Abstract
Ocean plastic pollution has resulted in a substantial accumulation of microplastics in the marine environment. Today, this plastic litter is ubiquitous in the oceans, including even remote habitats such as deep-sea sediments and polar sea ice, and it is believed to pose a threat to ecosystem health. However, the concentration of microplastics in the surface layer of the oceans is considerably lower than expected, given the ongoing replenishment of microplastics and the tendency of many plastic types to float. It has been hypothesized that microplastics leave the upper ocean by aggregation and subsequent sedimentation. We tested this hypothesis by investigating the interactions of microplastics with marine biogenic particles collected in the southwestern Baltic Sea. Our laboratory experiments revealed a large potential of microplastics to rapidly coagulate with biogenic particles, which substantiates this hypothesis. Together with the biogenic particles, the microplastics efficiently formed pronounced aggregates within a few days. The aggregation of microplastics and biogenic particles was significantly accelerated by microbial biofilms that had formed on the plastic surfaces. We assume that the demonstrated aggregation behaviour facilitates the export of microplastics from the surface layer of the oceans and plays an important role in the redistribution of microplastics in the oceans.
Keywords: marine plastic pollution, microbial biofilms, microplastics aggregation behaviour
1. Introduction
Mass production and usage of plastics combined with waste disposal and mismanagement have resulted in the accumulation of immense amounts of plastic litter in marine environments [1–6]. A dominant fraction of this plastic litter is distributed in the form of microplastics that are usually considered as plastic particles with a size less than 5 mm [7] and typically comprise the following types with different origins: (i) primary microplastics originating from factory discharge or transport spills of plastic granulates and pellets used for the production of larger plastic structures, (ii) primary microplastics from personal care products, and (iii) secondary microplastics originating from the degradation of macroplastic through weathering and mechanical forces [7,8]. Today, microplastics are present in all marine ecosystems including remotely located ones such as deep-sea sediments and polar seas including sea ice [7–17]. There is some indication for a significant increase in the concentrations of pelagic microplastics within the past decades [18]. However, a recent study, which confirmed the worldwide presence of microplastics at the surface of the open ocean, revealed that the concentrations of plastic in the surface ocean are considerably smaller than expected and that this discrepancy is particularly pronounced in the case of microplastics with a size of below 1 mm [19]. This indicates the existence of ubiquitous size-selective sinks that preferably remove plastic particles of this size range. Potential effective microplastics sinks comprise fragmentation, transfer to the ocean interior by food webs and ballasting processes [19]. Local accumulation and removal rates can be expected to critically determine ecosystem effects of microplastics, but, to the best of our knowledge, no information about aggregation and vertical export of microplastics in the marine water column is available. It is conceivable that microplastics impact marine particle dynamics by physically interacting with suspended biogenic particles and thereby influencing the natural particle size distribution and export dynamics of organic matter. Recently, microplastics were shown to be present in natural marine aggregates [20], and it was demonstrated in laboratory experiments that microplastics attach to and are incorporated in already existing aggregates [21,22]. Such processes can clearly increase the sinking rates of the microplastics and have been suggested acting as efficient microplastics sinks and resulting in the export of microplastics from the ocean surface layer [21–23]. However, the role of microplastics in the early formation of marine biogenic aggregates and their potential to even stimulate the formation of such aggregates have not been studied so far.
Aggregate formation strongly depends, among other factors, on the stickiness (or coagulation efficiency) of the organisms and particles involved [24]. Marine plastics are colonized by microorganisms and represent suitable substrates for the formation of biofilms [25–36]. Because biofilms typically feature a rather sticky matrix of extracellular polymeric substances [37–39], it is very likely that biofilm formation on the microplastics increases the stickiness of these artificial particles and thereby supports their adhesion to natural particles.
The aim of the present study was to systematically investigate the aggregation behaviour of microplastics by testing the following two hypotheses: (i) microplastics suspended in seawater are involved in the aggregation processes of natural particles and can stimulate them; (ii) biofilm formation on the microplastics surface enhances the aggregation potential of the microplastics. The tests were performed with laboratory aggregation experiments applying roller tanks and beads of polystyrene, a plastic type that is commonly found in marine environments [7,25,40] and whose density, which is comparable to that of seawater, makes it very appropriate for aggregation experiments.
2. Methods
To test the two hypotheses, we performed two types of experiments. (i) The aggregation potential of microplastics that had been chemically cleaned directly before the experiments (called ‘clean microplastics’ in the following) was analysed in natural coastal seawater from the Bay of Kiel in the southwestern Baltic Sea using polystyrene beads with a diameter of 700–900 µm (figure 1a). To see how the formed aggregates develop over time and if they are stable for longer time periods, these experiments had relatively long durations of 8 and 12 days. The seawater contained the natural biogenic particle community including phytoplankton that in all experiments was comprised mainly of dinoflagellates of the genus Ceratium and small diatoms. A bead concentration of 50 beads l−1 was applied. This concentration is higher than most microplastics concentrations that have been found in the oceans [4], but it is still in the range of microplastics concentrations observed in the Caribbean and Sargasso Seas [41] and in a coastal area of the southern North Sea [42]. As controls, identical aggregation analyses were performed simultaneously with (1) microplastics and filtered seawater from the Bay of Kiel containing dissolved organic compounds and small bacteria and (2) microplastics and sterile artificial seawater with a salinity that was identical to that of the natural seawater. In addition, the aggregation of microplastics and biogenic particles was compared with that of biogenic particles alone. (ii) To investigate the influence of microbial biofilms on the aggregation behaviour of the microplastics, 3-day-long experiments either with biofilm-covered microplastics or, as a control, with clean microplastics were carried out simultaneously in the same way and with the same controls as described for the aggregation experiments with clean microplastics. An overview of the different experiments and aggregation situations is given in the electronic supplementary material, table S1. All aspects regarding the preparation and the realization of the experiments are described in detail below.
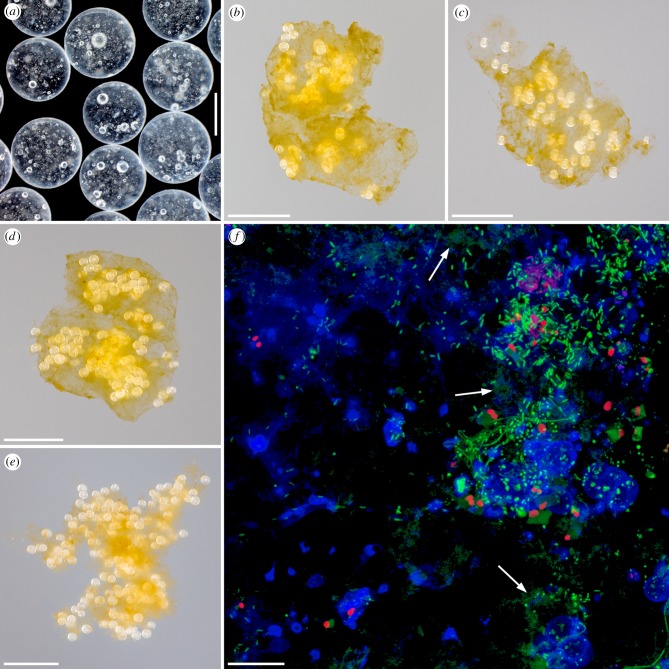
Figure 1.
Microplastics and experimentally formed aggregates consisting of biogenic particles and microplastics. (a) Stereo micrograph showing some of the polystyrene beads used for the experiments. (b–e) Photographs of exemplary aggregates that formed out of biogenic particles and microplastics during the experiments with clean microplastics (b) and with biofilm-covered microplastics (c–e). (f) Confocal laser scanning micrograph exhibiting a biofilm formed within five weeks on the surface of one of the polystyrene beads used for the experiments. Blue, polysaccharide-containing structures; green, structures containing nucleic acids; red, chlorophyll-containing structures. The arrows indicate exemplary structures containing extracellular DNA. Scale bars: 500 µm (a), 5 mm (b–e) and 20 µm (f).
(a). Collection, processing and preparation of seawater
Seawater was collected on board the research vessel ‘Littorina’ at the Boknis Eck Time Series site (54°31.2′ N, 10°02.5′ E) located at the mouth of the Eckernförde Bay in the Bay of Kiel in the southwestern Baltic Sea. The seawater pump of ‘Littorina’ was used to pump seawater from a depth of 2–2.5 m, which was located in the mixed surface layer, into a clean polyethylene tank in spring, late summer and autumn 2014 and in spring 2015. In the laboratory, the seawater containing the natural particle community (including the plankton community) was poured through a gauze with a mesh size of 500 µm (Hydrobios Apparatebau GmbH, Altenholz, Germany) to remove larger mesozooplankton organisms and to thereby clearly reduce the grazing mortality of the phytoplankton during the experiments. This seawater is called ‘unfiltered seawater’ in the following.
A part of the collected seawater was filtered using a peristaltic pump and a sterile Sartobran© P filter unit with a 0.2 µm cellulose acetate membrane (Sartorius Stedim Biotech GmbH, Göttingen, Germany). Artificial seawater was prepared with Milli-Q water and sodium chloride (greater than 99.8%, Carl Roth GmbH & Co. KG, Karlsruhe, Germany).
(b). Preparation of the microplastics
Polystyrene beads (called ‘microplastics’ in the following) with a diameter of 700–900 µm (Styropor© P 326; BASF SE, Ludwigshafen am Rhein, Germany) were cleaned in 10% hydrochloric acid overnight and subsequently rinsed thoroughly with Milli-Q water. These manufactured microplastics are typically used for producing expanded polystyrene (styrofoam) and contain inclusions of the blowing agent pentane (see the ‘bubbles’ in figure 1a) with slightly varying proportions resulting in a density range of 1.02–1.05 kg m−3. The cleaned microplastics were transferred to glass beakers filled with artificial seawater whose salinity was similar to that of the seawater used for the aggregation experiments. All neutrally buoyant microplastics were separated by pouring and pipetting away all microplastics swimming at the water surface and lying at the bottom of the beaker, respectively. This procedure was repeated three times, and only the remaining neutrally buoyant microplastics were used for the experiments.
(c). Monitoring of biofilm formation on plastics
To get information about how microbial biofilms form on plastic surfaces, lids of polystyrene Petri dishes (Greiner Bio-One GmbH, Frickenhausen, Germany) were put in (i) the Kiel Fjord (in the inner part of the Bay of Kiel) at a depth of 0.5 m in late summer 2012 for 8 and 12 days and (ii) an indoor mesocosm filled with seawater from the Kiel Fjord in autumn 2012 for 25 days.
(d). Preparation of the microplastics covered by microbial biofilms
For each experiment with biofilm-covered microplastics, neutrally buoyant cleaned microplastics were transferred to 2 l of the collected seawater kept in an open glass beaker (without extra aeration of the seawater), and the beaker was then stored in a climate chamber with a temperature of 11°C and a 24 h light–dark cycle (12 L : 12 D) for 5 weeks. After this period, the microplastics were covered with pronounced biofilms as evident from analyses with confocal laser scanning microscopy.
(e). Aggregation experiments
The aggregation experiments were performed using a custom-made roller table and custom-made cylindrical acrylic glass tanks (called ‘roller tanks’ in the following) as described earlier [43]. Each roller tank had a volume of 5 l. The following experiments were performed (see also electronic supplementary material, table S1). (1) Two 8-day-long experiments with clean microplastics in unfiltered seawater containing the natural particle community. As controls, roller tanks filled with (i) unfiltered seawater containing the natural particle community (but without microplastics), (ii) clean microplastics and filtered seawater, and (iii) clean microplastics and artificial seawater were placed and rotated on the roller table simultaneously. (2) Two 12-day-long experiments with the same approaches and the same controls as described in (1). (3) Four 3-day-long experiments, all with unfiltered seawater containing the natural particle community, with (a) biofilm-covered microplastics and (b) clean microplastics (for comparison). As controls, roller tanks filled with (i) biofilm-covered microplastics and filtered seawater, (ii) clean microplastics and filtered seawater, (iii) biofilm-covered microplastics and artificial seawater, and (iv) clean microplastics and artificial seawater were placed and rotated on the roller table simultaneously. All experiments were performed with three replicates and a microplastics concentration of 50 beads l−1. The microplastics were transferred into the roller tanks very carefully to prevent them from sticking together. All experiments took place in a dark climate chamber to avoid phytoplankton growth and an increase in the phytoplankton concentration and thereby in the density of biogenic particles during the experiments. Except for the first 8-day-long experiment, which for technical reasons was performed at a temperature of 7°C, all experiments were performed at a temperature of 11°C. These two temperatures are in the typical temperature range of the Baltic Sea during the spring phytoplankton bloom, and we assume that the relatively small temperature difference between the first experiment and all other experiments had a negligible effect. At the beginning of each experiment, the roller tanks were rotated with 3 revolutions per minute (rpm) for 1 h to bring the water body in the roller tanks in motion as fast as possible and to thereby avoid that the microplastics sank down to the tank wall and accumulated. Subsequently, the roller tanks were rotated with 1.5–2.5 rpm, which was an appropriate velocity to make sure that the water body in the tank rotated as fast as the tank wall and that the forming aggregates did not sink down to the tank wall but stayed in suspension. During the experiments, the aggregation development was monitored by visual inspection daily. At the end of each experiment, the roller tanks were carefully brought into a horizontal position. Immediately after the formed aggregates had sank down to the bottom of the roller tanks, water samples (one per tank) were taken through the lids of the tanks without damaging the aggregates and without removing microplastics from the tanks. Subsequently, the aggregates were photographed (see below), and the numbers of microplastics that were not included in the aggregates and still suspended in the water were determined. Owing to their size, the microplastics can easily be seen with the naked eye. Visual control could therefore make sure that no microplastics were removed from the tanks and included in the water samples and that all microplastics that were not included in the aggregates were counted properly. Based on the counting results, the proportions of microplastics included in the aggregates were calculated.
The water samples were analysed for the concentration of particulate organic carbon (POC). For this purpose, two aliquots with a volume of 250–500 ml were taken from each sample and filtered onto combusted (8 h, 500°C) GF/F glass fibre filters with a pore size of 0.7 µm (Whatman plc, Maidstone, UK) and stored at −20°C. For the analyses, the samples were dried at 60°C overnight and wrapped in tin foil. Subsequently, the POC concentrations were determined with a Euro EA CHNSO Elemental Analyser (HEKAtech GmbH, Wegberg, Germany) calibrated with an acetanilide standard. The means of the POC concentrations of the two aliquots were then used to calculate the proportions of POC included in the aggregates.
(f). Photographic and microscopic visualizations
The microplastics were visualized using the stereo microscope Leica M205 A equipped with a 1.6× PLANAPO objective, the digital camera Leica DFC420 and the software Leica Application Suite 3.7 (Leica Microsystems GmbH, Wetzlar, Germany). Photographs of the aggregates were taken using the objective AF-S VR Micro-Nikkor 105 mm 1 : 2.8 G IF-ED and the digital single-lens reflex cameras Nikon D750 and Nikon D800E (Nikon Corporation, Tokyo, Japan). The biofilms formed on the polystyrene surfaces were visualized using confocal laser scanning microscopy as described in the following: most biofilms were stained in the dark at room temperature for 20 min with sodium bicarbonate buffer (concentration: 0.1 mol l−1) containing SYTO© 83 (concentration: 5 µmol l−1; staining of nucleic acids; Thermo Fisher Scientific GmbH, Darmstadt, Germany) and a conjugate of the lectin Concanavalin A and the fluorescent dye Alexa Fluor© 633 (concentration: 0.1 mg ml−1; staining of polysaccharides; Thermo Fisher Scientific GmbH). The silica-containing structures of one single biofilm were stained with fluorescein isothiocyanate (FITC) bound to (3-aminopropyl)trimethoxysilane (both from Sigma-Aldrich Chemie GmbH, Steinheim, Germany) at 4°C in a dark fridge for 1 h as described previously [44–46]. Subsequently, the biofilm was thoroughly rinsed with sodium bicarbonate buffer (concentration: 0.1 mol l−1), and the structures containing nucleic acids and polysaccharides were stained as described above. After the staining, all biofilms were thoroughly rinsed with sodium bicarbonate buffer (concentration: 0.1 mol l−1). The biofilms on the polystyrene Petri dish lids were immersed in immersion oil type F (Leica Microsystems GmbH), while the biofilm-covered microplastics were transferred to BacLight mounting oil (Thermo Fisher Scientific GmbH) and mounted on an object slide using reinforcement rings (Herlitz PBS AG Papier-, Büro- und Schreibwaren, Berlin, Germany) [47] and high-precision coverslips (Carl Zeiss Microscopy GmbH, Jena, Germany). The fluorescences of the specimens were visualized with the confocal laser scanning microscope Leica TCS SP5 II and the objective Leica HC PL APO 20×/0.75 IMM CS2. In the case of the biofilms on the Petri dish lids, the objective was directly dipped in the immersion oil. The following wavelengths were used to excite the different fluorescences, and the following fluorescence wavelength ranges were detected for the visualization: FITC: 488 nm excitation, 500–550 nm emission; SYTO© 83: 543 nm excitation, 550–570 nm emission; Alexa Fluor© 633: 633 nm excitation, 640–670 nm emission; chlorophyll autofluorescence: 488 nm excitation, 640–740 nm emission.
(g). Data processing and statistical analyses
The data from all experiments with the same type of microplastics and/or the same aggregation situation (i.e. biofilm-covered or clean microplastics and unfiltered seawater, filtered seawater or artificial seawater) were pooled. By doing this, we achieved a replication of 6 for all experiments carried out for 8 or 12 days and a replication of 12 for all experiments carried out for 3 days.
Data on the proportion of microplastics included in the aggregates during the experiments that had been run for 8 and 12 days were analysed with a generalized linear model (GLM) of the family ‘Poisson’ using the factor ‘length of aggregation period’ with the levels ‘8 days’ and ‘12 days’ and the factor ‘situation’ with the levels ‘unfiltered seawater’, ‘filtered seawater’ and ‘artificial seawater’. The assumptions of normality of errors and homogeneity of variances were verified on the basis of residual plots.
To analyse the data on the proportion of POC that was present in the aggregates formed within 8 and 12 days, we applied a two-way analysis of variance using the factor ‘length of aggregation period’ with the levels ‘8 days’ and ‘12 days’ and the factor ‘presence of microplastics' with the levels ‘yes’ and ‘no’. The model assumptions were tested in the same way as for the GLM.
To test for a possible influence of the biofilm on the proportion of microplastics included in the aggregates that had formed in the different aggregation situations during the 3-day-long experiments, a two-way fully crossed model was calculated using the factor ‘biofilm’ with the levels ‘yes’ and ‘no’ and the factor ‘situation’ with the levels ‘unfiltered seawater’, ‘filtered seawater’ and ‘artificial seawater’. Since the variances in the experimental groups differed markedly between the levels of ‘situation’, we used a GLM of the family ‘Poisson’ for this analysis. After specifying the model, the assumptions of normality of errors and homogeneity of variances were verified on the basis of residual plots. In addition to this global test, Welch-adjusted t-tests were used as a post hoc procedure to identify significant differences between biofilm-covered and clean microplastics within the different levels of ‘situation’. The same was done to test for a significant influence of the biofilm on the proportion of POC that was present in the aggregates formed within the 3-day-long experiments. In this case, we verified the normality of the data with histograms.
All statistical analyses were performed with the freely available statistical computing software R v. 3.4.3 [48].
3. Results
(a). Aggregate formation
In all experiments with biogenic particles, the microplastics, both clean and biofilm-covered, interacted intensively and rapidly with the biogenic particles resulting in the formation of relatively large aggregates (in part with sizes of several millimetres) consisting of both microplastics and biogenic particles (figure 1b–e). A large fraction of the microplastics present in the aggregates was located in the inner aggregate parts, while only a few microplastics were attached to the outer aggregate surface. In the control experiments with clean microplastics and filtered or artificial seawater, the aggregates were never composed of more than three microplastics. In the control experiments with biofilm-covered microplastics and filtered or artificial seawater, most of the aggregates contained a maximum of three microplastics, and only single ones were composed of four or five microplastics.
(b). Aggregation behaviour of the clean microplastics
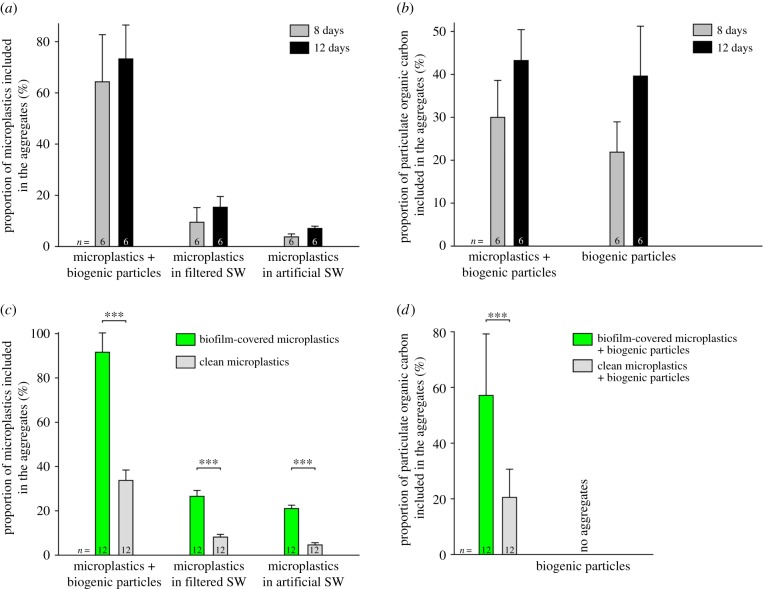
In the experiments with biogenic particles and clean microplastics, the first aggregates visible to the naked eye formed after about 1 day. During the longer experiments, after about 4 days, the aggregates had reached a size that was already very close to their final size at the end of the experiments after 8 and 12 days. By contrast, in the control experiments with biogenic particles only, the first aggregates typically formed after 3–4 days, and after 5–7 days, their size was very close to their final size after 8 and 12 days. At the end of the 3-day-long experiments, many of the clean microplastics (mean: 33.7%) were included in the aggregates (figure 2c). In the longer experiments, on average 64.3 and 73.3% of the microplastics were included in the aggregates after 8 and 12 days, respectively (figure 2a). In all corresponding control experiments with filtered and artificial seawater, much smaller proportions of the clean microplastics aggregated, and the proportions slightly increased with the length of the experiments and were always larger in experiments with filtered seawater than in experiments with artificial seawater (figure 2a). In all these longer experiments, the influence of the aggregation period and the influence of the aggregation situation on the proportion of microplastics included in the aggregates were significant (electronic supplementary material, table S2). The marginally significant interaction between ‘aggregation period’ and ‘situation’ (electronic supplementary material, table S2) is difficult to interpret, because the effect of ‘situation’ did not change with time. Regardless of the time that had elapsed since the start of the experiment, the proportion of aggregated microplastics was always the largest in the experiments with biogenic particles and the lowest in the experiments with artificial seawater (figure 2a).
Figure 2.
Aggregation behaviour of microplastics. (a) Incorporation of clean microplastics in aggregates as a function of the aggregation period and the aggregation situation (presence or absence of biogenic particles, aggregation in filtered or artificial seawater). (b) Incorporation of POC in aggregates as a function of the aggregation period and the aggregation situation (presence or absence of microplastics). (c) Incorporation of microplastics in aggregates as a function of the microplastics properties (clean or biofilm-covered) and the aggregation situation (presence or absence of biogenic particles, aggregation in filtered or artificial seawater). (d) Incorporation of POC in aggregates as a function of the microplastics properties (clean or biofilm-covered). In (a–d), the columns and error bars depict the means and standard deviations, respectively. SW, seawater; ***, significant differences revealed by the Welch-adjusted t-tests.
The proportion of POC that was included in the aggregates at the end of the longer experiments both with clean microplastics and biogenic particles and with biogenic particles only increased with an increase in the aggregation period. This proportion was always slightly larger when clean microplastics were present than when only biogenic particles aggregated (figure 2b). However, the latter influence was not statistically significant, while the length of the aggregation period had a significant influence on the proportion of POC included in the aggregates (electronic supplementary material, table S3).
(c). Formation of microbial biofilms on plastics
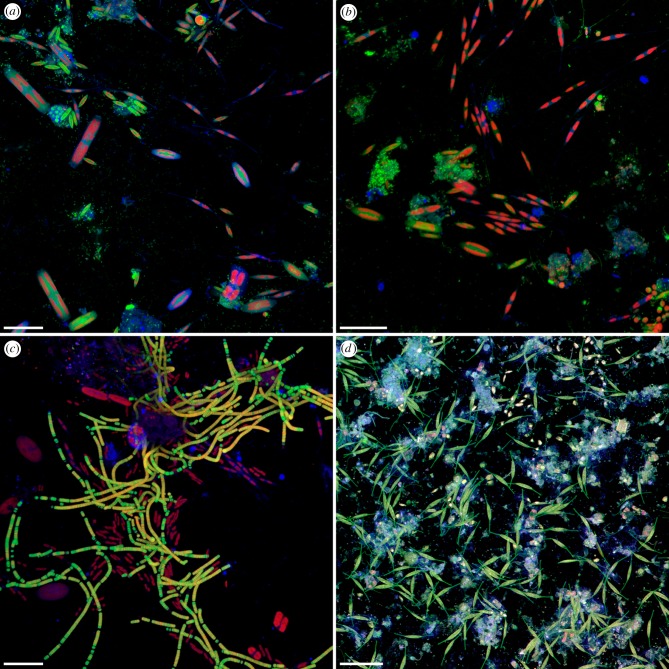
The confocal laser scanning microscopy analyses revealed that a considerable number of bacteria and microalgae was present on the plastic plates after a few days (figure 3a–c), and within a few weeks, pronounced biofilms had formed on the surfaces of the plastic plates (figure 3d).
Figure 3.
Biofilm formation on marine plastics. (a–d) Confocal laser scanning micrographs showing marine biofilms formed on the surface of polystyrene plates. (a–c) Biofilms formed within 8 (a,b) and 12 (c) days in the Kiel Fjord in late summer 2012. Blue, polysaccharide-containing structures; green, structures containing nucleic acids; red, chlorophyll-containing structures. (d) Biofilm formed within 25 days in an indoor mesocosm filled with seawater from the Kiel Fjord in late summer 2012. Blue, polysaccharide-containing structures; green, diatom frustules and structures containing nucleic acids; red, chlorophyll-containing structures; grey, protein-containing structures. Scale bars: 30 µm (a–c) and 50 µm (d).
The biofilms covering the microplastics that were used for the experiments had been formed by bacteria and microalgae and exhibited a pronounced matrix of extracellular polymeric substances including polysaccharides and DNA (figure 1f).
(d). Aggregation behaviour of the biofilm-covered microplastics
In the presence of biofilm-covered microplastics and biogenic particles (i.e. in unfiltered seawater), the first aggregates visible to the naked eye had formed already after a few hours, and after 1–2 days, the size of the aggregates was very close to the final aggregate size at the end of the experiments after 3 days. No obvious difference in the structure and composition was observed between the aggregates formed with biofilm-covered microplastics and those formed with clean microplastics (figure 1b–e). In the 3-day-long experiments in unfiltered seawater, nearly all (mean: 91.6%) biofilm-covered microplastics were included in the aggregates (figure 2c). After 3 days, the proportion of biofilm-covered microplastics included in the aggregates was significantly larger than the proportion of clean microplastics included in the aggregates, which was the case in all aggregation situations (Welch-adjusted t-test: ‘unfiltered seawater’: t = 20.18, p ≤ 0.001; ‘filtered seawater’: t = 21.76, p ≤ 0.001; ‘artificial seawater’: t = 31.13, p ≤ 0.001; figure 2c; electronic supplementary material, table S4). In addition, in each of the aggregation situations, the proportion of biofilm-covered microplastics included in the aggregates after 3 days was considerably larger than the both corresponding proportions of clean microplastics included in the aggregates after 8 and 12 days (figure 2a,c). The proportions of microplastics, biofilm-covered as well as clean, included in the aggregates were significantly different between the different aggregation situations (electronic supplementary material, table S4), and they increased from ‘artificial seawater’ via ‘filtered seawater’ to ‘unfiltered seawater’ (figure 2c). The significant interaction between ‘biofilm’ and ‘situation’ (electronic supplementary material, table S4) is again difficult to interpret, because the effect size of ‘biofilm’ (i.e. the difference in the proportions of aggregated microplastics) did not differ substantially between the aggregation situations nor did the direction of the biofilm effect vary between them.
The proportion of POC included in the aggregates after 3 days was significantly larger with biofilm-covered microplastics compared to the situation with clean microplastics (Welch-adjusted t-test: t = 5.22, p ≤ 0.001; figure 2d). Furthermore, in the experiments with biofilm-covered microplastics, the proportion of POC included in the aggregates after 3 days was considerably larger than those of the 8- and 12-day-long experiments carried out either with clean microplastics or with biogenic particles only (figure 2b,d).
4. Discussion
Our experiments clearly revealed that microplastics of the type we chose for our experiments have a high potential to aggregate with marine biogenic particles and, at least in the initial phase of the aggregate formation, to increase the natural particle aggregation rates. By contrast, they seem to have a relatively low potential to aggregate with themselves when biogenic particles are absent, even when they are covered by biofilms. This indicates that the presence of biogenic particles is essential for a pronounced microplastics aggregation.
Microplastics do not only interact with existing aggregates, as shown earlier [21,22], but are obviously also strongly involved in the formation of new aggregates. This is indicated by their presence in the inner parts of the formed aggregates and the fact that their addition to the system resulted in a much earlier, faster and more pronounced aggregate formation. Among the main factors responsible for the initial step of aggregate formation (i.e. the collision of particles) are particle abundance and size [49]. The addition of microplastics to the system increases both the total particle abundance and the number of, compared to most plankton cells, relatively large particles and thereby enhances the collision probability of all particles. This mechanism probably explains the increased particle aggregation rates in the presence of microplastics in the first days of aggregate formation observed in this study.
Another main factor is the stickiness of the particles involved in the aggregation, which has been known for a long time for biogenic particles [24] but is here demonstrated for microplastics for the first time. Polysaccharides and extracellular DNA, like those identified in the biofilm matrices in the present study, are known to be relatively sticky [38,39]. In aggregation experiments, extracellular polysaccharides in seawater were demonstrated to increase the coagulation efficiency of biogenic particles [50,51]. Accordingly, although the stickiness of the microplastics was not determined in the present study, it can be assumed that the biofilm-covered microplastics were considerably stickier than the clean ones and that this can explain the earlier onset of visible aggregate formation between biofilm-covered microplastics and biogenic particles and the significantly larger proportion of biofilm-covered microplastics included in the aggregates when compared with clean microplastics. Plastics that have been shown to be colonized by microorganisms and covered by biofilms in the oceans include various plastic types with different properties such as polyethylene [26,27,29,30–33,36,52], polyethylene terephthalate [28,31,33,35], polypropylene [27,29,33] and polystyrene [25,29,31–34]. The biofilm formation tests performed in the present study showed that the colonization of plastics by microorganisms can be relatively rapid, which is in accordance with the results of an earlier study [36]. These observations suggest that large proportions of the microplastics ending up in the oceans are covered by biofilms and become relatively sticky after short time periods. Consequently, biofilm-covered marine microplastics being sticky and featuring a large aggregation potential probably represent the typical situation in the oceans. If the aggregation behaviour of these microplastics is comparable with that observed in our experiments, different implications described in the following are conceivable.
Aggregation of microplastics with biogenic particles can influence the sinking rates and thereby the fate of microplastics in the ocean. Laboratory experiments of earlier studies showed that the incorporation of microplastics in existing aggregates can (i) increase or decrease the sinking rates of these aggregates, depending on the aggregates' composition and therewith mean density relative to that of the microplastics [21], and (ii) increase the sinking rates of the microplastics [22]. The latter can be assumed to take place also when microplastics aggregate with biogenic particles. If particles with a relatively high density, such as diatoms, are involved, the aggregation can result in higher effective microplastics sinking rates. The input of microplastics into the oceans typically takes place in the surface layer, and the described ballasting probably causes a fast sinking of microplastics out of this layer and thereby facilitates the distribution of microplastics into deeper ocean compartments. Such a removal from the surface layer can explain why the concentrations of microplastics in the surface ocean were observed to be lower than expected [19]. Our experiments clearly showed that aggregates composed of biogenic particles and microplastics can be stable for several days, which makes it conceivable that such aggregates can ‘survive’ the often relatively long-lasting sinking to great depths without breaking apart and becoming decomposed before they reach the ocean floor. Accordingly, besides a potential transport via the food web, sinking in aggregates is very likely to be the main transport pathway that causes the presence of microplastics in deep-sea sediments observed earlier [9,11,13]. Important implications of these processes are an increased availability of microplastics to benthic organisms and a long-term accumulation of microplastics in marine sediments.
Diatoms excrete polysaccharides that were shown to increase the particle coagulation efficiency [24,50]. Accordingly, the aggregation of diatoms and sticky microplastics is probably very efficient, and in ocean areas where diatoms dominate the phytoplankton community, aggregation can be assumed to result in relatively fast sinking microplastics and a rather pronounced removal of both microplastics and associated organic material from the surface layer.
As already pointed out above, the results of the present study indicate that the addition of microplastics to the system stimulates the aggregation and clearly increases the aggregation rates of organic material, at least sporadically. This suggests that microplastics can significantly modify the vertical export of biogenic particles in the marine water column and thereby alter globally important biogeochemical processes.
The formation of a biofilm on the plastic surface can reduce the hydrophobicity of the plastic and make plastic with a smaller density than that of seawater more neutrally buoyant [26]. This can bring plastics such as polyethylene, which typically floats at the surface when it enters the ocean in a fresh and clean state, into suspension, especially when the biofilms contain many relatively dense small diatoms. This effect is very likely to be intensified by aggregation of the plastic particles with denser biogenic particles.
Microbial communities colonizing plastics were shown to be distinct from those in the surrounding seawater and to differ between different plastic types and different geographical origins [27,28,33,35]. Accordingly, the aggregation of microplastics with biogenic particles might alter the microbial communities of the forming aggregates. In addition, through aggregation and subsequent aggregate sinking, these microbial communities present on microplastics are vertically distributed in the oceans and made available to the food web of deeper ocean layers. Because microbial communities on plastics can comprise pathogenic organisms as, for example, bacteria of the genus Vibrio [27,34], such aggregation and sinking processes probably increase the risk of organisms living in deeper ocean areas not only to become exposed to microplastics but also to become infected with pathogens.
In conclusion, this study demonstrates that microplastics rapidly aggregate with marine biogenic particles, which accelerates the gross aggregate formation including the incorporation of organic material. The microplastics' aggregation potential is significantly increased by the formation of biofilms on the plastic surfaces, which probably is the typical situation in the oceans where diverse bacterial communities colonize the microplastics surfaces. It is very likely that similar processes take place in the oceans and microplastics become strongly involved in the natural aggregation dynamics and thereby influence the particle size distribution and the export rates of organic matter. The experimentally demonstrated aggregation behaviour can explain the apparent removal of microplastics from the surface layer of the oceans and the presence of microplastics in deep-sea sediments. Future studies will have to further proof the existence of microplastics-containing aggregates in the marine water column using techniques such as sediment traps, marine snow catchers and underwater vision profilers.
Supplementary Material
Supplementary Material
Acknowledgements
We thank Tania Klüver for having performed the POC analyses and the captain and the crew of ‘Littorina’ for their help. Lars Heepe's comments on the statistical analyses are gratefully acknowledged. The polystyrene beads were kindly provided by BASF SE, Ludwigshafen am Rhein, Germany.
Data accessibility
All data are available in the electronic supplementary material.
Authors' contributions
J.M. and A.E. conceived and designed the study. K.W. contributed ideas. J.M. and A.S. performed the aggregation experiments and analysed the samples. J.M. performed all photography and microscopy analyses. M.L. performed the statistical analyses. J.M. drafted the manuscript. All authors discussed and revised the manuscript.
Competing interests
We have no competing interests.
Funding
This project was funded by the Cluster of Excellence 80 ‘The Future Ocean’, which is funded within the framework of the Excellence Initiative by the Deutsche Forschungsgemeinschaft (DFG) on behalf of the German federal and state governments. A.E. and K.W. were supported by the Helmholtz Association via the programmes OCEANS and PACES, respectively.
References
- 1.Derraik JGB. 2002. The pollution of the marine environment by plastic debris: a review. Mar. Poll. Bull. 44, 842–852. ( 10.1016/S0025-326X(02)00220-5) [DOI] [PubMed] [Google Scholar]
- 2.Barnes DKA, Galgani F, Thompson RC, Barlaz M. 2009. Accumulation and fragmentation of plastic debris in global environments. Phil. Trans. R. Soc. B 364, 1985–1998. ( 10.1098/rstb.2008.0205) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thompson RC, Swan SH, Moore CJ, vom Saal FS. 2009. Our plastic age. Phil. Trans. R. Soc. B 364, 1973–1976. ( 10.1098/rstb.2009.0054) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Avio CG, Gorbi S, Regoli F. 2017. Plastics and microplastics in the oceans: from emerging pollutants to emerged threat. Mar. Environ. Res. 128, 2–11. ( 10.1016/j.marenvres.2016.05.012) [DOI] [PubMed] [Google Scholar]
- 5.Jambeck JR, Geyer R, Wilcox C, Siegler TR, Perryman M, Andrady A, Narayan R, Law KL. 2015. Plastic waste inputs from land into the ocean. Science 347, 768–771. ( 10.1126/science.1260352) [DOI] [PubMed] [Google Scholar]
- 6.Law KL. 2017. Plastics in the marine environment. Annu. Rev. Mar. Sci. 9, 205–229. ( 10.1146/annurev-marine-010816-060409) [DOI] [PubMed] [Google Scholar]
- 7.Andrady AL. 2011. Microplastics in the marine environment. Mar. Poll. Bull. 62, 1596–1605. ( 10.1016/j.marpolbul.2011.05.030) [DOI] [PubMed] [Google Scholar]
- 8.Cole M, Lindeque P, Halsband C, Galloway TS. 2011. Microplastics as contaminants in the marine environment: a review. Mar. Poll. Bull. 62, 2588–2597. ( 10.1016/j.marpolbul.2011.09.025) [DOI] [PubMed] [Google Scholar]
- 9.Van Cauwenberghe L, Vanreusel A, Mees J, Janssen CR. 2013. Microplastic pollution in deep-sea sediments. Environ. Poll. 182, 495–499. ( 10.1016/j.envpol.2013.08.013) [DOI] [PubMed] [Google Scholar]
- 10.Obbard RW, Sadri S, Wong YQ, Khitun AA, Baker I, Thompson RC. 2014. Global warming releases microplastic legacy frozen in Arctic Sea ice. Earth's Future 2, 315–320. ( 10.1002/2014EF000240) [DOI] [Google Scholar]
- 11.Woodall LC, et al. 2014. The deep sea is a major sink for microplastic debris. R. Soc. open sci. 1, 140317 ( 10.1098/rsos.140317) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lusher AL, Tirelli V, O'Connor I, Officer R. 2015. Microplastics in Arctic polar waters: the first reported values of particles in surface and sub-surface samples. Sci. Rep. 5, 14947 ( 10.1038/srep14947) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bergmann M, Wirzberger V, Krumpen T, Lorenz C, Primpke S, Tekman MB, Gerdts G. 2017. High quantities of microplastic in Arctic deep-sea sediments from the HAUSGARTEN observatory. Environ. Sci. Technol. 51, 11 000–11 010. ( 10.1021/acs.est.7b03331) [DOI] [PubMed] [Google Scholar]
- 14.Obbard RW. 2018. Microplastics in polar regions: the role of long range transport. Curr. Opin. Environ. Sci. Health 1, 24–29. ( 10.1016/j.coesh.2017.10.004) [DOI] [Google Scholar]
- 15.Peeken I, Primpke S, Beyer B, Gütermann J, Katlein C, Krumpen T, Bergmann M, Hehemann L, Gerdts G. 2018. Arctic sea ice is an important temporal sink and means of transport for microplastic. Nat. Commun. 9, 1505 ( 10.1038/s41467-018-03825-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Waller CL, Griffiths HJ, Waluda CM, Thorpe SE, Loaiza I, Moreno B, Pacherres CO, Hughes KA. 2017. Microplastics in the Antarctic marine system: an emerging area of research. Sci. Total Environ. 598, 220–227. ( 10.1016/j.scitotenv.2017.03.283) [DOI] [PubMed] [Google Scholar]
- 17.Courtene-Jones W, Quinn B, Gary SF, Mogg AOM, Narayanaswamy BE. 2017. Microplastic pollution identified in deep-sea water and ingested by benthic invertebrates in the Rockall Trough, North Atlantic Ocean. Environ. Poll. 231, 271–280. ( 10.1016/j.envpol.2017.08.026) [DOI] [PubMed] [Google Scholar]
- 18.Thompson RC, et al. 2004. Lost at sea: where is all the plastic? Science 304, 838 ( 10.1126/science.1094559) [DOI] [PubMed] [Google Scholar]
- 19.Cózar A, et al. 2014. Plastic debris in the open ocean. Proc. Natl Acad. Sci. USA 111, 10 239–10 244. ( 10.1073/pnas.1314705111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao S, Danley M, Ward JE, Li D, Mincer TJ. 2017. An approach for extraction, characterization and quantitation of microplastic in natural marine snow using Raman microscopy. Anal. Methods 9, 1470–1478. ( 10.1039/C6AY02302A) [DOI] [Google Scholar]
- 21.Long M, Moriceau B, Gallinari M, Lambert C, Huvet A, Raffray J, Soudant P. 2015. Interactions between microplastics and phytoplankton aggregates: impact on their respective fates. Mar. Chem. 175, 39–46. ( 10.1016/j.marchem.2015.04.003) [DOI] [Google Scholar]
- 22.Porter A, Lyons BP, Galloway TS, Lewis C. 2018. Role of marine snows in microplastic fate and bioavailability. Environ. Sci. Technol. 52, 7111–7119. ( 10.1021/acs.est.8b01000) [DOI] [PubMed] [Google Scholar]
- 23.Galloway TS, Cole M, Lewis C. 2017. Interactions of microplastic debris throughout the marine ecosystem. Nat. Ecol. Evol. 1, 0116 ( 10.1038/s41559-017-0116) [DOI] [PubMed] [Google Scholar]
- 24.Kiørboe T, Andersen KP, Dam HG. 1990. Coagulation efficiency and aggregate formation in marine phytoplankton. Mar. Biol. 107, 235–245. ( 10.1007/BF01319822) [DOI] [Google Scholar]
- 25.Carpenter EJ, Anderson SJ, Harvey GR, Miklas HP, Peck BB. 1972. Polystyrene spherules in coastal waters. Science 178, 749–750. ( 10.1126/science.178.4062.749) [DOI] [PubMed] [Google Scholar]
- 26.Lobelle D, Cunliffe M. 2011. Early microbial biofilm formation on marine plastic debris. Mar. Poll. Bull. 62, 197–200. ( 10.1016/j.marpolbul.2010.10.013) [DOI] [PubMed] [Google Scholar]
- 27.Zettler ER, Mincer TJ, Amaral-Zettler LA. 2013. Life in the ‘plastisphere’: microbial communities on plastic marine debris. Environ. Sci. Technol. 47, 7137–7146. ( 10.1021/es401288x) [DOI] [PubMed] [Google Scholar]
- 28.Oberbeckmann S, Loeder MGJ, Gerdts G, Osborn AM. 2014. Spatial and seasonal variation in diversity and structure of microbial biofilms on marine plastics in Northern European waters. FEMS Microbiol. Ecol. 90, 478–492. ( 10.1111/1574-6941.12409) [DOI] [PubMed] [Google Scholar]
- 29.Reisser J, Shaw J, Hallegraeff G, Proietti M, Barnes DKA, Thums M, Wilcox C, Hardesty BD, Pattiaratchi C. 2014. Millimeter-sized marine plastics: a new pelagic habitat for microorganisms and invertebrates. PLoS ONE 9, e100289 ( 10.1371/journal.pone.0100289) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eich A, Mildenberger T, Laforsch C, Weber M. 2015. Biofilm and diatom succession on polyethylene (PE) and biodegradable plastic bags in two marine habitats: early signs of degradation in the pelagic and benthic zone? PLoS ONE 10, e0137201 ( 10.1371/journal.pone.0137201) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Debroas D, Mone A, Ter Halle A. 2017. Plastics in the North Atlantic garbage patch: a boat-microbe for hitchhikers and plastic degraders. Sci. Total Environ. 599–600, 1222–1232. ( 10.1016/j.scitotenv.2017.05.059) [DOI] [PubMed] [Google Scholar]
- 32.Oberbeckmann S, Kreikemeyer B, Labrenz M. 2018. Environmental factors support the formation of specific bacterial assemblages on microplastics. Front. Microbiol. 8, 2709 ( 10.3389/fmicb.2017.02709) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amaral-Zettler LA, Zettler ER, Slikas B, Boyd GD, Melvin DW, Morrall CE, Proskurowski G, Mincer TJ. 2015. The biogeography of the plastisphere: implications for policy. Front. Ecol. Environ. 13, 541–546. ( 10.1890/150017) [DOI] [Google Scholar]
- 34.Foulon V, Le Roux F, Lambert C, Huvet A, Soudant P, Paul-Pont I. 2016. Colonization of polystyrene microparticles by Vibrio crassostreae: light and electron microscopic investigation. Environ. Sci. Technol. 50, 10 988–10 996. ( 10.1021/acs.est.6b02720) [DOI] [PubMed] [Google Scholar]
- 35.Oberbeckmann S, Osborn AM, Duhaime MB. 2016. Microbes on a bottle: substrate, season and geography influence community composition of microbes colonizing marine plastic debris. PLoS ONE 11, e0159289 ( 10.1371/journal.pone.0159289) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harrison JP, Schratzberger M, Sapp M, Osborn AM. 2014. Rapid bacterial colonization of low-density polyethylene microplastics in coastal sediment microcosms. BMC Microbiol. 14, 232 ( 10.1186/s12866-014-0232-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sutherland IW. 2001. Biofilm exopolysaccharides: a strong and sticky framework. Microbiology 147, 3–9. ( 10.1099/00221287-147-1-3) [DOI] [PubMed] [Google Scholar]
- 38.Flemming H-C, Wingender J. 2010. The biofilm matrix. Nat. Rev. Microbiol. 8, 623–633. ( 10.1038/nrmicro2415) [DOI] [PubMed] [Google Scholar]
- 39.Petrova OE, Sauer K. 2012. Sticky situations: key components that control bacterial surface attachment. J. Bacteriol. 194, 2413–2425. ( 10.1128/JB.00003-12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gregory MR, Andrady AL. 2003. Plastics in the marine environment. In Plastics and the environment (ed. AL Andrady.), pp. 379–401. Hoboken, NJ: John Wiley & Sons. [Google Scholar]
- 41.Gordon RP.2000. The vertical and horizontal distribution of microplastics in the Caribbean and Sargasso Seas along the W-169 cruise track, May-June 2000. See http://people.oregonstate.edu/~gordonr/Ryan_Gordon/Downloads/Ryan_Gordon_files/Gordon_SEA_2000.pdf . (last accessed on 28 February 2014)
- 42.Dubaish F, Liebezeit G. 2013. Suspended microplastics and black carbon particles in the Jade system, southern North Sea. Water Air Soil Pollut. 224, 1352 ( 10.1007/s11270-012-1352-9) [DOI] [Google Scholar]
- 43.Shanks AL, Edmondson EW. 1989. Laboratory-made artificial marine snow: a biological model of the real thing. Mar. Biol. 101, 463–470. ( 10.1007/BF00541648) [DOI] [Google Scholar]
- 44.Desclés J, Vartanian M, El Harrak A, Quinet M, Bremond N, Sapriel G, Bibette J, Lopez PJ. 2008. New tools for labeling silica in living diatoms. New Phytol. 177, 822–829. ( 10.1111/j.1469-8137.2007.02303.x) [DOI] [PubMed] [Google Scholar]
- 45.Friedrichs L. 2013. A simple cleaning and fluorescent staining protocol for recent and fossil diatom frustules. Diatom Res. 28, 317–327. ( 10.1080/0269249X.2013.799525) [DOI] [Google Scholar]
- 46.Michels J, Vogt J, Simon P, Gorb SN. 2015. New insights into the complex architecture of siliceous copepod teeth. Zoology 118, 141–146. ( 10.1016/j.zool.2014.11.001) [DOI] [PubMed] [Google Scholar]
- 47.Michels J, Büntzow M. 2010. Assessment of Congo red as a fluorescence marker for the exoskeleton of small crustaceans and the cuticle of polychaetes. J. Microsc. 238, 95–101. ( 10.1111/j.1365-2818.2009.03360.x) [DOI] [PubMed] [Google Scholar]
- 48.R Core Team. 2017. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See https://www.r-project.org. [Google Scholar]
- 49.McCave IN. 1984. Size spectra and aggregation of suspended particles in the deep ocean. Deep-Sea Res. 31, 329–352. ( 10.1016/0198-0149(84)90088-8) [DOI] [Google Scholar]
- 50.Engel A. 2000. The role of transparent exopolymer particles (TEP) in the increase in apparent particle stickiness (α) during the decline of a diatom bloom. J. Plankton Res. 22, 485–497. ( 10.1093/plankt/22.3.485) [DOI] [Google Scholar]
- 51.Szlosek Chow J, Lee C, Engel A. 2015. The influence of extracellular polysaccharides, growth rate, and free coccoliths on the coagulation efficiency of Emiliania huxleyi. Mar. Chem. 175, 5–17. ( 10.1016/j.marchem.2015.04.010) [DOI] [Google Scholar]
- 52.Nauendorf A, Krause S, Bigalke NK, Gorb EV, Gorb SN, Haeckel M, Wahl M, Treude T. 2016. Microbial colonization and degradation of polyethylene and biodegradable plastic bags in temperate fine-grained organic-rich marine sediments. Mar. Poll. Bull. 103, 168–178. ( 10.1016/j.marpolbul.2015.12.024) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available in the electronic supplementary material.