Abstract
Caimanines are crocodylians currently restricted to South and Central America and the oldest members are from lower Palaeocene localities of the Salamanca Formation (Chubut Province, Argentina). We report here a new caimanine from this same unit represented by a skull roof and partial braincase. Its phylogenetic relationships were explored in a cladistic analysis using standard characters and a morphogeometric two-dimensional configuration of the skull roof. The phylogenetic results were used for an event-based supermodel quantitative palaeobiogeographic analysis. The new species is recovered as the most basal member of the South American caimanines, and the Cretaceous North American lineage ‘Brachychampsa and related forms' as the most basal Caimaninae. The biogeographic results estimated north-central North America as the ancestral area of Caimaninae, showing that the Cretaceous and Palaeocene species of the group were more widespread than thought and became regionally extinct in North America around the Cretaceous–Palaeocene boundary. A dispersal event from north-central North America during the middle Late Cretaceous explains the arrival of the group to South America. The Palaeogene assemblage of Patagonian crocodylians is composed of three lineages of caimanines as a consequence of independent dispersal events that occurred between North and South America and within South America around the Cretaceous–Palaeogene boundary.
Keywords: Alligatoridae, stem-Caimaninae, South America, palaeogene, phylogeny, palaeobiogeography
1. Introduction
Modern crocodylian faunas of South America are dominated by several species of caimanines that inhabit tropical and subtropical freshwater systems [1]. This group has a long history in the continent that spans most of the Cenozoic and achieved in some palaeoecosystems a considerably broader taxonomic diversity than today [2–5]. The evolutionary history of Caimaninae is mostly restricted to South America and two main events are recognized [4–7], a first appearance of the group with a limited morphological disparity in the early Palaeogene and a subsequent explosive taxonomic and ecomorphological diversification during the early Neogene [5]. The oldest caimanines are known from the early Palaeocene of the Argentinian Patagonia (i.e. Eocaiman palaeocenicus, Necrosuchus ionensis and Notocaiman stromeri) [2,3,6–10] and, although fragmentary, they provide key morphological information that sheds light on the origin of this clade. The closest relatives of the caimanines are Laurasian species and, as a result, their presence in the Palaeocene of South America has been explained by vicariance or dispersal events crossing marine barriers [2,4,7,11].
Here, we describe the new caimanine genus and species Protocaiman peligrensis from the earliest Palaeocene (Danian) of Patagonia, Argentina, based on a skull roof and partial braincase, regions of the skull previously unknown in the other Palaeocene Patagonian species. This new specimen has a direct and important impact on the phylogenetic relationships of basal caimanines and other alligatorids, and forces the re-evaluation of previous hypotheses of the early historical biogeography of these crocodylian lineages.
2. Material and methods
(a). Phylogenetic analysis
The phylogenetic position of P. peligrensis was tested using the data matrix of Brochu [6] as modified by Salas-Gismondi et al. [5]. The new taxon, three other Palaeocene South American caimanines (Eocaiman palaeocenicus, Eocaiman itaboraiensis and No. stromeri) and two allodaposuchids (Agaresuchus fontisensis and Allodaposuchus precedens) were added to this data matrix and Melanosuchus fisheri was excluded (see electronic supplementary material, text SIII). Scorings for Globidentosuchus and Centenariosuchus were modified following [12]. The skull roof of P. peligrensis closely resembles that of some North American alligatoroids and this region seems to be potentially very informative to reconstruct the phylogenetic relationships of, at least, this part of the crocodylian tree. As a result, the postorbital region of the skull roof in the dorsal view was sampled using two-dimensional landmarks for 38 alligatoroid species and used as a morphogeometric character [13] (see electronic supplementary material, text SIII, figure S5, tables S2 and S3). Two analyses were conducted, the first including all the standard characters and the second adding the morphogeometric configuration and deactivating its non-independent standard characters (electronic supplementary material, text SIII and figures S3 and S4). In addition, we conducted three alternative analyses merging Protocaiman with the other three Argentinian Palaeocene caimanines and using only standard characters (electronic supplementary material, text SIII). All the apomorphies listed below are unambiguous optimizations. Branch supports were calculated as Bremer supports, and absolute and GC (group present/contradicted) bootstrap frequencies.
(b). Biogeographic analysis
The historical biogeography of Caimaninae was estimated using an event-based quantitative analysis based on the phylogenetic results recovered here. The range of chronostratigraphic uncertainty of each species was listed in millions of years and their geographical range was sampled as palaeolatitudes and palaeolongitudes (electronic supplementary material, text SIV and tables S4 and S5). We conducted a k-means multivariate cluster analysis of 10 groupings based on the palaeocoordinates of 369 eusuchian specimens—representing all the occurrences stored in the PaleoBiology Database and the Global Biodiversity Information Facility—in order to determine the geographical areas to be used in the analysis. This cluster analysis was performed in R [14] with 1000 replicates. The following 10 areas were recovered and approximately comprise the following extant locations: (i) northern Central America + southern North America, (ii) north-eastern North America, (iii) eastern Africa + Madagascar, (iv) western Oceania, (v) Europe + northern Africa, (vi) north-western North America, (vii) south-central Asia, (viii) southern South America, (ix) northern South America and Panama, and (x) north-central North America (electronic supplementary material, figure S6).
The datasets were analysed using different likelihood versions of the following palaeobiogeographic models: dispersal–extinction–cladogenesis (DEC), DEC + J, dispersal vicariance analysis (DIVALIKE), DIVALIKE + J, BAYAREALIKE and BAYAREALIKE + J (+J models include founder-event speciation or long-distance dispersal; see [15]) using the R package BioGeoBEARS [15]. The analysis was conducted on a randomly selected, time-calibrated reduced MPT recovered from the data matrix including only standard characters and excluding Allognathosuchus polyodon (electronic supplementary material, text SIV, figure S7 and table S6).
3. Results
(a). Systematic palaeontology
Crocodyliformes [16]; Alligatoroidea [17]; Globidonta [4]; Caimaninae [4].
Protocaiman peligrensis, gen. et sp. nov.
Etymology: Protos, first (Greek); caiman (in reference to the extant genus Caiman); peligrensis (in reference to its type locality Punta Peligro).
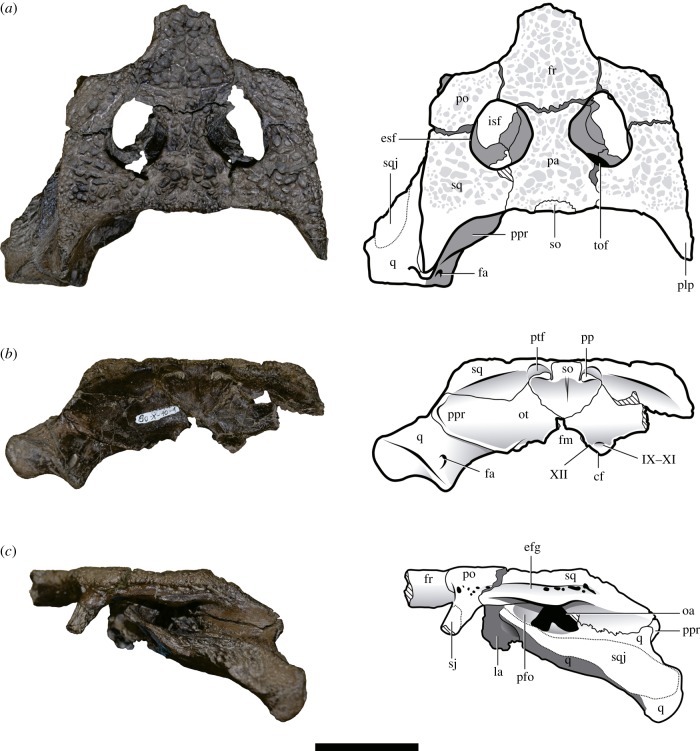
Holotype: Museo de La Plata (MLP) 80-X-10-1, partial skull, including the skull roof (missing the anterior end of the frontals and snout), lateral walls of the braincase (lacking the basicranium), most of the occipital table, and left quadrate (figure 1; electronic supplementary material, figures S2, S3 and S4c).
Figure 1.
Holotype of P. peligrensis nov. gen. et nov. sp. (MLP 80X-10-1) in (a) dorsal, (b) occipital and (c) left lateral views. Scale bar equals 5 cm. efg, ear-flap groove; esf, external supratemporal fenestra; fa, foramen aerum; fm, foramen magnum; fr, frontal; isf, internal supratemporal fenestra; la, laterosphenoid; oa, otic aperture; pa, parietal; pfo, preotic foramen; plp, posterolateral process of the squamosal; po, postorbital; pp, postoccipital process; ppr, paroccipital process; ptf, post-temporal fenestra; q, quadrate; sj, suture surface for the jugal; so, supraoccipital; sq, squamosal; sqj, suture surface for the quadratojugal; tof, temporo-orbital foramen; IX–XI, metotic foramen; XII, hypoglossal foramen. (Online version in colour.)
Locality and horizon: Punta Peligro locality, Patagonia, Chubut Province, Argentina, upper levels of the Salamanca Formation [18], upper Danian, lowermost Palaeocene [19] (electronic supplementary material, text SI and figure S1).
Diagnosis: Stem-caimanine diagnosed by the following autapomorphies: frontoparietal suture extends deeply within supratemporal fenestra, preventing broad contact between postorbital and parietal; squamosal extends ventrolaterally to lateral extent of paroccipital process; surface of the paroccipital process anterodorsally sloped, visible in the dorsal view; and ventral border of otoccipital convex and ventrally projected, hiding the posterior opening of the cranioquadrate passage in the occipital view.
Taxonomic nomenclature: We follow the original definition of Caimaninae as a stem-based clade that comprises Caiman and taxa closer to it than to Alligator [4].
General description: The ornamentation of the external surface of the preserved dermal cranial bones (parietal, postorbital, frontal and squamosal) resembles that of most alligatoroids. It consists of large pits in the central area of the bones and smaller pits on the lateral and posterior edges of the skull roof and the posterolateral process of the squamosal. The dorsal surface of the frontal and parietal possesses conspicuous depressions along their median line, forming small longitudinal median crests. The posteromedial margins of the external supratemporal fenestrae and orbits are formed by rounded edges that are flush with the rest of the skull roof surface (electronic supplementary material, figure S2 and table S1).
In the dorsal view, the skull roof is subrectangular, wider than long, with the lateral margins converging anteriorly, as occurs in some North American Cretaceous alligatoroids (e.g. Brachychampsa [20]; Stangerochampsa [21]; electronic supplementary material, figure S4). Most of the posterior margin of the skull roof is transversely straight, but the subtriangular posterolateral process of the squamosal is posterolaterally oriented (figure 1a). The interfenestral bridge is narrower than the interorbital bridge, as in Brachychampsa [20] and Stangerochampsa [21]. By contrast, the interfenestral bridge is broader than the interorbital bridge in the early caimanines Kuttanacaiman and Gnatusuchus [5]. Each external supratemporal fenestra is wider than the interfenestral bridge and oval, slightly longer than wide. The relative size of the external supratemporal fenestrae resembles that of Brachychampsa, Stangerochampsa and the enigmatic Culebrasuchus [22], but these openings are proportionally smaller in the early caimanines Gnatusuchus, Globidentosuchus and Kuttanacaiman [5]. The huge temporo-orbital foramen is observed through the external supratemporal fenestra (electronic supplementary material, figure S2) and opens at the posterior wall of the dorsally facing supratemporal fossa. The frontoparietal suture is placed on the interfenestral bridge and is slightly anteriorly concave. This suture extends ventrally through the medial wall of the supratemporal fossa, preventing a broad contact between the postorbital and parietal, which is a plesiomorphic condition of Eusuchia [5,6]. By contrast, a parietal–postorbital contact excludes the frontal from the border of the fenestra in the early caimanines Globidentosuchus, Gnatusuchus and Kuttanacaiman [5] (electronic supplementary material, figure S4). The supraoccipital extends slightly onto the posterior border of the skull roof and forms a semicircular suture with the parietal, as in other basal globidontans (figure 1a,b).
In the occipital view, the dorsal margin of the occipital table is mostly straight, but slightly elevated at the base of the squamosal posterolateral process. The surface of the occipital table (formed by the supraoccipital, squamosal and most of the otoccipital) is strongly concave. The surface of the otoccipital faces posterodorsally and forms a distinct change in slope with its posteroventrally facing ventral portion. The supraoccipital occupies most of the area above the foramen magnum, but it does not participate on its border. The ventral margin of the paroccipital process forms a conspicuous, rounded crest that projects ventrally beyond the ventromedial margin of the quadrate, a condition that seems to be unique among alligatoroids. The metotic foramen and part of the carotid canal are placed ventral and medial to the paroccipital process and lateral to the foramen magnum. Foramina for the passage of the CN XII1 (ventral and smaller) and CN XII2 (dorsal and medial) are medial to the metotic foramen (figure 1b; electronic supplementary material, figure S3).
The ventral articular end of the quadrate is transversely broad and anteroposteriorly short, similar to Brachychampsa and Stangerochampsa [20,21], but contrasting with the proportionally longer and medially bowed quadrate of Kuttanacaiman and modern caimanines [4,5]. As in other caimanines, the lateral condyle of the ventral end of the quadrate is broader than the medial one. The contact surface for the quadratojugal indicates that this bone may have formed part of the lateral ventral condyle of the quadrate (figure 1a,c; electronic supplementary material, figure S3).
(b). Phylogenetic analysis
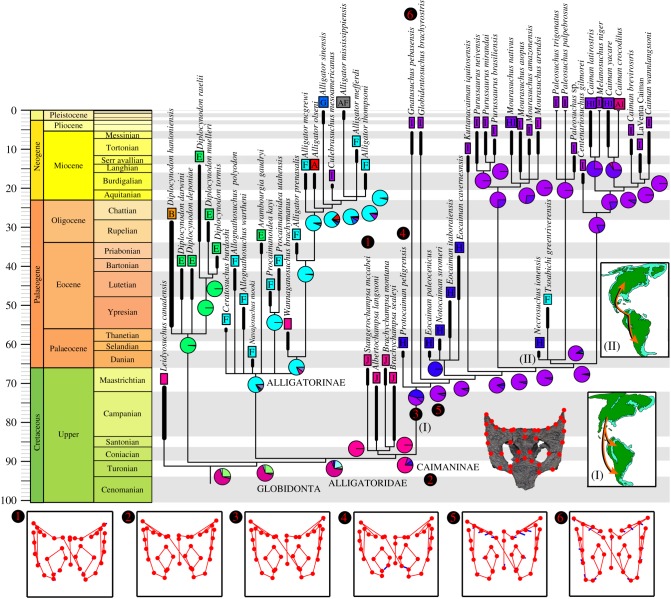
The analysis of the data matrix composed of standard characters found 60 MPTs of 728 steps and that including the morphogeometric configuration found 16 MPTs of 700.19881 steps (electronic supplementary material, figure S7 and text SIII). The topologies of the strict consensus trees of both analyses are generally consistent between each other and broadly congruent with the results of previous authors that used modified versions of this dataset (e.g. [5,6]). However, the inclusion of P. peligrensis generated some changes around the base of Alligatoroidea. The clade composed of the North American Brachychampsa (Brachychampsa montana and Brachychampsa sealeyi), Albertochampsa and Stangerochampsa (hereafter called ‘Brachychampsa and related forms’) is unambiguously recovered as the most basal caimanines sensu [4], being the sister taxon of all the South American species of the group (figure 2; electronic supplementary material, figures S6 and S7). The monophyly of Caimaninae—and the position of the four North American species within the clade—is supported by a proatlas without an anterior process (3: 0 → 1) and an angular not extended dorsally beyond the anterior end of the foramen intermandibularis caudalis (65: 0 → 1) in all trees and external nares that are dorsally facing (81: 0 → 1) in some trees. Protocaiman peligrensis is found as the sister taxon to all other South American caimanines because of the presence of dermal bones of skull roof overhanging the rim of the external supratemporal fenestrae (152: 0 → 1) and medial parietal wall of external supratemporal fenestra bearing foramina (154: 0 → 1). By contrast, the new species is excluded from the clade composed of Gnatusuchus pebasensis and more derived caimanines because it retains a small exposure of the supraoccipital on the skull roof, allowing the parietal to reach the posterior edge of the skull roof. These branches are also supported by landmark transformations shown in figure 2. Notocaiman and the three species of Eocamian are recovered in a monophyletic group of unresolved internal relationships, which is the sister clade of Kuttanacaiman and more derived caimanines.
Figure 2.
Time-calibrated strict consensus tree, from the analysis using only standard characters, showing the phylogenetic relationships of P. peligrensis nov. gen. et nov. sp. and other globidontans, and their estimated biogeographic history (pie charts). 1–6, optimization of the two-dimensional morphogeometric configuration of the skull roof in dorsal view in the analysis using combined characters. 1, Stangerochampsa; 2, hypothetical ancestor of Caimaninae; 3, hypothetical ancestor of South American caimanines; 4, Protocaiman; 5, hypothetical ancestor of South American caimanines excluding Protocaiman; 6, Gnatusuchus. Placement of landmarks exemplified in the partial skull roof of Protocaiman. Letters representing geographical areas are detailed in Material and methods. I–II, palaeogeography of the American continent and estimated dispersal events with their placement in the phylogeny during I, the Late Cretaceous and II, Palaeogene. (Online version in colour.)
(c). Biogeographic analysis
The biogeographic analysis found that DEC + J is the palaeobiogeographic model that best fit our data (electronic supplementary material, text SIV). North-central North America was estimated as the most probable ancestral geographical area of Alligatoroidea, Globidonta, Alligatoridae and Caimaninae (figure 2; electronic supplementary material, figure S13). The estimation of the latter clade is because of the geographical occurrence of Brachychampsa and related forms. A dispersal event (range switch) is estimated from ‘north-central North America’ to ‘north-western North America’ at the base of Alligatorinae. Within Caimaninae, a dispersal event from ‘north-central North America’ to South America (with a slightly higher probability to the southern region of the continent) is recovered during the middle Late Cretaceous (approx. 85–90 Ma). In the early evolutionary history of the South American caimanines, there are three dispersal events among the Palaeogene branches. Two independent dispersals from northern to southern South America to explain the occurrence of Necrosuchus in Patagonia and Eocaiman spp.—Notocaiman in Patagonia and central-east Brazil, and one or probably two from the area that includes northern South America and Panama to North America to account for the presence of Tsoabichi and Orthogenysuchus in the latter continent during the late Palaeogene [11].
4. Discussion
(a). The phylogenetic relationships of Cretaceous–Palaeogene caimanines
Protocaiman peligrensis lacks overlapping bones with the hypodigms of the two other crocodylian species from the Salamanca Formation (i.e. E. palaeocenicus and Ne. ionensis [7,9]). The phylogenetic position found for the new specimen is currently the only evidence that we have to decide on its taxonomy. Its placement as the most basal South American caimanine—whereas the other two species from the same unit are more derived—supports that it very likely belonged to a new genus and species (figure 2; electronic supplementary material, figures S3 and S4). The alternative analyses merging Protocaiman and the other Argentinian Palaocene caimanines into the same terminals found longer optimal trees than the first analysis (electronic supplementary material, figures S9–S11). This is a hypothesis that can be tested in the future with the discovery of other Palaeocene caimanines with overlapping bones. Despite the presence of typical caimanine apomorphies in Protocaiman, the overall morphology of its skull roof resembles more that of Brachychampsa and related forms rather than species traditionally included within Caimaninae [4,6] (figure 2; electronic supplementary material, figure S2). Indeed, the optimization of the morphogeometric configuration of the skull roof in the phylogeny shows that Protocaiman retains a skull roof morphology very similar to that of the ancestral node of Caimaninae, and distinct transformations occur in the node that includes Gnatusuchus and more derived members of the clade (e.g. narrower external supratemporal fenestrae and more posteriorly expanded median region of the skull roof) (figure 2: thick blue lines in the morphogeometric configurations). Recent phylogenetic analyses found Brachychampsa and related forms as either the most basal alligatorines or caimanines (e.g. [5,6]). Protocaiman bolsters the hypothesis of Brachychampsa and related forms as North American caimanines and here it is the first time that Mesozoic caimanines are recognized unambiguously.
(b). Biogeographic history of early caimanines
The general consensus among previous authors is that the earliest evolutionary and biogeographic history of Caimaninae was mostly restricted to the Cenozoic of South America [3–6,11]. However, contrasting with this traditional view, the results of our analyses indicate that the clade had a North American origin, was geographically more widespread than thought and North American caimanines become regionally extinct as victims of the Cretaceous–Palaeogene mass extinction. The earliest biogeographic event estimated here within Caimaninae is the dispersion of the clade into South America during the Late Cretaceous. It is interesting to note that the tempo and directionality of this biogeographic dispersal match those previously proposed for other terrestrial tetrapods, such as amphibians, squamates, testudines [23,24], hadrosauriform dinosaurs [25] and dryolestoid mammals [26]. The occurrence of dispersal events between North and South America during the latest Cretaceous has been explained through the establishment of an intercontinental land bridge that severed subsequently in the Palaeogene [24,27,28].
The ancestral geographical distribution of the caimanines more derived than P. peligrensis in the area that includes northern South America and Panama during the late Palaeogene and Neogene (e.g. [29]) can be explained by a dispersal event from southern South America or that the group was originally geographically widespread across the continent during the latest Cretaceous–early Palaeogene. The phylogenetic relationships of basal caimanines indicate that the Palaeogene assemblage of Patagonian crocodylians is composed of three independent lineages without evidence of a sympatric diversification. As a result, the taxonomic diversity of Patagonian caimanines is a consequence of independent dispersal events that occurred between North and South America and within South America before and/or immediately after the Cretaceous–Palaeogene boundary. Previous studies suggested a positive correlation between global mean temperature and latitudinal range in crocodylians [30]. The presence of caimanines in the high southern latitudes of Patagonia in the early Palaeogene may have been prompted by the high global temperatures reconstructed around the late Palaeocene thermal maximum and the early Eocene climatic optimum [31].
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank M. Reguero for access to the MLP collection; J. Desojo, G. Mendes-Cidade and D. Riff for specimen photographs. We thank C. Brochu, A. Hastings and two anonymous reviewers for their comments, which improved the manuscript. We are much indebted to H. Herrera for collecting the holotype of Protocaiman during fieldwork conducted by the MLP at the beginning of the 1980s.
Ethics
The authors declare that have followed all the ethical policies requested by the journal.
Data accessibility
Coordinates for cluster analysis, phylogenetic taxon-character matrix, TPS file of landmark coordinates and biogeographic taxon-area matrix are available as electronic supplementary material and in the Dryad Digital Repository at http://dx.doi.org/10.5061/dryad.r4b158n [32].
Authors' contributions
P.B., F.B. and M.D.E. designed the research. P.B. and M.D.E. wrote the main part of manuscript; P.B., M.D.E. and F.B. performed anatomical descriptions and the systematic and biogeographic research. M.V.F.B. performed the geometric morphometric analysis and the final edition of the manuscript. All authors contributed to the final writing, interpretation of results, discussion and design of figures.
Competing interests
The authors declare no competing interests.
Funding
The study was supported by CONICET (PIP 112201301-00733) and ANPCyT (PICT 2016-0159) to P.B.
References
- 1.Rueda-Almonacid JV, et al. 2007. Las tortugas y los cocodrilianos de los países andinos del trópico. Conservación Internacional. Serie de guías tropicales de campo 6. Colombia, South America: Editorial Panamericana. [Google Scholar]
- 2.Langston W. 1965. Fossil crocodilians from Colombia and the Cenozoic history of the crocodilia in South America. Berkeley, CA: University of California Press. [Google Scholar]
- 3.Gasparini Z. 1996. Biogeographic evolution of the South American crocodilians. Münchner Geowiss. Abh. 30, 159–184. [Google Scholar]
- 4.Brochu CA. 1999. Phylogenetics, taxonomy, and historical biogeography of Alligatoroidea. J. Vertebr. Paleontol. 19, 9–100. ( 10.1080/02724634.1999.10011201) [DOI] [Google Scholar]
- 5.Salas-Gismondi R, Flynn JJ, Baby P, Tejada-Lara JV, Wesselingh FP, Antoine PO. 2015. A Miocene hyperdiverse crocodylian community reveals peculiar trophic dynamics in proto-Amazonian mega-wetlands. Proc. R. Soc. B 282, 20142490 ( 10.1098/rspb.2014.2490) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brochu CA. 2011. Phylogenetic relationships of Necrosuchus ionensis Simpson, 1937 and the early history of caimanines. Zool. J. Linn. Soc. 163, S228–S256. ( 10.1111/j.1096-3642.2011.00716.x) [DOI] [Google Scholar]
- 7.Bona P. 2007. Una nueva especie de Eocaiman Simpson (Crocodylia. Alligatoridae) del Paleoceno inferior de Patagonia. Ameghiniana 44, 435–445. [Google Scholar]
- 8.Simpson GG. 1933. A new crocodilian from the Notostylops beds of Patagonia. Am. Mus. Novit. 623, 1–9. [Google Scholar]
- 9.Simpson GG. 1937. An ancient eusuchian crocodile from Patagonia. Am. Mus. Novit. 965, 1–20. [Google Scholar]
- 10.Rusconi C. 1937. Nuevo aligatorino del Paleoceno de Patagonia. Bol. Paleontol. Buenos Aires 8, 1–5. [Google Scholar]
- 11.Brochu CA. 2010. A new alligatorid from the lower Eocene Green River formation of Wyoming and the origin of caimans. J. Vertebr. Paleontol. 30, 1109–1126. ( 10.1080/02724634.2010.483569) [DOI] [Google Scholar]
- 12.Hastings AK, Reisser M, Scheyer TM. 2016. Character evolution and the origin of Caimaninae (Crocodylia) in the New World Tropics: new evidence from the Miocene of Panama and Venezuela. J. Paleontol. 90, 317–332. ( 10.1017/jpa.2016.37) [DOI] [Google Scholar]
- 13.Goloboff PA, Catalano SA. 2016. TNT version 1.5, including a full implementation of phylogenetic morphometries. Cladistics 32, 221–238. ( 10.1111/cla.12160) [DOI] [PubMed] [Google Scholar]
- 14.R Development Core Team. 2017. R: a language and environment for statistical computing, software. Vienna, Austria: R Foundation for Statistical Computing; See https://www.R-project.org/ [Google Scholar]
- 15.Matzke N. 2013. Probabilistic historical biogeography: new models for founder-event speciation, imperfect detection, and fossils allow improved accuracy and model-testing. Front. Biogeogr. 5, 242–248. [Google Scholar]
- 16.Hay OP. 1930. Second bibliography and catalogue of the fossil vertebrata of North America. Washington, DC: Carnegie Institute of Washington Publication. [Google Scholar]
- 17.Gray JE. 1844. Catalogue of the tortoises, crocodiles and amphisbaenians in the collection of the British Museum. London, UK: Edward Newman. [Google Scholar]
- 18.Lesta P, Ferello R. 1972. Región extraandina de Chubut y norte de Santa Cruz. In Geología regional Argentina (ed. Leanza A.), pp. 601–654. Córdoba, Spain: Academia Nacional de Ciencias. [Google Scholar]
- 19.Andreis RR. 1977. Geología del área de Cañadón Hondo. Departamento de Escalante, Provincia del Chubut, República Argentina. Rev. Mus. La Plata, Obra del Centenario. Geologia 4, 77–102. [Google Scholar]
- 20.Norell MA, Clark JM, Hutchison JH. 1994. The Late Cretaceous alligatorid Brachychampsa montana (Crocodylia): new material and putative relationships. Am. Mus. Novit. 3116, 1–26. [Google Scholar]
- 21.Wu XC, Brinkman DB, Russell AP. 1996. A new alligator from the Upper Cretaceous of Canada and the relationships of early eusuchians. Palaeontology 39, 351–375. [Google Scholar]
- 22.Hastings AK, Bloch JI, Jaramillo CA, Rincon AF, MacFadden BJ. 2013. Systematics and biogeography of crocodylians from the Miocene of Panama. J. Vertebr. Paleontol. 33, 239–263. ( 10.1080/02724634.2012.713814) [DOI] [Google Scholar]
- 23.Estes R, Báez A. 1985. Herpetofaunas of North and South America during the Late Cretaceous and Cenozoic: evidence for interchange? In The great American biotic interchange. Topics in geobiology (eds Stehli FG, Webb SD), pp. 139–197. Boston, MA: Springer. [Google Scholar]
- 24.Vanzolini PE, Heyer WR. 1985. The American herpetofauna and the interchange. In The great American biotic interchange. Topics in geobiology (eds Stehli FG, Webb SD), pp. 475–487. Boston, MA: Springer. [Google Scholar]
- 25.Prieto-Marquez A. 2010. Global historical biogeography of hadrosaurid dinosaurs. Zool. J. Linn. Soc. Lond. 159, 503–525. ( 10.1111/j.1096-3642.2010.00642.x) [DOI] [Google Scholar]
- 26.Rougier GW, Apesteguía S, Gaetano LC. 2011. Highly specialized mammalian skulls from the Late Cretaceous of South America. Nature 479, 98–102. ( 10.1038/nature10591) [DOI] [PubMed] [Google Scholar]
- 27.Bonaparte. 1984. Nuevas pruebas de la conexión física entre Sudamérica y Norteamérica durante el Cretácico tardío (Campaniano). In Actas II Congreso Argentino de paleontología y Bioestratigrafía, pp. 141–149.
- 28.Krause DW, Prasad GVR, von Koengswald W, Sahni A, Grine FE. 1997. Cosmopolitan among Late Cretaceous Gondwanan mammals. Nature 390, 504–507. ( 10.1038/37343) [DOI] [Google Scholar]
- 29.Scheyer TM, Delfino M. 2016. The late Miocene caimanine fauna (Crocodylia: Alligatoroidea) of the Urumaco Formation, Venezuela. Palaeontol. Electron. 19, 1–57. [Google Scholar]
- 30.Mannion PD, Benson RBJ, Carrano MT, Tennant JP, Judd J, Butler RJ. 2015. Climate constrains the evolutionary history and biodiversity of crocodylians. Nat. Commun. 6, 8438 ( 10.1038/ncomms9438) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zachos J, Pagani M, Sloan L, Thomas E, Billups K. 2001. Trends, rhythms, and aberrations in global climate 65 Ma to present. Science 292, 686–693. ( 10.1126/science.1059412) [DOI] [PubMed] [Google Scholar]
- 32.Bona P, Ezcurra MD, Barrios F, Fernandez Blanco MV. 2018. Data from: A new Palaeocene crocodylian from southern Argentina sheds light on the early history of caimanines Dryad Digital Repository. ( 10.5061/dryad.r4b158n) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Bona P, Ezcurra MD, Barrios F, Fernandez Blanco MV. 2018. Data from: A new Palaeocene crocodylian from southern Argentina sheds light on the early history of caimanines Dryad Digital Repository. ( 10.5061/dryad.r4b158n) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Coordinates for cluster analysis, phylogenetic taxon-character matrix, TPS file of landmark coordinates and biogeographic taxon-area matrix are available as electronic supplementary material and in the Dryad Digital Repository at http://dx.doi.org/10.5061/dryad.r4b158n [32].