Abstract
Pollinators in agroecosystems are often exposed to pesticide mixtures. Even at low concentrations, the effects of these mixtures on bee populations are difficult to predict due to potential synergistic interactions. In this paper, we orally exposed newly emerged females of the solitary bee Osmia bicornis to environmentally realistic levels of clothianidin (neonicotinoid insecticide) and propiconazole (fungicide), singly and in combination. The amount of feeding solution consumed was highest in bees exposed to the neonicotinoid, and lowest in bees exposed to the pesticide mixture. Ovary maturation and longevity of bees of the neonicotinoid and the fungicide treatments did not differ from those of control bees. By contrast, bees exposed to the pesticide mixture showed slow ovary maturation and decreased longevity. We found a synergistic interaction between the neonicotinoid and the fungicide on survival probability. We also found an interaction between treatment and emergence time (an indicator of physiological condition) on longevity. Longevity was negatively correlated to physiological condition only in the fungicide and the mixture treatments. Delayed ovary maturation and premature death imply a shortened nesting period (highly correlated to fecundity in Osmia). Our findings provide a mechanism to explain the observed dynamics of solitary bee populations exposed to multiple chemical residues in agricultural environments.
Keywords: neonicotinoids, pesticide cocktail, ergosterol-biosynthesis-inhibiting fungicide, synergism, ecotoxicology, Osmia bicornis
1. Introduction
The last decades have seen significant declines in wild bee diversity at local and regional scales [1–3], together with abnormal honeybee colony losses in various parts of the world [4,5]. Although these declines are undoubtedly caused by a combination of factors, pesticides in general, and neonicotinoid insecticides in particular, have often been signalled as one of the main drivers of the population declines experienced by both wild and managed species. For this reason, the use of neonicotinoids has been recently restricted in the European Union [6]. Nonetheless, neonicotinoids are still used on a wide range of crops and account for more than 30% of the global insecticide market [7]. Neonicotinoids are highly toxic to insects [8–10]. However, studies testing lethal and sublethal effects of neonicotinoids on bees often yield inconsistent results [11–14]. There are several important challenges when assessing the potential hazards of pesticides on bees. First, inasmuch as possible, bees should be subjected to realistic exposure conditions, likely to be experienced in field situations. In relation to this, some studies have been criticized based on allegedly overestimated exposure in terms of concentration and duration (e.g. studies testing acute exposure to high doses rather than chronic exposure to low doses) [15]. Second, in agricultural environments, bees are often exposed to combinations of chemicals [16]. This is important because certain pesticide mixtures have been shown to produce synergistic effects [17–19]. Yet, with some exceptions (e.g. [17–20]), ecotoxicological studies usually test single compounds. Third, sensitivity to pesticides may be highly influenced by the physiological condition of the bee. A recent review [21] shows that response to pesticide exposure in honeybees is highly variable at the individual level and dependent on several endogenous factors such as genetic background, body size and age. Fourth, the effects of pesticides may be species-dependent. Most bee ecotoxicological studies have been conducted on a single species, the western honeybee, Apis mellifera [16,22]. However, there is increasing evidence that solitary bees (Osmia bicornis) are more sensitive to certain pesticide treatments than honeybees and bumblebees [12,13,18,23].
In this study, we tested the effects of environmentally realistic oral exposure to clothianidin (a neonicotinoid insecticide) and propiconazole (an ergosterol-biosynthesis-inhibiting (EBI) fungicide), singly and in combination, in the solitary bee O. bicornis. In agricultural environments, bees are likely to be exposed simultaneously to both compounds because these two groups of agrochemicals are commonly applied to various crops [24,25].
A key question in ecotoxicological studies is whether the test doses applied in the laboratory can be considered to be field realistic. However, estimating field-realistic pesticide doses is not easy. The amount of nectar collected in a foraging bout by a nesting Osmia female can be estimated from the literature [26], and concentrations of pesticides in nectar can be measured (e.g. [27,28]). However, it is difficult to establish how much of the nectar collected is actually ingested by the foraging female versus regurgitated onto the larval food provision. Nonetheless, we know that upon emergence out of the natal nest, and prior to engaging in nesting activities, Osmia females collect nectar exclusively for their own consumption [29]. Therefore, we provided newly emerged Osmia females in the laboratory with ad libitum feeding solution to simulate this ‘first nectar meal’. To account for the physiological condition of the bees, we measured body size and emergence time. Adult body size in Osmia is strongly correlated to the amount of food ingested during the larval period [30]. Large bees have higher lipid content [31], and are more likely to survive the winter [32]. As for emergence time, Osmia females lose approximately 7.5% of their body weight during the process of emerging out of the cocoon [31]. Previous studies have shown that the probability to start a nest and reproduce decreases with emergence time [33], indicating that females that take longer to emerge are less vigorous than females that emerge promptly.
Upon feeding at the flowers, newly emerged Osmia females undergo a short period (2–4 days) during which they complete ovary maturation prior to initiating nesting activities [33,34]. During this period, ovary size and vitellogenin concentration in the haemolymph increase in parallel for up to 6 days [35]. On average, individual Osmia females live for about 20 days, and their fecundity is low (10–20 eggs) and highly correlated to the duration of the nesting period [33,34]. Therefore, any effects on ovary maturation during this pre-nesting phase may significantly delay the onset of nesting activities, with important consequences on reproductive success. Consequently, we measured vitellogenin levels, ovary maturation and longevity in females exposed to the neonicotinoid insecticide and the EBI fungicide, singly and in combination. Based on previous studies showing synergistic mortality effects between clothianidin and propiconazole [18], we hypothesize lower vitellogenin levels, slower ovary maturation and shorter lifespan in newly emerged O. bicornis females taking their first meal on the neonicotinoid–fungicide mixture. We also hypothesize that these effects will be stronger on bees in poor physiological condition (smaller bees and/or bees taking longer to emerge).
2. Material and methods
(a). Bee population and treatments
Osmia bicornis cocoons were obtained from a population nesting in a pesticide-free area in Kazimierz Landscape Park, Poland. In January 2016, wintering adults within their cocoons were shipped to the CREA-AA in Bologna, Italy, where they were transferred to a 3°C cabinet. In early April 2016, cocoons were taken to the laboratory of Agricultural Entomology at the University of Bologna. In mid-April 2016, cocoons presumed to contain females (generally larger than those containing males) were incubated at 21 ± 2°C and 55 ± 10% RH under natural light. Emergence was checked daily. As most males emerge a few days before females, any emerging males were discarded. We recorded the days each female took to emerge out of the cocoon following incubation (henceforth emergence time). Upon emergence, females were transferred to a Plexiglas laboratory cage (50 × 50 × 50 cm) to allow them to deposit the meconium. Females emerging on any given day were equally distributed among four treatments: control (feeding solution with 1% acetone, CON), propiconazole (PRO), clothianidin (CLO) and mixture (propiconazole + clothianidin, MIX). Throughout the study, bees were maintained at 21–23°C, 40–50% RH under natural light.
(b). Test solution preparation
We used clothianidin active ingredient (purity 99%) from Dr Ehrenstorfer Gmbh. A stock solution was prepared by dissolving technical grade clothianidin (99% pure) in acetone at a nominal concentration of 1000 mg l−1 (actual concentration: 1090 mg l−1), which was then diluted to 1 mg l−1 (actual concentration: 0.983 mg l−1). The stock solution was then diluted in a 38% w : v (33% w : w) sugar + distilled water solution to achieve the desired concentration of 10 µg l−1 (corresponding to 8.6 µg kg−1). This concentration is within the range of clothianidin residues found in nectar collected from flowers of oilseed rape grown from clothianidin-coated seeds (6.7–16 µg l−1 [12]; 5–16 µg kg−1 [24]; 2.3–10.1 µg kg−1 [36]; less than 0.7–13.2 µg kg−1 [37]).
We tested a propiconazole concentration of 62.5 mg l−1. This concentration corresponds to the field application rate of the commercial formulation Protil ® EC (250 g l−1 of a.i.) in orchards (25 ml hl−1 or 0.25 l ha−1). To obtain this concentration, we prepared a stock solution with a propiconazole concentration of 25 g l−1 by dissolving Protil® EC in distilled water. The stock solution was then diluted with 38% w : v (33% w : w) sugar solution to achieve the desired concentration.
The final concentration of acetone in the feeding solution was adjusted to 1% (v : v) with pure acetone in all treatments.
(c). Exposure phase
Previous studies have shown that upon emergence out of the cocoon, Osmia females take about 1 day to come out of their natal nest [38]. Therefore, 24 h after emergence, meconium-free females were individually housed in small plastic cylinders (width: 3.5 cm; height: 5.5 cm) with a transparent plastic lid through which a feeder made with a 1 ml syringe was inserted. Each feeder contained approximately 150 µl of feeding solution (33% sucrose concentration w : w) with or without pesticides. A flower petal (Euryops, Asteraceae) was attached to the tip of the syringe to ensure the bees located the feeder quickly (see [18,39] for details). To simulate a first nectar meal, bees were maintained in these cylinders for 4 h. Preliminary trials showed that extending this exposure phase up to 8 h did not result in increased solution consumption. To measure the amount of solution ingested by each bee, syringes were weighed before and after the exposure phase. Three cages without bees served as controls to account for potential evaporation. Only bees that fed were included in the statistical analyses. In natural conditions, newly emerged bees have to fly to reach flowers on which to sip nectar. In our laboratory set-up, bees only had to walk a very short distance to have access to a feeding solution source. Therefore, if anything, our method can be assumed to underestimate the amount of nectar and chemical residue ingested by a newly emerged bee in her first nectar meal. Sample size was 35–50 bees per treatment.
(d). Experiment 1
After the exposure phase, each bee was individually transferred to a plastic ice cream cup (width: 5.5–8 cm; height: 7 cm) with a transparent lid through which a 2.5 ml syringe filled with sucrose solution (33% sugar concentration, w : w) was inserted. Again, a flower petal was attached to the tip of the feeder to ensure the bees located the feeder quickly. Bees were allowed to feed ad libitum and the sucrose solution in the feeder was renewed every 3 days. Solution consumption was visually assessed every day. Mortality was monitored daily until all bees died. Upon death, the head width of each bee was measured under a stereomicroscope at 32×. Head size is strongly correlated to body weight in Osmia [30]. Sample sizes were approximately 30 bees per treatment.
(e). Experiment 2
We followed the same procedure as experiment 1 with two modifications. First, because pollen consumption enhances ovary maturation in Osmia [40], bees of this experiment were provided with a source of pollen throughout the post-exposure phase. In each ice cream cup, we provided approximately 55 mg of pollen in a 1.5 ml Eppendorf tube cap. Pollen was obtained from nests of an O. bicornis population nesting in a pear/apple orchard near Bologna. Several provision masses (pollen mixed with nectar) from various nests were mixed to obtain a common homogeneous pollen source from which 55 mg portions were taken. Samples of this pollen source were subjected to palynological and chemical multi-residue analyses (see details in the electronic supplementary material). Chemical analyses revealed that the provisions contained several pesticide residues, including insecticides, fungicides and herbicides at very low concentrations (electronic supplementary material, table S1). Although unplanned, the presence of these residues resulted in a more realistic exposure, congruent with the co-occurrence of multiple compounds in pollen-nectar matrices in agricultural environments [41,42]. Importantly, no obvious negative effects were observed in the nesting O. bicornis population from which the provisions were taken, or its progeny.
Second, in this experiment, the post-exposure phase was interrupted after 3 days to measure vitellogenin levels in the haemolymph and ovary maturation. Details of vitellogenin and ovary maturation measurements are available in the electronic supplementary material.
All statistical analyses are described in the electronic supplementary material.
3. Results
(a). Exposure phase feeding
The amount of feeding solution ingested during the 4 h exposure phase differed among treatments (table 1). Bees of the CLO treatment fed significantly more than bees of the other treatments, and feeding levels were lowest in the MIX treatment (figure 1). Solution ingestion during this phase also depended on body size (larger bees ingested more syrup), but not on emergence time (table 1). However, the interaction between treatment and emergence time was significant. As emergence time increased, feeding increased in CLO bees, whereas it decreased in PRO and MIX bees, and did not change in CON bees (electronic supplementary material, figure S1).
Table 1.
Best selected (ΔAICc < 2) general linear models explaining the effects of treatment (Tr), emergence time (ET), head size (HS) and the interactions between treatment and emergence time and treatment and head size on each response variable. Significant predictors (p < 0.05) are in bold, marginally significant predictors (p = 0.05–0.1) are in italics. Positive and negative signs in parentheses denote the direction of the relationship.
| Response variable | model components | AICc | ΔAICc | wi | R2 (%) | ||
|---|---|---|---|---|---|---|---|
| exposure phase | exposure feeding | 1 | Tr + ET (+) + HS (+) + Tr:ET | 1376.7 | 0.00 | 0.592 | 22 |
| 2 | Tr + HS (+) | 1378.4 | 1.73 | 0.249 | 17 | ||
| experiment 1 | post-exposure feeding rate | 1 | Tr + ET (−) + HS (+) | 707.1 | 0.00 | 0.463 | 21 |
| 2 | ET (−) + HS (+) | 707.5 | 0.44 | 0.371 | 14 | ||
| longevity (sqrt-transformed) | 1 | Tr + ET (+) + Tr:ET | 380.3 | 0.00 | 0.358 | 26 | |
| 2 | Tr + ET (−) + HS (+) + Tr:ET | 381.3 | 0.99 | 0.218 | 27 | ||
| 3 | Tr + ET (−) + HS (+) | 381.9 | 1.62 | 0.159 | 21 | ||
| 4 | Tr + ET (−) | 382.2 | 1.89 | 0.139 | 19 | ||
| experiment 2 | post-exposure feeding rate | 1 | Tr | 647.5 | 0.00 | 0.562 | 22 |
| oocyte length | 1 | Tr + HS (+) | −51.0 | 0.00 | 0.667 | 37 | |
| 2 | Tr + ET (+) + HS (+) | −49.3 | 1.78 | 0.273 | 38 | ||
| vitellogenin concentration (sqrt-transformed) | 1 | HS (+) | 123.1 | 0.00 | 0.467 | 27 | |
| 2 | ET (−) + HS (+) | 123.1 | 0.03 | 0.460 | 31 |
Figure 1.
Mean + s.e. test solution ingested during the 4 h exposure phase in O. bicornis females orally exposed to four treatments (CON, control; CLO, clothianidin; PRO, propiconazole; MIX, clothianidin + propiconazole mixture). Different letters denote significant differences (Fisher's LSD post hoc, p < 0.05).
(b). Experiment 1
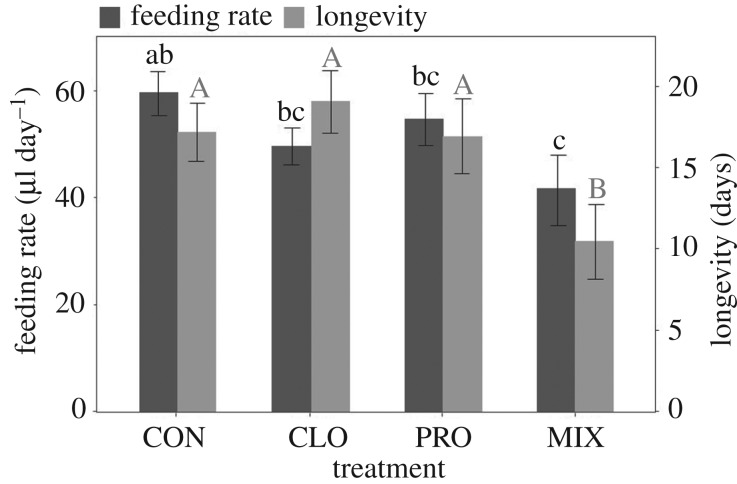
Differences among treatments in feeding rate (microlitres of syrup per day) during the post-exposure phase approached significance (table 1), again with bees of the MIX treatment tending to feed less (figure 2). Both body size and emergence time affected post-exposure feeding (table 1). Feeding rates were higher in larger bees and lower in bees that took longer to emerge.
Figure 2.
Experiment 1—mean + s.e. post-exposure feeding rate and longevity in O. bicornis females orally exposed to four treatments (CON, control; CLO, clothianidin; PRO, propiconazole; MIX, clothianidin + propiconazole mixture). Different letters denote significant differences (Fisher's LSD post hoc, p < 0.05).
Cumulative survival curves differed significantly among treatments (d.f. = 3, χ2 = 12.99, p = 0.005) (figure 3). Throughout the first days following exposure, mortality in the MIX treatment was much greater than mortality in the other treatments, yielding a significant synergistic interaction between clothianidin and propiconazole on day 4 (day 4: p = 0.045; day 8: p = 0.075; day 17: p = 0.44). That is, the CLO–PRO combination was significantly more toxic than the sum of the toxicity of the two compounds separately. Consequently, longevity differed significantly across treatments (table 1), and was shortest in the MIX treatment (figure 2). Body size had no effect on longevity, but bees that took longer to emerge tended to have shorter longevity (table 1). In addition, there was a significant interaction between treatment and emergence time. As emergence time increased, longevity decreased in PRO and MIX bees, but did not change in CON and CLO bees (table 1; electronic supplementary material, figure S2).
Figure 3.

Experiment 1—cumulative survival probability of O. bicornis females orally exposed to four treatments (CON, control; CLO, clothianidin; PRO, propiconazole; MIX, clothianidin + propiconazole mixture). Synergistic interactions between CLO and PRO treatments (p < 0.05; one-tailed binomial proportion test; assessment times: 4, 8 and 17 days) are marked with an asterisk. (Online version in colour.)
(c). Experiment 2
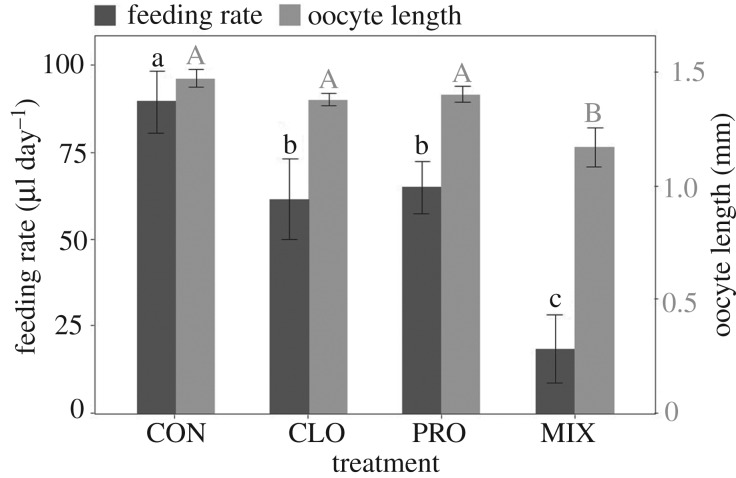
Nectar feeding rate during the 3-day post-exposure phase significantly differed among treatments (table 1). As in experiment 1, it was highest in the CON treatment and lowest in the MIX treatment (figure 4). In contrast with experiment 1, body size and emergence time did not affect post-exposure feeding (table 1), but it is important to note that the post-exposure phase lasted only 3 days in this experiment. We repeatedly observed O. bicornis females feeding on the pollen provided. However, the amount of pollen consumed could not be measured because bees spread the pollen all over the hoarding cage.
Figure 4.
Experiment 2—mean + s.e. post-exposure feeding rate and basal oocyte length in O. bicornis females orally exposed to four different treatments (CON, control; CLO, clothianidin; PRO, propiconazole; MIX, clothianidin + propiconazole mixture). Different letters denote significant differences (Fisher's LSD post hoc, p < 0.05).
Three-day cumulative survival curves differed among treatments (d.f. = 3, χ2 = 45.72, p < 0.001). Survival was again lowest in the MIX treatment (figure 5), and there was a significant synergistic interaction between clothianidin and propiconazole on all three assessment time points (day 1: p < 0.001; day 2: p < 0.001; day 3: p = 0.002). Oocyte length and vitellogenin concentration were measured in all the bees that survived the 3-day post-exposure period (n = 55). We found significant differences among treatments in basal oocyte mean length (table 1), with bees of the MIX treatment having shorter oocytes than bees of the other treatments (figure 4). Oocyte length was positively related to head size, but was not related to emergence time (table 1). We found no differences among treatments in vitellogenin concentration (table 1). Larger bees had higher vitellogenin concentrations, but emergence time did not affect vitellogenin levels (table 1). No interactions between treatment and head size or emergence time were apparent in this experiment (table 1).
Figure 5.

Experiment 2—cumulative survival probability of O. bicornis females orally exposed to four treatments (CON, control; CLO, clothianidin; PRO, propiconazole; MIX, clothianidin + propiconazole mixture). Synergistic interactions between CLO and PRO treatments (p < 0.05; one-tailed binomial proportion test; assessment times: 1, 2 and 3 days) are marked with an asterisk. (Online version in colour.)
4. Discussion
Wild and managed bees are exposed to pesticide mixtures in agricultural and urban areas [41,43–45]. Neonicotinoids and EBI fungicides, in particular, are routinely used on many crops [24,25], and have often been found together in the nectar and pollen of both cultivated and wild flowers [37,41], in honeybee-collected pollen and on bee body surfaces [41,46,47]. In a previous study [18], we showed synergistic mortality effects in honeybees, bumblebees and solitary bees (O. bicornis) acutely exposed to sublethal doses of CLO (0.63 ng bee−1) and PRO (7 µg bee−1) in a fixed amount of syrup (10 µl). The amount of CLO ingested by bees in that study was within the range of CLO potentially ingested in a foraging bout. However, the tested concentration (63 µg l−1 of CLO) was higher than concentrations likely to be found in nectar (< 0.7–16 µg l−1) [12,24,36,37,48]. On the other hand, considering the honey stomach capacity of honeybees (approximately 30 µl) and bumblebees (80 µl) [49,50], it is conceivable that a bee could ingest more than 10 µl of nectar in a single foraging bout. At any rate, given the difficulty to estimate what proportion of the nectar collected by a nesting female bee is ingested versus regurgitated in the nest, in this study we worked with pre-nesting females, which consume all the nectar they collect. Our study provides first-time evidence that oral exposure to field-relevant concentrations of an insecticide and a fungicide mixture affect feeding behaviour, ovary maturation and longevity in a solitary bee.
Results of syrup consumption during the exposure phase show that O. bicornis females not only did not avoid, but even preferred neonicotinoid-laced syrup. This behaviour has also been observed in bumblebees and honeybees [51,52]. Interestingly, syrup consumption during this phase was lowest in bees of the MIX treatment, indicating that the attractiveness of clothianidin was lost when propiconazole was added. Post-exposure feeding rate (microlitres of syrup consumed per day) was also lowest in the MIX treatment in both experiments (although differences among treatments narrowly failed significance in experiment 1), suggesting that the clothianidin–propiconazole combination alters the feeding behaviour of O. bicornis.
Vitellogenin is a fat-body-synthesized glycolipophosphoprotein that constitutes a significant part of the yolk protein of insect eggs [53]. In Osmia, vitellogenin concentration in the haemolymph increases with ovary maturation, reaching maximum levels 3–6 days after adult emergence and gradually declining thereafter [35]. Studies on honeybee and bumblebee queens have reported a strong upregulation of vitellogenin genes [54] but slower ovary maturation following experimental neonicotinoid exposure [52,55]. Because pollen feeding enhances ovary development in bumblebees [56], Baron et al. [52] hypothesized a reduction in pollen consumption in bees exposed to neonicotinoids. Osmia females also require pollen to mature their oocytes [40]. Our bees clearly fed on the pollen supplied in experiment 2, but we could not establish whether pollen consumption differed among treatments because bees spread the pollen over the hoarding cages. At any rate, we did not find differences in vitellogenin concentration or ovary maturation between clothianidin-exposed and control bees. On the other hand, we found that ovary maturation was slowest in bees of the MIX treatment, even if this reduction was not accompanied by increased levels of vitellogenin concentration.
In experiment 1, the longevity of propiconazole- and clothianidin-exposed bees (mean: 17 and 19 days, respectively) did not differ from that of control bees (mean: 17.5 days). These lifespans are similar to those recorded in field and greenhouse populations (17.5–24 days [33,34,57], although mean longevity can be extended up to 30.5 days under bad weather conditions [34]). Bees of the CLO treatment consumed larger amounts of feeding solution, thus ingesting greater amounts of sugar, which could have buffered any negative effect of clothianidin [58]. By contrast, exposure to the MIX treatment resulted in significantly reduced longevity. The lifespan of bees of the MIX treatment in experiment 1 was 10 days, that is, 0.5–0.6 times shorter than that of control bees and bees exposed to single compounds. The negative effect of the pesticide mixture was further evidenced by the comparison of the survival curves of the various treatments, revealing a synergistic interaction between clothianidin and propiconazole on survival probability in both experiments. Three days after exposure, mortality in the MIX treatment of experiment 2 was 78%, more than twice higher than expected under additive (non-synergistic) effects (36%). Bees of experiment 2 were fed pollen during the post-exposure phase, whereas bees of experiment 1 were not, and the pollen supplied was contaminated with pesticide residues (electronic supplementary material, table S1). This pollen was obtained from O. bicornis provisions from a population nesting in a pear/apple orchard that was sprayed during bloom with boscalid. This fungicide was the main chemical residue found in the pollen, but four other chemicals that were not sprayed in the orchard were also found. Pollen analysis of the provisions revealed that O. bicornis females foraged mostly on wild plants (Quercus robur (39%), Ranunculus spp. (27%), Cercis spp. (25%), apple/pear (2%)). Thus, our study provides further evidence of pesticide exposure affecting not only bees foraging on sprayed crops, but also those foraging on the accompanying flora [13,59,60].
The differences between experiments 1 and 2 in survival probability at day 3 were very small for the CON (87 versus 87%) and PRO (82 versus 88%) treatments. By contrast, these differences were very pronounced for the CLO (93 versus 73%) and the MIX treatments (48 and 22%), suggesting that, even at the low concentrations recorded, the presence of additional pesticides in the pollen supplied in experiment 2 interacted with the clothianidin ingested during the exposure phase.
We used body size and timing of emergence as proxies of physiological condition. Not surprisingly, large bees consumed more feeding solution during the exposure phase and during the post-exposure phase of experiment 1. No such relationship was found in experiment 2, but the post-exposure phase of this experiment lasted only 3 days. Larger bees also had higher levels of vitellogenin in the haemolymph and, in agreement with previous studies [33], produced larger oocytes. However, large bees did not live longer than small bees. Studies on Osmia populations nesting in field and greenhouse conditions have also failed to find a relationship between female body size and longevity (or nesting period) [33,34,61–63].
Emergence time affected post-exposure feeding solution consumption rate and longevity in experiment 1, both of which were lower in females with long emergence periods. These results are congruent with the reduced ability of bees that take longer to emerge to start nesting activities [33]. As with body size, such a relationship was not apparent in experiment 2, possibly due to the short post-exposure phase of this experiment. Despite their lower feeding solution consumption, we did not find lower vitellogenin levels or slower ovary maturation in bees with long emergence times.
Physiological condition may influence sensitivity to pesticides [21]. Our results show that the negative effects of emergence time on longevity occurred only in the MIX and PRO treatments. The suboptimal physiological condition of bees with long pre-emergence periods could have reduced their detoxification capacity making them more vulnerable to these two treatments. To our knowledge, this is the first time an effect of physiological condition on sensitivity to pesticides is shown for a solitary bee. Ecotoxicological studies are often carried out under conditions that are assumed to be optimal for the test organisms (e.g. healthy individuals kept at adequate temperatures with ad libitum feeding). In the field, however, bees may be exposed to various stress factors, such as parasites, diseases and limiting food resources, which could magnify the negative effects of pesticides. In their review, Holmstrup et al. [64] argue that synergistic interactions between toxic compounds and natural stressors are frequent and should be considered in risk assessment schemes.
Our study shows that a single meal with a cocktail of pesticides at sublethal doses and realistic concentrations during the pre-nesting period affects feeding behaviour, ovary maturation and longevity in a solitary bee. Importantly, none of these effects were observed when bees were exposed to either compound singly. The pre-nesting period is a critical stage in the life cycle of solitary bees for two reasons. First, females in poor physiological condition are less likely to start nesting activities and reproduce [33]. Our results show that the nesting success of these weakened females may be further compromised by exposure to pesticide mixtures at realistic field concentrations. Second, fecundity of females that do successfully nest is highly correlated to the duration of the nesting period [33,34], which is constrained by ovary maturation at one end [33,35] and by death at the other end. Our insecticide–fungicide mixture had negative effects on both ovary maturation and longevity, thus affecting the duration of the nesting period at both ends. Under field conditions, Osmia females live approximately 20 days on average [34]. Of this time, approximately 5 days are spent maturing the ovaries [35], prior to the initiation of nesting activities (pre-nesting period) [33,34]. During the rest of their lifetime (nesting period), females build and provision nest cells and lay eggs at a rate of approximately 0.7 day−1 [34]. If we assume that mean longevities recorded in our study are representative of longevities under field conditions, females of our MIX treatment would have laid a mean of 3.5 eggs compared to 8.4 in control bees. We conclude that our findings have direct repercussions on the reproductive success of solitary bees, and provide a potential mechanism to explain observed negative dynamics of Osmia populations in agricultural environments [12,13,65]. Our study has also important implications for pesticide regulation. Current risk assessment schemes rely on tests of single compounds [27,28]. Our results underscore the need to consider pesticide combinations likely to occur in agricultural environments.
Supplementary Material
Acknowledgements
We thank Michela Boi, Francesca Grillenzoni and Francesca Corvucci for the chemical and palynological analysis of pollen samples. We are grateful to Enea Ferlizza for the technical support in the vitellogenin analysis. We also thank several students, especially Andrea Cocchi and Maria Meloni, for their help with laboratory data collection. Finally, we appreciate the helpful comments of two anonymous reviewers and of James Cresswell (University of Exeter, UK) on an early draft of the manuscript.
Data accessibility
Data available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.895pn6p [66].
Authors' contributions
F.S and J.B. conceived the experiments. F.S, J.B., R.C., G.I., D.T. and P.M. designed the experiments. F.S. and R.C. collected the data. X.A. analysed the data. F.S. and J.B. took the lead in writing the manuscript.
Competing interests
We have no competing interests.
Funding
This study was partially funded by the Council for Agricultural Research and Economics, Agriculture and Environment Research Centre and Alma Mater Studiorum, University of Bologna (RFO 2014-SGOLASTRA FABIO, RFO 2014_2015- ISANI GLORIA). R.C. was supported by a grant from the Eva Crane Trust (ECTA_20161205). X.A. was supported by a Ramón y Cajal research contract by the Spanish Ministry of Economy and Competitiveness (RYC-2015-18448). J.B. was supported by a DURSI-GDR (Catalan Government) grant SGR SGR2005-2008-046.
References
- 1.Biesmeijer JC, et al. 2006. Parallel declines in pollinators and insect-pollinated plants in Britain and the Netherlands. Science 313, 351–354. ( 10.1126/science.1127863) [DOI] [PubMed] [Google Scholar]
- 2.Bartomeus I, Ascher JS, Gibbs J, Danforth BN, Wagner DL, Hedtke SM, Winfree R. 2013. Historical changes in northeastern US bee pollinators related to shared ecological traits. Proc. Natl Acad. Sci. USA 110, 4656–4660. ( 10.1073/pnas.1218503110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ollerton J, Erenler H, Edwards M, Crockett R. 2014. Extinctions of aculeate pollinators in Britain and the role of large-scale agricultural changes. Science 346, 1360–1362. ( 10.1126/science.1257259) [DOI] [PubMed] [Google Scholar]
- 4.Chauzat MP, Cauquil L, Roy L, Franco S, Hendrikx P, Ribière-Chabert M. 2013. Demographics of the European apicultural industry. PLoS ONE 8, e79018 ( 10.1371/journal.pone.0079018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee KV, et al. 2015. A national survey of managed honey bee 2013–2014 annual colony losses in the USA. Apidologie 46, 292–305. ( 10.1007/s13592-015-0356-z) [DOI] [Google Scholar]
- 6.EU. 2013. Commission implementing Regulation (EU) No 485/2013 of 24 May 2013 amending Implementing Regulation (EU) No 540/2011, as regards the conditions of approval of the active substances clothianidin, thiamethoxam and imidacloprid, and prohibiting the use and sale of seeds treated with plant protection products containing those active substances. Off. J. Eur. Union 139, 12–26. [Google Scholar]
- 7.Simon-Delso N, et al. 2015. Systemic insecticides (neonicotinoids and fipronil): trends, uses, mode of action and metabolites. Environ. Sci. Pollut. Res. Int. 22, 5–34. ( 10.1007/s11356-014-3470-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blacquière T, Smagghe G, van Gestel CM, Mommaerts V. 2012. Neonicotinoids in bees: a review on concentrations, side-effects and risk assessment. Ecotoxicology 21, 973–992. ( 10.1007/s10646-012-0863-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Godfray HCJ, Blacquière T, Field LM, Hails RS, Petrokofsky G, Potts SG, Raine NE, Vanbergen AJ, McLean AR. 2014. A restatement of the natural science evidence base concerning neonicotinoid insecticides and insect pollinators. Proc. R. Soc. B 281, 20140558 ( 10.1098/rspb.2014.0558) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pisa LW, et al. 2015. Effects of neonicotinoids and fipronil on non-target invertebrates. Environ. Sci. Pollut. Res. Int. 22, 68–102. ( 10.1007/s11356-014-3471-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walters K. 2013. Data, data everywhere but we don't know what to think? Neonicotinoid insecticides and pollinators. Outlooks Pest Manag. 24, 151–555. ( 10.1564/v24) [DOI] [Google Scholar]
- 12.Rundlöf M, et al. 2015. Seed coating with a neonicotinoid insecticide negatively affects wild bees. Nature 521, 77–80. ( 10.1038/nature14420) [DOI] [PubMed] [Google Scholar]
- 13.Woodcock BA, et al. 2017. Country-specific effects of neonicotinoid pesticides on honey bees and wild bees. Science 356, 1393–1395. ( 10.1126/science.aaa1190) [DOI] [PubMed] [Google Scholar]
- 14.Potts SG, et al. 2016. Summary for policymakers of the assessment report of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services on pollinators, pollination and food production, pp. 1–30. Bonn, Germany: IPBES.
- 15.Carreck NL, Ratnieksi FLW. 2014. The dose makes the poison: have ‘field realistic’ rates of exposure of bees to neonicotinoid insecticides been overestimated in laboratory studies? J. Apicult. Res. 53, 607–614. ( 10.3896/IBRA.1.53.5.08) [DOI] [Google Scholar]
- 16.Lundin O, Rundlöf M, Smith HG, Fries I, Bommarco R. 2015. Neonicotinoid insecticides and their impacts on bees: a systematic review of research approaches and identification of knowledge gaps. PLoS ONE 10, e0136928 ( 10.1371/journal.pone.0136928) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thompson HM, Fryday SL, Harkin S, Milner S. 2014. Potential impacts of synergism in honeybees (Apis mellifera) of exposure to neonicotinoids and sprayed fungicides in crops. Apidologie 45, 545–553 ( 10.1007/s13592-014-0273-6) [DOI] [Google Scholar]
- 18.Sgolastra F, et al. 2017. Synergistic mortality between a neonicotinoid insecticide and an ergosterol-biosynthesis-inhibiting fungicide in three bee species. Pest Manag. Sci. 73, 1236–1243. ( 10.1002/ps.4449) [DOI] [PubMed] [Google Scholar]
- 19.Sgolastra F, Blasioli S, Renzi T, Tosi S, Medrzycki P, Molowny-Horas R, Porrini C, Braschi I. 2018. Lethal effects of Cr(III) alone and in combination with propiconazole and clothianidin in honey bees. Chemosphere 191, 365–372. ( 10.1016/j.chemosphere.2017.10.068) [DOI] [PubMed] [Google Scholar]
- 20.Gill RJ, Ramos-Rodriguez O, Raine NE. 2012. Combined pesticide exposure severely affects individual- and colony-level traits in bees. Nature 491, 105–108. ( 10.1038/nature11585) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poquet Y, Vidau C, Alaux C. 2016. Modulation of pesticide response in honeybees. Apidologie 47: 412–426. ( 10.1007/s13592-016-0429-7) [DOI] [Google Scholar]
- 22.Sgolastra F, Hinarejos S, Pitts Singer T, Joseph T, Luckmann J, Raine N. 2018. Pesticide exposure assessment paradigm for solitary bees. Environ. Entomol. in press ( 10.1093/ee/nvy105) [DOI] [PubMed] [Google Scholar]
- 23.Arena M, Sgolastra F. 2014. A meta-analysis comparing the sensitivity of bees to pesticides. Ecotoxicology 23, 324–334. ( 10.1007/s10646-014-1190-1) [DOI] [PubMed] [Google Scholar]
- 24.EFSA. 2013. Conclusion on the peer review of the pesticide risk assessment for bees for the active substance clothianidin. EFSA J. 11, 3066 ( 10.2903/j.efsa.2013.3066 ) [DOI] [Google Scholar]
- 25.EFSA. 2015. Reasoned opinion on the review of the existing maximum residue levels (MRLs) for propiconazole according to Article 12 of Regulation (EC) No. 396/2005. EFSA J. 13, 3975 ( 10.2903/j.efsa.2015.3975) [DOI] [Google Scholar]
- 26.Bosch J. 1994. The nesting behaviour of the mason bee Osmia cornuta (Latr) with special reference to its pollinating potential (Hymenoptera, Megachilidae). Apidologie 25, 84–93. ( 10.1051/apido:19940109) [DOI] [Google Scholar]
- 27.EFSA. 2012. Scientific Opinion on the science behind the development of a risk assessment of Plant Protection Products on bees (Apis mellifera, Bombus spp. and solitary bees). EFSA J. 10, 2668 ( 10.2903/j.efsa.2012.2668). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.EPA. 2012. White paper in support of the proposed risk assessment process for bees. Submitted to the FIFRA Scientific Advisory Panel for Review and Comment Office of Chemical Safety and Pollution Prevention Office of Pesticide Programs Environmental Fate and Effects, pp. 1–275.
- 29.Torchio PF. 1987. Use of non-honey bee species as pollinators of crops. Proc. Entomol. Soc. Ont. 118, 111–124. [Google Scholar]
- 30.Bosch J, Vicens N. 2002. Body size as an estimator of production costs in a solitary bee. Ecol. Entomol. 27, 129–137. ( 10.1046/j.1365-2311.2002.00406.x) [DOI] [Google Scholar]
- 31.Sgolastra F, Kemp WP, Buckner JS, Pitts-Singer TL, Maini S, Bosch J. 2011. The long summer: pre-wintering temperatures affect metabolic expenditure and winter survival in a solitary bee. J. Insect Physiol. 57, 1651–1659. ( 10.1016/j.jinsphys.2011.08.017) [DOI] [PubMed] [Google Scholar]
- 32.Bosch J, Kemp WP. 2004. Effect of pre-wintering and wintering temperature regimes on weight loss, survival, and emergence time in the mason bee Osmia cornuta (Hymenoptera: Megachilidae). Apidologie 35, 469–479. ( 10.1051/apido:2004035) [DOI] [Google Scholar]
- 33.Sgolastra F, Arnan X, Pitts-Singer TL, Maini S, Kemp WP, Bosch J. 2016. Pre-wintering conditions and post-winter performance in a solitary bee: does diapause impose an energetic cost on reproductive success? Ecol. Entomol. 41, 201–210. ( 10.1111/een.12292) [DOI] [Google Scholar]
- 34.Bosch J, Vicens N. 2006. Relationship between body size, provisioning rate, longevity and reproductive success in females of the solitary bee Osmia cornuta. Behav. Ecol. Sociobiol. 60, 26–33. ( 10.1007/s00265-005-0134-4) [DOI] [Google Scholar]
- 35.Lee KY, Lee KS, Yoon HJ, Jin BR. 2015. Ovarian development and secretion of vitellogenin protein during the wintering period and after emergence in the hornfaced bee, Osmia cornifrons. J. Asia-Pac. Entomol. 18, 515–523. ( 10.1016/j.aspen.2015.07.002) [DOI] [Google Scholar]
- 36.Pohorecka K, et al. 2012. Residues of neonicotinoid insecticides in bee collected plant materials from oilseed rape crops and their effect on bee colonies. J. Apic. Sci. 56,115–134. [Google Scholar]
- 37.Botías C, David A, Horwood J, Abdul-Sada A, Nicholls E, Hill EM, Goulson D. 2015. Neonicotinoid residues in wildflowers, a potential route of chronic exposure for bees. Environ. Sci. Technol. 49, 12 731–12 740. ( 10.1021/acs.est.5b03459) [DOI] [PubMed] [Google Scholar]
- 38.Pitts-Singer T, Bosch J, Kemp WP, Trostle GE. 2008. Field use of an incubation box for improved emergence timing of Osmia lignaria populations used for orchard pollination. Apidologie 39, 235–246 ( 10.1051/apido:2007061) [DOI] [Google Scholar]
- 39.Hinarejos S, Domene X, Bosch J. 2015. Oral toxicity of dimethoate to adult Osmia cornuta using an improved laboratory feeding method for solitary bees In 12th Int. Symp. of ICP-PR Hazards of Pesticides to Bees, Ghent, Belgium, 15–17 September 2014 Julius-Kuhn-Arch.450, 192. [Google Scholar]
- 40.Cane JH. 2016. Adult pollen diet essential for egg maturation by a solitary Osmia bee. J. Insect Physiol. 95, 105–109. ( 10.1016/j.jinsphys.2016.09.011) [DOI] [PubMed] [Google Scholar]
- 41.David A, Botias C, Abdul-Sada A, Nicholls E, Rotheray EL, Hill EM, Goulson D. 2016. Widespread contamination of wildflower and bee-collected pollen with complex mixtures of neonicotinoids and fungicides commonly applied to crops. Environ. Int. 88, 169–178. ( 10.1016/j.envint.2015.12.011) [DOI] [PubMed] [Google Scholar]
- 42.Porrini C, et al. 2016. The status of honey bee health in Italy: results from the nationwide bee monitoring network. PLoS ONE 11, e0155411 ( 10.1371/journal.pone.0155411) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hladik ML, Vandever M, Smalling KL. 2016. Exposure of native bees foraging in an agricultural landscape to current-use pesticides. Sci. Total Environ. 542, 469–477. ( 10.1016/j.scitotenv.2015.10.077) [DOI] [PubMed] [Google Scholar]
- 44.Botías C, David A, Hill EM, Goulson D. 2017. Quantifying exposure of wild bumblebees to mixtures of agrochemicals in agricultural and urban landscapes. Environ. Pollut. 222, 73–82. ( 10.1016/j.envpol.2017.01.001) [DOI] [PubMed] [Google Scholar]
- 45.Samson-Robert O, Labrie G, Chagnon M, Fournier V. 2017. Planting of neonicotinoid-coated corn raises honey bee mortality and sets back colony development. PeerJ 5, e3670 (doi10.7717/peerj.3670) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kiljanek T, Niewiadowska A, Gaweł M, Semeniuk S, Borze M, Posyniak A, Pohorecka K. 2017. Multiple pesticide residues in live and poisoned honeybees—preliminary exposure assessment. Chemosphere 175, 36–44. ( 10.1016/j.chemosphere.2017.02.028) [DOI] [PubMed] [Google Scholar]
- 47.Tosi S, Costa C, Vesco U, Quaglia G, Guido G. 2018. A 3-year survey of Italian honey bee-collected pollen reveals widespread contamination by agricultural pesticides. Sci. Total Environ. 615, 208–218. ( 10.1016/j.scitotenv.2017.09.226) [DOI] [PubMed] [Google Scholar]
- 48.Bonmatin J-M, et al. 2015. Environmental fate and exposure; neonicotinoids and fipronil. Environ. Sci. Pollut. Res. Int. 22, 35–67. ( 10.1007/s11356-014-3332-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ribbands C. 1953. The behaviour and social life of honeybees. London, UK: Bee Research Association. [Google Scholar]
- 50.Heinrich B. 1979. Bumblebee economics. Cambridge, MA: Harvard University Press. [Google Scholar]
- 51.Kessler SC, Tiedeken EJ, Simcock KL, Derveau S, Mitchell J, Softley S, Stout JC, Wright GA. 2015. Bees prefer foods containing neonicotinoid pesticides. Nature 521, 74–76. ( 10.1038/nature14414) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baron GL, Raine NE, Brown MJF. 2017. General and species-specific impacts of a neonicotinoid insecticide on the ovary development and feeding of wild bumblebee queens. Proc. R. Soc. B 284, 20170123 ( 10.1098/rspb.2017.0123) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chapman RF. 2013. The insects: structure and function (eds Simpson SJ, Douglas AE), 5th edn New York, NY: Cambridge University Press. [Google Scholar]
- 54.Christen V, Mittner F, Fent K. 2016. Molecular effects of neonicotinoids in honey bees (Apis mellifera). Environ. Sci. Technol. 50, 4081 ( 10.1021/acs.est.6b00678) [DOI] [PubMed] [Google Scholar]
- 55.Williams GR, Troxler A, Retschnig G, Roth K, Yañez O, Shutler D, Neumann P, Gauthier L. 2015. Neonicotinoid pesticides severely affect honey bee queens. Sci. Rep. 5, 14621 ( 10.1038/srep1462) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Duchateau MJ, Velthuis H. 1989. Ovarian development and egg laying in workers of Bombus terrestris. Entomol. Exp. Appl. 51, 199–213. ( 10.1111/j.1570-7458.1989.tb01231.x.) [DOI] [Google Scholar]
- 57.Sandrock C, Tanadini LG, Pettis JS, Biesmeijer JC, Potts SG, Neumann P. 2014. Sublethal neonicotinoid insecticide exposure reduces solitary bee reproductive success. Agric. For. Entomol. 16, 119–128. ( 10.1111/afe.12041) [DOI] [Google Scholar]
- 58.Tosi S, Nieh JC, Sgolastra F, Cabbri R, Medrzycki P. 2017. Neonicotinoid pesticides and nutritional stress synergistically reduce survival in honeybees. Proc. R. Soc. B 284, 20171711 ( 10.1098/rspb.2017.1711) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McArt SH, Fersch AA, Milano NJ, Truitt LL, Böröczky K. 2017. High pesticide risk to honey bees despite low focal crop pollen collection during pollination of a mass blooming crop. Sci. Rep. 7, 46554 ( 10.1038/srep46554) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tsvetkov N, Samson-Robert O, Sood K, Patel HS, Malena DA, Gajiwala PH, Maciukiewicz P, Fournier V, Zayed A. 2017. Chronic exposure to neonicotinoids reduces honey-bee health near corn crops. Science 356, 1395–1397. ( 10.1126/science.aam7470) [DOI] [PubMed] [Google Scholar]
- 61.Tepedino VJ, Torchio PF. 1982. Phenotypic variability in the nesting success among Osmia lignaria propinqua females in a glasshouse environment (Hymenoptera: Megachilidae). Ecol. Entomol. 7, 453–462. ( 10.1111/j.1365-2311.1982.tb00688.x) [DOI] [Google Scholar]
- 62.Frohlich DR, Tepedino VJ. 1986. Sex ratio, parental investment, and interparent variability in nesting success in a solitary bee. Evolution 40, 142–151. ( 10.1111/j.1558-5646.1986.tb05725.x) [DOI] [PubMed] [Google Scholar]
- 63.Sugiura N, Maeta Y. 1989. Parental investment and offspring sex ratio in a solitary mason bee, Osmia cornifrons (Radoszkowski) (Hymenoptera. Megachilidae). Jpn. J. Entomol. 57, 861–875. [Google Scholar]
- 64.Holmstrup M, et al. 2010. Interactions between effects of environmental chemicals and natural stressors: a review. Sci. Total Environ. 408, 3746–3762. ( 10.1016/j.scitotenv.2009.10.067) [DOI] [PubMed] [Google Scholar]
- 65.Ladurner E, Bosch J, Kemp WP, Maini S. 2005. Assessing delayed and acute toxicity of five formulated fungicides to Osmia lignaria Say and Apis mellifera. Apidologie 36, 449–460. ( 10.1051/apido:2005032) [DOI] [Google Scholar]
- 66.Sgolastra F, Arnan X, Cabbri R, Isani G, Medrzycki P, Teper D, Bosch J.2018. Data from: Combined exposure to sublethal concentrations of an insecticide and a fungicide affect feeding, ovary development and longevity in a solitary bee. Dryad Digital Repository. (http://dx.doi:10.5061/dryad.895pn6p. ) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Sgolastra F, Arnan X, Cabbri R, Isani G, Medrzycki P, Teper D, Bosch J.2018. Data from: Combined exposure to sublethal concentrations of an insecticide and a fungicide affect feeding, ovary development and longevity in a solitary bee. Dryad Digital Repository. (http://dx.doi:10.5061/dryad.895pn6p. ) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.895pn6p [66].