourhood interactions drive overyielding in mixed-species tree communities. Nat. Commun. 9, 1144 ( 10.1038/s41467-018-03529-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Castro-Izaguirre N, Chi XL, Baruffol M, Tang ZY, Ma KP, Schmid B, Niklaus PA. 2016. Tree diversity enhances stand carbon storage but not leaf area in a subtropical forest. PLoS ONE 11, e0167771 ( 10.1371/journal.pone.0167771) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sullivan MJP, et al. 2017. Diversity and carbon storage across the tropical forest biome. Sci. Rep. 7, 39102 ( 10.1038/srep39102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van der Perre F, et al. 2018. Reconciling biodiversity and carbon stock conservation in an afrotropical forest landscape. Sci. Adv. 4, eaar6603 ( 10.1126/sciadv.aar6603) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chisholm RA, et al. 2013. Scale-dependent relationships between tree species richness and ecosystem function in forests. J. Ecol. 101, 1214–1224 ( 10.1111/1365-2745.12132) [DOI] [Google Scholar]
- 36.Stephenson NL, et al. 2014. Rate of tree carbon accumulation increases continuously with tree size. Nature 507, 90–93. ( 10.1038/nature12914) [DOI] [PubMed] [Google Scholar]
- 37.Lasky JR, Uriarte M, Boukili VK, Erickson DL, Kress WJ, Chazdon RL. 2014. The relationship between tree biodiversity and biomass dynamics changes with tropical forest succession. Ecol. Lett. 17, 1158–1167. ( 10.1111/ele.12322) [DOI] [PubMed] [Google Scholar]
- 38.Nuchel J, Svenning JC. 2017. Recent tree cover increases in eastern China linked to low, declining human pressure, steep topography, and climatic conditions favoring tree growth. PLoS ONE 12, e0177552 ( 10.1371/journal.pone.0177552) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hua F, Wang X, Zheng X, Fisher B, Wang L, Zhu J, Tang Y, Yu DW, Wilcove DS. 2016. Opportunities for biodiversity gains under the world's largest reforestation programme. Nat. Commun. 7, 12717 ( 10.1038/ncomms12717) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bruelheide H, et al. 2011. Community assembly during secondary forest succession in a Chinese subtropical forest. Ecol. Monogr. 81, 25–41. ( 10.1890/09-2172.1) [DOI] [Google Scholar]
- 41.Lai JS, Mi XC, Ren HB, Ma KP. 2009. Species–habitat associations change in a subtropical forest of China. J. Veg. Sci. 20, 415–423. ( 10.1111/j.1654-1103.2009.01065.x) [DOI] [Google Scholar]
- 42.Walsh C, Nally RM.2013. hier.part: Hierarchical partitioning. See https://cran.r-project.org/web/packages/hier.part/index.html .
- 43.Rosseel Y. 2012. lavaan: an R package for structural equation modeling. J. Stat. Softw. 48, 1–36. (doi:10.18637/jss.v048.i02) [Google Scholar]
- 44.R Core Team. 2016. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See https://www.R-project.org/. [Google Scholar]
- 45.Zeng Z-Q, Wang S-L, Zhang C-M, Gong C, Hu Q. 2013. Carbon storage in evergreen broad-leaf forests in mid-subtropical region of China at four succession stages. J. Forestry Res. 24, 677–682. ( 10.1890/09-2172.1) [DOI] [Google Scholar]
- 46.Zhang K, Xu X, Wang Q, Liu B. 2010. Biomass, and carbon and nitrogen pools in a subtropical evergreen broad-leaved forest in eastern China. J. Forest Res.-Jpn 15, 274–282. ( 10.1007/s10310-009-0175-z) [DOI] [Google Scholar]
- 47.Chen LC, Wang SL, Wang QK. 2016. Ecosystem carbon stocks in a forest chronosequence in Hunan Province, South China. Plant Soil 409, 217–228. ( 10.1007/s11104-016-2950-x) [DOI] [Google Scholar]
- 48.Russell MB, Fraver S, Aakala T, Gove JH, Woodall CW, D'Amato AW, Ducey MJ. 2015. Quantifying carbon stores and decomposition in dead wood: a review. Forest Ecol. Manag. 350, 107–128. ( 10.1016/j.foreco.2015.04.033) [DOI] [Google Scholar]
- 49.Barrufol M, Schmid B, Bruelheide H, Chi X, Hector A, Ma K, Michalski S, Tang Z, Niklaus PA. 2013. Biodiversity promotes tree growth during succession in subtropical forest. PLoS ONE 8, e0081246 ( 10.1371/journal.pone.0081246) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sun Z, Liu X, Schmid B, Bruelheide H, Bu W, Ma K. 2017. Positive effects of tree species richness on fine-root production in a subtropical forest in SE-China. J. Plant Ecol. 10, 146–157. ( 10.1093/jpe/rtw094) [DOI] [Google Scholar]
- 51.Niklaus PA, Baruffol M, He JS, Ma K, Schmid B. 2017. Can niche plasticity promote biodiversity-productivity relationships through increased complementarity? Ecology 98, 1104–1116. ( 10.1002/ecy.1748) [DOI] [PubMed] [Google Scholar]
- 52.Both S, Fang T, Bohnke M, Bruelheide H, Geissler C, Kuhn P, Scholten T, Trogisch S, Erfmeier A. 2011. Lack of tree layer control on herb layer characteristics in a subtropical forest, China. J. Veg. Sci. 22, 1120–1131. ( 10.1111/j.1654-1103.2011.01324.x) [DOI] [Google Scholar]
- 53.Ma Z, Chen HY. 2016. Effects of species diversity on fine root productivity in diverse ecosystems: a global meta-analysis. Glob. Ecol. Biogeogr. 25, 1387–1396. ( 10.1111/geb.12488) [DOI] [Google Scholar]
- 54.Wirth C, Gleixner G, Heimann M. 2009. Old-growth forests: function, fate and value—an overview. In Old-growth forests (eds Wirth C, Gleixner G, Heimann M), pp. 3–10. Berlin, Germany: Springer. [Google Scholar]
- 55.Tang X, Wang Y-P, Zhou G, Zhang D, Liu S, Liu S, Zhang Q, Liu J, Yan J. 2011. Different patterns of ecosystem carbon accumulation between a young and an old-growth subtropical forest in Southern China. Plant Ecol. 212, 1385–1395. ( 10.1007/s11258-011-9914-2) [DOI] [Google Scholar]
- 56.Guo Z, Hu H, Li P, Li N, Fang J. 2013. Spatio-temporal changes in biomass carbon sinks in China's forests from 1977 to 2008. Sci. China Life Sci. 56, 661–671. ( 10.1007/s11427-013-4492-2) [DOI] [PubMed] [Google Scholar]
- 57.Liu X, et al. 2018. Data from: Tree species richness increases ecosystem carbon storage in subtropical forests Dryad Digital Repository. ( 10.5061/dryad.t9t0sc4) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Liu X, et al. 2018. Data from: Tree species richness increases ecosystem carbon storage in subtropical forests Dryad Digital Repository. ( 10.5061/dryad.t9t0sc4) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
The data supporting the findings of this study are deposited in the Dryad Digital Repository at: http://dx.doi.org/10.5061/dryad.t9t0sc4 [57].