Abstract
Prey animals have evolved a wide variety of behaviours to combat the threat of predation, and these have been generally well studied. However, one of the most common and taxonomically widespread antipredator behaviours of all has, remarkably, received almost no experimental attention: so-called ‘protean’ behaviour. This is behaviour that is sufficiently unpredictable to prevent a predator anticipating in detail the future position or actions of its prey. In this study, we used human ‘predators’ participating in 3D virtual reality simulations to test how protean (i.e. unpredictable) variation in prey movement affects participants' ability to visually target them as they move (a key determinant of successful predation). We found that targeting accuracy was significantly predicted by prey movement path complexity, although, surprisingly, there was little evidence that high levels of unpredictability in the underlying movement rules equated directly to decreased predator performance. Instead, the specific movement rules differed in how they impacted on targeting accuracy, with the efficacy of protean variation in one element depending on the values of the remaining elements. These findings provide important insights into the understudied phenomenon of protean antipredator behaviour, which are directly applicable to predator–prey dynamics within a broad range of taxa.
Keywords: predator–prey interactions, anti-predator defence, unpredictability, virtual reality
1. Introduction
Prey organisms have evolved a wide diversity of behavioural mechanisms to combat the threat of predation. These range from avoiding detection (for example through nocturnality [1–3], cryptic coloration [4] or living underground [5,6]), to actively warding off attack (for example via thanatosis [7] or startle displays [8–10]), to fleeing away from a predator [11]. Many antipredator behaviours, including those described above have received considerable empirical and theoretical attention and are generally well understood in terms of their function and mechanistic underpinning [12]. However, one of the most commonly observed and taxonomically widespread antipredator behaviours of all has, remarkably, received almost no experimental investigation: so-called ‘protean’ behaviour [13].
Protean behaviour is broadly defined as behaviour that is sufficiently unpredictable to prevent a predator from anticipating the future position or actions of its prey [13], and there are many anecdotal examples of animals engaging in this behaviour upon the detection of a predator. For instance, the erratic ‘zig-zagging’ behaviour observed in the dwarf blaasop pufferfish (Torquigener flavimaculosus) [14] and the wedge-snouted desert lizard (Meroles cuneirostris) [15], or the sharp turns and powered dives by the male budwing mantis (Parasphendale agrionina) [16] have all been hypothesized to make it harder for a predator to anticipate the animal's subsequent location, and hence make it harder to catch [13]. These are potential examples of active protean movement (i.e. behaviour in which prey engage when they are aware of an immediate predatory threat), although, protean behaviour may also be displayed in a passive context as ‘insurance’. By continuously displaying protean movement, prey animals may deter or unknowingly evade attacks from undetected predators [13]; for example many fly and butterfly species incorporate protean-like elements in their normal flight [17,18]. However, despite the almost universal presence of putatively protean behaviour in the animal kingdom, only one study has empirically investigated whether this behaviour actually increases the chance of escaping [19].
In their study, Jones et al. [19] found, using human subjects ‘preying upon’ computer-generated moving prey, that individual prey items were harder to catch when their turning angles were drawn randomly from a relatively wide angular range (which they classed as ‘protean’) than when their turn angles were selected (also randomly) from a relatively narrow angular range (which they classed as ‘predictable’). This elegant study therefore provides clear evidence that incorporating protean elements into an animal's movement can have positive anti-predator benefits, although by focusing solely on turning angle it does not consider that an animal's movement could be considered protean in various different ways. For example, animals may show unpredictable changes in speed or the distance travelled before turning, alongside (or even instead of) unpredictable turning angles; both of which would be predicted to make an animal's future position harder to predict. Furthermore, because in Jones et al.'s [19] study all prey items incorporated some element of unpredictability into their turns, it is unclear what would happen if prey moved in predictable, but non-trivial, ways, such as spiralling. This has been highlighted as a putatively protean escape behaviour in the take-off flight of chironomid midges [13] and could occur, for instance, if movement parameters such as turning angle had fixed, rather than protean, values. Pulling apart the effects of these different movement elements is crucial to furthering our understanding of how a broad range of species respond to potential, and real, threats of predation.
In this study, we used human ‘predators’ playing a 3D virtual reality (VR) simulation to test how protean variation in one or more of these three movement elements (speed, the distance travelled between turns, and turn angle) influenced a predator's ability to target the prey item as it moved (a key determinant of successful predation; [20]), relative to prey that exhibited movement elements with fixed (and hence potentially predictable) values. We predicted that, as the number of movement elements that exhibited protean variation increased, this would result in increasingly unpredictable prey movement paths which would be more difficult to target.
2. Methods
(a). Simulations
All simulations were created in the Unity3D game engine (Unity Technologies, San Francisco, USA), and built to run on a Samsung Galaxy S7 smartphone using the Samsung Gear VR system. Unlike simulations on a standard computer screen, where movement is confined to a restricted 2D space, within VR the participant can observe a full 360° 3D environment. This allows both a greater range of motion (e.g. objects can potentially move behind as well as in front of the participant) and, crucially, the third dimension (allowing objects to be perceived as moving away from the participant). Simulations consisted of a black sphere (the ‘prey’) moving in a 3D virtual space centred on the participant. The prey had a radius of 0.1 m and was presented against a homogeneous white background to maximize contrast. The high contrast between the prey item and its background, combined with the lack of visual clutter in the virtual environment, minimizes the likelihood of attentional lapses (e.g. by excluding the possibility that attention is involuntarily drawn to salient features of the background) [21].
Prey movement consisted of a series of steps during each of which it travelled in a straight line in 3D space before turning and moving off on a different trajectory. This pattern of movement is commonly used in animal movement models and is characteristic of the movement patterns of a wide variety of species [22–24]. Movement of prey in the simulation was therefore determined by three parameters: the distance travelled in a straight line between turns (hereafter termed ‘distance’), the time taken to travel over this distance (‘speed’) and the angle turned within a cone centred on the prey's direction of travel (‘angle’). We considered that each of these parameters could be either ‘fixed’ (that is, the value assigned to a given prey item was randomly chosen but remained constant throughout a trial; see below) or ‘protean’ (the parameter value was randomly chosen each time the prey performed a particular behaviour, e.g. each time it turned). The specific values used were based on those obtained from pilot experiments, and were as follows: distance could take fixed values of either 1 m or 5 m (termed ‘short’ and ‘long’, respectively) or a protean value drawn from a uniform distribution on (1 m, 5 m); speed could take fixed values of either 1 ms−1 or 3 ms−1 (termed ‘slow’ and ‘fast’, respectively) or a protean value drawn from a uniform distribution on (1 ms−1, 3 ms−1); and angle could take fixed values of either 0.1π radians or 0.5π radians (termed ‘narrow’ and ‘wide’, respectively) or a protean value drawn from a uniform distribution on (0.1π radians, 0.5π radians). In total, this resulted in 27 possible combinations of fixed/protean movement elements (e.g. short distance, fast speed and protean angle, and so on).
Within the simulation, participants were free to look around the virtual environment. A small, red circle (the reticle) was superimposed onto the centre of the participants' field of view and provided a point of reference for the participant to facilitate targeting, allowing them to interact with moving prey objects in real time. We use the term ‘targeting’ to emphasize the similarities between this process and, for example, maintaining a target within a rifle's sights (a process that requires the participant to move their head to maintain alignment with the target), although note that eye movements will be required to fine-tune tracking accuracy [21,25]. Quantifying targeting accuracy using head movements alone is therefore likely to suffer from reduced stability (greater jitter), result in slightly slower response times, and be less sensitive to minor attentional lapses than when also considering eye movements [21], although importantly our simulated prey were not making subtle movements that could be tracked solely with the eyes (cf. [21,25,26]). Instead, they moved rapidly around the virtual environment, requiring participants to constantly move their head in order to keep the prey within their field of view. Targeting, as measured using head movements, therefore provides a useful overall measure of a participant's ability to follow a fast moving prey item, while providing a measure of biological realism in the context of predator–prey interactions (where animals often align their head with the target before attack; e.g. [27,28]).
(b). Experimental protocol
A total of n = 40 participants took part in this study (20 females and 20 males, with a mean age of 20.7 (range, 18 to 28)), all of whom were students of the University of Lincoln. Before providing consent to take part in the study, participants were given written information on the general aims of the study (although not the specific hypotheses being tested), what they would be asked to do, and the approximate time required to complete the study. Their age and gender were noted, but not linked to their experimental data.
When participants put on the headset to begin the simulation they were presented with a series of simple text instructions to familiarize them with the VR environment and demonstrate how to interact with objects within it. Each experimental trial presented the participant with one prey item to target. At the start of each trial, the prey was coloured red and appeared at a fixed default position (5 m directly in front of the participant) and trajectory (facing directly away from the participant). To start each trial, the participant used their head movements to position the reticle over the prey for 3 s. The prey item then turned from red to black to indicate that the trial had started, and began to move based on the combination of fixed/protean movement rules it had been allocated for that particular trial. Participants were instructed that their task was to constantly target the prey item, by maintaining the reticle as close to its centre as possible as it moved around the virtual environment. Each trial lasted 10 s and there were 27 trials in total per participant (one for each possible combination of fixed/protean parameter values). The order of these trials was randomized for each participant.
(c). Data collection
Data on prey location (its Cartesian coordinates in 3D space) and the participant's head orientation (a 3D vector passing through a point between the participant's eyes and towards the reticle) were collected every 0.02 s throughout each trial, and stored in anonymised text files. At each time step, we subsequently calculated the minimum distance between a 3D point representing the centre of the prey and a ray indicating the participant's head orientation; if the reticle was directly over the centre of the prey this distance would be 0, and would increase with as the reticle moved further away from the prey's centre. This distribution of distance values was used to calculate the mean distance from the centre of the prey over the 10 s of each trial, as a measure of overall targeting accuracy (where a lower mean distance indicates better overall accuracy) and therefore the overall effectiveness of prey ‘behaviour’ in terms of avoiding predation.
We also used the data on prey location to compute a measure of prey movement path complexity in each trial, using the information-theoretic approach described by Herbert-Read et al. [29]. This method assigns a numeric value to each path, such that more complex paths receive higher values, and so provides an objective measure of how ‘protean’ each movement path was. In brief, we constructed an embedding matrix  containing the 3D positions of the prey over the time window
containing the 3D positions of the prey over the time window  (where here n was simply the total number of positions recorded during each 10 s trial). The x component of the embedding matrix
(where here n was simply the total number of positions recorded during each 10 s trial). The x component of the embedding matrix 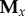 was derived from the x coordinates of the positions, such that
was derived from the x coordinates of the positions, such that
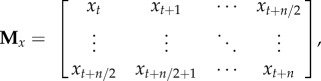 |
2.1 |
with 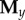 and
and 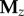 derived similarly from the y and z coordinates, respectively. The full embedding matrix is then simply given by
derived similarly from the y and z coordinates, respectively. The full embedding matrix is then simply given by 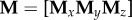 . We next subtracted the mean from each column of
. We next subtracted the mean from each column of  , before extracting the vector of singular values s from its singular value decomposition. Each singular value was normalized by dividing it by the sum of all singular values, to give
, before extracting the vector of singular values s from its singular value decomposition. Each singular value was normalized by dividing it by the sum of all singular values, to give  , and the complexity of the movement path, H, taken as the entropy of the distribution of the singular values
, and the complexity of the movement path, H, taken as the entropy of the distribution of the singular values
| 2.2 |
Representative movement paths, of varying complexity, are given in figure 1.
Figure 1.
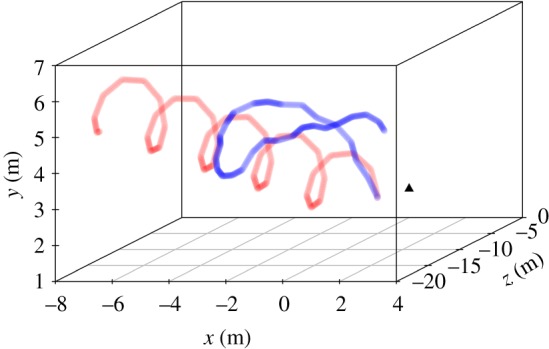
Representative movement paths from a prey with all fixed movement parameters (red; which has a path complexity of 1.53) and a prey with all protean movement parameters (blue; which has a path complexity of 2.29). The black triangle denotes the location of the participant's head in each case, and all prey start from the same position.
(d). Statistical analysis
All analyses were conducted using general linear mixed-effects models (glmm) in R version 3.3.2, using the lmer function in the lme4 package [30]. We first tested whether path complexity predicted targeting accuracy, regardless of the specific movement rules underpinning each path. Log10-transformed targeting accuracy was included as the dependent variable, with path complexity as a continuous predictor and trial order as a covariate to control for possible learning or fatigue effects over consecutive trials. Each participant's anonymous identifier was included as a random effect to control for repeated data from the same individual. Significance was determined by comparing the full model to a reduced model lacking the term of interest using a likelihood ratio test [31]. The validity of the model assumptions was confirmed by visually assessing the normality of the model residuals.
We next considered how the number of protean elements making up the movement rules for each path (which could range from 0, when all three movement parameters had fixed values, to 3, when all three parameters were protean) affected both path complexity and participant performance. Either log10-transformed targeting accuracy or log10-transformed path complexity was included as the dependent variable, with the number of protean movement elements as a fixed factor. As above, we also included trial order as a covariate and each participant's anonymous identifier as a random effect. As we would predict systematic trends in the dependent variable as the number of protean movement elements increased, we additionally fitted polynomial (linear, quadratic and cubic) contrasts over successive levels of the fixed factor. For the analysis involving targeting accuracy, we tested whether the mean targeting distance was significantly different from 0.1 (the radius of the prey's body) by including an offset of 0.1 in the model and testing the significance of the intercept.
Finally, we considered whether the values assigned to the movement parameters predicted participant performance. Each model included log10-transformed targeting accuracy as the dependent variable, and the three movement parameters (distance, speed and angle, each with three levels), along with their three- and two-way interactions, as fixed factors. As above, we included trial order as a covariate and each participant's anonymous identifier as a random effect. In each case, a global model was initially fitted containing all explanatory variables and their interactions. A final model was then determined by stepwise exclusion of the least significant terms, starting with the non-significant highest order interactions and then non-significant main effects. The resulting minimum adequate model is presented. For significant factors we also tested for differences between factor levels using planned treatment contrasts, in which protean movement (the reference group) was compared to each of the other two levels. This allowed us to specifically test the relative efficacy of protean, compared to fixed, movement strategies.
3. Results
(a). Path complexity
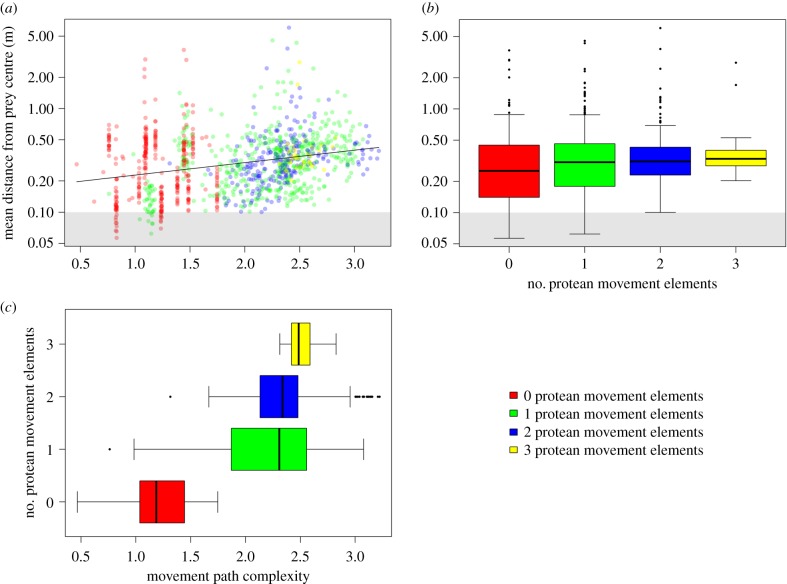
The complexity of prey movement paths significantly predicted participant performance, with participants exhibiting poorer accuracy (i.e. having a greater mean distance from the prey's centre) as path complexity increased (glmm: 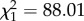 , p < 0.001; figure 2a). Moreover, path complexity itself was significantly predicted by the number of protean elements in the movement rules underpinning it (
, p < 0.001; figure 2a). Moreover, path complexity itself was significantly predicted by the number of protean elements in the movement rules underpinning it (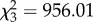 , p < 0.001), with an increasing number of protean elements resulting in increased path complexity (cubic contrasts: p < 0.001; figure 2a,b). This in turn had a significant (although modest) effect on participants' ability to accurately target prey (
, p < 0.001), with an increasing number of protean elements resulting in increased path complexity (cubic contrasts: p < 0.001; figure 2a,b). This in turn had a significant (although modest) effect on participants' ability to accurately target prey (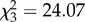 , p < 0.001; figure 2a,c), with the mean distance from the prey's centre increasing linearly (and targeting accuracy thereby reducing linearly) as the number of protean movement elements rose (linear contrasts: p = 0.002; figure 2c). There was, however, considerable variation within these categories. In particular, even though prey with 0, 1 or 2 protean movement elements contained exemplars that were comparatively easy to target (i.e. on average participants were able to maintain the targeting reticle within the prey's ‘body’; figure 2c), targeting accuracy was comparatively poor for the majority of prey items across all categories (including the category with 0 protean movement elements). As such, the mean targeting distance was considerably outside the prey's body in each category, on average (all p < 0.001; figure 2c). This suggests that rather than targeting accuracy being simply a function of movement path complexity, the specific movement rules underpinning them may be important.
, p < 0.001; figure 2a,c), with the mean distance from the prey's centre increasing linearly (and targeting accuracy thereby reducing linearly) as the number of protean movement elements rose (linear contrasts: p = 0.002; figure 2c). There was, however, considerable variation within these categories. In particular, even though prey with 0, 1 or 2 protean movement elements contained exemplars that were comparatively easy to target (i.e. on average participants were able to maintain the targeting reticle within the prey's ‘body’; figure 2c), targeting accuracy was comparatively poor for the majority of prey items across all categories (including the category with 0 protean movement elements). As such, the mean targeting distance was considerably outside the prey's body in each category, on average (all p < 0.001; figure 2c). This suggests that rather than targeting accuracy being simply a function of movement path complexity, the specific movement rules underpinning them may be important.
Figure 2.
(a) Targeting accuracy (measured as the mean distance from the centre of the prey item over the course of a trial) as a function of movement path complexity. Higher values along the x-axis denote more complex movement paths, while higher values along the y-axis denote poorer targeting accuracy. Note the log scale on the y-axis. Each data point represents a single simulated prey item, and is coloured according to how many protean movement elements it had. The solid line denotes the glmm model fit, and the grey shaded area indicates distances within the ‘body’ of the prey item. For any data point within this shaded area, participants therefore managed to maintain the targeting reticle over the prey's body throughout the entire trial, on average. (b) Movement path complexity as a function of the number of protean movement elements, and (c) targeting accuracy as a function of the number of protean movement elements. Thick lines denote the median, boxes the interquartile range, lines the range of the data, and dots denote potential outliers.
(b). Movement rules
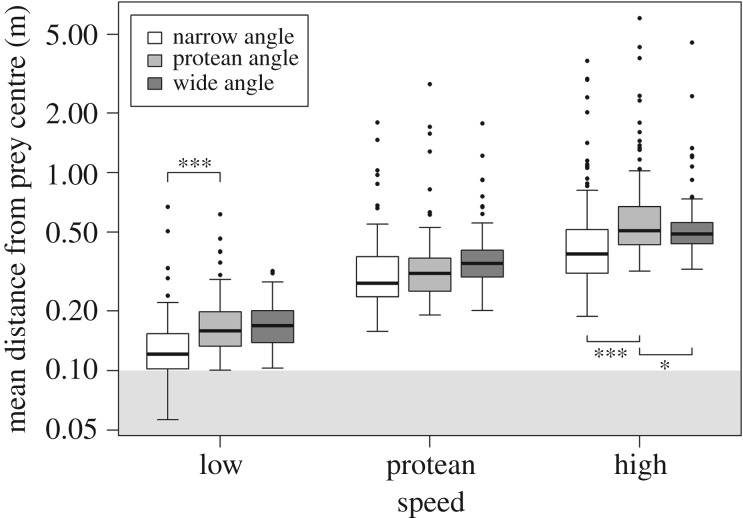
When considering the specific movement rules underpinning prey movement, and hence contributing to the observed variation in path complexity, targeting accuracy was significantly predicted by a single interaction between the speed at which the prey moved and the angle at which it turned (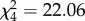 , p < 0.001). Specifically, regardless of whether the turning angle was narrow, protean or wide, accuracy was always significantly poorer for prey moving at high speeds than those exhibiting protean variation in speed (treatment contrasts: all p < 0.001) and significantly poorer for protean speeds compared to low speeds (all p < 0.001) (figure 3). However, the relationship between targeting accuracy and turning angle differed depending on the speed of movement: at low speeds, accuracy was significantly poorer when prey turned at protean compared to narrow angles (p < 0.001); at protean speeds, there was no difference in accuracy between turn angles; while at high speeds accuracy was significantly poorer when prey turned at protean angles compared to both narrow (p < 0.001) and wide angles (p = 0.024) (figure 3).
, p < 0.001). Specifically, regardless of whether the turning angle was narrow, protean or wide, accuracy was always significantly poorer for prey moving at high speeds than those exhibiting protean variation in speed (treatment contrasts: all p < 0.001) and significantly poorer for protean speeds compared to low speeds (all p < 0.001) (figure 3). However, the relationship between targeting accuracy and turning angle differed depending on the speed of movement: at low speeds, accuracy was significantly poorer when prey turned at protean compared to narrow angles (p < 0.001); at protean speeds, there was no difference in accuracy between turn angles; while at high speeds accuracy was significantly poorer when prey turned at protean angles compared to both narrow (p < 0.001) and wide angles (p = 0.024) (figure 3).
Figure 3.
Targeting accuracy (measured as the mean distance from the centre of the prey item over the course of a trial) as a function of speed (which was categorized as low, protean or high) and angle (which could be either narrow, protean or wide); please see text for full details. Higher values along the y-axis denote poorer targeting accuracy (note the log scale). Thick lines denote the median, boxes the interquartile range, lines the range of the data, and dots denote potential outliers. The grey shaded area indicates distances within the ‘body’ of the prey item. Asterisks (*) denote significant differences between levels of angle at each given level of speed: *p < 0.05; ***p < 0.001.
4. Discussion
Previous studies have found that prey exhibit increased movement path complexity following a simulated threat (e.g. [29,32]) with the (untested) assumption being that this increased complexity makes targeting the prey harder, resulting in a reduced chance of predation. Here, we tested this assumption directly by quantifying the ability of human predators to target virtual prey which differed in the unpredictability of their underlying movement rules, and hence exhibited variation in their resultant movement path complexity. Our results provide direct empirical support for the overall prediction that increased path complexity results in a reduced ability to accurately target prey, although, surprisingly, there was little evidence that high levels of unpredictability in the underlying movement rules equated directly to decreased predator performance. Indeed, prey items that displayed no protean variation in their movement elements at all (and which typically travelled along a putatively ‘predictable’ spiralling path; e.g. see figure 1) were found to be as difficult to target as prey exhibiting protean variation in all three movement elements (which moved along far more tortuous paths). This may explain the evolution of spiralling take-off behaviours observed in some insect species [13], which may be as effective as the more classically ‘protean’ erratic zig-zag-type behaviours in evading predators. It also suggests that the mathematical predictability of movement (as encompassed here by our measure of movement path complexity), while a good general predictor of predator performance, ignores the importance of specific movement parameters. Interestingly, here we found that the interaction between movement speed and turn angle was the best predictor of predator performance, while the distance between turns was of limited importance (and not included in the minimum adequate model). More specifically, the relative efficacy of turning behaviour (i.e. whether turns were narrow, protean or wide) differed as a function of speed, with the most effective protean behaviour involving a mix of protean and fixed elements (in this case high speeds and protean turn angles, regardless of distance travelled). This demonstrates that in terms of efficacy, the ‘most protean’ behaviour may not always be as effective as combinations of protean and fixed elements.
Our understanding of prey escape decisions has been advanced greatly by considering the fitness costs and benefits of escape, and economic models of escape behaviour have been used to provide qualitative predictions about aspects of escape behaviour [33]. In these models, the costs of escaping typically refer to the lost opportunities of engaging in other behaviours (such as feeding and engaging in social activities including courtship, mating and territorial defence), and the costs of escape are often considered relatively insignificant [34]. However, the energetic and/or cognitive costs of maintaining behaviours at the extremes of an animal's abilities, such as travelling at high speeds or turning at wide angles [34–36], or, in the case of protean behaviour, behaving unpredictably [19,37] could be considerable. Animals may therefore be expected to optimize the trade-off between the increased chances of avoiding predation and the costs of engaging in protean behaviour. Our results suggest that engaging in escape behaviour that is potentially less cognitively or energetically challenging, but equally efficacious in terms of predator avoidance (such as spiralling), may offer animals a solution to this trade-off. However, the specific ecological conditions that allow the evolution of these different types of behaviour are still to be established.
Literature examples of real-world predator–prey pursuits show a great variation in strategies that vary based on several factors (e.g. the type of predator (solitary or pack hunters) or the difference in size between predator and prey). For example, prey pursued by a single predator tend to use sharp turns [38] while prey fleeing from multiple predators will often make few or no turns and try to outrun them [39,40]. However, active evasion of predators may not be the only successful strategy: for example, in a recent study Combes et al. [17] reported that fruit flies (Drosophila melanogaster) attacked on the wing by dragonflies (Libellula cyanea) rarely responded with evasive manoeuvres; instead, the flies performed routine, erratic turns during flight (i.e. passive protean behaviour; sensu [13]) which were responsible for more failed predation attempts than active evasive manoeuvres. We note, though, that whether prey adopt a constitutive or induced anti-predator strategy may depend strongly on the prevailing environmental conditions: the former is likely to be better when predation pressure is constant, or at least predictable; the latter when predation is variable or difficult to predict. The fact that the results from our virtual study into protean behaviour are in agreement with those from a real-life system highlights the benefits of a virtual approach in the study of adaptive prey behaviour. For example, the use of easily manipulable artificial prey circumvents animal welfare concerns and allows the rapid generation of large sample sizes. Furthermore, our novel approach to this study through the use of VR allowed targeting within a 3D space, allowing prey to flee away from a predator (the most common behavioural response of a fleeing animal [12]), thereby conferring a greater degree of realism over previous 2D approaches (e.g. [19]), at least for simulated animals that ‘fly’ or ‘swim’ within a 3D environment. In our study, participant performance was assessed by their ability to consistently and accurately target moving prey items using head movements alone, although in humans (and most likely many other animals) visual attention is in fact a function of both head movements and accompanying eye movements [26,41]. Our approach, while providing sufficient resolution to uncover clear relationships between protean movement and participant performance, may nonetheless benefit by simultaneously considering the movement of the eyes, particularly in terms of reducing noise, recording faster response latencies, and detecting subtle attentional lapses of the sort that may be important in the precise local tracking of an erratically moving target [26].
In summary, we can draw several general conclusions about protean behaviour from this study. Firstly, incorporating protean variation into a prey's movement can improve the chances of escaping predators; however, more important with respect to avoiding predation were the interactions between these different movement rules. Interestingly, here we found that the ‘most protean’ behaviour was not the most effective at avoiding predation. In fact the most effective behavioural strategy incorporated a combination of protean and fixed elements. To put the results of this study into a broader context, here we have provided strong experimental support for the widely held assumption that protean strategies can reduce chances of predation, and have determined how the individual behavioural rules that make up prey movement can interact to affect the overall efficacy of protean behaviour. Our virtual methodology into the study of adaptive behaviour, combined with the parallels between our results and those from real-world systems demonstrates the utility of this approach.
Acknowledgements
We thank the two anonymous referees for their helpful comments.
Ethics
This project was approved by the College of Science ethics committee at the University of Lincoln (reference CoSREC265).
Data accessibility
Data used in the analyses reported here are available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.9h95737 [42].
Authors' contributions
All authors designed the study, G.R. collected the data, G.R. and T.W.P. conducted the statistical analysis, and all authors wrote the paper.
Competing interests
The authors declare that they have no competing interests.
Funding
G.R. was supported by a scholarship from the School of Life Sciences, University of Lincoln.
References
- 1.Culp JM, Glozier NE, Scrimgeour GJ. 1991. Reduction of predation risk under the cover of darkness—avoidance responses of mayfly larvae to a benthic fish. Oecologia 86, 163–169. ( 10.1007/Bf00317527) [DOI] [PubMed] [Google Scholar]
- 2.Duverge PL, Jones G, Rydell J, Ransome RD. 2000. Functional significance of emergence timing in bats. Ecography 23, 32–40. ( 10.1034/j.1600-0587.2000.230104.x) [DOI] [Google Scholar]
- 3.Mougeot F, Bretagnolle V. 2000. Predation risk and moonlight avoidance in nocturnal seabirds. J. Avian Biol. 31, 376–386. ( 10.1034/j.1600-048X.2000.310314.x) [DOI] [Google Scholar]
- 4.Stevens M, Merilaita S. 2009. Animal camouflage: current issues and new perspectives. Phil. Trans. R. Soc. B 364, 423–427. ( 10.1098/rstb.2008.0217) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hemmi JM. 2005. Predator avoidance in fiddler crabs: 1. Escape decisions in relation to the risk of predation. Anim. Behav. 69, 603–614. ( 10.1016/j.anbehav.2004.06.018) [DOI] [Google Scholar]
- 6.Langerhans R.B. 2007. Evolutionary consequences of predation: avoidance, escape, reproduction, and diversification. In Predation in organisms (ed. AMT Elewa), pp. 177–220 Berlin, Germany: Springer. [Google Scholar]
- 7.Miyatake T, Katayama K, Takeda Y, Nakashima A, Sugita A, Mizumoto M. 2004. Is death-feigning adaptive? Heritable variation in fitness difference of death-feigning behaviour. Proc. R. Soc. Lond. B 271, 2293–2296. ( 10.1098/rspb.2004.2858) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martins M. 1989. Deimatic behavior in Pleurodema brachyops. J. Herpetol. 23, 305–307. ( 10.2307/1564457) [DOI] [Google Scholar]
- 9.Umbers KDL, De Bona S, White TE, Lehtonen J, Mappes J, Endler JA. 2017. Deimatism: a neglected component of antipredator defence. Biol. Lett. 13 ( 10.1098/Rsbl.2016.0936) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vallin A, Jakobsson S, Wiklund C. 2007. An eye for an eye? On the generality of the intimidating quality of eyespots in a butterfly and a hawkmoth. Behav. Ecol. Sociobiol. 61, 1419–1424. ( 10.1007/s00265-007-0374-6) [DOI] [Google Scholar]
- 11.Edmunds M. 1974. Defence in animals: a survey of anti-predator defences. Burnt Mill, UK: Longman. [Google Scholar]
- 12.Krause J, Ruxton GD. 2002. Living in groups. Oxford, UK: Oxford University Press. [Google Scholar]
- 13.Humphries DA, Driver PM. 1970. Protean defence by prey animals. Oecologia 5, 285–302. ( 10.1007/Bf00815496) [DOI] [PubMed] [Google Scholar]
- 14.Bilecenoğlu M. 2005. Observations on the burrowing behaviour of the dwarf blaasop, Torquigener flavimaculosus (Osteichthyes: Tetraodontidae) along the coast of Fethiye, Turkey. Zool. Middle East 35, 29–34. [Google Scholar]
- 15.Eifler D, Eifler M. 2014. Escape tactics in the lizard Meroles cuneirostris. Amphib-reptil. 35, 383–389. ( 10.1163/15685381-00002963) [DOI] [Google Scholar]
- 16.Yager DD, May ML, Fenton MB. 1990. Ultrasound-triggered, flight-gated evasive maneuvers in the praying mantis Parasphendale agrionina. 1. Free flight. J. Exp. Biol. 152, 17–39. [DOI] [PubMed] [Google Scholar]
- 17.Combes SA, Rundle DE, Iwasaki JM, Crall JD. 2012. Linking biomechanics and ecology through predator–prey interactions: flight performance of dragonflies and their prey. J. Exp. Biol. 215, 903–913. ( 10.1242/jeb.059394) [DOI] [PubMed] [Google Scholar]
- 18.Dudley R. 1990. Biomechanics of flight in neotropical butterflies—morphometrics and kinematics. J. Exp. Biol. 150, 37–53. [Google Scholar]
- 19.Jones KA, Jackson AL, Ruxton GD. 2011. Prey jitters: protean behaviour in grouped prey. Behav. Ecol. 22, 831–836. ( 10.1093/beheco/arr062) [DOI] [Google Scholar]
- 20.Olberg RM, Worthington AH, Venator KR. 2000. Prey pursuit and interception in dragonflies. J. Comp. Physiol. A 186, 155–162. ( 10.1007/s003590050015) [DOI] [PubMed] [Google Scholar]
- 21.Borji A, Sihite DN, Itti L. 2013. What stands out in a scene? A study of human explicit saliency judgment. Vision Res. 91, 62–77. ( 10.1016/j.visres.2013.07.016) [DOI] [PubMed] [Google Scholar]
- 22.Bovet P, Benhamou S. 1988. Spatial analysis of animals movements using a correlated random-walk model. J. Theor. Biol. 131, 419–433. ( 10.1016/S0022-5193(88)80038-9) [DOI] [Google Scholar]
- 23.Couzin ID, Krause J, James R, Ruxton GD, Franks NR. 2002. Collective memory and spatial sorting in animal groups. J. Theor. Biol. 218, 1–11. ( 10.1006/yjtbi.3065) [DOI] [PubMed] [Google Scholar]
- 24.Kareiva PM, Shigesada N. 1983. Analyzing insect movement as a correlated random-walk. Oecologia 56, 234–238. ( 10.1007/Bf00379695) [DOI] [PubMed] [Google Scholar]
- 25.Land MF, Lee DN. 1994. Where we look when we steer. Nature 369, 742–744. ( 10.1038/369742a0) [DOI] [PubMed] [Google Scholar]
- 26.Gilchrist ID, Brown V, Findlay JM, Clarke MP. 1998. Using the eye-movement system to control the head. Proc. R. Soc Lond. B 265, 1831–1836. ( 10.1098/rspb.1998.0509) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rossel S. 1980. Foveal fixation and tracking in the praying mantis. J. Comp. Physiol. A 139, 307–331. ( 10.1007/Bf00610462) [DOI] [Google Scholar]
- 28.Westhoff G, Boetig M, Bleckmann H, Young BA. 2010. Target tracking during venom ‘spitting’ by cobras. J. Exp. Biol. 213, 1797–1802. ( 10.1242/jeb.037135) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herbert-Read JE, Ward AJW, Sumpter DJT, Mann RP. 2017. Escape path complexity and its context dependency in Pacific blue-eyes (Pseudomugil signifer). J. Exp. Biol. 220, 2076–2081. ( 10.1242/jeb.154534) [DOI] [PubMed] [Google Scholar]
- 30.Bates D, Machler M, Bolker BM, Walker SC. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. [Google Scholar]
- 31.Crawley MJ. 2005. Statistics: an introduction using R. Chichester, UK: John Wiley & Sons. [Google Scholar]
- 32.Schaerf TM, Dillingham PW, Ward AJW. 2017. The effects of external cues on individual and collective behavior of shoaling fish. Sci. Adv. 3, e1603201 ( 10.1126/sciadv.1603201) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cooper WE, Blumstein DT. 2015. Escaping from predators: an integrative view of escape decisions. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 34.Cooper WE, Frederick WG. 2007. Optimal flight initiation distance. J. Theor. Biol. 244, 59–67. ( 10.1016/j.jtbi.2006.07.011) [DOI] [PubMed] [Google Scholar]
- 35.Taylor CR, Schmidtn K, Raab JL. 1970. Scaling of energetic cost of running to body size in mammals. Am. J. Physiol. 219, 1104. [DOI] [PubMed] [Google Scholar]
- 36.Wilson RP, Griffiths IW, Legg PA, Friswell MI, Bidder OR, Halsey LG, Lambertucci SA, Shepard ELC. 2013. Turn costs change the value of animal search paths. Ecol. Lett. 16, 1145–1150. ( 10.1111/ele.12149) [DOI] [PubMed] [Google Scholar]
- 37.Domenici P, Booth D, Blagburn JM, Bacon JP. 2009. Cockroaches keep predators guessing by using preferred escape trajectories. Comp. Biochem. Physiol., Part A Mol. Integr. Physiol. 153, S153 ( 10.1016/j.cbpa.2009.04.300) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cooke SJ. 2008. Biotelemetry and biologging in endangered species research and animal conservation: relevance to regional, national, and IUCN Red List threat assessments. Endanger. Species Res. 4, 165–185. [Google Scholar]
- 39.Handcock RN, Swain DL, Bishop-Hurley GJ, Patison KP, Wark T, Valencia P, Corke P, O'Neill CJ. 2009. Monitoring animal behaviour and environmental interactions using wireless sensor networks, GPS collars and satellite remote sensing. Sensors-Basel 9, 3586–3603. ( 10.3390/s90503586) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saunders G, Kay B, Nicol H. 1993. Factors affecting bait uptake and trapping success for feral pigs (Sus scrofa) in Kosciusko National Park. Wildl. Res. 20, 653–665. ( 10.1071/Wr9930653) [DOI] [Google Scholar]
- 41.Freedman EG. 2008. Coordination of the eyes and head during visual orienting. Exp. Brain Res. 190, 369–387. ( 10.1007/s00221-008-1504-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Richardson G, Dickinson P, Burman OHP, Pike TW. 2018. Data from: Unpredictable movement as an anti-predator strategy Dryad Digital Repository. ( 10.5061/dryad.9h95737) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Richardson G, Dickinson P, Burman OHP, Pike TW. 2018. Data from: Unpredictable movement as an anti-predator strategy Dryad Digital Repository. ( 10.5061/dryad.9h95737) [DOI] [PMC free article] [PubMed]
Data Availability Statement
Data used in the analyses reported here are available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.9h95737 [42].