Abstract
We hypothesized differences in molecular strategies for similar journeys that migrants undertake to reproduce in spring and to overwinter in autumn. We tested this in redheaded buntings (Emberiza bruniceps) photoinduced into spring and autumn migratory states, with winter and summer non-migratory states as controls. Compared with controls, buntings fattened, gained weight and showed Zugunruhe (nocturnal migratory restlessness) in the migratory state. Spring migration was associated with greater fat and body mass, and higher intensity of Zugunruhe, compared with autumn migration. Circulating corticosterone levels were higher in spring, while T3 levels were higher in autumn. Hypothalamic expression of thyroid hormone-responsive (dio2, dio3), light-responsive (per2, cry1, adcyap1) and th (tyrosine hydroxylase, involved in dopamine biosynthesis) genes showed significant changes with transition from non-migratory to the migratory state. There were significantly higher mRNA expressions in autumn, except for higher th levels in the spring. Furthermore, the expression patterns of dnmt3a (not dnmt3b) and tet2 genes suggested an epigenetic difference between the non-migrant and migrant periods, and the spring and autumn migrant periods. These results demonstrate for the first time seasonal transition in hypothalamic gene expressions, and suggest differences in regulatory strategies at the transcriptional level for spring and autumn migrations in songbirds.
Keywords: bird, corticosterone, gene expression, hormone, migration, seasonal
1. Background
Millions of birds migrate along a latitudinal gradient twice a year: they fly south to wintering grounds in autumn (autumn migration) and begin return flight towards north to breeding grounds in spring (spring migration). Both seasonal migrations occur within a discrete window of time and involve striking alterations in the physiology and behaviour, as regulated by the prevailing photoperiod [1,2]. The specific photoperiod-induced changes linked with migration include fat deposition and weight gain, nocturnal Zugunruhe (migratory restlessness) and elevated circulating levels of triglycerides, free fatty acids, triiodothyronine (T3) and corticosterone (Cort) [1–4].
The two migrations differ in several ways. First, spring and autumn migrations placed before and after the breeding season, respectively, differ in context. Spring migration is relevant for the timely arrival of migrants at the breeding grounds, while the autumn migration is essentially for the wintering habitat with an adequate food resource. Further, migrating male birds have a stronger drive to reach their breeding territories and begin to migrate significantly earlier than females in spring, but not in autumn [5]. Thus, the timing and speed differ between two migratory journeys; overall, compared with the autumn, spring migrants have shorter timing, hence higher speed, longer flight duration and shorter stopovers [1,5,6]. Second, migrants differ in the physiological state between two migratory seasons: they are sensitive and refractory to the long-day photostimulation at the beginning of the spring and autumn migrations, respectively [7]. Third, there is a difference in the direction of photoperiod change during the course of two migrations; the migrants experience increasing and decreasing photoperiods during the spring and autumn journeys, respectively.
The anatomical localization of the regulatory site and genetic underpinnings of the seasonal migrations have not been clearly delineated, as yet. There is substantial evidence, however, for the mediobasal hypothalamus (MBH) as the regulatory site of the photoperiod-induced seasonal responses in long-day birds [8,9]. In most birds studied to date, the exposure to stimulatory long photoperiods causes a rapid reciprocal switching of genes coding for type 2 and type 3 deiodinases (dio2 and dio3). In turn, dio2 and dio3 mediate the conversion of local T4 (thyroxine) into its active (T3, triiodothyronine) and inactive (reverse T3) forms, respectively, and control the photoperiod-induced seasonal responses [9]. Intriguingly, dio3 seems to be a critical regulator for an appropriate level of the hypothalamic thyroid hormones [10,11]. It undergoes a dynamic transition in its expression with the photoperiod change, as shown by significantly decreased dio3 mRNA expressions in photosensitive and photorefractory redheaded buntings (Emberiza bruniceps) after their exposure to a day of the long and short photoperiods, respectively [12]. Furthermore, DNA methyltransferases (DNMTs) catalysed reversible DNA methylation can act as the molecular switch for dio3-regulated seasonal response, as evidenced by a study on Siberian hamsters, Phodopus sungorus [13]. De novo DNMT3a and DNMT3b carryout promoter methylation and regulate tissue-specific gene expressions [14], while ten-eleven translocase (TET) enzymes mediate the concurrent demethylation [15].
The hypothalamic expression of light-responsive core clock genes (bmal1, clock, per2, cry1) shows seasonal alterations in their 24 h expression patterns. For example, there were significant changes in the amplitude and/ or phase of the 24 h rhythm of these clock genes in the hypothalamus, pineal, retina, muscle and liver of migratory blackheaded buntings (Emberiza melanocephala) when they were photoinduced from winter non-migratory to the spring migratory state [16]. Similarly, hypothalamic expression of adcyap1 (adenylate cyclase activating peptide 1 gene that encodes for pituitary adenylate cyclase activating peptide, PACAP) has been found associated with the development of migratory restlessness in European blackcaps, Sylvia atricapilla [17]. This is consistent with the suggested role of PACAP in connecting circadian clock with the photoperiodic environment. When administered in vitro, PACAP caused a significant decrease in clock and cry1 mRNA levels in chicken pineal gland [18]. Furthermore, seasonal differences in the temporal relationship of dopamine with serotonin have been implicated in the hypothalamic regulation of gonadal growth in birds [19].
How avian migrants differentially prepare for two similar journeys that they undertake to reproduce in the spring and to overwinter in the autumn is poorly understood. However, the important neural structures possibly involved in the regulation of migratory behaviour include interconnected pathways between hypothalamic nuclei that detect, transmit and integrate photoperiodic cues for the seasonal timing of migration in birds [20]. Therefore, we hypothesized differences in the hypothalamic transcription of regulatory genes with transition from non-migration to the migration state, and between two (spring and autumn) migrations during the year. Palaearctic-Indian migratory redheaded bunting (Emberiza bruniceps) with breeding grounds in West Asia and wintering grounds in India was an ideal model system to test this, since seasonal states could be easily and reproducibly induced within few weeks in buntings under artificial photoperiods [21]. Here, we measured and predicted differences in the mRNA expression pattern of thyroid hormone-responsive (dio2, dio3) and light-responsive (per2, cry1, adcyap1) genes, and th gene that codes for tyrosine hydroxylase, the rate-limiting enzyme of dopamine biosynthesis, in redheaded buntings that were photoperiodically induced into the non-migratory (winter or summer) and migratory (spring or autumn) states. To further ascertain seasonal differences at the epigenetic level, we measured the hypothalamic expression of dnmt3a, dnmt3b and tet2 genes during both non-migration and migration periods in redheaded buntings.
2. Methods
(a). Animals and experiment
This study on redheaded buntings was conducted in accordance with guidelines of the Institutional Animal Ethics Committee (IAEC) of the University of Delhi, India. Buntings are a migrant songbird species that arrive in India during late September/early October, overwinter, and begin to return to their breeding grounds located in west Asia and southeast Europe during late March/early April [22]. Bunting exhibits seasonal life-history states under programmed photoperiods [21]. For this study, birds were procured in late February, acclimated to captive conditions under natural photoperiod and temperature conditions for about two weeks, and subsequently exposed for about 24 weeks under short days (SD; 10 h light and 14 h darkness, hereafter 10 L : 14 D) or long days (LD; 14 h light and 10 h darkness, hereafter 14 L : 10 D) in temperature controlled photoperiodic cubicles (22 ± 2°C). Under a non-stimulatory SD, buntings maintain unstimulated photosensitive state, which is comparable to the winter non-migratory (wnM) LHS in the wild. However, under a stimulatory LD, buntings undergo growth-regression cycle. They fatten, gain weight and exhibit Zugunruhe; this mimics the spring migration (SM) LHS in the wild. On prolonged exposure to LD, buntings show fat depletion, become lean and cease to exhibit Zugunruhe; this photorefractory state is comparable to the post-reproductive summer non-migratory (snM) LHS in the wild [22]. Whereas SD-photosensitive birds exhibit SM LHS under LD [22], the LD-photorefractory birds exhibit AM (autumn migration) LHS when exposed to a shorter photoperiod [23]. Here, we chose SD and LD conditions with light periods close to that buntings experience during winter and summer months when held captive at the wintering grounds and exhibited seasonal LHSs [21].
The experiment used 22 males each from the wnM and snM states maintained under 10 L : 14 D and 14 L : 10 D, respectively. Birds were singly housed in activity cages (size = 42 cm × 30 cm × 52 cm) that were individually placed in photoperiodic boxes (size = 70 cm × 50 cm × 70 cm), and for a week were exposed to photoperiodic conditions, as before. Then, 12 birds from each state were subjected to a 3 h photoperiod change; i.e. wnM and snM birds were exposed to 13 L : 11 D and 11 L : 13 D to induce the SM and AM LHSs, respectively. Birds remaining in wnM and snM states (n = 10 each) served as non-migrant period controls for photostimulated SM and AM states, respectively (figure 1a). After five nights of Zugunruhe in SM and AM groups (i.e. when an individual has shown five consecutive nights of activity), blood samples were collected from birds of all four groups during the middle of the day and night (aligned to ZT5 and ZT17 in wnM, to ZT6.5 and ZT18.5 in SM, to ZT7 and ZT19 in snM, and to ZT5.5 and ZT17.5 in AM LHSs) for hormone assays, and brains for the measurement of hypothalamic gene expressions. We chose 5-night Zugunruhe as a criterion for the migrant period, based on our previous studies of simulated migratory state in captive migratory blackheaded buntings, an allied species of redheaded buntings [8,16].
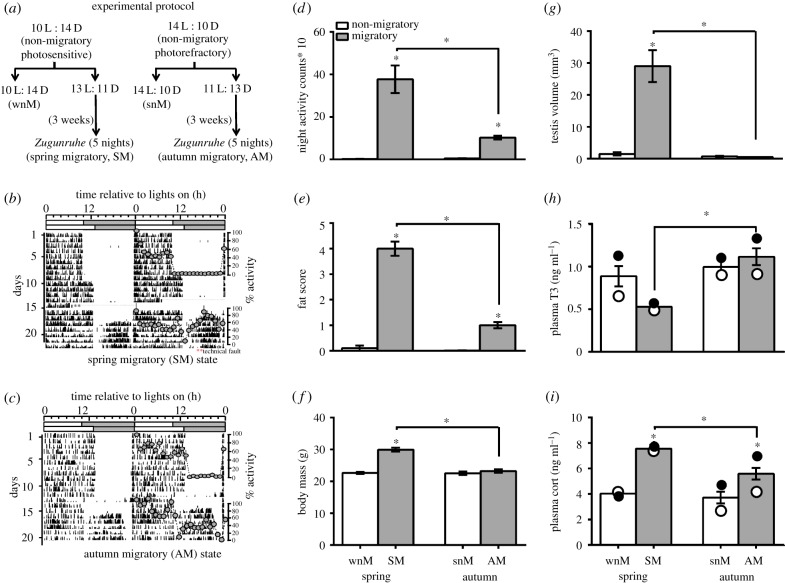
Figure 1.
Measures of behavioural and physiological phenotypes in simulated non-migratory and migratory life-history states (LHSs) during spring and autumn in migratory redheaded buntings (Emberiza bruniceps). (a) Experimental protocol: we used photosensitive and photorefractory birds (n = 22 each) that were maintained under 10 h of light and 14 h of darkness (10 L : 14 D) and 14 L : 10 D, and represented the winter non-migratory (wnM) and summer non-migratory (snM) LHSs, respectively. Twelve birds of each state were subjected to 3 h photoperiod change: birds of wnM state were exposed to a 3 h longer light period (13 L : 11 D), while those of snM state were exposed to a 3 h shorter light period (11 L : 13 D). The experiment ran for a period of 3–4 weeks during which all individuals exhibited 5 nights nocturnal Zugunruhe, a reliable index of the development of migratory state in buntings. (b,c) Representative double plotted 24 h pattern of activity behaviour of an individual showing (b) spring (SM) and (c) autumn (AM) migration LHS. Superimposed on the actogram is 24 h hourly activity profile with reference to the time of light on representing for both the non-migrant and migrant periods. (d–i) Bar diagrams show mean (±s.e.) night activity counts (d), fat score (e), body mass (f), testicular volume (g), and baseline plasma levels of T3 (h) and Cort (i) of all four groups. Open and closed circles within the bar represent mean hormone levels (h,i) during the day and night, respectively. An asterisk (*) on the bar indicates a significant difference between the non-migratory and migratory states within a season and asterisk over the line indicates a significant difference between the spring (SM) and autumn (AM) LHSs (p < 0.05, Bonferroni post-test).
(b). Measurement of 24 h activity pattern, fat deposition, weight gain and testis size
A passive infrared motion sensor (DSC, LC100 PI digital PIR detector, Canada) continuously detected the bird's movement in the cage. The collection, analysis and presentation of 24 h activity records were done using the Chronobiology Kit software from Stanford Software Systems, USA. We also presented hourly profile over 24 h for randomly chosen 5 individuals from each group. For this, we first averaged hourly activity counts for each individual from four consecutive days, and then normalized the hourly averages relative to the hour with highest average activity which was given a score of 100. The normalized hourly activity scores from five birds of each state were taken to calculate the mean (±s.e.) 24 h activity profile for each state. We also recorded changes in the subcutaneous fat deposition, body mass and testis size, as per procedure routinely carried out in our laboratory and published [24–26] (for further details please refer to the supplementary methods section).
(c). Measurement of circulating T3 and Cort levels
We measured T3 and Cort levels in blood samples (approx. 150 µl) collected into heparinized capillary tubes by puncturing the wing vein. The handling and blood collection were completed in less than 1 min to avoid stress-induced effects [27]. Blood was centrifuged for 10 min at 5000 r.p.m. at 4°C, and plasma was separated and stored at −20°C until assayed for hormones. Plasma T3 and Cort levels were measured by ELISA, and used immunoassay kits and protocols that have been validated and used in our earlier studies [24,26]. Plasma T3 assay used an immunoassay kit from Accubind-monobind (Lake Forest, CA; cat. no. 125–300) with an intra-assay variability and sensitivity of 5.4% and 0.04 ng ml−1, respectively. Similarly, plasma Cort assay used an immunoassay kit from Enzo Life Sciences (Ann Arbor, MI, cat no. ADI-900-097) with an intra-assay variability and sensitivity of 8.4% and 0.02 ng ml−1, respectively. We ran ELISAs as per the manufacturer's protocol. Day and night T3 and Cort values from each bird were averaged for further comparisons. More details have been given in the electronic supplementary material, methods section.
(d). Measurement of hypothalamic gene expressions
Hypothalamus was quickly excised out, kept at 4°C in RNAlater overnight and stored at −80°C until processed for gene expression assays, as described in detail in the supplementary methods section. Briefly, the total RNA was extracted using Trizol (Ambion, AM9738) and reverse transcribed using Revert Aid first strand cDNA synthesis Kit (Thermoscientific, K1622). We used gene-specific primers for the measurement of mRNA expression levels of dio2, dio3, per2, cry1 and th genes, as published earlier [12,16]. For adcyap1, dnmt3a, dnmt3b, and tet2, we first used degenerate primers to amplify the candidate genes, and then gene-specific primers were designed from cloned partial sequences (electronic supplementary material, table S1). We measured the mRNA expression by qRT-PCR run on Applied Biosystems StepOne Plus thermal cycler using SYBR green chemistry. Although we tested three potential reference genes (β-actin, hprt1 and ppia), we preferred and used β-actin as the endogenous control (reference) gene for qPCR, because of its highest stability value as assessed by BestKeeper [28], Genorm [29] and NormFinder [30] MS Excel based algorithms. (see supplementary methods section; electronic supplementary material, table S2). Both the samples and reference were run in duplicates, and the relative mRNA expression level was determined as 2–ΔΔCt. We first calculated ΔCt value by subtracting threshold cycle (Ct) of the target gene from reference gene (Ct[target] − Ct[reference]), and then ΔCt was normalized against ΔCt of a calibrator sample (consisting cDNA mix of all four groups, wnM, snM, SM and AM); this gave the ΔΔCt value. The negative value of ΔΔCt powered to 2 (2−ΔΔct) was plotted.
(e). Statistical analyses
Two-way analysis of variance (2-way ANOVA) tested the effects of two factors (season: spring/autumn; phenotype: non-migratory/ migratory; time of day: midday/midnight, as appropriate) at a time on all datasets, except fat score for which we used the ordinal logistic regression analysis (OLRA). Pearson's correlation coefficient was calculated to show the relationship between the measurements of interest. All statistical analyses were performed by GraphPad Prism (version 5.0) software, except for OLRA, for which we used IBM SPSS Statistics version 20 software.
3. Results
(a). LHS-dependent changes in behaviour and physiology
At the beginning of the experiment, birds of all four groups had non-migratory phenotype with normal body mass (20–24 g), no subcutaneous fat deposition and reproductively inactive small testes (testis volume = 0.33–0.52 mm3). Whereas wnM and snM control groups maintained the unstimulated non-migratory phenotypes, the SM and AM groups developed spring and autumn migratory phenotypes during 3–4 weeks of exposure to 13 l and 11 l photoperiods, respectively. Thus, there were significant differences between the non-migrant and migrant phenotypes within a season (wnM versus SM, and snM versus AM) in the behaviour and physiology, as shown in activity at night (F1,24 = 53.62, p < 0.0001; 2-way ANOVA), fat score (Wald's χ2 = 20.652, p = 0.0001, OLRA) and body mass (F1,40 = 54.06, p < 0.0001; 2-way ANOVA). Interestingly, we found a significant difference in all measures of behaviour and physiology between spring and autumn (night activity: F1,24 = 16.84, p = 0.0004, 2-way ANOVA; fat score: Wald's χ2 = 18.701, p = 0.0001, OLRA; body mass: F1,40 = 39.51, p < 0.0001; 2-way ANOVA). Overall, there were significantly increased fat stores, higher body mass and more intense Zugunruhe in SM, compared with the AM state (p < 0.05, Bonferroni post-test; figure 1b–f). As expected, testes were recrudesced, hence significantly larger, in SM, compared with all the other states (p < 0.05, Bonferroni post-test; figure 1g).
Further, we found changes in plasma Cort, but not T3, levels with the development of migration state in both spring and autumn. However, plasma T3 levels were markedly low in spring during migrant, compared with the non-migrant phenotype. There was also a diurnal variation in Cort secretion in autumn, but not spring, with plasma levels significantly higher at night than during the day (p < 0.05, Bonferroni post-test; figure 1i). Between SM and AM states, however, both hormones showed an opposite trend: T3 was significantly lower and Cort was significantly higher in SM than in AM LHS (figure 1h,i). Overall, we found a significant effect of the season (T3: F1,40 = 12.0, p = 0.0013; Cort: F1,40 = 9.341, p = 0.0040), the phenotype (Cort: F1,40 = 53.35, p < 0.0001) as well as the season×phenotype interaction (T3: F1,40 = 5.615, p = 0.0227; Cort: F1,40 = 5.122, p = 0.0291; 2-way ANOVA) on plasma hormone levels.
(b). LHS-dependent hypothalamic gene expression patterns
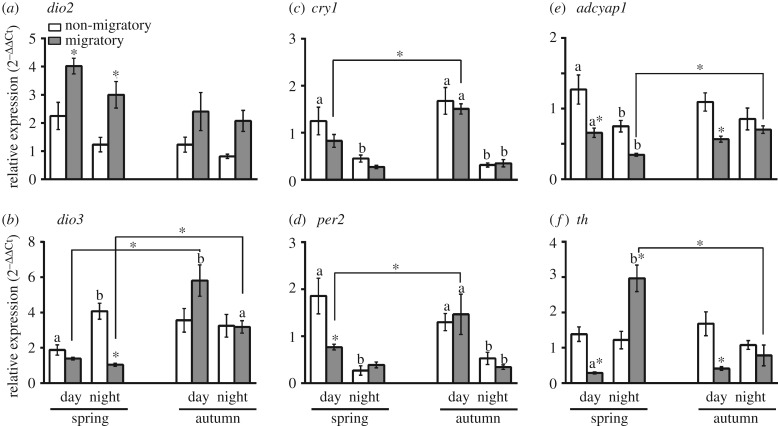
Figure 2 shows mRNA levels of the thyroid hormone-responsive (dio2, dio3) and light-responsive (per2, cry1, adcyap1) genes, and th gene involved in the dopamine biosynthesis during both non-migrant and migrant periods of the spring and autumn seasons. Within a season, we found a significant effect of the time of day, the phenotype and the time of day×phenotype interaction on gene expressions, with few exceptions (p < 0.05, 2-way ANOVA; table 1, figure 2). For example, there was no effect of the time of day on dio2, adcyap1 and th expressions in autumn, and of phenotype on the expression of cry1 and th in spring, and of dio3, per2 and cry1 in the autumn (table 1, figure 2). Notably in spring, not autumn, dio2 mRNA levels were significantly higher during migrant, compared to the non-migrant period; conversely, dio3 (night alone) mRNA levels were significantly higher during non-migrant, compared to the migrant period (p < 0.05, Bonferroni post-test; figure 2a,b). However, the overall pattern that dio2 expression was higher during migrant than non-migrant period was consistent in both seasons. Similarly, we found a consistent pattern in adcyap1 expression with higher mRNA levels in the non-migrant than in the migrant phenotype in both seasons. However, daytime adcyap1 and th levels were significantly lower during migrant period, compared to the non-migrant period in both spring and autumn seasons (p < 0.05, Bonferroni post-test; figure 2d,e). The spring night th levels were also significantly increased during the migrant compared with the non-migrant period.
Figure 2.
Hypothalamic expression of genes in simulated non-migratory and migratory life-history states (LHSs) during spring and autumn in migratory redheaded buntings (Emberiza bruniceps). Mean (±s.e.) mRNA expression levels of thyroid hormone-responsive genes (a, dio2; b, dio3), light-responsive genes (c, cry1; d, per2; e, adcyap1) and th (tyrosine hydroxylase) gene involved in dopamine biosynthesis (f) during middle of the day and night in spring and autumn. An asterisk on the bar indicates a significant difference in mRNA levels between non-migrant and migrant periods within a season (wnM versus SM or snM versus AM), an asterisk over the line indicates a significant difference in mRNA levels between SM and AM states, and different alphabets on bars indicate a signficant difference between midday and midnight mRNA levels within a state (p < 0.05, Bonferroni post-test). For statistical significance, alpha was set at 0.05.
Table 1.
Results of 2-way ANOVA: comparison of gene expression in migratory redheaded buntings (Emberiza bruniceps) photoperiod-induced into different seasonal states. (a) Winter non-migratory versus spring migratory; (b) summer non-migratory versus autumn migratory; (c) spring migratory versus autumn migratory; (d) spring versus autumn seasons. Asterisks indicate a significant effect of the factor/interaction at p < 0.05 level.
| (a) winter non-migratory versus spring migratory (wnM/SM) state (factor 1—phenotype; factor 2—time of day) | |||
|---|---|---|---|
| gene | phenotype (wnM/SM) | time of day (midday/midnight) | interaction |
| dio2 |
F1,18 = 20.68 p = 0.0002* |
F1,18 = 6.863 p = 0.0174* |
F1,18 1.086 e-005 p = 0.9974 |
| dio3 |
F1,18 = 48.97 p < 0.0001* |
F1,18 = 13.52 p = 0.0017* |
F1,18 = 25.60 p < 0.0001* |
| cry1 |
F1,18 = 3.625 p = 0.0730 |
F1,18 = 18.47 p = 0.0004* |
F1,18 = 0.5976 p = 0.4495 |
| per2 |
F1,18 = 7.007 p = 0.0164* |
F1,18 = 28.80 p < 0.0001* |
F1,18 = 10.85 p = 0.0040* |
| adcyap1 |
F1,18 = 22.50 p = 0.0002* |
F1,18 = 15.08 p = 0.0011* |
F1,18 = 0.9395 p = 0.3452 |
| th |
F1,18 = 1.632 p = 0.2176 |
F1,18 = 24.44 p = 0.0001* |
F1,18 = 31.33 p < 0.0001* |
| (b) summer non-migratory versus autumn migratory (snM/AM) state (factor 1—phenotype; factor 2—time of day) | |||
|---|---|---|---|
| gene | phenotype (snM/AM) | time of day (midday/midnight) | interaction |
| dio2 |
F1,18 = 7.466 p = 0.0137* |
F1,18 = 0.7082 p = 0.4111 |
F1,18 = 0.009117 p = 0.9250 |
| dio3 |
F1,18 = 2.627 p = 0.1224 |
F1,18 = 4.766 p = 0.0425* |
F1,18 = 2.977 p = 0.1016 |
| cry1 |
F1,18 = 0.1934 p = 0.6653 |
F1,18 = 70.70 p < 0.0001* |
F1,18 = 0.4817 p = 0.4965 |
| per2 |
F1,18 = 1.365 p = 0.2579 |
F1,18 = 34.84 p < 0.0001* |
F1,18 = 0.04359 p = 0.8370 |
| adcyap1 |
F1,18 = 11.89 p = 0.0029* |
F1,18 = 0.2842 p = 0.6005 |
F1,18 = 3.654 p = 0.0720 |
| th |
F1,18 = 11.43 p = 0.0033* |
F1,18 = 0.2500 p = 0.6231 |
F1,18 = 4.444 p = 0.0493* |
| (c) spring migratory versus autumn migratory (SM/AM) state (factor 1—season; factor 2—time of day) | |||
|---|---|---|---|
| gene | season (SM/AM) | time of day (midday/midnight) | interaction |
| dio2 |
F1,20 = 7.224 p = 0.0142* |
F1,20 = 2.038 p = 0.1688 |
F1,20 = 0.5261 p = 0.4766 |
| dio3 |
F1,20 = 46.66 p < 0.0001* |
F1,20 = 9.634 p = 0.0056* |
F1,20 = 5.667 p = 0.0273* |
| cry1 |
F1,20 = 77.43 p < 0.0001* |
F1,20 = 15.21 p = 0.0009* |
F1,20 = 9.528 p = 0.0058* |
| per2 |
F1,20 = 3.694 p = 0.0690 |
F1,20 = 42.91 p < 0.0001* |
F1,20 = 5.905 p = 0.0246* |
| adcyap1 |
F1,20 = 7.492 p = 0.0127* |
F1,20 = 3.330 p = 0.0830 |
F1,20 = 21.35 p = 0.0002* |
| th |
F1,20 = 18.35 p = 0.0004* |
F1,20 = 40.33 p < 0.0001* |
F1,20 = 23.09 p = 0.0001* |
| (d) spring versus autumn season (factor 1—season; factor 2—phenotype) | |||
|---|---|---|---|
| gene | season (spring/autumn) | phenotype (non-migratory/migratory) | interaction |
| dnmt3a |
F1,40 = 4.822 p = 0.0340* |
F1,40 = 26.64 p < 0.0001* |
F1,40 = 2.572 p = 0.1166 |
| dnmt3b |
F1,40 = 0.4561 p = 0.5034 |
F1,40 = 7.146 p = 0.0108* |
F1,40 = 0.9250 p = 0.3419 |
| tet2 |
F1,40 = 0.01166 p = 0.9146 |
F1,40 = 3.879 p = 0.0528* |
F1,40 = 8.449 p = 0.0059* |
We further compared gene expression patterns during migrant periods of spring and autumn. Overall, there was a significant effect of the season, the time of day and the season×time of day interaction on the expression of all but few genes; e.g. there was no effect of migratory season on per2 and of time of day on dio2 and adcyap1 expressions (table 1, figure 2). Particularly, both day and night dio3, day cry1 and per2 and night adcyap1 mRNA levels were significantly higher in AM than the SM state (p < 0.05, Bonferroni post-test; figure 2b–e). By contrast, nocturnal th mRNA levels were significantly higher (almost threefold higher) in SM than the AM state (p < 0.05, Bonferroni post-test; table 1, figure 2f).
(c). Epigenetic regulation of seasonal states
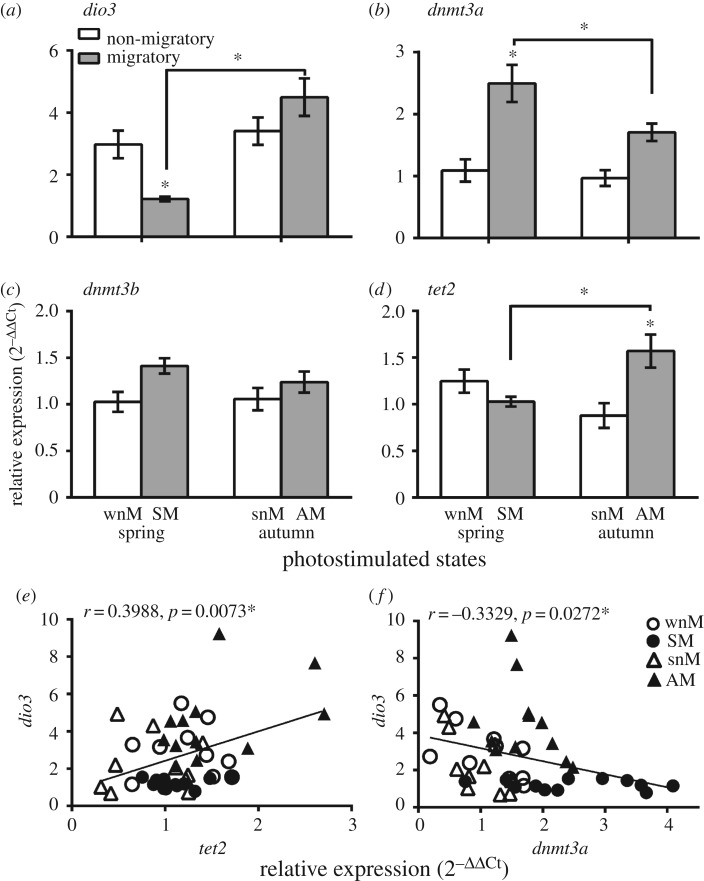
Figure 3 plots an overall mRNA expression of dio3, dnmt3a, dnmt3b and tet2 genes. Overall, there was a significantly lower dio3 mRNA levels during the migrant, compared with the non-migrant, period in spring; however, dio3 levels did not differ and were high in both, the non-migrant and migrant periods in autumn (figure 3a). Consistent with this, we found significantly higher dnmt3a, not dnmt3b, and tet2 mRNA expressions during the migrant, compared with the non-migrant, period in spring and autumn, respectively (p < 0.05, Bonferroni post-test, figure 3b,d). Between two migrant periods, we found a significantly higher dnmt3a in spring and tet2 mRNA levels in the autumn (p < 0.05, Bonferroni post-test; figure 3b,d). Furthermore, we found a significant negative correlation of dnmt3a (r = −0.3329, r2 = 0.1108, p = 0.0272) and a positive correlation of tet2 (r = 0.3988, r2 = 0.1590, p = 0.0073) on dio3 expression in buntings (figure 3e,f).
Figure 3.
Hypothalamic expression of epigenetic markers in simulated non-migratory and migratory life-history states (LHSs) during spring and autumn in migratory redheaded buntings (Emberiza bruniceps). (a–d) Overall mRNA expression level of dio3 (a), dnmt3a (b), dnmt3b (c) and tet2 (d) in non-migratory and migratory LHSs during spring and autumn. An asterisk on bar indicates a significant difference between non-migrant and migrant periods within a season (wnM versus SM or snM versus AM), and an asterisk over the line indicates a significant difference in mRNA levels between SM and AM states (p < 0.05, Bonferroni post-test). (e,f) Scatter plots with Pearson's correlation coefficient (r-value) and significance levels (p-value) to show the relationship of tet2 or dnmt3a mRNA with dio3 levels. A solid line in scatter plots denotes linear regression between gene expression levels, as indicated on x and y axes.
4. Discussion
To our knowledge, this is the first demonstration of differences in the hypothalamic expression of regulatory genes between the non-migrant and migrant periods as well as between the spring and autumn migration periods in a captive songbird migrant. Three important conclusions have emerged from our study. First, compared with autumn, spring migration in buntings occurred at a faster pace, as evidenced by the longer duration and enhanced nocturnal Zugunruhe levels during the SM state, consistent with similar findings in other migrants like white-crowned sparrows, Zonotrichia leucophrys gambelii [1,4], migratory swifts, Apus apus [31] and long-distance nocturnal passerines migrants passing across the Scandinavian Peninsula [32]. Perhaps buntings with more intense Zugunruhe had higher motivation to migrate in spring, compared to those with less Zugunruhe in autumn. In fact, a study on northern wheatears (Oenanthe oenanthe) supports this, showing a linkage of Zugunruhe levels with the motivation to migrate [33]. Wheatear migrants with little Zugunruhe remained at stopover sites for longer than those individuals with more intense Zugunruhe [33]. Buntings also had a higher body mass and fat fuel load in spring than in autumn, consistent with other songbirds including seventeen neotropical migratory land birds [4–6,34]. This suggested an overall time-minimizing physiological strategy for the spring migration which is perhaps linked to appetitive phase (i.e. sexual motivation) of the sexual behaviour in male birds [35]. Such a control strategy appears to have a selective advantage since an early arrival at the breeding grounds would confer an advantage to individuals in establishing territories and competing for mates, the critical components of successful reproduction [6].
Second, we found seasonal changes in circulating T3 and Cort levels. T3 levels were higher in snM and AM than the SM, although did not differ between the non-migrant wnM and SM states. This indicated an enhanced metabolism during autumn when buntings were lean with relatively little or no subcutaneous fat, consistent with high surface body temperature in photorefractory migratory blackheaded buntings suggesting increased metabolism during autumn in latitudinal migrants [36]. However, T3 levels could also be correlated with the gonadal state: low T3 and enlarged testes during SM, and high T3 and small testes during AM state. In fact, there were low T3 levels during testis recrudescence phase (May–June) in redheaded buntings held captives under natural photoperiods at their wintering grounds, 25°N [37], and high T3 levels during testicular regression phase in Japanese quails, Coturnix c. japonica [38]. Further, high Cort levels seemed to be associated with an increased fat fuel load during spring migration, consistent with findings in arctic western sandpipers, Calidris mauri [39] and red knots, Calidris canutus [40]. Increased Cort levels in migrant compared with non-migrant period suggested the role of Cort in seasonal migration of redheaded buntings. Elevated night Cort levels have been linked to the preparation for departure from a stopover site in migratory godwits, Limosa lapponica [41] and to the migratory restlessness in captive migratory garden warblers, Sylvia borin [42]. An early-night Cort peak has also been found during pre-migrant and migrant periods in Gambel's white-crowned sparrows (Zonotrichia leucophrys gambelii [43]) and blackheaded buntings [24].
Third, and most importantly, we suggest the transcriptional basis for differences between seasonal migrations, as revealed by significant changes with transition from non-migratory to migratory state during the year (wnM versus SM or snM versus AM) and by significant difference between the spring and autumn migration periods in gene expression patterns in redheaded buntings. All genes showed a diurnal expression pattern (except dio2) and interesting differences between the non-migrant and migrant periods. Particularly, we found that the overall pattern of dio2 and adcyap1 expressions were consistent in both seasons although with an opposite trend: dio2 expression was higher in the migrant phenotype while adcyap1 expression was higher in non-migrants. Differences in adcyap1 expression between the spring and autumn migrant period, with a higher mRNA expression during the day than at night in spring, but not the autumn, indicated its association with the pattern and level of Zugunruhe in redheaded buntings, as also suggested in migratory blackcaps [17]. The expression of adcyap1 may also be linked to that of per2 and cry1 [18], and hence to the seasonal responsiveness of the circadian photoperiodic mechanism(s). Both, per2 and cry1 mRNA levels were significantly higher during the day than at night in all states, except SM when day and night levels were similar. Similarly, high nocturnal th mRNA levels may suggest an enhanced hypothalamic dopamine-mediated pituitary activation during the SM state [19]. High SM th levels might also reflect an enhanced drive linked to sexual reproduction as buntings at this time had fully recrudesced testes, although this remains purely speculative at this time. Nonetheless, a linkage of androgens with such a drive has been suggested in zebra finches (Taeniopygia guttata) in which androgen-deprivation decreased catecholamine content and turnover in the preoptic area, paraventricular nucleus and area X, the brain areas linked with sexual reproduction [44].
We suggest the role of dio3 as a gatekeeper of the photoperiodic responsiveness [10] in mediating epigenetic changes associated with seasonal transition in the behaviour and physiology in migratory buntings, as suggested in seasonally breeding mammals [10,13]. Consistent with this, we found a significant difference in the expression of dnmt3a, not dnmt3b, and tet2 genes between the SM and AM states. Particularly, the hypothalamic expression of (i) dnmt3a that suppresses the target gene expression by methylation of cytosine residues in the promoter region was found negatively correlated with that of the dio3 mRNA levels, and (ii) tet2 gene that codes for enzyme responsible for an initial step during demethylation process was positively correlated with that of dio3 expression levels. We speculate that epigenetic modifications are integral to the seasonal transcriptional regulation, and are part of a conserved mechanism for seasonal rhythms [45].
5. Conclusion
We for the first time demonstrate differences in the hypothalamic gene expressions concurrently with changes in the behaviour and physiology between the non-migrant and migrant periods within a season, and between the photoinduced spring and autumn migration states under controlled experimental conditions in a latitudinal songbird migrant. These results give insights into how avian migrants could employ different adaptive strategies at the transcriptional level in accomplishing two similar journeys that they undertake during the year. Although we have shown seasonal differences in the expression pattern of genes involved in the epigenetic modification, future studies need to confirm whether the photoperiod-induced seasonal transition involves methylation-dependent mechanism(s) in migratory songbirds.
Supplementary Material
Supplementary Material
Supplementary Material
Ethics
All procedures were approved and carried out in accordance with guidelines of the Institutional Animal Ethics Committee (IAEC) of Department of Zoology, University of Delhi, India (Institutional Ethical Approval number: DU/ZOOL/IAECR/2015/04).
Data accessibility
The mRNA sequences with their partial CDS can be accessed using GenBank accession numbers as provided in electronic supplementary material, table S1.
Authors' contribution
V.K. conceived the idea. V.K., D.S., N.J.G. and S.R. designed the study. D.S., A.S. and S.M. performed experiments and carried out sampling. D.S. and A.S. performed hormone assays. A.S. and D.S. performed gene expression assays. A.S. analysed data and prepared final figures. V.K. and A.S. wrote the manuscript and carried out extensive revision. N.J.G. and S.R. provided the animal resources; V.K. provided all chemical and other laboratory resources. All authors gave final approval for publication.
Competing interests
Authors have no competing interests.
Funding
The funds were provided by the Department of Biotechnology, New Delhi through a research grant (BT/PR4984/MED/30/752/2012) to V.K. A.S. received a Junior Research Fellowship from the Council of Scientific and Industrial Research, New Delhi. The experimental facility used for experiments was built with the support from the Science and Engineering Research Board, New Delhi under IRHPA grant to V.K.
References
- 1.Newton I. 2007. The migration ecology of birds. London, UK: Academic Press. [Google Scholar]
- 2.Berthold P. 1996. Control of bird migration. London, UK: Chapman & Hall. [Google Scholar]
- 3.Wingfield JC, Schwabl H, Mattocks PW Jr. 1990. Endocrine mechanisms of migration. In Bird migration, physiology and ecophysiology (ed. Gwinner E.), pp. 232–256. Berlin, Germany: Springer. [Google Scholar]
- 4.Agatsuma R, Ramenofsky M. 2006. Migratory behaviour of captive white-crowned sparrows, Zonotrichia leucophrys gambelii, differs during autumn and spring migration. Behaviour 143, 1219–1240. ( 10.1163/156853906778691586) [DOI] [Google Scholar]
- 5.Tryjanowski P, Yosef R. 2002. Differences between the spring and autumn migration of the red-backed shrike Lanius collurio: record from the Eilat Stopover (Israel). Acta Ornithol. 37, 85–90. ( 10.3161/068.037.0204) [DOI] [Google Scholar]
- 6.Nilsson C, Klaassen RHG, Alerstam T. 2013. Differences in speed and duration of bird migration between spring and autumn. Am. Nat. 181, 837–845. ( 10.1086/670335) [DOI] [PubMed] [Google Scholar]
- 7.Cornelius JM, Boswell T, Eiermann SJ, Breuner CW, Ramenofsky M. 2013. Contribution of endocrinology to the migration life history of birds. Gen. Comp. Endocrinol. 190, 47–60. ( 10.1016/j.ygcen.2013.03.027) [DOI] [PubMed] [Google Scholar]
- 8.Rastogi A, Kumari Y, Rani S, Kumar V. 2013. Neural correlates of migration: activation of hypothalamic clock(s) in and out of migratory state in the blackheaded bunting (Emberiza melanocephala). PLoS ONE 8, e70065 ( 10.1371/journal.pone.0070065) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakane Y, Yoshimura T. 2014. Universality and diversity in the signal transduction pathway that regulates seasonal reproduction in vertebrates. Front. Neurosci. 8, 1–7. ( 10.3389/fnins.2014.00115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barrett P, et al. 2007. Hypothalamic thyroid hormone catabolism acts as a gatekeeper for the seasonal control of body weight and reproduction. Endocrinology 148, 3608–3617. [DOI] [PubMed] [Google Scholar]
- 11.Stevenson TJ, Lincoln GA. 2017. Epigenetic mechanism regulation circannual rhythms. In Biological timekeeping: clocks, rhythms and behaviour (ed. Kumar Vinod.), pp. 607–623. New Delhi, India: Springer. [Google Scholar]
- 12.Majumdar G, Rani S, Kumar V. 2015. Hypothalamic gene switches control transitions between seasonal life history states in a night migratory photoperiodic songbird. Mol. Cell. Endocrinol. 399, 110–121. ( 10.1016/j.mce.2014.09.020) [DOI] [PubMed] [Google Scholar]
- 13.Stevenson TJ, Prendergast BJ. 2013. Reversible DNA methylation regulates seasonal photoperiodic time measurement. Proc. Natl Acad. Sci. USA 110, 16 651–16 656. ( 10.1073/pnas.1310643110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moore LD, Le T, Fan G. 2013. DNA methylation and its basic functions. Neuropsychopharmacology 38, 23–38. ( 10.1038/npp.2012.112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ito S, D'Alessio AC, Taranova OV, Hong K, Sowers LC, Zhang Y. 2010. Role of Tet proteins in 5mC to 5hmC conversion, ES-cell self-renewal and inner cell mass specification. Nature 466, 1129–1133. ( 10.1038/nature09303) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh D, Trivedi AK, Rani S, Panda S, Kumar V. 2015. Circadian timing in central and peripheral tissues in a migratory songbird: dependence on annual life-history states. FASEB J. 29, 4248–4255. ( 10.1096/fj.15-275339) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mueller JC, Pulido F, Kempenaers B. 2011. Identification of a gene associated with avian migratory behaviour. Proc. R. Soc. B 278, 2848–2856. ( 10.1098/rspb.2010.2567) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagy AD, Csernus VJ. 2007. The role of PACAP in the control of circadian expression of clock genes in the chicken pineal gland. Peptides 28, 1767–1774. [DOI] [PubMed] [Google Scholar]
- 19.Chaturvedi CM, Yadav S. 2013. Influence of temporal relationship between serotonergic and dopaminergic precursors on the regulation of gonadal development in birds. Gen. Comp. Endocrinol. 190, 203–213. ( 10.1016/j.ygcen.2013.06.019) [DOI] [PubMed] [Google Scholar]
- 20.Stevenson TJ, Kumar V. 2017. Neural control of daily and seasonal timing of songbird migration. J. Comp. Physiol. A 203, 399–409. ( 10.1007/s00359-017-1193-5) [DOI] [PubMed] [Google Scholar]
- 21.Rani S, Singh S, Misra M, Malik S, Singh BP, Kumar V. 2005. Daily light regulates seasonal responses in the migratory male redheaded bunting (Emberiza bruniceps). J. Exp. Zool. 303, 541–550. [DOI] [PubMed] [Google Scholar]
- 22.Ali S, Ripley SD. 1974. Handbooks of the birds of India and Pakistan, vol. 10 New York: Oxford University Press. [Google Scholar]
- 23.Totzke U, Hȕbinger A, Dittami J, Bairlein F. 2000. The autumn fattening of the long-distance migratory garden warbler (Sylvia borin) is simulated by intermittent fasting. J. Comp. Physiol. B 170, 627–631. ( 10.1007/s003600000143) [DOI] [PubMed] [Google Scholar]
- 24.Mishra I, Singh D, Kumar V. 2017. Daily levels and rhythm in circulating Cort and insulin are altered with photostimulated seasonal states in night-migratory blackheaded buntings. Horm. Behav. 94, 114–123. ( 10.1016/j.yhbeh.2017.07.004) [DOI] [PubMed] [Google Scholar]
- 25.Kumar V, Kumar BS, Singh BP, Sarkar A. 1991. A common functional basis for the photoperiodic mechanism regulating reproductive and metabolic responses in the migratory redheaded bunting. Period. Biol. 93, 169–174. [Google Scholar]
- 26.Mishra I, Bhardwaj S, Malik S, Kumar V. 2017. Concurrent hypothalamic gene expression under acute and chronic long days: Implications for initiation and maintenance of photoperiodic response in migratory songbirds. Mol. Cell. Endocrinol. 439, 81–94. ( 10.1016/j.mce.2016.10.023) [DOI] [PubMed] [Google Scholar]
- 27.Wagner DN, Green DJ, Cooper JM, Love OP, Williams TD. 2014. Variation in plasma corticosterone in migratory songbirds: a test of the migration-modulation hypothesis. Physiol. Biochem. Zool. 87, 695–703. ( 10.1086/676937) [DOI] [PubMed] [Google Scholar]
- 28.Pfaffl MW, Tichopad A, Prgomet C, Neuvians TP. 2004. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper—Excel-based tool using pair-wise correlations. Biotechnol. Lett. 26, 509–515. [DOI] [PubMed] [Google Scholar]
- 29.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. 2002. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 3, R0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andersen CL, Jensen JL, Orntoft TF. 2004. Normalization of real-time quantitative reverse transcription- PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 64, 5245–5250. ( 10.1158/0008-5472.CAN-04-0496) [DOI] [PubMed] [Google Scholar]
- 31.Henningsson P, Karlsson H, Bäckman J, Alerstam T, Hedenstrom A. 2009. Flight speeds of swifts (Apus apus): seasonal differences smaller than expected. Proc. R. Soc. B 276, 2395–2401. ( 10.1098/rspb.2009.0195) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karlsson H, Nilsson C, Bäckman J, Alerstam T. 2012. Nocturnal passerine migrants fly faster in spring than in autumn: a test of the time minimization hypothesis. Anim. Behav. 83, 87–93. ( 10.1016/j.anbehav.2011.10.009) [DOI] [Google Scholar]
- 33.Eikenaar C, Klinner T, Szostek KL, Bairlein F. 2014. Migratory restlessness in captive individuals predicts actual departure in the wild. Biol. Lett. 10, 20140154 ( 10.1098/rsbl.2014.0154) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bayly NJ, Gómez C. 2011. Comparison of autumn and spring migration strategies of Neotropical migratory landbirds in northeast Belize. J. Field Ornithol. 82, 117–131. ( 10.1111/j.1557-9263.2011.00314.x) [DOI] [Google Scholar]
- 35.Balthazart J, Ball GF. 2007. Topography in the preoptic region: differential regulation of appetitive and consummatory male sexual behaviors. Front. Neuroendocrinol. 28, 161–178. (https://doi.org./10.1016/j.yfrne.2007.05.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singh D. 2013. Molecular analysis of daily and seasonal timekeeping in photoperiodic songbirds. PhD thesis, Department of Zoology, University of Delhi, India. [Google Scholar]
- 37.Pathak VK, Chandola A. 1982. Involvement of thyroid gland in the development of migratory disposition in the redheaded bunting, Emberiza bruniceps . Horm. Behav. 16, 46–58. [DOI] [PubMed] [Google Scholar]
- 38.Ikegami K, et al. 2015. Low temperature-induced circulating triiodothyronine accelerates seasonal testicular regression. Endocrinology 156, 647–659. ( 10.1210/en.2014-1741) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O'Reilly KM, Wingfield JC. 1995. Spring and autumn migration in Arctic shorebirds: same distance, different strategies. Am. Zool. 35, 222–233. [Google Scholar]
- 40.Piersma T, Reneerkens J, Ramenofsky M. 2000. Baseline corticosterone peaks in shorebirds with maximal energy stores for migration: a general preparatory mechanism for rapid behavioral and metabolic transitions? Gen. Comp. Endocrinol. 120, 118–126. ( 10.1006/gcen.2000.7543) [DOI] [PubMed] [Google Scholar]
- 41.Landys MM, Ramenofsky M, Piersma T, Jukema J, Wingfield JC. 2002. Baseline and stress-induced plasma corticosterone during long-distance migration in the bar-tailed godwit, Limosa lapponica. Physiol. Biochem. Zool. 75, 101–110. ( 10.1086/338285) [DOI] [PubMed] [Google Scholar]
- 42.Schwabl H, Bairlein F, Gwinner E. 1991. Basal and stress-induced corticosterone levels of garden warblers, Sylvia borin, during migration. J. Comp. Physiol. B 161, 576–580. ( 10.1007/BF00260747) [DOI] [Google Scholar]
- 43.Landys MM, Wingfield JC, Ramenofsky M. 2004. Plasma corticosterone increases during migratory restlessness in the captive white-crowned sparrow Zonotrichia leucophrys gambelii. Horm. Behav. 46, 574–581. ( 10.1016/j.yhbeh.2004.06.006) [DOI] [PubMed] [Google Scholar]
- 44.Barclay SR, Harding CF. 1988. Androstenedione modulation of monoamine levels and turnover in hypothalamic and vocal control nuclei in the male zebra finch: steroid effects on brain monoamines. Brain Res. 459, 333–343. [DOI] [PubMed] [Google Scholar]
- 45.Stevenson TJ. 2018. Epigenetic regulation of biological rhythms: an evolutionary ancient molecular timer. Trends Genet. 34, 90–100. ( 10.1016/j.tig.2017.11.003) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The mRNA sequences with their partial CDS can be accessed using GenBank accession numbers as provided in electronic supplementary material, table S1.