Abstract
The evolution of helping behaviour in species that breed cooperatively in family groups is typically attributed to kin selection alone. However, in many species, helpers go on to inherit breeding positions in their natal groups, but the extent to which this contributes to selection for helping is unclear as the future reproductive success of helpers is often unknown. To quantify the role of future reproduction in the evolution of helping, we compared the helping effort of female and male retained offspring across cooperative birds. The kin selected benefits of helping are equivalent between female and male helpers—they are equally related to the younger siblings they help raise—but the future reproductive benefits of helping differ because of sex differences in the likelihood of breeding in the natal group. We found that the sex which is more likely to breed in its natal group invests more in helping, suggesting that in addition to kin selection, helping in family groups is shaped by future reproduction.
Keywords: cooperative breeding, sex differences, comparative meta-analysis, helping effort, future reproduction, kin selection
1. Introduction
Cooperative breeding in family groups is characterized by a reproductive division of labour where a breeding pair is accompanied by adult offspring that help raise younger siblings [1–3]. There is clear evidence that kin selection favours helping behaviour in family groups [4–8] but in many cooperative breeders, there is also the possibility that helpers will inherit breeding positions in their natal groups [1,9,10]. This provides a further incentive to help, as helping can improve the chances of survival (pay-to-stay) and augment the size and success of the natal group [10–14]. The contribution of future reproduction to selection for helping is, however, poorly determined: the reproductive success of helpers in most species is often unknown, being realized years into the future [1,3,15], making it difficult to tease apart the roles of future reproduction and kin selection in the evolution of helping.
Patterns of investment in cooperative behaviour that vary independently of relatedness provide an opportunity for detecting whether future reproduction contributes to selection for helping behaviour in family groups. In many cooperative breeders, female and male retained offspring consistently differ in their helping effort [16,17]. Since female and male helpers are equally related to their younger siblings, the kin selected benefits of helping are equivalent between the sexes and, therefore, cannot explain sex differences in helping effort. Instead, variation in helping effort between the sexes is hypothesized to have evolved in response to variation in future reproductive benefits that result from sex differences in the likelihood of inheriting breeding positions in the natal group [9,17].
Reproduction within groups is not always monopolized by the dominant breeding pair. In some species, natal helpers reproduce, for example, when there is turnover of the dominant opposite sex breeder [18] or by co-breeding [19]. This may lead to sex differences in helping effort, not in response to future reproduction, but because one sex is investing in raising its own offspring. Although subordinate reproduction needs to be considered when examining the effect of future reproduction on helping effort, the fitness benefits of breeding as a subordinate are likely to be limited compared with those obtained through breeding position inheritance for two reasons. Firstly, breeding by subordinates in family groups is typically rare, being limited by access to unrelated mating partners [20]. Secondly, successful reproduction by subordinates is likely to be greatly exceeded by the sustained reproductive output of an established dominant breeding pair [21–23].
In this study, we use phylogenetic meta-analyses of female and male helping effort across 20 species of cooperatively breeding birds to quantify the role of future reproduction in the evolution of helping in family groups. Sex differences in helping effort were measured by calculating a statistical effect size of the difference in how much female and male helpers invest in raising their younger siblings from published studies. We then tested whether sex differences in helping effort are associated with differences between the sexes in the likelihood of breeding in the natal group and sex differences in subordinate reproduction. We do not analyse differences in the probability that females and males help, as this cannot be used to tease apart the roles of future reproduction and kin selection in the evolution of helping (figure 1).
Figure 1.
Disentangling the roles of future reproduction and kin selection in the evolution of helping in family groups. Individuals face two decisions regarding helping behaviour. (1) Help in the natal group? Sex differences in the probability of helping cannot be used to separate indirect and future direct fitness benefits as individuals stay and help potentially for both types of fitness benefits. (2) How much to help? Relatedness of female and male retained natals to siblings is equal; therefore, variation in helping effort is hypothesized to be explained by sex differences in future breeding opportunities in the natal group. Image by P. Barden (wikimedia.org).
2. Material and methods
(a). Data collection
(i). Sex differences in helping effort
To measure how much female and male helpers invest in cooperative behaviour, we searched the published literature using Scopus and the Web of Knowledge for studies measuring helping effort in cooperatively breeding birds, using the following topic search term: ‘(feed* OR provision* OR help* OR defen*) AND species name’. We started with the species that breed cooperatively in family groups listed by Riehl [24] and updated this to include newly recognized cooperative breeders and searched both common and scientific species names including known synonyms. Cooperative breeders which do not have delayed dispersal of both sexes and breeding in the natal group by at least one sex were excluded (see electronic supplementary material, table S1 for the species excluded from the analysis and justification for exclusion). For well-studied species for which no relevant studies could be found, we contacted individual researchers to request data (electronic supplementary material, tables S1 and S2). Effect sizes were extracted following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis statement [25,26].
Our effect size is the mean sex difference in helping effort, calculated as Hedges' d [27,28]:
Here,  is the mean female investment in helping,
is the mean female investment in helping,  is mean male investment in helping (most commonly the average number of feeding trips made per hour by female and male helpers in their natal groups),
is mean male investment in helping (most commonly the average number of feeding trips made per hour by female and male helpers in their natal groups), 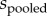 is the pooled sample variance, and J is a correction to account for small sample sizes. To account for differences in sampling effort between studies, each effect size was weighted by its sampling variance:
is the pooled sample variance, and J is a correction to account for small sample sizes. To account for differences in sampling effort between studies, each effect size was weighted by its sampling variance:
where  and
and  are the number of female and male helpers studied.
are the number of female and male helpers studied.
Group members that did not help were included in the estimate of investment in helping for each sex. By estimating the difference in the average contributions made by female and male helpers, our effect size is independent of the number of helpers of each sex, the likelihood of helping, and the total contribution to help. Positive values indicate that males invest more, negative values that females invest more, and a value of zero indicates no difference in helping effort between the sexes.
Our final sample size included 51 effect sizes from 23 studies representing 20 species (electronic supplementary material, figure S1 and table S2). Multiple effect sizes were extracted for 11 of the 20 species in our sample. Multiple effect sizes were calculated when, for example, female and male helping effort were measured in different age classes, in different group sizes, and at different levels of relatedness (e.g. full sibling and half sibling). We reduced our dataset to a single effect size per species by taking weighted averages.
We used Egger's regression method to explore publication bias [29,30]. We regressed the mean sex difference in helping effort against the inverse standard error of the effect sizes using the MCMCglmm R package [31,32], with phylogeny included as a random effect. There was no relationship between the mean sex difference in helping effort and the inverse of the standard error: β (slope estimate) = −0.03, credible interval (CI) = −0.24 to 0.14, Nspecies = 20 (electronic supplementary material, figure S2). We also conducted a trim and fill analysis in the ‘metafor’ R package [33] which estimated that our sample does not require any extra effect sizes to generate a symmetric funnel plot.
(ii). Sex differences in future reproduction
For each species in our sample, we determined whether female or male helpers are more likely to obtain breeding positions in their natal groups based on longitudinal data collected from the same study populations as our effect sizes. First, we collected data on the percentage of breeding positions that are filled by female and male helpers that remained in their natal groups, as opposed to the percentage of breeding positions that are filled by dispersing individuals (data were available for 16 species—for four species inheritance rates were not available, although inheritance occurs in these species; electronic supplementary material, table S3). We then weighted these values by the percentage of individuals of each sex that delayed dispersal to estimate the probability that helping will lead to the inheritance of a breeding position in the natal group for each sex. For example, few breeding positions may be filled by retained offspring, but if relatively few individuals delay dispersal and help and those that do have a high probability of breeding in their natal groups, we would expect their investment in helping to be high, despite low rates of inheritance at the population level. Therefore, sex differences in the likelihood of breeding in the natal group were calculated as follows:
where  is the percentage of females that become breeders by remaining in their natal groups,
is the percentage of females that become breeders by remaining in their natal groups,  is the percentage of females that delay dispersal,
is the percentage of females that delay dispersal,  is the percentage of males that become breeders by remaining in their natal groups, and
is the percentage of males that become breeders by remaining in their natal groups, and  is the percentage of males that delay dispersal. We calculated the sex difference in future reproduction for 15 of the 16 species for which we had data on inheritance rates. For one species, the percentage of individuals of each sex that delayed dispersal was unknown (electronic supplementary material, table S3).
is the percentage of males that delay dispersal. We calculated the sex difference in future reproduction for 15 of the 16 species for which we had data on inheritance rates. For one species, the percentage of individuals of each sex that delayed dispersal was unknown (electronic supplementary material, table S3).
(iii). Sex differences in subordinate reproduction
To account for the possibility that female and male subordinates reproduce in their natal groups and, therefore, may be investing in raising their own offspring rather than helping to raise siblings, we searched for data on subordinate parentage for the 20 species for which we obtained effect sizes. Data on the percentage of offspring in a sampled population whose parent was a female or male subordinate were available for 14 species (electronic supplementary material, table S3). Again, we weighted these values by the percentage of individuals of each sex that delayed dispersal to estimate the relative fitness payoffs of this strategy (for two species, we did not have data on the percentage of individuals of each sex that delayed dispersal, reducing our sample size to 12 species). Therefore, sex differences in subordinate parentage were calculated as follows:
 |
where  is the percentage of offspring whose parent was a female helper,
is the percentage of offspring whose parent was a female helper,  is the percentage of females that delay dispersal,
is the percentage of females that delay dispersal,  is the percentage of offspring whose parent was a male helper, and
is the percentage of offspring whose parent was a male helper, and  is the percentage of males that delay dispersal. Positive values indicate that male subordinates are more likely to be raising their own offspring than female subordinates, negative values indicate the opposite, and a value of zero indicates no difference between the sexes. For 11 of these 12 species, we also had data on sex differences in future reproduction.
is the percentage of males that delay dispersal. Positive values indicate that male subordinates are more likely to be raising their own offspring than female subordinates, negative values indicate the opposite, and a value of zero indicates no difference between the sexes. For 11 of these 12 species, we also had data on sex differences in future reproduction.
(b). Confounding factors
(i). Age and relatedness
Sex differences in the frequency and timing of natal dispersal can lead to sex differences in the age structure of helpers and the relatedness of helpers to the offspring in their group (e.g. if individuals of one sex are more likely to join non-natal groups as immigrant helpers [34]). As helping effort typically increases with age and relatedness in cooperatively breeding birds [35–39], this could bias our effect size calculation. To account for this, for all species in our sample we compared the helping effort of females and males of similar relatedness—either because helping effort was studied with respect to relatedness or because immigrant helpers are rare (electronic supplementary material, table S3—‘effect size confounds' section). In all but two species, we compared female and male helpers of similar age. We could not be certain that we compared female and male helpers of similar ages in the white-browed sparrow weaver and the grey-backed fiscal shrike. As we lacked data on sex differences in future and subordinate reproduction for these species, they were excluded from our analyses. Finally, although extra-pair mating by the breeding female and breeder turnover can also lead to reduced relatedness between group members [40,41], this will affect the sexes equally if female and male helpers of similar ages helping in their natal groups are compared.
(ii). Different measures of helping effort
All our effect sizes measured sex differences in offspring provisioning: 31 measured differences in provisioning rates, 14 measured differences in the percentage share of provisioning, and six measured differences in the total biomass delivered to nestlings (electronic supplementary material, table S2).
(c). Analyses
(i). General approach: phylogenetic meta-analysis
We used Bayesian phylogenetic mixed models (BPMMs) implemented in the MCMCglmm R package for our analyses [31]. These models allow non-independence between data points arising due to shared evolutionary history to be quantified and allow each data point to be weighted by sampling effort to account for differences in sample sizes between studies. We assessed model convergence by assessing plots of chain mixing and levels of autocorrelation. Parameter estimates are reported as the posterior mode (β) and CI of the posterior distribution of the Markov chain. Relationships were considered significantly different from 0 where the credible interval of the posterior mode did not include 0 [42]. Full details of burn-in, run length, priors as well as the model formula, including error structures, are reported in the electronic supplementary material (the R script).
To account for phylogenetic uncertainty in our BPMMs, we marginalized over the posterior distribution of bird trees published by Jetz et al. [43]. This was done by calculating the parameter estimates for the BPMM based on 1 300 different phylogenetic covariance matrices included sequentially as random effects at successive iterations of the Markov chain in each model, with the first 300 discarded as a burn-in. Each time we updated the phylogenetic covariance matrix in the model, we used the values of the latent variables and variance components calculated using the last covariance matrix as starting values for the next tree in the sequence. For further details, see the electronic supplementary material (the R script) and the methods section of Ross et al. [44].
(ii). Sex differences in helping effort
To test whether sex differences in helping effort are associated with differences between the sexes in the likelihood of breeding in the natal group, we constructed a BPMM with the mean sex difference in helping effort as the response variable with a Gaussian error distribution, with sex differences in future reproduction (z-transformed) included as a fixed effect and each effect size weighted by its sampling variance (Nspecies = 15). To test whether differences between the sexes in subordinate reproduction explain variation between the sexes in helping effort, we repeated this model, but replacing sex differences in future reproduction with sex differences in subordinate reproduction (z-transformed) as the fixed effect (Nspecies = 12).
We modelled the effects of future and subordinate reproduction on sex differences in helping effort separately as these variables are likely to be correlated [18,45]. When fixed effects are correlated, estimates of parameter variance may be biased [46], making the independent effect of each variable on the mean sex difference in helping effort difficult to assess. To check for collinearity, we modelled the relationship between sex differences in future reproduction (response variable) and sex differences in subordinate parentage (explanatory variable). We also modelled the relationship between the mean sex difference in helping effort (response variable) and both sex differences in future reproduction (z-transformed) and subordinate reproduction (z-transformed) included as fixed effects (Nspecies = 11).
Finally, we explored the relationship between female and male helper routes to breeding. Given that inbreeding is rare in bird species that breed cooperatively in family groups [20], we might expect females and males within a species to adopt opposite strategies for obtaining breeding positions—when one sex remains to breed in the natal group, the other sex will disperse to breed. To do this, we constructed a multi-response BPMM with the absolute difference between the sexes in each species in the percentage of breeding positions filled by helpers and the absolute difference between the sexes in each species in subordinate parentage as response variables (both with a Gaussian error distribution) with the intercept for each trait estimated as fixed effects.
3. Results
(a). Sex differences in helping effort are associated with future breeding opportunities
Species varied considerably in the amount female and male helpers invested in helping behaviour—in seven species females helped more, in four species there was little difference between the sexes in helping effort, and in nine species males helped more (figure 2). Similarly, there was considerable variation between the sexes in the percentage of breeding positions that are filled by retained offspring (figure 3a). In 12 species, more males than females inherited breeding positions in their natal groups while in four species more females than males inherited breeding positions. In addition, when the percentage of males that obtained breeding positions in the natal group was high, the percentage of females that did so was low and vice versa, which was generally true across species (βdifference = 23.7, CI = −2.05 to 53.98, Nspecies = 16; electronic supplementary material, table S4).
Figure 2.
Variation in helping effort between the sexes across species. Points indicate the mean sex difference in helping effort and are bracketed by their 95% credible intervals. Negative values indicate that females help more, positive values that males help more.
Figure 3.
(a) The percentage of breeding positions that are filled by retained helpers of each sex. Values for female and male helpers of the same species are connected by dotted lines. (b) Helping effort and sex differences in the likelihood of breeding in the natal group—the more likely each sex is to breed in the natal group relative to the other sex, the greater their investment in helping. Weighted mean effect sizes for each species are plotted with regression lines and 95% credible intervals estimated from the BPMM.
Sex differences in helping effort were significantly related to sex differences in future reproduction (βslope = 0.30, CI = 0.07 to 0.58, Nspecies = 15; figure 3b; electronic supplementary material, table S4). When females that delayed dispersal were more likely to obtain breeding positions in their natal groups than males, they helped more, whereas when males that delayed dispersal were more likely to obtain breeding positions in their natal groups than females, they helped more. Furthermore, when the sexes were equally likely to breed in their natal groups in the future, females and males did not differ in how much they helped (βintercept = 0.15, CI = −0.16 to 0.48, Nspecies = 15; electronic supplementary material, table S4).
(b). Subordinate reproduction is associated with future reproduction and sex differences in helping effort
Breeding within-groups was monopolized by dominant individuals in most species, with subordinates obtaining a relatively small share of total parentage (figure 4a). In seven species, females did not breed in their natal groups as subordinates and in five species males did not breed in their natal groups as subordinates. In the species where subordinates bred in their natal groups, only one sex tended to do so (βdifference = 4.6, CI = −0.64 to 9.12, Nspecies = 14; electronic supplementary material, table S4).
Figure 4.
(a) The percentage of offspring in each species whose parent was a subordinate. Values for female and male helpers of the same species are connected by dotted lines. (b) Helping effort and sex differences in subordinate reproduction—the sex that is more likely to be investing in raising its own offspring helps more. Weighted mean effect sizes for each species are plotted with regression lines and 95% credible intervals estimated from the BPMM.
Sex differences in subordinate reproduction were positively associated with sex differences in future reproduction (βslope = 1.55, CI = 0.07 to 3.38; Nspecies = 11; electronic supplementary material, table S4). As expected given their collinearity, neither of these variables explained sex differences in helping effort when considered together as explanatory variables (subordinate reproduction: βslope = 0.30, CI = −0.13 to 0.64; future reproduction: βslope = 0.17, CI = −0.25 to 0.46; Nspecies = 11; electronic supplementary material, table S4). However, sex differences in subordinate reproduction were significantly associated with sex differences in helping effort when sex differences in future reproduction were excluded as an explanatory variable (βslope = 0.34, CI = 0.07 to 0.62, Nspecies = 12; figure 4b; electronic supplementary material, table S4), with the sex that was more likely to breed as a subordinate helping more. When there was no difference between the sexes in subordinate reproduction, females and males helped equally (βintercept = 0.16, CI = −0.22 to 0.54, Nspecies = 12; electronic supplementary material, table S4).
4. Discussion
Sex differences in helping effort across species of cooperatively breeding birds (figure 2) provide a tool for disentangling selection for helping behaviour that results from future reproduction and kin selection in family groups. By exploiting these differences, we show that future breeding opportunities in the natal group are associated with investment decisions of helpers. The sex that is more likely to obtain a breeding position in its natal group typically invests more in cooperative behaviour than the sex which disperses to breed (figure 3b). Our results contribute to growing evidence that future reproduction provides a strong incentive to help [9,10,47–50] and highlight that this is not only the case in groups of unrelated individuals—future reproduction also shapes helping behaviour in family groups.
Future breeding opportunities in the natal group have been argued to play a negligible role in the evolution helping behaviour in cooperative birds [51], most likely because few helpers were thought to obtain breeding positions in this way. However, we found that future reproduction in the natal group can be very common: from 7 to 55% of breeding positions are filled by retained offspring in our sample of species (figure 3a—averaged across the sexes). Furthermore, the number of helpers observed breeding in their natal groups is strongly dependent on the length of the field study (Spearman's rank correlation between the number of helpers breeding in the natal group and study duration: ρ = 0.77, p < 0.001; Nspecies = 18; electronic supplementary material, table S3), suggesting that inheritance may be even more common than currently estimated.
Even if future reproduction in the natal group is rare, it can still lead to considerable fitness gains. For example, in an 18-year study of the Florida scrub-jay, the descendants of just four male breeders went on to occupy over 30 territories [22]. The fitness payoffs of acquiring a breeding position are even more evident in some social mammals. In naked mole rats, fewer than 0.1% of females become queens in the wild [23] and evidence from captive colonies suggests that breeding females may produce hundreds of offspring throughout their lifetimes—the record is 900 pups over an 11-year breeding tenure [52]. The type of group an individual is born into is also likely to influence dispersal decisions. In green wood hoopoes, individuals that breed on high-quality territories leave behind more descendants than individuals breeding on low-quality territories [53] and in stripe-backed wrens, reproductive success is highest when breeding in a large group [54]. Individuals born into successful groups are therefore likely to delay dispersal as the potential fitness returns of breeding in such groups can be substantial. This seems to be the case in brown jays where females that breed in their natal groups have higher reproductive success than females breeding as immigrants [55].
Kin selection has played a key role in the evolution of family-based cooperative systems [4–8]. However, as long as the opportunity for future reproduction remains possible, helpers have to trade-off the current kin selected benefits of helping against their potential future direct fitness [56]. This trade-off is known to shape helping behaviour in two cooperative wasps where helpers next in line to inherit a breeding position reduce their investment in helping compared to helpers that have a low probability of inheriting [47,48]. In these species, helpers decrease their investment in care, which demonstrates that across disparate taxa, future fitness considerations drive variation in the value that helpers place on indirect fitness benefits, but that the direction of change is dependent on the biology of the system in question.
Several mechanisms have been suggested to explain why investment in helping is related to the likelihood of breeding in the natal group. For example, helpers may have to pay-to-stay for this privilege or they may choose to work harder to augment the size of their future breeding group [12,14]. Previous research on the mechanisms via which direct fitness benefits are accrued in cooperatively breeding species has focused on non-kin groups, such as the cooperative fish, Neolamprologus pulcher, as kin selection cannot provide an explanation for helping behaviour [57,58]. Our results highlight that in family groups, the mechanisms driving increased investment in helping in response to direct fitness benefits require further empirical attention alongside the study of kin selected benefits.
Sex differences in subordinate parentage within the natal group also explained some of the variation between the sexes in helping effort (figure 4b). This effect was largely driven by the two species with the highest rates of female-biased philopatry and male-biased dispersal in our sample—the Seychelles warbler and the brown jay. In these species, breeding opportunities for subordinate females represent an important component of direct fitness [34,55]. In most of the species in our analysis, however, future reproduction as a dominant breeder is often the only way for individuals to make a direct genetic contribution to the next generation (figure 4a). Since the lifetime fitness gains of obtaining a breeding position in the natal group can be considerable, as discussed above, the fitness payoffs of obtaining a dominant breeding position are likely to greatly exceed those of breeding as a subordinate and, therefore, have a stronger effect on selection for helping. Quantifying the relative contributions of these two components of direct fitness to selection for helping requires further study.
In summary, our results highlight the importance of detailed long-term ecological field studies across different species of cooperative breeders. The natural variation observed across bird species in both helping behaviour and the way fitness is obtained provides a unique opportunity to test social evolutionary theory that is not possible in experimental settings or in other taxa. For example, in other model systems for studying social evolution, such as some eusocial insects, variation in helping behaviour and reproductive opportunities is either absent or sex-limited [59,60]. More specifically, our study illustrates that such variation can be key to addressing questions that are empirically challenging, such as disentangling selection for helping behaviour arising through future reproduction and kin selection. To this end, in groups of relatives where kin selected benefits have provided the clear explanation for helping behaviour, it is now evident that future reproductive benefits are also important, and that the evolution of helping behaviour can be the result of multiple selective forces.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
Many thanks to Lyanne Brouwer and Jonathan Wright for providing raw data and to Geoff Wild for comments on the manuscript.
Data accessibility
Data are available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.gp14p82 [61].
Authors' contributions
A.S.G., P.A.D., and C.K.C. conceived the study. P.A.D. designed the study. P.A.D. and C.K.C. analysed the data and all authors contributed to writing the manuscript.
Competing interests
We have no competing interests.
Funding
We thank the Swedish Research Council (VR), the Wallenberg Academy, the Royal Society and Lund University for funding.
References
- 1.Stacey PB, Koenig WD. 1990. Cooperative breeding in birds: long-term studies of ecology and behavior. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 2.Jennions MD, Macdonald DW. 1994. Cooperative breeding in mammals. Trends Ecol. Evol. 9, 89–93. ( 10.1016/0169-5347(94)90202-X) [DOI] [PubMed] [Google Scholar]
- 3.Koenig WD, Dickinson JL. 2016. Cooperative breeding in vertebrates: studies of ecology, evolution and behavior. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 4.Metcalf RA, Whitt GS. 1977. Relative inclusive fitness in the social wasp Polistes metricus. Behav. Ecol. Sociobiol. 2, 353–360. ( 10.1007/BF00299505) [DOI] [Google Scholar]
- 5.Emlen ST, Wrege PH. 1989. A test of alternate hypotheses for helping behavior in white-fronted bee-eaters of Kenya. Behav. Ecol. Sociobiol. 25, 303–319. ( 10.1007/BF00302988) [DOI] [Google Scholar]
- 6.Griffin AS, West SA. 2003. Kin discrimination and the benefit of helping in cooperatively breeding vertebrates. Science 302, 634–636. ( 10.1126/science.1089402) [DOI] [PubMed] [Google Scholar]
- 7.Bourke AFG. 2014. Hamilton's rule and the causes of social evolution. Phil. Trans. R. Soc. B 369, 20130362 ( 10.1073/pnas.1103457108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hatchwell BJ, Gullett PR, Adams MJ. 2014. Helping in cooperatively breeding long-tailed tits: a test of Hamilton's rule. Phil. Trans. R. Soc. B 369, 20130565 ( 10.1101/sqb.2009.74.041) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woolfenden GE, Fitzpatrick JW. 1978. The inheritance of territory in group-breeding birds. Bioscience 28, 104–108. ( 10.2307/1307423) [DOI] [Google Scholar]
- 10.Wiley RH, Rabenold KN. 1984. The evolution of cooperative breeding by delayed reciprocity and queuing for favorable social positions. Evolution 38, 609–621. ( 10.2307/2408710) [DOI] [PubMed] [Google Scholar]
- 11.Pen I, Weissing FJ. 2000. Towards a unified theory of cooperative breeding: the role of ecology and life history re-examined. Proc. R. Soc. Lond. B 267, 2411–2418. ( 10.1098/rspb.2000.1299) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kokko H, Johnstone RA. 2001. The evolution of cooperative breeding through group augmentation. Proc. R. Soc. Lond. B 268, 187–196. ( 10.1098/rspb.2000.1349) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kingma SA, Hall ML, Peters A. 2011. Multiple benefits drive helping behavior in a cooperatively breeding bird: an integrated analysis. Am. Nat. 177, 486–495. ( 10.1086/658989) [DOI] [PubMed] [Google Scholar]
- 14.Kingma SA, Santema P, Taborsky M, Komdeur J. 2014. Group augmentation and the evolution of cooperation. Trends Ecol. Evol. 29, 476–484. ( 10.1016/j.tree.2014.05.013) [DOI] [PubMed] [Google Scholar]
- 15.Downing PA, Cornwallis CK, Griffin AS. 2015. Sex, long life and the evolutionary transition to cooperative breeding in birds. Proc. R. Soc. B 282, 20151663 ( 10.1126/science.1205140) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cockburn A. 1998. Evolution of helping behavior in cooperatively breeding birds. Annu. Rev. Ecol. Syst. 29, 141–177. ( 10.1146/annurev.ecolsys.29.1.141) [DOI] [Google Scholar]
- 17.Clutton-Brock TH, Russell AF, Sharpe LL, Young AJ, Balmforth Z, McIlrath GM. 2002. Evolution and development of sex differences in cooperative behavior in meerkats. Science 297, 253–256. ( 10.1126/science.1071412) [DOI] [PubMed] [Google Scholar]
- 18.Rabenold PP, Rabenold KN, Piper WH, Haydock J, Zack SW. 1990. Shared paternity revealed by genetic analysis in cooperatively breeding tropical wrens. Nature 348, 538–540. ( 10.1038/348538a0) [DOI] [Google Scholar]
- 19.Richardson DS, Jury FL, Blaakmeer K, Komdeur J, Burke T. 2001. Parentage assignment and extra-group paternity in a cooperative breeder: the Seychelles warbler (Acrocephalus sechellensis). Mol. Ecol. 10, 2263–2273. ( 10.1046/j.0962-1083.2001.01355.x) [DOI] [PubMed] [Google Scholar]
- 20.Riehl C. 2017. Kinship and incest avoidance drive patterns of reproductive skew in cooperatively breeding birds. Am. Nat. 190, 774–785. ( 10.1086/694411) [DOI] [PubMed] [Google Scholar]
- 21.Clutton-Brock TH, Hodge SJ, Spong G, Russell AF, Jordan NR, Bennett NC, Sharpe LL, Manser MB. 2006. Intrasexual competition and sexual selection in cooperative mammals. Nature 444, 1065–1068. ( 10.1038/nature05386) [DOI] [PubMed] [Google Scholar]
- 22.Woolfenden GE, Fitzpatrick JW. 1990. Florida scrub jays: a synopsis after 18 years. In Cooperative breeding in birds: long-term studies of ecology and behavior (eds Stacey PB, Koenig WD), pp. 239–266. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 23.Faulkes CG, Bennett NC. 2016. Damaraland and naked mole-rats: convergence of social evolution. In Cooperative breeding in vertebrates (eds Koenig WD, Dickinson JL), pp. 338–352. Cambridge: Cambridge University Press. [Google Scholar]
- 24.Riehl C. 2013. Evolutionary routes to non-kin cooperative breeding in birds. Proc. R. Soc. B 280, 20132245 ( 10.1016/j.cub.2010.09.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liberati A, et al. 2009. The PRISMA Statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 6, e1000100 ( 10.1371/journal.pmed.1000100.s002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. 2009. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 6, e1000097 ( 10.1371/journal.pmed.1000097) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hedges LV, Olkin I. 1985. Statistical methods for meta-analysis. New York: Academic Press. [Google Scholar]
- 28.Nakagawa S, Cuthill IC. 2007. Effect size, confidence interval and statistical significance: a practical guide for biologists. Biol. Rev. 82, 591–605. ( 10.1111/j.1469-185X.2007.00027.x) [DOI] [PubMed] [Google Scholar]
- 29.Egger M, Smith GD, Schneider M, Minder C. 1997. Bias in meta-analysis detected by a simple, graphical test. Br. Med. J. 315, 629–634. ( 10.1136/bmj.315.7109.629) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moreno SG, Sutton AJ, Ades AE, Stanley TD, Abrams KR, Peters JL, Cooper NJ. 2009. Assessment of regression-based methods to adjust for publication bias through a comprehensive simulation study. BMC Med. Res. Methodol. 9, 2 ( 10.1186/1471-2288-9-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hadfield JD. 2010. MCMC methods for multi-response generalized linear mixed models: the MCMCglmm R package. J. Stat. Softw. 33, 1–22. (doi:10.18637/jss.v033.i02)20808728 [Google Scholar]
- 32.R Core Team. 2013. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 33.Viechtbauer W. 2010. Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 36, 1–48. (doi:10.18637/jss.v036.i03) [Google Scholar]
- 34.Richardson DS, Burke T, Komdeur J. 2002. Direct benefits and the evolution of female-biased cooperative breeding in Seychelles warblers. Evolution 56, 2313–2321. ( 10.1111/j.0014-3820.2002.tb00154.x) [DOI] [PubMed] [Google Scholar]
- 35.Rabenold KN. 1985. Cooperation in breeding by nonreproductive wrens: kinship, reciprocity, and demography. Behav. Ecol. Sociobiol. 17, 1–17. ( 10.1007/BF00299422) [DOI] [Google Scholar]
- 36.Curry RL. 1988. Influence of kinship on helping behavior in Galapagos mockingbirds. Behav. Ecol. Sociobiol. 22, 141–152. ( 10.1007/BF00303549) [DOI] [Google Scholar]
- 37.McGowan KJ, Woolfenden GE. 1990. Contributions to fledgling feeding in the Florida Scrub Jay. J. Anim. Ecol. 59, 691–707. ( 10.2307/4889) [DOI] [Google Scholar]
- 38.Legge S. 2000. Helper contributions in the cooperatively breeding laughing kookaburra: feeding young is no laughing matter. Anim. Behav. 59, 1009–1018. ( 10.1006/anbe.2000.1382) [DOI] [PubMed] [Google Scholar]
- 39.Woxvold IA, Mulder RA, Magrath MJ. L. 2006. Contributions to care vary with age, sex, breeding status and group size in the cooperatively breeding apostlebird. Anim. Behav. 72, 63–73. ( 10.1016/j.anbehav.2005.08.016) [DOI] [Google Scholar]
- 40.Mulder RA, Dunn PO, Cockburn A, Lazenby-Cohen KA, Howell MJ. 1994. Helpers liberate female fairy-wrens from constraints on extra-pair mate choice. Proc. R. Soc. Lond. B 255, 223–229. ( 10.1098/rspb.1994.0032) [DOI] [Google Scholar]
- 41.Warrington MH, Rollins LA, Russell AF, Griffith SC. 2015. Sequential polyandry through divorce and re-pairing in a cooperatively breeding bird reduces helper-offspring relatedness. Behav. Ecol. Sociobiol. 69, 1311–1321. ( 10.1007/s00265-015-1944-7) [DOI] [Google Scholar]
- 42.Hadfield JD.2017. MCMCglmm Course Notes. 1–144. https://cran.r-project.org/web/packages/MCMCglmm/vignettes/CourseNotes.pdf .
- 43.Jetz W, Thomas GH, Joy JB, Hartmann K, Mooers AO. 2012. The global diversity of birds in space and time. Nature 491, 444–448. ( 10.1038/nature11631) [DOI] [PubMed] [Google Scholar]
- 44.Ross L, Gardner A, Hardy N, West SA. 2013. Ecology, not the genetics of sex determination, determines who helps in eusocial populations. Curr. Biol. 23, 2383–2387. ( 10.1016/j.cub.2013.10.013) [DOI] [PubMed] [Google Scholar]
- 45.Richardson DS, Burke T, Komdeur J. 2007. Grandparent helpers: the adaptive significance of older, postdominant helpers in the Seychelles warbler. Evolution 61, 2790–2800. ( 10.1111/j.1558-5646.2007.00222.x) [DOI] [PubMed] [Google Scholar]
- 46.Freckleton R. 2011. Dealing with collinearity in behavioural and ecological data: model averaging and the problems of measurement error. Behav. Ecol. Sociobiol. 65, 91–101. ( 10.1007/s00265-010-1045-6) [DOI] [Google Scholar]
- 47.Cant MA, Field J. 2001. Helping effort and future fitness in cooperative animal societies. Proc. R. Soc. Lond. B 268, 1959–1964. ( 10.1098/rspb.2001.1754) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Field J, Cronin A, Bridge C. 2006. Future fitness and helping in social queues. Nature 441, 214–217. ( 10.1038/nature04560) [DOI] [PubMed] [Google Scholar]
- 49.Johns PM, Howard KJ, Breisch NL, Rivera A, Thorne BL. 2009. Nonrelatives inherit colony resources in a primitive termite. Proc. Natl Acad. Sci. USA 106, 17 452–17 456. ( 10.1073/pnas.0907961106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kingma SA. 2017. Direct benefits explain interspecific variation in helping behaviour among cooperatively breeding birds. Nat. Commun. 8, 1–7. ( 10.1038/s41467-017-01299-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ekman J, Dickinson JL, Hatchwell BJ, Griesser M. 2004. Delayed dispersal. In Ecology and evolution of cooperative breeding in birds (eds Koenig WD, Dickinson JL), pp. 35–47. Cambridge: Cambridge University Press. [Google Scholar]
- 52.Buffenstein R. 2008. Negligible senescence in the longest living rodent, the naked mole-rat: insights from a successfully aging species. J. Comp. Physiol. B 178, 439–445. ( 10.1007/s00360-007-0237-5) [DOI] [PubMed] [Google Scholar]
- 53.Ligon JD, Ligon SH. 1990. Green Woodhoopoes: life history traits and sociality. In Cooperative breeding in birds: long-term studies of ecology and behavior (eds Koenig WD, Dickinson JL), pp. 31–66. Cambridge: Cambridge University Press. [Google Scholar]
- 54.Rabenold KN. 1990. Campylorhynchus wrens: the ecology of delayed dispersal and cooperation in the Venezuelan savanna. In Cooperative breeding in birds: long-term studies of ecology and behavior (eds Stacey PB, Koenig WD), pp. 157–196. Cambridge: Cambridge University Press. [Google Scholar]
- 55.Williams DA, Rabenold KN. 2005. Male-biased dispersal, female philopatry, and routes to fitness in a social corvid. J. Anim. Ecol. 74, 150–159. ( 10.1111/j.1365-2656.2004.00907.x) [DOI] [Google Scholar]
- 56.Downing PA, Cornwallis CK, Griffin AS. 2016. How to make a sterile helper. Bioessays 39, e201600136 ( 10.1002/bies.201600136) [DOI] [PubMed] [Google Scholar]
- 57.Bergmüller R, Heg D, Taborsky M. 2005. Helpers in a cooperatively breeding cichlid stay and pay or disperse and breed, depending on ecological constraints. Proc. R. Soc. B 272, 325–331. ( 10.1098/rspb.2004.2960) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fischer S, Zöttl M, Groenewoud F, Taborsky B. 2014. Group-size-dependent punishment of idle subordinates in a cooperative breeder where helpers pay to stay. Proc. R. Soc. B 281, 20140184 ( 10.1098/rspb.2014.0184) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wilson EO. 1971. The insect societies. Cambridge, MA: Belknap Press. [Google Scholar]
- 60.Bignell DE, Roisin Y, Lo N. 2010. Biology of termites: a modern synthesis. London: Springer. [Google Scholar]
- 61.Downing PA, Griffin AS, Cornwallis CK. 2018. Data from: Sex differences in helping effort reveal the effect of future reproduction on cooperative behaviour in birds Dryad Digital Repository. ( 10.5061/dryad.gp14p82) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Downing PA, Griffin AS, Cornwallis CK. 2018. Data from: Sex differences in helping effort reveal the effect of future reproduction on cooperative behaviour in birds Dryad Digital Repository. ( 10.5061/dryad.gp14p82) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data are available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.gp14p82 [61].






