Abstract
Chemical contaminants (e.g. metals, pesticides, pharmaceuticals) are changing ecosystems via effects on wildlife. Indeed, recent work explicitly performed under environmentally realistic conditions reveals that chemical contaminants can have both direct and indirect effects at multiple levels of organization by influencing animal behaviour. Altered behaviour reflects multiple physiological changes and links individual- to population-level processes, thereby representing a sensitive tool for holistically assessing impacts of environmentally relevant contaminant concentrations. Here, we show that even if direct effects of contaminants on behavioural responses are reasonably well documented, there are significant knowledge gaps in understanding both the plasticity (i.e. individual variation) and evolution of contaminant-induced behavioural changes. We explore implications of multi-level processes by developing a conceptual framework that integrates direct and indirect effects on behaviour under environmentally realistic contexts. Our framework illustrates how sublethal behavioural effects of contaminants can be both negative and positive, varying dynamically within the same individuals and populations. This is because linkages within communities will act indirectly to alter and even magnify contaminant-induced effects. Given the increasing pressure on wildlife and ecosystems from chemical pollution, we argue there is a need to incorporate existing knowledge in ecology and evolution to improve ecological hazard and risk assessments.
Keywords: behavioural ecology, endocrine-disrupting chemicals, predator-prey dynamics, plasticity, sublethal
1. Introduction
Contamination of the environment with diverse inorganic and organic compounds, such as pesticides, pharmaceuticals and metals, represents one of the main environmental challenges driven by anthropogenic activity. In 2010, the global chemical industry's value was US$4.12 trillion, having risen 54% over a decade [1]. In addition, the trend towards global urbanization is concentrating chemical consumption in cities faster than environmental interventions and remediation systems can be implemented, including in developing countries near biodiversity hotspots [2]. The increasing production and release of chemicals means that wildlife, humans and ecosystems are continuously exposed to chemical contaminants. While large-scale mortality events of wildlife represent an obvious, if rare, sign of chemical releases, chemical contaminants can elicit more subtle but nevertheless important and harmful ecological impacts [3]. Further, chemical contamination of the environment is certainly not limited to short-term, acute exposures. Effects of long-term, low-level chronic exposures can be equally deleterious, though less obvious for human observers. In this review, we develop a conceptual framework that integrates concepts and approaches from multiple disciplines to investigate how chemical contaminants can alter animal behaviour, with resultant impacts on short- (e.g. individual and community) and long-term (e.g. evolutionary) responses, potentially leading to population declines.
Research on chemical contaminants conventionally recorded a limited range of endpoints, most commonly by studying mortality following exposure in the laboratory and/or by testing the impact of a single contaminant on a single species under standardized laboratory conditions ([4], but see [5]). These approaches are logistically tractable and repeatable but are criticized for their simplicity, particularly when such experiments neither take chemical nor biological complexity into account [6]. Behaviour, on the other hand, is the result of numerous complex developmental and physiological processes, and so connects physiological function and ecological processes [7]. Thus, behavioural change provides a comprehensive measure of both direct and indirect effects of chemical contaminants on individuals, linking to population-level processes [8–10] and, importantly, is often impacted at much lower contaminant concentrations than are traditional toxicological endpoints [11]. Here, we illustrate how behavioural responses can represent a powerful, highly quantifiable and biologically relevant indicator of environmental impacts.
Chemical contaminants can affect animal behaviour both directly and indirectly. Direct effects on behaviour in wildlife—here, we focus mostly on vertebrates—are caused by contaminants acting on the physiology of an animal (e.g. impaired sensory or cognitive abilities, altered endocrine/neural signalling, metabolic dysfunction). To date, research in behavioural ecotoxicology has largely focused on direct effects of contaminants on individuals (e.g. activity) (see §2). In contrast, indirect effects, when contaminant-induced changes to animal behaviour in one organism or species have cascading effects on other organisms and species in the exposed system, have received far less attention [12–15]. Indirect effects are most pronounced when a contaminant affects exposed organisms differentially, such as when one species is more sensitive and another more resistant (i.e. asymmetrical effects; [12,14,16]). While the importance of investigating both direct and indirect effects of contaminants is evident, this multi-directional approach has rarely been applied in ecotoxicology (but see [15,17]).
In this review, we focus exclusively on studies conducted under ‘natural’ conditions, specifically measuring behavioural responses following contaminant exposures in the wild or at environmentally relevant concentrations in the laboratory. We first critically examine existing literature on the role of chemical contaminants in mediating direct effects on individual behaviour (§2). In contrast with previous reviews [14,17], our focus centres on sublethal effects, particularly those induced by emerging contaminants, such as pharmaceuticals. Moreover, as well as considering short-term, mean behavioural responses to exposure, we discuss how chemical contaminants can alter trait variance (i.e. plasticity) and act as potent evolutionary forces. Moving from effects on individuals, we investigate how chemical contaminants can alter interspecific interactions indirectly via changes in behaviour of susceptible species (§3). By integrating these collective effects, we develop a conceptual framework to identify ways in which animal behaviour can be affected by chemical contaminants (§4). In doing so, we use predator–prey interactions as a case study to demonstrate how our conceptual framework has real-world impact. While we highlight the challenges of scale and complexity involved with predicting ecological effects of chemical contaminants (§5), we also provide directions for future research (§6). Finally, the overarching aim of this review is to improve research practices by increasing the ecological relevance of research approaches employed, in order to uncover global hazards and risks posed by chemical contaminants.
2. Direct effects on individual behaviour
Here, we discuss why, in a rapidly changing world, we need to expand our concept of direct effects—perhaps more accurately ‘mean behavioural responses'—to incorporate the potential for chemical contaminants to affect both plasticity in, and evolution of, behavioural responses.
(a). Direct effects
Exposure to chemical contaminants can result in direct effects on a range of both ‘general’ behaviours (e.g. activity levels)—changes in which can have knock-on effects on multiple fitness-related traits—and specific mechanisms underpinning specific behaviours. Given that behaviour is the product of interconnected physiological, anatomical and neurological processes, and, in the wild, organisms are usually exposed to chemical cocktails rather than single contaminants, pinpointing mechanistic pathways between exposure to a contaminant and a behavioural change can be challenging. For example, round gobies (Neogobius melanostomus) collected from heavily contaminated industrial sites (e.g. polychlorinated biphenyls (PCBs), PAHs, metals) [18] or exposed to municipal wastewater effluent [19] both showed reduced aggression, even though the contaminant mixtures were very different.
Disruption of reproductive behaviours resulting from exposure to chemical contaminants has been increasingly studied in both laboratory and field settings because of the obvious population-level consequences [8]. Mechanisms underlying such behavioural changes include contaminant actions on endocrine and neural signalling, via changes to receptors, enzymes and/or transporters [20–22]. For instance, environmental exposures to organochlorine pesticides reduce parental care behaviour in predatory birds [23]. Studies on fish have demonstrated that exposure to municipal wastewater treatment plant effluent (e.g. [19]), and the active ingredients in (and metabolites of) the oral contraceptive pill, reduce nest building and courtship behaviours (reviewed in [20]). Furthermore, exposure to the insecticide endosulfan disrupts pheromonal communication between the sexes in red-spotted newts (Notophthalmus viridescens), leading to disrupted mate choice and depressed mating success [24]. Apparently subtle changes in reproductive behaviour could potentially be as devastating for fitness as major malformations of reproductive morphology, because an animal that fails to attract a mate or care for offspring appropriately will accrue zero fitness.
Changes in animal movement (e.g. frequency and speed) following contaminant exposure are common behavioural endpoints in ecotoxicological studies [25,26]. For example, small-scale activity, which is often measured in the laboratory, has high ecological importance because it increases encounter rates with both resources (e.g. food, potential mates) and risks (e.g. predators, diseases). Activity also underlies individual dispersal and migration tendencies [27,28], although smaller scale movements measured in the laboratory do not automatically reflect larger scale movements in the field. Chemical contaminants can alter these movement behaviours by disrupting either sensory capabilities used to locate suitable environments and resources (e.g. inability to detect chemical cues [29–31]) or physiological pathways governing and supporting movement (e.g. neural/endocrine disruption, metabolic dysfunction [32,33]). Contaminants can, for instance, directly impair movement, making animals less adept at capturing prey and/or escaping predators, as has been noted in vertebrates exposed to acetylcholinesterase-inhibiting pesticides [34]. So far, only a handful of studies have connected these measures to dispersal or migration in the wild. One such study showed that Atlantic salmon (Salmo salar) smolts exposed to the anxiolytic pharmaceutical oxazepam migrate faster both in laboratory migration pools and down a river [35]. By contrast, while round gobies collected from heavily contaminated environments dispersed more slowly in a laboratory maze, there was no evidence that dispersal was affected in the wild [36]. Recent work has also demonstrated that exposure of European starlings (Sturnus vulgaris) to a PCB mixture in the laboratory resulted in reduced activity and incorrect orientation for migration [37], indicating that exposed birds might migrate later and less accurately in the wild. Overall, activity seems to be a sensitive and relatively easily measured endpoint, but its potential to indicate individual fitness or population-level processes is assumed rather than proven, in most cases.
Chemical contaminants can also interfere with complex behaviours, such as predator-avoidance, grouping and aggression, which have direct implications for fitness and population dynamics. By acting on the sensory system, contaminants can affect an organism's responses to conspecifics or predators by, for example, reducing their ability to detect stimuli, but also rendering them less active or motivated to respond [29]. If receivers are unable to detect prey, predators or signals from conspecifics, or alternatively if signallers emit altered signals, this could lead to ineffective communication [38]. The resulting disruption of group interactions and coordination could potentially reduce the anti-predator and food-location benefits of grouping [39]. By impacting conspecific detection pathways, chemical contaminants can also alter aggression and dominance hierarchies among individuals. For example, captive rainbow trout (Oncorhynchus mykiss) exposed to cadmium, which damages the olfactory epithelium, were less aggressive towards an unexposed rival and, therefore, formed dominance hierarchies faster [40].
Interestingly, some chemicals, such as psychoactive pharmaceuticals, have actually been designed to modulate adaptive stress or fear responses. Thus, they have great potential to impact foraging and anti-predator responses of wild animals (e.g. [41–44]). Indeed, recent studies have shown that exposure of fish to environmentally relevant concentrations of the antidepressant fluoxetine can extend the duration of ‘freezing’ behaviour [44] after predatory attack and increase activity levels regardless of the presence of a predator [43]. Because natural selection favours individuals that can quickly and accurately detect and assess risk, any disruption of this fine-tuned system is likely to have important implications for individual fitness [45] (see electronic supplementary material for more on predator–prey effects).
(b). Plasticity
Individuals can adjust their behaviour in response to chemical contaminants, i.e. they show phenotypic plasticity [7]. This ‘plasticity’ in behaviours has been the subject of much interest in behavioural ecology, because of its role in enabling species to cope with rapid environmental change [46,47]. However, most ecotoxicological studies so far have focused primarily on the mean behavioural responses of the contaminated population, with little to no mention of the variance in the trait. To date, we are unaware of any research explicitly investigating how contaminants can modulate behavioural plasticity or flexibility (i.e. how responsive individuals are to environmental variation) (but see [41]; §5). Predictions as to how plasticity will be modulated by chemical contaminants are not straightforward. If a behaviour is attenuated by a contaminant by, for example, all individuals becoming inactive regardless of environmental conditions, this could erode plasticity. Thus, there would be no benefit to individuals having variable responses to environmental changes, because they would never be expressed. Consequently, over time, this could decrease the intensity of selection for plasticity. In turn, this could reduce population variation in responsiveness to environmental change, reflecting a decrease in variance in behavioural responsiveness of all individuals. Conversely, one study found that exposure of jumping spiders (Eris militaris) to pesticides led to an increase in within-individual behavioural variability, while not changing the population's average level of predatory behaviour [48]. There is a clear need to integrate new experimental designs, technologies and statistical approaches (e.g. [35,47–50]) from behavioural ecology to measure individual behavioural responses under varying environmental conditions, such as, for example, multi-stressor studies, to better understand the consequences of contaminant exposure.
(c). Chemical contamination drives evolution
There is growing interest in the long-term, multi-generational consequences of chemical contamination and how contaminants might modulate population persistence and evolutionary trajectories. Our current focus is on how selection can act directly on exposed organisms, although it is important to acknowledge that selection may also operate indirectly via impacts of chemical contaminants on, for example, a species' prey, or competitors (see §4).
It is established that exposure to chemical contaminants can result in the evolution of physiological resistance, with perhaps the best-studied example being the micro-evolution of resistance in populations exposed to metal pollution (see [51,52]). By contrast, far less is known about how this resistance might affect the subsequent behavioural responses of exposed organisms. Adaptive physiological adjustments could reduce the likelihood that downstream behaviours are maladaptive. On the other hand, changes in physiology can also have negative effects on behaviour and life histories via the reallocation of resources required for growth and reproduction. For example, laboratory selection for cadmium resistance in least killifish (Heterandria formosa) resulted in decreased fecundity, female life expectancy and brood size [53]. Whether such trade-offs also impinge on behaviour remains to be tested.
Even in the absence of physiological resistance, organisms can simply change their behaviour, for example altering their diet, to avoid contaminants. However, it is often unclear whether these behavioural changes reflect plasticity or evolved responses [54,55]. Studies have shown spatial avoidance of contaminated sediments and water by aquatic invertebrates [55] and vertebrates [54,55], as well as adjustment of migration routes by salmon in response to metal pollution [56]. Other species show temporal avoidance of potential contaminant exposure by employing a faster life history or changing reproductive timing [52]. An interesting hypothesis is that the adaptive potential of an organism to respond rapidly to strong selection favouring earlier maturation and reproduction could, in turn, facilitate adaptations to novel stressors, such as chemical contaminants [57].
If organisms have neither evolved physiological tolerance nor behavioural compensation, exposure to chemical contaminants can result in drastic population declines [58]. This potentially creates a destructive feedback loop where a reduction in population size leads to further loss of genetic diversity, thus restricting the adaptive potential of populations [59,60], including adaptive behavioural responses. Chemical contaminants (e.g. persistent organic pollutants) can also affect mutation rate (e.g. [61]), which may either compensate for the loss of genetic diversity during population bottlenecks (e.g. marsh frogs, Rana ridibunda [62]) or otherwise alter population responses to contaminants [63]. However, most contaminant-induced mutations are likely to be deleterious [64]. Thus, adaptive behaviour that shields genotypes from otherwise harsh selection imposed by chemical contaminants could allow for population persistence and the maintenance of adequate levels of standing genetic variation crucial for further adaptation [65].
Chemical contaminants can also impact the strength and targets of selection via their direct effects on behaviour. For example, because sexually selected behaviours can affect the rate and trajectory of evolution (e.g. [66]), contaminants that interfere with sexual selection (e.g. endocrine-disrupting chemicals, EDCs; [67]) have considerable potential to affect subsequent evolution. For example, in European starlings, treatment with an EDC mixture resulted in males producing longer and more complex songs that are preferred by females, despite exposed males also having suppressed immune responses [68]. Whereas, in guppies (Poecilia reticulata), exposure to the agricultural contaminant 17β-trenbolone increased the occurrence of coercive copulatory behaviour in males, thus circumventing female mate choice [69]. While such changes that weaken sexual selection could further contribute to population decline [70], some studies find the opposite effect, whereby sexual selection enhances the evolution of mechanisms to cope with contaminants, presumably resulting in population growth. For example, flour beetles (Tribolium castaneum) evolved resistance to a pyrethroid pesticide faster when sexual selection was allowed to occur compared with when it was experimentally precluded [71].
Given the importance of evolution in facilitating population persistence, a key question is: what might limit the ability of organisms to evolve adaptive physiological or behavioural responses to contaminants? One possibility is that it may be difficult to adaptively respond simultaneously to multiple contaminants, or, more broadly, multiple stressors that exert conflicting selection pressures [72]. Resistance to a single class of contaminants, such as pesticides, can evolve very quickly, but evolving resistance to cocktails of contaminants with different modes of action is likely to be much slower. Here, the ability to cope with a particular contaminant could make it more difficult to deal with another [63]. A complementary idea emphasizes the role of evolutionary history—i.e. the notion that organisms often have greater difficulty coping with stressors that are truly ‘novel’, as opposed to those that are mechanistically similar to those that are familiar [73]. Clearly, there is a need for a deeper mechanistic understanding of when and why plastic or evolutionary responses to one contaminant should facilitate or conflict with responses to another.
3. Indirect effects of chemical contaminants on behaviour via interspecies interactions
Contaminants can, as outlined above, exert direct effects on the behaviour of species, which often results in decreases in organism abundance. However, species and their behaviours can also be altered indirectly because changes in behaviour (or abundance) of susceptible species will lead to cascading indirect effects—even on resistant species—at all trophic levels within a community. One of the most commonly documented indirect effects of contamination is predator responses to reduced prey abundance caused by contaminant-induced direct lethality or reproductive failure in their prey species. A population crash of fathead minnows (Pimephales promelas), caused by experimental EE2-exposure of a whole lake, led to cascading indirect effects: zooplankton populations in the exposed lake increased without minnow predation, while the biomass of larger lake trout (Salvelinus namaycush) decreased without minnows as a prey item [14]. Indirect effects can also reduce the efficacy of ecosystem services provided by wildlife. For instance, population crashes of Gyps vultures in India due to diclofenac toxicity resulted in an increase in feral dogs scavenging on decaying carcasses and a consequent increase in human rabies infections from dog bites [74]. By contrast, examples of indirect effects caused specifically by changes to animal behaviour are rare in the literature [16]. For example, mummichog (Fundulus heteroclitus) from industrial sites were less active and less adept at capturing prey grass shrimp (Palaemonetes paludosus) than were fish at pristine sites, allowing these prey to grow larger and become more abundant [75]. We predict that contaminant-induced increases in boldness or aggression in one species, for example, will change the competition and predation pressures on, and thus alter the behaviour of, other species within a community (figure 1). Contaminant-disrupted courtship leading to declines in abundance is predicted to have cascading effects on the interspecies interactions across a community. Here, we use cascading effects as a tool to illustrate the importance of indirect effects in ecological risk assessment, although other indirect effects such as keystone predator effects and exploitative competition can also be locally important [76]. The key point, here, is the need to understand the mechanism, i.e. the contaminant-induced change in behaviour(s), initiating the cascade.
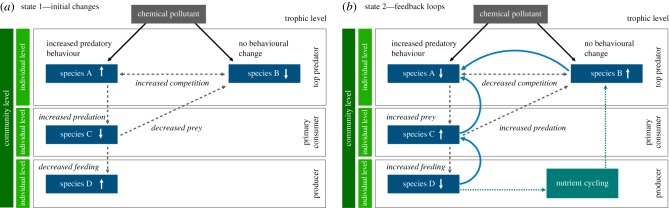
Figure 1.
Outline of our conceptual framework modelling the direct and indirect effects of a chemical contaminant using predator–prey dynamics as a case study. Two predatory species (A and B) are exposed to a chemical contaminant. (a) State 1 shows initial changes to species in the food web at the individual and community levels; (b) state 2 includes feedback loops, which show dynamic interactions between species in time and space. Increases and decreases in population size for each species are indicated by arrows. The solid arrows indicate direct effects, dashed arrows indirect effects, dotted arrows nutrient cycling and blue arrows species interactions.
Given the complexity of studying multi-species responses to contaminants [12], it is not surprising that indirect community effects, particularly those acting via changed behaviours, have not yet been broadly studied and quantified. First, multiple organisms must be studied simultaneously in real time using environmentally realistic mesocosms or field-based studies. Second, the system often must be studied for longer durations than are typical of laboratory exposures (i.e. several months to years). One might argue that studying indirect effects is redundant because the net effect on the community is the ultimate endpoint. However, because species compositions differ between most environments and reactions to contaminants can be highly species-specific, the net effect on a mesocosm community will only provide the outcome for that particular community. Without a mechanistic understanding of which behaviours in which species are affected and how, the generality, and, as such, the predictive power of mesocosm studies for risk assessment of particular contaminants, is limited at best. Knowledge of indirect effects is also crucial for modelling ecological risk, a promising and cost-effective tool that will help to reduce the number of animals required for ecotoxicological testing.
4. Conceptual framework for understanding the ecological and evolutionary impacts of chemical contaminants
Here, we have developed a conceptual framework that can be used by researchers aiming to design experiments or research programmes that move away from the ‘one chemical–one species–one (usually lethal) endpoint’ style of ecotoxicology (but see [71]) towards a more holistic approach. Specifically, our framework demonstrates the direct and indirect effects of chemical contaminants on the behaviour of individuals within a population, and of species within communities. We draw upon knowledge and literature from ecology and lay out potential scenarios of community-level effects caused by chemical contaminants (figure 1). As communities are composed of interconnected populations overlapping in time and space, the effects of chemical contaminants on communities necessarily manifest in the interactions within and among populations [72]. For example, some of the most salient interactions shaping ecological communities worldwide are between prey and their predators [72,73]. All animals are either prey or predators at some point in their lives and this interaction often has considerable consequences on individual fitness and population size [74].
Imagine that a chemical contaminant is introduced into an ecosystem. This chemical does not change the behaviour of top predator ‘species B’, but does increase the boldness of a second top predator ‘species A’, resulting in species A taking more risks, spending longer foraging and less time avoiding predators. ‘Species C’, the prey of species A, which is resistant to the contaminant, is indirectly affected because of the increased time and energy spent on anti-predator behaviours, but it is still consumed at a higher rate than when the ecosystem was uncontaminated. Thus, prey species C decreases in numbers, which, in turn, causes its own plant prey ‘species D’ to proliferate, thereby shifting the nutrient cycling and changing the ecosystem for all species (figure 1a). Notably, if the contaminant's action was conserved across taxa, such that species C also became bolder, its population would rapidly decline by predation-induced mortality from species A. Further, the decreased numbers of prey species C could potentially result in predator species B changing its foraging preference to alternative prey. The risky behaviour of species A will increase its own probability of being preyed upon, attacked by competitor species B and/or eating novel but toxic or infected foods. This would, in turn, decrease the predation pressure from predator species A on species C, and could potentially decrease competition between species A and B (figure 1b) [72]. We have included dynamic feedback loops to magnify the actions of the chemical contaminant on both directly and indirectly affected species, which, in turn, have community-level consequences and can alter ecosystem functioning (figure 1b).
Importantly, indirect effects due to contaminant-induced behavioural shifts could cause systems to respond far more strongly and quickly than an assessment of direct effects alone, or simply monitoring changes in the abundance of key predators, would predict [73]. Moreover, contaminant-mediated effects could yield novel forms of ecological interactions by, for example, inducing prey-switching due to changes in predatory behaviour and/or changes in prey abundance or quality, or by differentially altering the vulnerability of individuals or species to parasites [75]. Also, we have focused on the top-down effects, but some contaminants will affect primary productivity and so will have bottom-up impacts. These can be difficult to predict but, again, could have indirect, sublethal effects by increasing competition for food and/or necessitating greater foraging distances. Such a framework allows us to integrate and go beyond individual experiments and encourages researchers to assess behavioural change within its environmental context. By understanding the behavioural mechanism underpinning multi-level changes, modelling, for example, can be used to predict the impacts of contaminants with similar modes of action for enhanced environmental risk assessments [77]. As an implementation plan, we provide figure 2, which directs researchers to consider which experimental design (laboratory, mesocosm or whole ecosystem manipulations) and level (individual, species or community) or modelling approaches are required, and which endpoints should or could be tested. Our basic framework can, therefore, be applied to specific behaviours and/or interspecific interactions, as well as to different levels of organisation, as required.
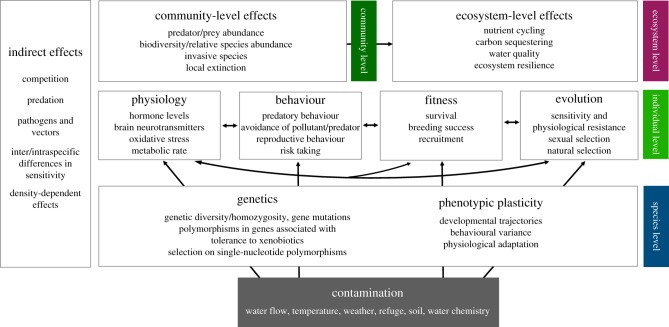
Figure 2.
Implementation plan suggesting methodological approaches for utilizing our conceptual framework to identify the routes by which animal behaviour is affected by chemical contaminants. For each level of biological organization (individual, species, community and ecosystem), we highlight some of the factors that should or could be quantified or experimentally manipulated.
5. Problems of scale and complexity: predicting effects in the wild from effects in the laboratory
Predicting the ecological effects and behavioural perturbations caused by chemical contaminants is valuable for guiding legislation and policy to protect wildlife, but it is also challenging for many reasons. Behaviour is inherently variable—although so are many of the physiological endpoints currently measured—and how organisms respond to any given contaminant may vary across an individual's lifetime, between sexes, among individuals of the same species, and across species with different life histories, habitat use, trophic position and/or physiology [7,10,33,75,78].
Most earlier standardized ecotoxicological tests used model species that are easily cultured with simple, measurable endpoints [4], which allowed direct comparisons of toxicity among different compounds. This long-used approach has efficiently generated hazard and risk assessments for many chemical contaminants under the premise that similar species are equally affected by the contaminant. Of course, the ‘all species are the same’ argument does not hold for the effects of many contaminants (e.g. pharmaceuticals [79]). Inter- and intraspecies differences in physiology, behaviour and life history, when coupled with differential metabolism, generate substantial differences among species and individuals in susceptibility and responses to chemical contaminants. Unfortunately, our understanding of comparative mechanistic responses to contaminants still remains quite limited, even for model laboratory organisms.
Susceptibility differences between species are one of the key challenges in ecotoxicology. For example, studies have shown that small wild-caught prey fish are more sensitive to the anxiolytic effects of the pharmaceutical oxazepam than are larger predatory fish or laboratory-reared fish [5,80,81]. This could be due to species differences in the rate and extent of pharmaceuticals being taken up, metabolized and concentrated. Indeed, bioconcentration of pharmaceuticals in fish tissues can differ by several orders of magnitude between species [82], and even across life history stages [83]. Therefore, two species inhabiting the same polluted system can be exposed to very different internal concentrations of contaminants [81]. Moreover, tests including a less vulnerable life-stage might underestimate ecological risk [83]. Such differential exposures, and the associated effects, make it very difficult to predict the ecological effects of chemical contaminants in the environment [16].
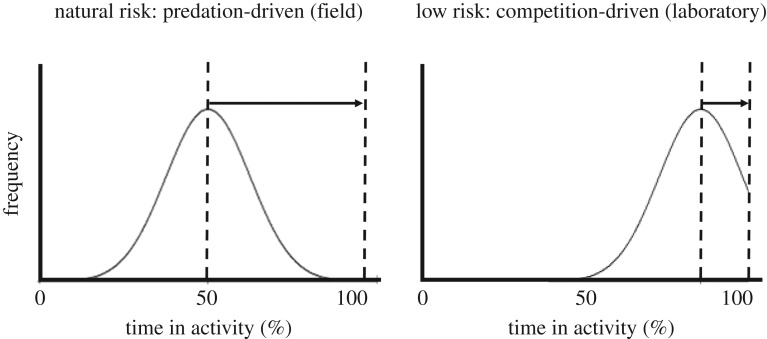
Differential behavioural responses to chemical contaminants in laboratory-reared versus wild species have also been explained by the lack of predation risk or high competition in laboratory environments, which selects for inherited behavioural phenotypes that are often bolder, more aggressive and less responsive to predators than wild-type individuals [84]. For example, in assessing the risk of chemicals that potentially modify anti-predator behaviour, using a laboratory fish model that may exhibit a suppressed basal behavioural response to predators may greatly underestimate actual risk in the field (figure 3). Also, the distribution of behavioural traits studied should be characterized within each test group [83]. This consideration is critically important because a contaminant that acts to increase activity and/or boldness will more probably generate behavioural change in individuals originating from a (wild-type) population of low competition/high predation, compared with a (laboratory-reared) high-competition/low-predation population that contains many active and bold individuals (figure 3). Even in the wild, populations of the same species under different predation pressures are known to have evolved different physiology, morphology and behaviours [84]. In terms of our conceptual framework, such population-level differences in behavioural responses will alter both the state of a community before contamination, and the magnitude of feedback loops triggered by a contaminant. Such differences between populations, generated by differing selection regimes, have received very little attention despite clearly being important considerations when assessing contaminant vulnerability.
Figure 3.
The distribution of expressions of a trait (here, activity) in two populations from environments with different levels of predation risk. (a) Population collected from the field (high predation); (b) laboratory-bred population (low predation). Black arrows illustrate the potential for contaminant-induced increases in activity in the populations (the longer the arrow, the greater the potential change).
6. Future directions
The use of behavioural studies enables us to link the effects of contaminants at multiple levels of organization, from individual to ecosystem. This is an invaluable asset, because chemical contaminants have a wide range of actions and effects. At the individual level, the fields of behavioural ecology and so-called ‘personalized medicine’ are increasingly realizing the need to analyse inter-individual variation in responses, not just population means [46]. Far from being ‘noise’, plasticity in responses in itself represents a trait that can shape the capacity of individuals and populations to cope with environmental change in the short term. In this review, we illustrate that chemical contaminants can impact the capacity of populations to persist into the future by altering the strength and targets of evolutionary selection, for example, via direct effects of behaviour. To date, a mechanistic understanding of how evolutionary and plastic responses interact to facilitate population persistence is lacking. This also limits our ability to predict how populations will respond if legislation succeeds in reducing concentrations of specific chemical contaminants. Consequently, we have identified avenues to fill the knowledge gaps and challenge the often simplistic assessment of direct effects of contaminants, specifically in terms of how behaviour and other endpoints should be measured, analysed and interpreted.
With the rise in emerging contaminants, many of which are designed to exert sublethal effects on evolutionarily conserved physiological systems at ecologically realistic concentrations, it is important to update existing frameworks for studying their short- and long-term consequences. Sublethal behavioural effects can be both ‘positive’ and ‘negative’ for individuals, populations and communities. As illustrated by our conceptual framework (figure 1), effects can vary dynamically within the same individuals and populations. Indeed, this could be described as a key feature of emerging or dilute contaminants. Importantly, behavioural effects can lead to top-down and/or bottom-up effects. For example, changes at a lower trophic level could have sublethal effects by increasing competition for food and/or necessitating greater foraging distances. This is because linkages within communities will act indirectly to alter and even magnify contaminant-induced effects. Future work, integrating modelling, remote sensors and tracking technologies and statistical analyses, should focus on quantifying changes on the individual level and how the linkages within these networks are affected by contaminants. We argue that understanding the behavioural and ecological mechanisms underpinning contaminant-induced population changes will greatly increase the accuracy and power of environmental risk assessment to protect wildlife and ecosystems from disturbance by chemical contaminants.
Supplementary Material
Supplementary Material
Acknowledgements
We thank the attendees of the ‘Behavioural responses to human-induced environmental change’ workshop at the 16th International Society for Behavioural Ecology Congress 2016 for their input and Anna Hatzisavas for editing the figures.
Data accessibility
This article has no additional data.
Authors' contributions
M.S., T.B. and K.E.A. organized the symposia on which this paper is based, developed the conceptual framework, edited the manuscript and created figures. All authors contributed to publication writing. All authors gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
Support for this review was provided by Academy of Finland Postdoctoral Researcher Fellowship (265629) (M.S.), Swedish Research Council Formas (2013-4431) (T.B.), NSERC Discovery Grant (S.B.), Australian Postgraduate Award Scholarship (M.G.B.), US National Science Foundation (Project: CHE-1339637) and US Environmental Protection Agency (B.W.B.), National Science Foundation Graduate Research Fellowship (S.M.E.), Wenner-Gren Foundation Postdoctoral Fellowship (E.S.M.), US National Science Foundation (IOS 1456724) (A.S.), the Swedish Research Council Formas (2013-947) (J.S.), Discovery Grant from the Australian Research Council (DP160100372) (B.B.M.W.) and University of York grant (K.E.A.).
References
- 1.UNEP. 2013. Global chemicals outlook—towards sound management of chemicals, pp. 11–15. Geneva, Switzerland: United Nations Environment Programme. [Google Scholar]
- 2.Kookana RS, et al. 2014. Potential ecological footprints of active pharmaceutical ingredients: an examination of risk factors in low-, middle- and high-income countries. Phil. Trans. R. Soc. B 369, 20130586 ( 10.1098/rstb.2013.0586) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hellou J. 2011. Behavioural ecotoxicology, an ‘early warning’ signal to assess environmental quality. Environ. Sci. Pollut. Res. 18, 1–11. ( 10.1007/s11356-010-0367-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.OECD. 2012. test No. 229: fish short term reproduction assay, pp. 1–40. Paris, France: OECD Publishing. [Google Scholar]
- 5.Klaminder J, Hellström G, Fahlman J, Jonsson M, Fick J, Lagesson A, Bergman E, Brodin T. 2016. Drug-induced behavioral changes: using laboratory observations to predict field observations. Front. Environ. Sci. 4, 81 ( 10.3389/fenvs.2016.00081) [DOI] [Google Scholar]
- 6.Levin SA, Harwell MA, Kelly JR, Kimball KD. 1989. Ecotoxicology: problems and approaches. New York, NY: Springer. [Google Scholar]
- 7.Wong BB.M, Candolin U. 2015. Behavioral responses to changing environments. Behav. Ecol. 26, 665–673. ( 10.1093/beheco/aru183) [DOI] [Google Scholar]
- 8.Clotfelter ED, Bell AM, Levering KR. 2004. The role of animal behaviour in the study of endocrine-disrupting chemicals. Anim. Behav. 68, 665–676. ( 10.1016/j.anbehav.2004.05.004) [DOI] [Google Scholar]
- 9.Zala SM, Penn DJ. 2004. Abnormal behaviours induced by chemical pollution: a review of the evidence and new challenges. Anim. Behav. 68, 649–664. ( 10.1016/j.anbehav.2004.01.005) [DOI] [Google Scholar]
- 10.Melvin SD, Wilson SP. 2013. The utility of behavioral studies for aquatic toxicology testing: a meta-analysis. Chemosphere 93, 2217–2223. ( 10.1016/j.chemosphere.2013.07.036) [DOI] [PubMed] [Google Scholar]
- 11.Arnold KE, Brown AR, Ankley GT, Sumpter JP. 2014. Medicating the environment: assessing risks of pharmaceuticals to wildlife and ecosystems. Phil. Trans. R. Soc. B 369, 20130569 ( 10.1098/rstb.2013.0569) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fleeger JW, Carman KR, Nisbet RM. 2003. Indirect effects of contaminants in aquatic ecosystems. Sci. Total Environ. 317, 207–233. ( 10.1016/S0048-9697(03)00141-4) [DOI] [PubMed] [Google Scholar]
- 13.Clements WH, Rohr JR. 2009. Community responses to contaminants: using basic ecological principles to predict ecotoxicological effects. Environ. Toxicol. Chem. 28, 1789–1800. ( 10.1897/09-140.1) [DOI] [PubMed] [Google Scholar]
- 14.Kidd KA, Paterson MJ, Rennie MD, Podemski CL, Findlay DL, Blanchfield PJ, Liber K. 2014. Direct and indirect responses of a freshwater food web to a potent synthetic oestrogen. Phil. Trans. R. Soc. B 369, 20130578 ( 10.1098/rstb.2013.0578) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rohr JR, Kerby JL, Sih A. 2006. Community ecology as a framework for predicting contaminant effects. Trends Ecol. Evol. 21, 606–613. ( 10.1016/j.tree.2006.07.002) [DOI] [PubMed] [Google Scholar]
- 16.Brodin T, Heynen M, Fick J, Klaminder J, Piovano S, Jonsson M. 2014. Inconspicuous effects of pharmaceuticals in aquatic systems—ecological impacts through behavioural modifications at dilute concentrations. Phil. Trans. R. Soc. B 369, 20130580 ( 10.1098/rstb.2013.0580) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Halstead N, McMahon T., Johnson S., Raffel T., Romansic J., Crumrine P., Rohr J, Fussmann G. 2014. Community ecology theory predicts the effects of agrochemical mixtures on aquatic biodiversity and ecosystem properties. Ecol. Lett. 17, 932–941. ( 10.1111/ele.12295) [DOI] [PubMed] [Google Scholar]
- 18.Sopinka N, Marentette J, Balshine S. 2010. Impact of contaminant exposure on resource contests in an invasive fish. Behav. Ecol. Sociobiol. 64, 1947–1958. ( 10.1007/s00265-010-1005-1) [DOI] [Google Scholar]
- 19.McCallum ES, Krutzelmann E, Brodin T, Fick J, Sundelin A, Balshine S. 2017. Exposure to wastewater effluent affects fish behaviour and tissue-specific uptake of pharmaceuticals. Sci. Total Environ. 605–606, 578–588. ( 10.1016/j.scitotenv.2017.06.073) [DOI] [PubMed] [Google Scholar]
- 20.Soeffker M, Tyler CR. 2012. Endocrine disrupting chemicals and sexual behaviors in fish—a critical review on effects and possible consequences. Crit. Rev. Toxicol. 42, 653–668. ( 10.3109/10408444.2012.692114) [DOI] [PubMed] [Google Scholar]
- 21.Hotchkiss AK, Rider CV, Blystone CR, Wilson VS, Hartig PC, Ankley GT, Foster PM, Gray CL, Gray LE. 2008. Fifteen years after “Wingspread”—environmental endocrine disrupters and human and wildlife health: where we are today and where we need to go. Toxicol. Sci. 105, 235–259. ( 10.1093/toxsci/kfn030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lopez-Antia A, Ortiz-Santaliestra ME, Mougeot F, Mateo R. 2013. Experimental exposure of red-legged partridges (Alectoris rufa) to seeds coated with imidacloprid, thiram and difenoconazole. Ecotoxicology 22, 125–138. ( 10.1007/s10646-012-1009-x) [DOI] [PubMed] [Google Scholar]
- 23.Grue CE, Gibert PL, Seeley ME. 1997. Neurophysiological and behavioral changes in non-target wildlife exposed to organophosphate and carbamate pesticides: thermoregulation, food consumption, and reproduction. Am. Zool. 37, 369–388. ( 10.1093/icb/37.4.369) [DOI] [Google Scholar]
- 24.Park D, Hempleman SC, Propper CR. 2001. Endosulfan exposure disrupts pheromonal systems in the red-spotted newt: a mechanism for subtle effects of environmental chemicals. Environ. Health Perspect. 109, 669–673. ( 10.1289/ehp.01109669) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Little EE, Finger SE. 1990. Swimming behavior as an indicator of sublethal toxicity in fish. Environ. Toxicol. Chem. 9, 13–19. ( 10.1002/etc.5620090103) [DOI] [Google Scholar]
- 26.Robinson PD. 2009. Behavioural toxicity of organic chemical contaminants in fish: application to ecological risk assessments (ERAs). Can. J. Fish. Aquat. Sci. 66, 1179–1188. ( 10.1139/F09-069) [DOI] [Google Scholar]
- 27.Cote J, Clobert J, Brodin T, Fogarty S, Sih A. 2010. Personality-dependent dispersal: characterization, ontogeny and consequences for spatially structured populations. Phil. Trans. R. Soc. B 365, 4065–4076. ( 10.1098/rstb.2010.0176) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herborn KA, Macleod R, Miles WTS, Schofield AN.B., Alexander L, Arnold KE. 2010. Personality in captivity reflects personality in the wild. Anim. Behav. 79, 835–843. ( 10.1016/j.anbehav.2009.12.026) [DOI] [Google Scholar]
- 29.Lürling M, Scheffer M. 2007. Info-disruption: pollution and the transfer of chemical information between organisms. Trends Ecol. Evol. 22, 374–379. ( 10.1016/j.tree.2007.04.002) [DOI] [PubMed] [Google Scholar]
- 30.Scholz NL, Truelove NK, French BL, Berejikian BA, Quinn TP, Casillas E, Collier TK. 2000. Diazinon disrupts antipredator and homing behaviors in chinook salmon (Oncorhynchus tshawytscha). Can. J. Fish. Aquat. Sci. 57, 1911–1918. ( 10.1139/f00-147) [DOI] [Google Scholar]
- 31.van der Sluijs I, et al. 2011. Communication in troubled waters: responses of fish communication systems to changing environments. Evol. Ecol. 25, 623–640. ( 10.1007/s10682-010-9450-x) [DOI] [Google Scholar]
- 32.Sloman KA, Lepage O, Rogers JT, Wood CM, Winberg S. 2005. Socially-mediated differences in brain monoamines in rainbow trout: effects of trace metal contaminants. Aquat. Toxicol. 71, 237–247. ( 10.1016/j.aquatox.2004.11.008) [DOI] [PubMed] [Google Scholar]
- 33.Scott GR, Sloman KA. 2004. The effects of environmental pollutants on complex fish behaviour: integrating behavioural and physiological indicators of toxicity. Aquat. Toxicol. 68, 369–392. ( 10.1016/j.aquatox.2004.03.016) [DOI] [PubMed] [Google Scholar]
- 34.DuRant SE, Hopkins WA, Talent LG. 2007. Impaired terrestrial and arboreal locomotor performance in the western fence lizard (Sceloporus occidentalis) after exposure to an AChE-inhibiting pesticide. Environ. Pollut 149, 18–24. ( 10.1016/j.envpol.2006.12.025) [DOI] [PubMed] [Google Scholar]
- 35.Hellström G, Klaminder J, Finn F, Persson L, Alanärä A., Jonsson M, Fick J, Brodin T. 2016. GABAergic anxiolytic drug in water increases migration behaviour in salmon. Nat. Commun. 7, 13460 ( 10.1038/ncomms13460) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marentette JR, Tong S, Wang G, Sopinka NM, Taves MD, Koops MA, Balshine S. 2012. Behavior as biomarker? Laboratory versus field movement in round goby (Neogobius melanostomus) from highly contaminated habitats. Ecotoxicology 21, 1003–1012. ( 10.1007/s10646-012-0854-y) [DOI] [PubMed] [Google Scholar]
- 37.Flahr LM, Michel NL, Zahara AR.D., Jones PD, Morrissey CA. 2015. Developmental exposure to Aroclor 1254 alters migratory behavior in juvenile European starlings (Sturnus vulgaris). Environ. Sci. Technol. 49, 6274–6283. ( 10.1021/acs.est.5b01185) [DOI] [PubMed] [Google Scholar]
- 38.Ward AJW, Duff AJ, Horsfall JS, Currie S. 2008. Scents and scents-ability: pollution disrupts chemical social recognition and shoaling in fish. Proc. R. Soc. B 275, 101–105. ( 10.1098/rspb.2007.1283) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dew WA, Azizishirazi A, Pyle GG. 2014. Contaminant-specific targeting of olfactory sensory neuron classes: connecting neuron class impairment with behavioural deficits. Chemosphere 112, 519–525. ( 10.1016/j.chemosphere.2014.02.047) [DOI] [PubMed] [Google Scholar]
- 40.Sloman KA. 2007. Effects of trace metals on salmonid fish: the role of social hierarchies. App. Anim. Behav. Sci. 104, 326–345. ( 10.1016/j.applanim.2006.09.003) [DOI] [Google Scholar]
- 41.Bean TG, Boxall ABA, Lane J, Herborn KA, Pietravalle S, Arnold KE. 2014. Behavioural and physiological responses of birds to environmentally relevant concentrations of an antidepressant. Phil. Trans. R. Soc. B 369, 20130575 ( 10.1098/rstb.2013.0575) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brodin T, Fick J, Jonsson M, Klaminder J. 2013. Dilute concentrations of a psychiatric drug alter behavior of fish from natural populations. Science 339, 814–815. ( 10.1126/science.1226850) [DOI] [PubMed] [Google Scholar]
- 43.Martin JM, Saaristo M, Bertram MG, Lewis PJ, Coggan TL, Clarke BO, Wong BBM. 2017. The psychoactive pollutant fluoxetine compromises antipredator behaviour in fish. Environ. Pollut. 222, 592–599. ( 10.1016/j.envpol.2016.10.010) [DOI] [PubMed] [Google Scholar]
- 44.Saaristo M, McLennan A, Johnstone CP, Clarke BO, Wong BB.M. 2017. Impacts of the antidepressant fluoxetine on the anti-predator behaviours of wild guppies (Poecilia reticulata). Aquat. Toxicol. 183, 38–45. ( 10.1016/j.aquatox.2016.12.007) [DOI] [PubMed] [Google Scholar]
- 45.Cresswell W. 2008. Non-lethal effects of predation in birds. Ibis 150, 3–17. ( 10.1111/j.1474-919X.2007.00793.x) [DOI] [Google Scholar]
- 46.Dingemanse NJ, Kazem AJ.N., Reale D, Wright J. 2010. Behavioural reaction norms: animal personality meets individual plasticity. Trends Ecol. Evol. 25, 81–89. ( 10.1016/j.tree.2009.07.013) [DOI] [PubMed] [Google Scholar]
- 47.Herborn KA, Heidinger BJ, Alexander L, Arnold KE. 2014. Personality predicts behavioral flexibility in a fluctuating, natural environment. Behav. Ecol. 25, 1374–1379. ( 10.1093/beheco/aru131) [DOI] [Google Scholar]
- 48.Royauté R., Buddle CM, Vincent C. 2015. Under the influence: sublethal exposure to an insecticide affects personality expression in a jumping spider. Funct. Ecol. 29, 962–970. ( 10.1111/1365-2435.12413) [DOI] [Google Scholar]
- 49.Cleasby IR, Nakagawa S, Schielzeth H, Hadfield J. 2015. Quantifying the predictability of behaviour: statistical approaches for the study of between-individual variation in the within-individual variance. Methods Ecol. Evol. 6, 27–37. ( 10.1111/2041-210X.12281) [DOI] [Google Scholar]
- 50.Snijders L, Blumstein DT, Stanley CR, Franks DW. 2017. Animal social network theory can help wildlife conservation. Trends Ecol. Evol. 32, 567–577. ( 10.1016/j.tree.2017.05.005) [DOI] [PubMed] [Google Scholar]
- 51.Medina MH, Correa JA, Barata C. 2007. Micro-evolution due to pollution: possible consequences for ecosystem responses to toxic stress. Chemosphere 67, 2105–2114. ( 10.1016/j.chemosphere.2006.12.024) [DOI] [PubMed] [Google Scholar]
- 52.Hamilton PB, Rolshausen G, Webster TM.U, Tyler CR. 2017. Adaptive capabilities and fitness consequences associated with pollution exposure in fish. Phil. Trans. R. Soc. B 372, 20160042 ( 10.1098/rstb.2016.0042) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xie LT, Klerks PL. 2004. Changes in cadmium accumulation as a mechanism for cadmium resistance in the least killifish Heterandria formosa. Aquat. Toxicol. 66, 73–81. ( 10.1016/j.aquatox.2003.08.003) [DOI] [PubMed] [Google Scholar]
- 54.Silva D, Araujo CVM, Lopez-Doval JC, Neto MB, Silva FT, Paiva TCB, Pompeo MLM. 2017. Potential effects of triclosan on spatial displacement and local population decline of the fish Poecilia reticulata using a non-forced system. Chemosphere 184, 329–336. ( 10.1016/j.chemosphere.2017.06.002) [DOI] [PubMed] [Google Scholar]
- 55.Araujo CVM, Moreira-Santos M, Ribeiro R. 2016. Active and passive spatial avoidance by aquatic organisms from environmental stressors: a complementary perspective and a critical review. Environ. Int. 92–93, 405–415. ( 10.1016/j.envint.2016.04.031) [DOI] [PubMed] [Google Scholar]
- 56.Saunders RL, Sprague JB. 1967. Effects of copper-zinc mining pollution on a spawning migration of Atlantic salmon. Water Res. 1, 419 ( 10.1016/0043-1354(67)90051-6) [DOI] [Google Scholar]
- 57.Rolshausen G, et al. 2015. Do stressful conditions make adaptation difficult? Guppies in the oil-polluted environments of southern Trinidad. Evol App. 8, 854–870. ( 10.1111/eva.12289) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oaks JL, Gilbert M, Virani MZ, Watson RT, Meteyer CU, Rideout BA, Shivaprasad HL. et al. . 2004. Diclofenac residues as the cause of vulture population decline in Pakistan. Nature 427, 630–633. ( 10.1038/nature02317) [DOI] [PubMed] [Google Scholar]
- 59.Willi Y, Van Buskirk J, Hoffmann AA. 2006. Limits to the adaptive potential of small populations. Annu. Rev. Ecol. Evol. Syst. 37, 433–458. ( 10.1146/annurev.ecolsys.37.091305.110145) [DOI] [Google Scholar]
- 60.Blanquart F, Gandon S, Nuismer SL. 2012. The effects of migration and drift on local adaptation to a heterogeneous environment. J. Evol. Biol. 25, 1351–1363. ( 10.1111/j.1420-9101.2012.02524.x) [DOI] [PubMed] [Google Scholar]
- 61.Cachot J, Law M, Pottier D, Peluhet L, Norris M, Budzinski H, Winn R. 2007. Characterization of toxic effects of sediment-associated organic pollutants using the lambda transgenic medaka. Environ. Sci. Technol. 41, 7830–7836. ( 10.1021/es071082v) [DOI] [PubMed] [Google Scholar]
- 62.Matson CW, Lambert MM, McDonald TJ, Autenrieth RL, Donnelly KC, Islamzadeh A, Politov DI, Bickham JW. 2006. Evolutionary toxicology: population-level effects of chronic contaminant exposure on the marsh frogs (Rana ridibunda) of Azerbaijan. Environ. Health Perspect. 114, 547–552. ( 10.1289/ehp.8404) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Oziolor EM, De Schamphelaere K, Matson CW. 2016. Evolutionary toxicology: meta-analysis of evolutionary events in response to chemical stressors. Ecotoxicology 25, 1858–1866. ( 10.1007/s10646-016-1735-6) [DOI] [PubMed] [Google Scholar]
- 64.Loewe L, Hill WG. 2010. The population genetics of mutations: good, bad and indifferent. Phil. Trans. R. Soc. B 365, 1153–1167. ( 10.1098/rstb.2009.0317) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chevin LM, Lande R. 2010. When do adaptive plasticity and genetic evolution prevent extinction of a density-regulated population? Evolution 64, 1143–1150. ( 10.1111/j.1558-5646.2009.00875.x) [DOI] [PubMed] [Google Scholar]
- 66.Maan ME, Seehausen O. 2011. Ecology, sexual selection and speciation. Ecol. Lett. 14, 591–602. ( 10.1111/j.1461-0248.2011.01606.x) [DOI] [PubMed] [Google Scholar]
- 67.Gore AC, Holley AM, Crews D. 2017. Mate choice, sexual selection, and endocrine-disrupting chemicals. Horm. Behav. 101, 3–12. ( 10.1016/j.yhbeh.2017.09.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Markman S, Leitner S, Catchpole C, Barnsley S, Muller CT, Pascoe D, Buchanan KL. 2008. Pollutants increase song complexity and the volume of the brain area HVC in a songbird. PLoS ONE 3, e0001674 ( 10.1371/journal.pone.0001674) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bertram MG, Saaristo M, Baumgartner JB, Johnstone CP, Allinson M, Allinson G, Wong BBM. 2015. Sex in troubled waters: widespread agricultural contaminant disrupts reproductive behaviour in fish. Horm. Behav. 70, 85–91. ( 10.1016/j.yhbeh.2015.03.002) [DOI] [PubMed] [Google Scholar]
- 70.Martinez-Ruiz C, Knell RJ. 2017. Sexual selection can both increase and decrease extinction probability: reconciling demographic and evolutionary factors. J. Anim. Ecol. 86, 117–127. ( 10.1111/1365-2656.12601) [DOI] [PubMed] [Google Scholar]
- 71.Jacomb F, Marsh J, Holman L. 2016. Sexual selection expedites the evolution of pesticide resistance. Evolution 70, 2746–2751. ( 10.1111/evo.13074) [DOI] [PubMed] [Google Scholar]
- 72.Whitehead A, Clark BW, Reid NM, Hahn ME, Nacci D. 2017. When evolution is the solution to pollution: key principles, and lessons from rapid repeated adaptation of killifish (Fundulus heteroclitus) populations. Evol. App. 10, 762–783. ( 10.1111/eva.12470) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sih A, Trimmer PC, Ehlman SM. 2016. A conceptual framework for understanding behavioral responses to HIREC. Curr. Opin. Behav. Sci. 12, 109–114. ( 10.1016/j.cobeha.2016.09.014) [DOI] [Google Scholar]
- 74.Markandya A, Taylor T, Longo A, Murty MN, Murty S, Dhavala K. 2008. Counting the cost of vulture decline—an appraisal of the human health and other benefits of vultures in India. Ecol. Econ. 67, 194–204. ( 10.1016/j.ecolecon.2008.04.020) [DOI] [Google Scholar]
- 75.Weis J, Candelmo A. 2012. Pollutants and fish predator/prey behavior: a review of laboratory and field approaches. Curr. Zool. 58, 9–20. ( 10.1093/czoolo/58.1.9) [DOI] [Google Scholar]
- 76.Oksanen L, Fretwell SD, Arruda J, Niemela P. 1981. Exploitation ecosystems in gradients of primary productivity. Am. Nat. 118, 240–261. ( 10.1086/283817) [DOI] [Google Scholar]
- 77.Ankley GT, et al. 2010. Adverse outcome pathways: a conceptual framework to support ecotoxicology research and risk assessment. Environ. Toxicol. Chem. 29, 730–741. ( 10.1002/etc.34) [DOI] [PubMed] [Google Scholar]
- 78.Windsor FM, Ormerod SJ, Tyler CR. 2017. Endocrine disruption in aquatic systems: up-scaling research to address ecological consequences. Biol. Rev. 93, 626–641. ( 10.1111/brv.12360) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brown AR, Gunnarsson L, Kristiansson E, Tyler C. 2014. Assessing variation in the potential susceptibility of fish to pharmaceuticals, considering evolutionary differences in their physiology and ecology. Phil. Trans. R. Soc. B 369, 20130576 ( 10.1098/rstb.2013.0576) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Huerta B, Rodriguez-Mozaz S, Barcelo D. 2012. Pharmaceuticals in biota in the aquatic environment: analytical methods and environmental implications. Anal. Bioanal. Chem. 404, 2611–2624. ( 10.1007/s00216-012-6144-y) [DOI] [PubMed] [Google Scholar]
- 81.Brodin T, Nordling J, Lagesson A, Klaminder J, Hellstrom G, Christensen B, Fick J. 2017. Environmental relevant levels of a benzodiazepine (oxazepam) alters important behavioral traits in a common planktivorous fish (Rutilus rutilus). J. Toxicol. Environ. Health A 80, 963–970. ( 10.1080/15287394.2017.1352214) [DOI] [PubMed] [Google Scholar]
- 82.Lagesson A, Fahlman J, Brodin T, Fick J, Jonsson M, Bystrom P, Klaminder J. 2016. Bioaccumulation of five pharmaceuticals at multiple trophic levels in an aquatic food web—insights from a field experiment. Sci. Tot. Environ. 568, 208–215. ( 10.1016/j.scitotenv.2016.05.206) [DOI] [PubMed] [Google Scholar]
- 83.Kristofco LA, Cruz LC, Haddad SP, Behra ML, Chambliss CK, Brooks BW. 2016. Age matters: developmental stage of Danio rerio larvae influences photomotor response thresholds to diazinon or diphenhydramine. Aquat. Toxicol. 170, 344–354. ( 10.1016/j.aquatox.2015.09.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Huntingford FA. 2004. Implications of domestication and rearing conditions for the behaviour of cultivated fishes. J. Fish Biol. 65, 122–142. ( 10.1111/j.0022-1112.2004.00562.x) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This article has no additional data.