Abstract
Animals are thought to use achromatic signals to detect small (or distant) objects and chromatic signals for large (or nearby) objects. While the spatial resolution of the achromatic channel has been widely studied, the spatial resolution of the chromatic channel has rarely been estimated. Using an operant conditioning method, we determined (i) the achromatic contrast sensitivity function and (ii) the spatial resolution of the chromatic channel of a diurnal raptor, the Harris's hawk Parabuteo unicinctus. The maximal spatial resolution for achromatic gratings was 62.3 c deg−1, but the contrast sensitivity was relatively low (10.8–12.7). The spatial resolution for isoluminant red-green gratings was 21.6 c deg−1—lower than that of the achromatic channel, but the highest found in the animal kingdom to date. Our study reveals that Harris's hawks have high spatial resolving power for both achromatic and chromatic vision, suggesting the importance of colour vision for foraging. By contrast, similar to other bird species, Harris's hawks have low contrast sensitivity possibly suggesting a trade-off with chromatic sensitivity. The result is interesting in the light of the recent finding that double cones—thought to mediate high-resolution vision in birds—are absent in the central fovea of raptors.
Keywords: colour vision, contrast sensitivity, foraging, raptors, spatial resolution
1. Background
Vertebrate vision has been extensively studied and debated since the important early works of Walls and Rochon-Duvigneaud [1,2]. One debated question is whether animals analyse intensity and colour (i.e. achromatic and chromatic) information of a visual scene combined or separately, and what each of them is used for. It is now assumed that many animals use achromatic signals for detection of small objects or fine details, and chromatic signals for large objects or coarse features [3–5].
While acuity is usually determined using achromatic gratings with high contrast (e.g. black and white bars) [6], in natural situations, objects of interest often differ in both contrast and colour to the background. Thus, the visual acuity threshold provides only partial information about visual capabilities of an animal as it reveals only the upper limit of spatial resolution for objects of maximum contrast. The spatial resolution of the achromatic channel has been estimated in numerous species, but contrast sensitivity remains poorly understood [7], and the spatial resolution of the chromatic channel is known only in three animal species: humans [8], honeybees Apis mellifera [3] and budgerigars Melopsittacus undulatus [9]. In all three species, it is much lower than the spatial resolution of the achromatic channel. Humans, for example, have been shown to resolve achromatic gratings with 30–60 cycles per degree (c deg−1), but isoluminant red-green and blue-yellow gratings of less than 10 c deg−1 [8]. In budgerigars, the threshold was close to 4.5 c deg−1 for both red-green and blue-green gratings [9].
Diurnal raptors (accipitriform and falconiform birds, hereafter called raptors), renowned for their extraordinarily sharp eyesight, have fascinated scientists for decades [6]. Among all animals studied to date, some raptors, such as the wedge-tailed eagle Aquila audax [10], the Indian vulture Gyps indicus [11] or the brown falcon Falco berigora [12], have the most acute vision. High-acuity vision, probably the most important sensory modality for hunting raptors [13], results from the large eye size and high cone density in their most acute zone of vision, the central fovea. Yet, besides visual acuity, very little is known about raptor vision. Contrast sensitivity has been studied only in two species, the wedge-tailed eagle [14] and the American kestrel Falco sparverius [15] (and only in one individual of each species). Surprisingly, while these two raptors have high spatial resolution (142 c deg−1 for the wedge-tailed eagle and 42 c deg−1 for the American kestrel) compared with non-raptorial birds, such as the budgerigar (10 c deg−1), their contrast sensitivity is similarly low (e.g. 13.6 for the wedge-tailed eagle and 10.2 for the budgerigar) [9,14].
Raptors, like other birds, have four spectrally distinct types of single cones (violet-sensitive, VS; short-wavelength-sensitive, SWS; medium-wavelength-sensitive, MWS; long-wavelength-sensitive, LWS) and one type of double cones in their retinae, but several raptor species have been shown to lack double cones in the central fovea [10,12,16]. These findings challenge the common assumption that raptors, as other birds, use double cones for achromatic vision and single cones for chromatic vision [17,18]. Instead, these data suggest that raptors may use single cones for high achromatic resolution, and that they may possess highly resolved tetrachromatic vision [16]. However, the spatial resolution of the chromatic channel has never been estimated in any raptor species.
In this study, we behaviourally determined (i) the achromatic contrast sensitivity function (CSF) and (ii) the spatial resolution limit of the chromatic channel in a diurnal raptor, the Harris's hawk Parabuteo unicinctus. This species mainly hunts live mammals, and high spatial resolution may be important to detect prey at distance. Some recently studied aspects of its vision suggest that Harris's hawks are highly visually specialized to their foraging demands [19,20]. They have a broad binocular field (47°), a deep central and a shallow temporal fovea, and high spatial resolution of achromatic vision (the maximum visual acuity measured in one animal was 43.7 c deg−1) [19]. Thus, Harris's hawks have a similar achromatic acuity as humans [21] allowing for an interesting comparison of the chromatic spatial resolution and the CSF.
2. Methods
(a). Experimental subjects
Experimental animals were three healthy adult female Harris's hawks (subjects A, B and C), belonging to the French falconry park Les Ailes de l'Urga that were used in a previous study for visual acuity estimation [19]. All three individuals were raised in the raptor facility and used for public shows in the summer season. During the period of this study (Experiment 1, contrast sensitivity function: 25 April to 3 July 2017; Experiment 2, chromatic spatial resolution: 5 October to 26 November 2017), the birds’ body weight was controlled every day and maintained at about 90% of their free feeding weight. Water was provided ad libitum, whereas food (chicken meat) was given only during the experiments. If a bird did not perform well in a daily session, it received more food for every correct choice the day after. Training and experimentation took place 5 days per week. The hawks were housed together in an aviary and hand-fed by the falconer when no experiments took place. During the experiment, they were placed outside their aviaries and attached to an adapted falconry perch.
(b). Experimental room and aviary
The CSF (Experiment 1) was measured outdoors, in an aviary of 8 m width, 7.5 m length and 3 m height. A diffusing tarpaulin was placed on the top of the aviary. The aviary wall behind the monitors used to present the stimuli was covered with a grey tarpaulin. The experiments were conducted from 10 to 13 h in order to avoid the birds facing the sun while flying. No experiments were performed under very cloudy or rainy conditions. Before every session, we measured the illuminance at the starting perch, using an LCD Digital light meter (Tasi HS1010). The average illuminance was 9400 ± 1200 lx (mean ± s.e.) and ranged from 3730 to 17780 lx, which corresponds to full daylight (but without direct sun).
The chromatic spatial resolution measurements (Experiment 2) were conducted in a room of 7.5 m width, 6 m length and 3 m height. A neutral-white LED lamp (flicker frequency 100 Hz; Xanlite, France) was used to light up the room. The illuminance at the starting perch was 210 lx.
In both experiments, two monitors for stimulus presentation were positioned on one side of the room, at 5 m distance from each other. Under each of the monitors, a perch was attached to a feeding box, which had ten compartments with a piece of chicken meat in each. Individual compartments could be opened remotely using an electric motor to expose the meat as a reward for a correct choice [19]. A starting perch was positioned 5 m from the screens on the other side of the room.
(c). Stimuli
Stimuli were created in R v. 3.4.1 (R Development Core Team, 2017) and presented using Microsoft Office PowerPoint 2016 on two computer monitors (display size 476 × 266 mm; Samsung S22C300H). The stimuli (238 × 133 mm) were presented in the centre of the screens and subtended 2.7 × 1.5 degrees of visual angle, when observed from the starting perch. We presented the stimuli only in the central part of the monitor where the luminance was most uniform (175 cd m−2 measured with Hagner ScreenMaster, B. Hagner, Solna, Sweden).
Negative stimuli were achromatic square-wave gratings of different spatial frequencies and contrasts (Experiment 1) or red-green gratings of different spatial frequencies and fixed colour contrast (Experiment 2). The positive stimulus was a grating with very high spatial frequency (175.8 c deg−1) of the same achromatic (Experiment 1) or chromatic (Experiment 2) contrast and mean luminance as the simultaneously presented negative stimulus. Gratings of such high spatial frequency should appear as uniform field for a Harris's hawk, because the maximum visual acuity determined previously in this species was 43.7 c deg−1 (97% Michelson contrast [19]). The radiance spectra of the red and green bars of the chromatic grating were measured with a spectroradiometer RSP900-R (International Light).
The chromatic stimuli (red-green gratings, Experiment 2) were designed to have high chromatic and low achromatic contrast. We assumed that chromatic vision is driven by signals from single cones, while double cones mediate achromatic vision [7,18]. Because no data on the Harris's hawks retina exist, the red-green gratings were created based on the relative cone abundances and spectral sensitivities of the common buzzard Buteo buteo [17], another closely related member of the Accipitridae family, and the receptor noise-limited model of colour discrimination [22].
The cone spectral sensitivities and achromatic contrasts of the stimuli were modelled as described in detail by Lind et al. [17] (see electronic supplementary material, table S1 and figure S1). Briefly, the peak wavelength (λmax) of the sws1 pigment sensitivity (405 nm in common buzzard [23]) was used to predict λmax of the pigments of other cone types and oil droplet transmittance spectra [24]; ocular media transmittance (OMT; t50 at 375 nm), pigment absorption coefficient (0.035 µm−1), cone outer segment length (10 µm), the Weber fraction of the LWS mechanism (0.1) and the cone abundance ratio of 1 : 2 : 2 : 4 (VS : SWS : MWS : LWS) were taken from Lind et al. [17]. The photoreceptor spectral sensitivities were modelled using a visual pigment template [25]. Based on this established method, the chromatic contrast between the red and green bars was 25 JNDs (just noticeable differences; the discrimination threshold is 1 JND). Spectral reflectances and quantum catches of all cone types for these colours are given in the excel-file in the electronic supplementary material.
The achromatic contrast was calculated as Michelson contrast [26] for the double cones. Because pigmentation of the oil droplet of raptor double cones is unknown and cannot be predicted by a model [24], we calculated a range of achromatic contrasts by varying the cut-off wavelength of the double cone oil droplet λcut from 400 to 500 nm (see electronic supplementary material, figure S1). The double cone contrast between red and green bars (varying from 8.6% to 2.7%, respectively) was lower than or very similar to the minimum achromatic contrast threshold (see results of Experiment 1).
As the use of only the double cones for achromatic vision is hypothetical and other raptors have been shown to lack them in the fovea [16], we also calculated the Michelson contrast between red and green bar colours, for each single cone type (see electronic supplementary material, table S1).
(d). Behavioural experiment
The CSF (contrast sensitivity refers to the inverse of Michelson Contrast) and the spatial resolution of the chromatic channel of the Harris's hawks were measured using an operant conditioning technique, involving two phases as described below.
(i). Conditioning
Sitting on the starting perch, the birds were required to choose between the positive (rewarded) and a negative (unrewarded) stimulus. As negative stimuli, gratings were used with low spatial frequency (either 1.1 or 2.9 c deg−1) and high achromatic (69% Michelson contrast; Experiment 1) or high chromatic contrast (25 JND; Experiment 2).
The side of the positive and negative stimuli was changed in a pseudo-random order (i.e. the positive stimulus was not presented on the same side for more than three consecutive trials). A session consisted of 40 trials, and the positive stimulus was presented 20 times on each side.
When the bird opened the wings to leave the starting perch, the monitors were switched off to ensure that the bird could not change the decision on its way. If the bird chose the positive stimulus, a compartment with meat was opened after the bird landed on the perch. The experimenter was hiding in a cabin to avoid any visual contact and influence on the bird's choice. Two training sessions were conducted daily. When a bird reached 80% correct choices in two consecutive sessions, the training phase ended and the test phase began.
(ii). Testing
As in the conditioning phase, two sessions of 40 trials were conducted with each bird every day, and the side of the positive stimulus was varied pseudo-randomly. Before each test session, we presented five low-frequency gratings (either 1.1 or 2.9 c deg−1) to ensure that the bird was still conditioned.
For Experiment 1, achromatic gratings of six spatial frequencies (1.1, 2.9, 5.9, 11.7, 22.0, 35.2 c deg−1) were used. Each frequency was tested with six to nine different Michelson contrasts (from 69, 53, 29, 25, 20, 18, 15, 9, 6 and 3%). High-frequency gratings were tested with fewer different contrasts. During a single session, only one spatial frequency was presented, but with all contrasts. Tests were repeated until each bird had completed 40 choices for each combination of spatial frequency and contrast needed to establish the CSF.
For Experiment 2, eight spatial frequencies (1.1, 2.9, 5.9, 7.3, 11.7, 22.0, 35.2 and 44.0 c deg−1) of the red-green gratings were used and tested in each session. The tests were repeated until each bird had completed 40 choices for each spatial frequency.
(e). Data analysis
All analyses were performed with R v. 3.4.1 using {psyphy} [27] and {ggplot2} [28] packages. Psychometric functions were fitted to the choice frequencies from each bird in each test. From these functions, the detection threshold (72.5% correct choices, binomial test, n = 40, p < 0.01) was determined. A double-exponential function was fitted to the contrast sensitivity data using a method of least squares [29].
3. Results
Three individuals were used in both experiments, but while Harris's hawk B performed in both experiments, we obtained only the CSF for Harris's hawk A, and only the chromatic spatial resolution for Harris's hawk C.
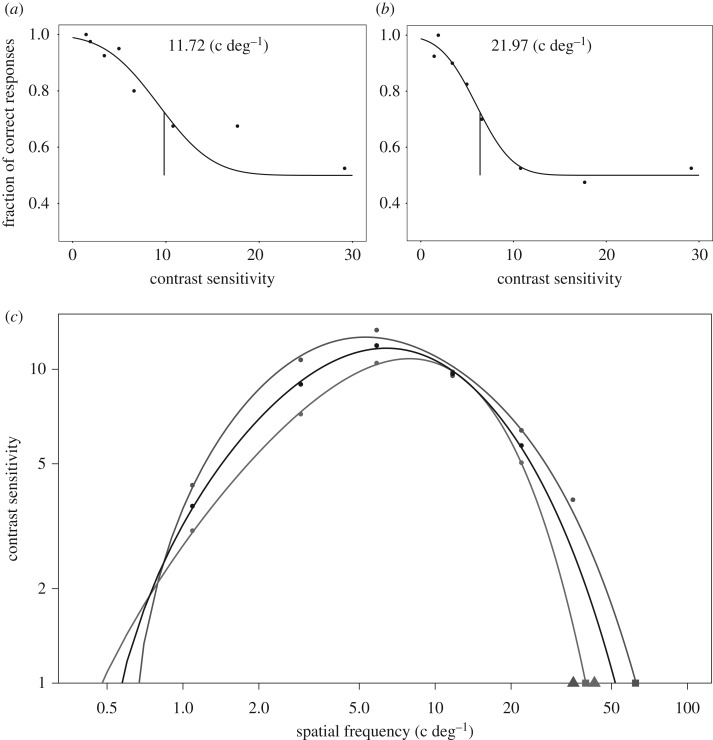
To build the CSF, the contrast sensitivity threshold was interpolated for each spatial frequency using psychometric functions (figure 1a,b for examples). Contrast sensitivity is given as the inverse of the stimulus contrast, at which discrimination performance was at threshold level (72.5%; figure 1c). We found maximum contrast sensitivities of 10.8 and 12.7 at spatial frequencies of 7.9 and 5.3 c deg−1 for Harris's hawk A and B, respectively. These sensitivities correspond to Michelson contrasts of 9.3% and 7.9%. The extrapolated spatial resolution at highest contrast (contrast sensitivity = 1) was 39.5 c deg−1 for Harris's hawk A and 62.3 c deg−1 for Harris's hawk B.
Figure 1.
The behavioural contrast sensitivity function of Harris's hawks. (a,b) Examples of two psychometric functions from contrast threshold tests of (a) Harris's hawk A and (b) Harris's hawk B with different spatial frequencies. Each circle represents 40 choices made by one bird. Vertical lines are threshold values interpolated from logistic functions that were fitted to the data. All curves are given in the electronic supplementary material, figure S2. (c) Contrast sensitivity, defined as the inverse of contrast threshold, as a function of spatial frequency. Sensitivity values were fitted to a double exponential function (see methods). Red, Harris's hawk A; blue, Harris's hawk B; black, the pooled data. Filled squares at the baseline represent the spatial resolution threshold extrapolated from the contrast sensitivity function. Triangles represent the spatial resolution threshold of the same two individuals determined in the study of Potier et al. [19]. (Online version in colour.)
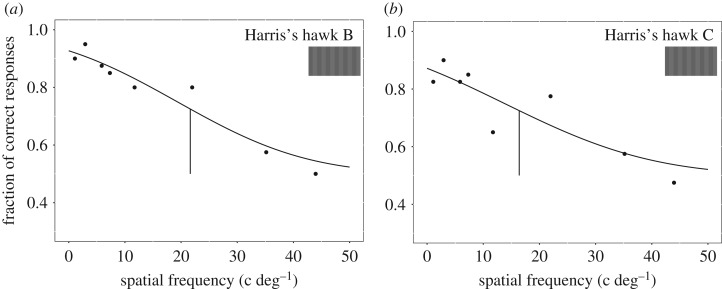
With the red-green gratings, we obtained the spatial resolution of the chromatic channel for Harris's hawks B and C. The threshold was 21.6 c deg−1 for Harris's hawk B and 16.4 c deg−1 for Harris's hawk C (figure 2).
Figure 2.
Psychometric functions used to determine the chromatic spatial resolution of (a) Harris's hawk B and (b) Harris's hawk C. Each circle represents 40 choices made by one bird. Vertical segments are threshold values, which were interpolated from logistic functions that were fitted to the data. (Online version in colour.)
4. Discussion
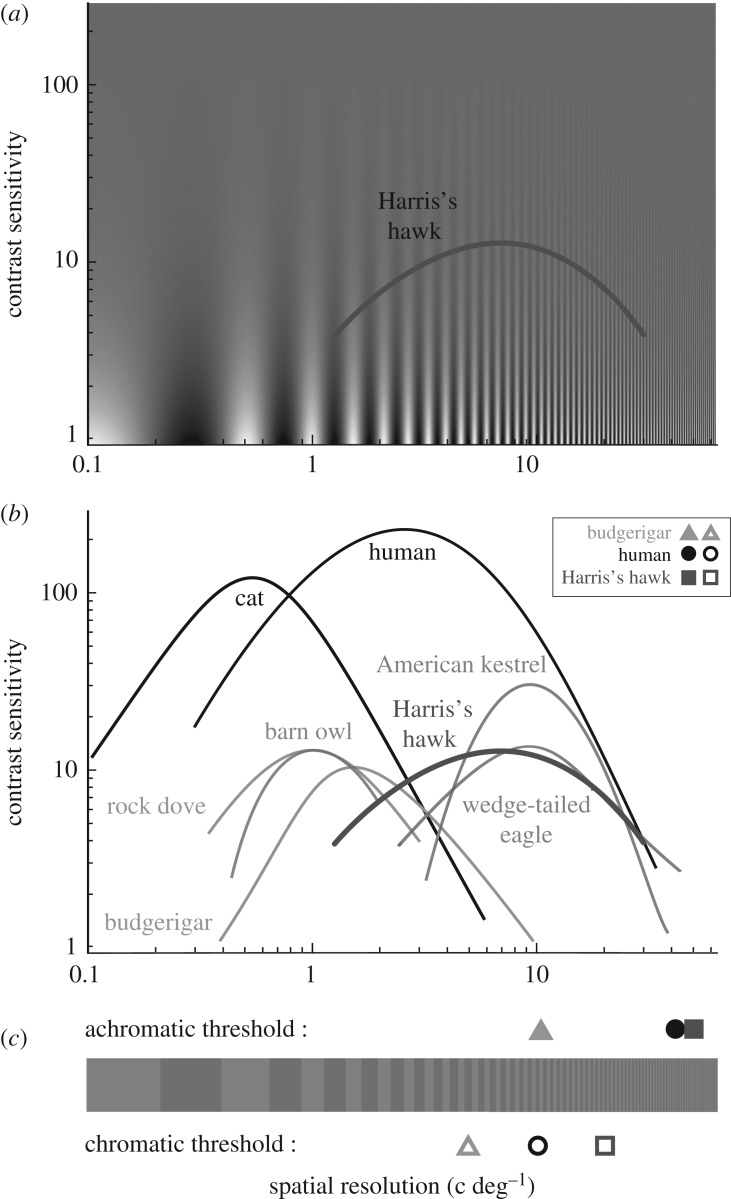
We determined the achromatic CSF function and the spatial resolution of the chromatic (red-green) channel of Harris's hawks, an actively hunting diurnal raptor species. While the highest achromatic spatial resolution of Harris's hawks (40–60 c deg−1) is in a similar range as in humans [21], the highest contrast sensitivity (11–12) is approximately ten times lower than that in humans [21], and the resolution for red-green gratings (16–22 c deg−1) is twice as high (figure 3).
Figure 3.
Comparison of the vision of Harris's hawks and other animals. (a) Contrast sensitivity of Harris's hawks (average of two birds). (b) Comparison of the contrast sensitivity function of Harris's hawks (red-brown) and other animals (diurnal raptors in blue, nocturnal raptor in green, non-raptorial birds in pink and human in black). The spatial resolution thresholds of achromatic (filled symbols) vision are represented for budgerigars, humans and Harris's hawks. (c) Spatial resolution thresholds of chromatic vision of budgerigars, humans and Harris's hawks (open symbols). References: humans [8,21], American kestrel [15], wedge-tailed eagle [14], barn owl [30] rock dove [31] and budgerigar [9]. (Online version in colour.)
(a). Achromatic contrast sensitivity function
The shape of the CSF in Harris's hawks is similar to that of other raptors [14,15] and other vertebrates tested so far [9,29,30,32,33]. The maximum contrast sensitivity of 12.7 is close to the CS found in the most closely related species studied to date, the wedge-tailed eagle (13.6) [14], but lower than in the American kestrel (≈30) [15]. While the maximum contrast sensitivity of these birds occurred at a spatial resolution of ≈10 c deg−1 [14,15], the maximum contrast sensitivity of Harris's hawks was found at 5.3 and 7.9 c deg−1. This suggests that maximum contrast sensitivity and visual acuity are not directly related in raptors. It is unclear why raptors—and generally all birds tested so far—have such low contrast sensitivity, but it has been suggested that birds may trade contrast sensitivity for other visual abilities, such as chromatic sensitivity [33], which would be in agreement with the high chromatic spatial resolution found in this study.
From the CSF, we extrapolated the maximum resolving power of Harris's hawks. The visual acuity of the same two individuals has also been determined in a previous study [19]. The spatial resolution of 40 to 60 c deg−1 agrees well with an anatomical estimation based on eye size alone [19] or presumed focal length and a hexagonal cone mosaic with cone centre-to-centre distances of 2 to 2.5 µm, which is slightly larger than the 1.6 µm cone centre-to-centre distances determined anatomically in the deep fovea of the wedge-tailed eagle [11]. If all cone types contributed to achromatic vision and no spatial summation took place in Harris's hawks’ fovea, this would indicate relatively moderate maximum densities of ≈200 000 cones mm−2.
For Harris's hawk A, the extrapolated resolving power is similar to the value from an earlier direct measurement of resolution (39.5 versus 42.8 c deg−1 [19]), as has been found by similar extrapolations in other bird species [9,30,34]. By contrast, the results for Harris's hawk B differ markedly (62.3 versus 35.3 c deg−1 [19]), which is surprising. In the previous experiment [19], individuals that made more horizontal head movements before choosing a stimulus showed a higher visual acuity. In that study, Harris's hawk B made very few head movements before each choice (1.6 ± 0.2; mean ± s.e.), suggesting that its visual acuity may have been underestimated. In the present study, Harris's hawk B was more attentive and made more head movements (S.P. 2017, personal observation), which may explain the higher visual acuity threshold. This suggests that readers should rely more on the maximum, not the average of behaviourally determined visual acuity for a species, because individuals differ in attention not only between conditioning experiments, but even from session to session.
Finally, all these values and interpretations need to be taken with caution. Using stimuli with a luminance of 175 cd m−2, we may have slightly underestimated the absolute maximum of resolution. We do not, however, think the difference could be large. In the wedge-tailed eagle, the resolution determined at 200 cd m−2 was 128 c deg−1, compared with 136 c deg−1 at 2000 cd m−2, thus an increase of less than 10% with a 10-fold increase in luminance [11].
(b). Chromatic spatial resolution
In Harris's hawks, similar to budgerigars [9], the spatial resolution of the chromatic channel is lower than that of the achromatic channel. This indicates that they can detect prey providing maximum achromatic contrast to the background from a larger distance than prey only providing chromatic contrast. The chromatic spatial resolution of Harris's hawks for red-green stimuli with high colour contrast is the highest found to date among animals, twice as high as measured in humans (below 10 c deg−1) [8] and five times higher than in budgerigars (≈4 c deg−1) [9]. Both previous studies found the same resolution threshold for red-green gratings as for other colour gratings, blue-green for budgerigars [9] and blue-yellow for humans [8]. Although we cannot be sure, we are therefore rather confident that our result is not specific for this particular colour combination, either. Because raptors lack double cones in their fovea, it has recently been suggested that they may have high chromatic spatial resolution [16]. Our study provides the first evidence that this is true for one raptor species.
With the eye the size of Harris's hawks [11], the resolution of ≈20 c deg−1 requires a cone centre-to-centre distance of ≈6 µm. A cone abundance ratio of 1 : 2 : 2 : 4 (VS : SWS : MWS : LWS) and the estimated total density of ≈200 000 cones mm−2 contributing the achromatic resolution would mean that the rarest cone type (VS) would have cone centre-to-centre distances of ≈6 µm and thus determine the chromatic resolution limit. This estimation is rather conservative. As nothing is known about the specific opponent channels underlying bird colour vision, it assumes that resolution of the chromatic channel is limited by the cone type with lowest density in the retina. The red-green gratings did have high contrast for the SWS, MWS and LWS cone types; therefore, we cannot completely exclude the possibility that the threshold is set by some achromatic mechanism involving only single cones.
We used red-green gratings that were isoluminant for the double cones, assuming receptor properties reported in the literature for another accipitriform bird, the common buzzard. However, these assumptions come with some uncertainty. For example, in the visual streak of the wedge-tailed shearwater Puffinus pacificus, the oil droplet coloration is greatly reduced and no yellow or red oil droplets are present [35]. It is unknown whether anything similar is the case in the fovea of raptors. However, even if all oil droplets were transparent (λcut at 300 nm), our stimulus would still generate a high chromatic contrast (10.93 JNDs) and sub-threshold achromatic contrast (8.6%). Therefore, we consider that the red-green grating used in this study is isoluminant for the double cones.
(c). Vision and foraging ecology of Harris's hawks
How do spatial resolution and CS relate to the ecological needs of Harris's hawks? Harris's hawks live mainly in dry environments and forage mainly on mammals [36]. From a foraging perspective, the contrast between prey and background may be important and while the maximum contrast sensitivity of the birds is relatively low, it is certainly sufficient to detect and catch the prey. In addition, while it has been shown that raptors cannot use UV cues for prey detection [17], the high spatial resolution of chromatic vision found in our study suggests a potentially important role of colour vision for foraging. Furthermore, it is possible that contrast sensitivity is higher for moving stimuli, as found in budgerigars [37]. In another raptor species, the American kestrel, prey motion has been found to be a better predictor of prey detection than prey size [38].
Finally, it is important to note that all three species of raptors studied so far for CSF live in open habitats, where–at least on a sunny day—achromatic contrasts, caused for instance by sharp shadows, are higher and thus may be more important than in closed environments (such as dense forest; Dan-E. Nilsson 2018, personal communication). It would be interesting to estimate the CSF of a raptor that lives in a closed habitat to see whether living in a different environment leads to higher contrast sensitivities.
5. Conclusion
Because raptors are considered to be mainly visually guided foragers, and some species have high visual acuity [13], it has long been suggested that raptors have generally superior visual abilities compared to other animals. In this study, we showed that Harris's hawks have the highest chromatic visual acuity threshold found to date, suggesting that they can discriminate an object (e.g. a prey) that is isoluminant but differs in colour from the background at long distance. However, while its achromatic visual acuity is indeed high for its body size, the maximum contrast sensitivity is similar to that of other birds (figure 3; and see [30] for a review). This illustrates that the perfect eye is not necessarily an eye with high performance in every domain, but an eye adapted to the behaviour and ecology of a species [39,40]. For Harris's hawks, this involves having high chromatic and achromatic spatial resolution and relatively low contrast sensitivity.
Supplementary Material
Supplementary Material
Acknowledgements
We thank P. Potier and N. Descarsin from Les Ailes de l'Urga for allowing to perform experiments with their birds. We also thank M. Lieuvin for her help with the fieldwork. Thanks to O. Lind and P. Olsson for the help with modelling, stimulus preparation and fitting of the contrast sensitivity function.
Ethics
The study was conducted under a formal agreement between the animal rearing facility Les Ailes de l'Urga (France) and Lund University (Sweden). In agreement with French law, the birds were handled by their usual trainers under the permit of Les Ailes de l'Urga (national certificate to maintain birds ‘Certificat de capacite’ delivered to the director of the falconry, Patrice Potier, on 20 June 2006).
Data accessibility
The datasets supporting this article have been uploaded electronic supplementary material.
Authors' contributions
S.P., M.M. and A.K. designed the study. S.P. performed the experiments, analysed the data and wrote the manuscript with contributions by all authors.
Competing interests
We have no competing interests.
Funding
This study was financially support by the Swedish Research Council (2016-03298) and the K. & A. Wallenberg Foundation (Ultimate Vision).
References
- 1.Rochon-Duvigneaud A. 1943. Les yeux et la vision des vertébrés. Paris, France: Masson. [PubMed] [Google Scholar]
- 2.Walls GL. 1942. The vertebrate eye and its adaptive radiation. New York, NY: Hafner Publishing Co (fascimile of 1942 edition). [Google Scholar]
- 3.Giurfa M, Vorobyev M, Brandt R, Posner B, Menzel R. 1997. Discrimination of coloured stimuli by honeybees: alternative use of achromatic and chromatic signals. J. Comp. Physiol. A 180, 235–243. ( 10.1007/s003590050044) [DOI] [Google Scholar]
- 4.Osorio D, Miklósi A, Gonda Z. 1999. Visual ecology and perception of coloration patterns by domestic chicks. Evol. Ecol. 13, 673–689. ( 10.1023/A:1011059715610) [DOI] [Google Scholar]
- 5.Spaethe J, Tautz J, Chittka L. 2001. Visual constraints in foraging bumblebees: flower size and color affect search time and flight behavior. Proc. Natl Acad. Sci. USA 98, 3898–3903. ( 10.1073/pnas.071053098) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mitkus M, Potier S, Martin GR, Duriez O, Kelber A. 2018. Raptors vision. In Oxford research encyclopedia of neuroscience. Oxford, UK: Oxford University Press. See http://neuroscience.oxfordre.com. [Google Scholar]
- 7.Olsson P, Lind O, Kelber A. 2018. Chromatic and achromatic vision: parameter choice and limitations for reliable model predictions. Behav. Ecol. 29, 273–282. ( 10.1093/beheco/arx133) [DOI] [Google Scholar]
- 8.Mullen KT. 1985. The contrast sensitivity of human colour vision to red-green and blue-yellow chromatic gratings. J. Physiol. 359, 381–400. ( 10.1113/jphysiol.1985.sp015591) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lind O, Kelber A. 2011. The spatial tuning of achromatic and chromatic vision in budgerigars. J. Vision 11, 1–9. ( 10.1167/11.7.2) [DOI] [PubMed] [Google Scholar]
- 10.Reymond L. 1985. Spatial visual acuity of the eagle Aquila audax: a behavioural, optical and anatomical investigation. Vision Res. 25, 1477–1491. ( 10.1016/0042-6989(85)90226-3) [DOI] [PubMed] [Google Scholar]
- 11.Fischer AB. 1969. Laboruntersuchungen und Freilandbeobachtungen Zum Sehvermögen und Verhalten Von Altweltgeiern. Zool. Jahrb. Syst. 96, 81–132. [Google Scholar]
- 12.Reymond L. 1987. Spatial visual acuity of the falcon, Falco berigora: a behavioural, optical and anatomical investigation. Vision Res. 27, 1859–1874. ( 10.1016/0042-6989(87)90114-3) [DOI] [PubMed] [Google Scholar]
- 13.Jones MP, Pierce KE, Ward D. 2007. Avian vision: a review of form and function with special consideration to birds of prey. J. Exo. Pet. Med. 16, 69–87. ( 10.1053/j.jepm.2007.03.012) [DOI] [Google Scholar]
- 14.Reymond L, Wolfe J. 1981. Behavioural determination of the contrast sensitivity function of the eagle Aquila audax. Vision Res. 21, 263–271. ( 10.1016/0042-6989(81)90120-6) [DOI] [PubMed] [Google Scholar]
- 15.Hirsch J. 1982. Falcon visual sensitivity to grating contrast. Nature 300, 57–58. ( 10.1038/300057a0) [DOI] [Google Scholar]
- 16.Mitkus M, Olsson P, Toomey MB, Corbo JC, Kelber A. 2017. Specialized photoreceptor composition in the raptor fovea. J. Comp. Neurol. 525, 2152–2163. ( 10.1002/cne.24190) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lind O, Mitkus M, Olsson P, Kelber A. 2013. Ultraviolet sensitivity and colour vision in raptor foraging. J. Exp. Biol. 216, 1819–1826. ( 10.1242/jeb.082834) [DOI] [PubMed] [Google Scholar]
- 18.Martin G, Osorio D. 2008. Vision in birds. In The senses: a comprehensive reference, vol. 1 (eds Basbaum AI, Kaneko A, Shepherd GM, Westheimer G), pp. 25–52. Amsterdam, The Netherlands: Elsevier. [Google Scholar]
- 19.Potier S, Bonadonna F, Kelber A, Martin GR, Isard P-F, Dulaurent T, Duriez O. 2016. Visual abilities in two raptors with different ecology. J. Exp. Biol. 291, 2639–2649. ( 10.1242/jeb.142083) [DOI] [PubMed] [Google Scholar]
- 20.Potier S, Mitkus M, Bonadonna F, Duriez O, Isard P-F, Dulaurent T, Mentek M, Kelber A. 2017. Eye size, fovea, and foraging ecology in accipitriform raptors. Brain Behav. Evol. 90, 232–242. ( 10.1159/000479783) [DOI] [PubMed] [Google Scholar]
- 21.Berkley M. 1976. Cat visual psychophysics: Neural correlates and comparison with man. In Progress in psychobiology and physiological psychology, vol. 6 (eds Sprague J, Epsteine A), pp. 63–119. London, UK: Academic Press. [Google Scholar]
- 22.Vorobyev M, Osorio D. 1998. Receptor noise as a determinant of colour thresholds. Proc. R. Soc. Lond. B 265, 351–358. ( 10.1098/rspb.1998.0302) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ödeen A, Håstad O. 2003. Complex distribution of avian color vision systems revealed by sequencing the SWS1 opsin from total DNA. Mol. Biol. Evol. 20, 855–861. ( 10.1093/molbev/msg108) [DOI] [PubMed] [Google Scholar]
- 24.Hart NS, Vorobyev M. 2005. Modelling oil droplet absorption spectra and spectral sensitivities of bird cone photoreceptors. J. Comp. Physiol. A 191, 381–392. ( 10.1007/s00359-004-0595-3) [DOI] [PubMed] [Google Scholar]
- 25.Govardovskii VI, Fyhrquist N, Reuter T, Kuzmin DG, Donner K. 2000. In search of the visual pigment template. Vis. Neurosci. 17, 509–528. ( 10.1017/S0952523800174036) [DOI] [PubMed] [Google Scholar]
- 26.Michelson A. 1927. Studies in optics. Chicago, IL: University of Chicago Press. [Google Scholar]
- 27.Knoblauch K. 2007. psyphy: Functions for analyzing psychophysical data in R. R package version 00–5. See http://cran/R-project.org/package=psyphy.
- 28.Wickham H, Chang W. 2014. ggplot2: an implementation of the grammar of graphics. See www.rdocumentation.org/packages/ggplot2/versions/0.9.0.
- 29.Uhlrich DJ, Essock EA, Lehmkuhle S. 1981. Cross-species correspondence of spatial contrast sensitivity functions. Behav. Brain Res. 2, 291–299. ( 10.1016/0166-4328(81)90013-9) [DOI] [PubMed] [Google Scholar]
- 30.Harmening WM, Nikolay P, Orlowski J, Wagner H. 2009. Spatial contrast sensitivity and grating acuity of barn owls. J. Vision 9, 13 ( 10.1167/9.7.13) [DOI] [PubMed] [Google Scholar]
- 31.Hodos W, Ghim MM, Potocki A, Fields JN, Storm T. 2002. Contrast sensitivity in pigeons: a comparison of behavioral and pattern ERG methods. Doc. Ophthalmol. 104, 107–118. ( 10.1023/A:1014427615636) [DOI] [PubMed] [Google Scholar]
- 32.De Valois RL, De Valois KK. 1990. Spatial vision. New York, NY: Oxford University Press. [Google Scholar]
- 33.Ghim MM, Hodos W. 2006. Spatial contrast sensitivity of birds. J. Comp. Physiol. A 192, 523–534. ( 10.1007/s00359-005-0090-5) [DOI] [PubMed] [Google Scholar]
- 34.Lind O, Sunesson T, Mitkus M, Kelber A. 2012. Luminance-dependence of spatial vision in budgerigars (Melopsittacus undulatus) and Bourke's parrots (Neopsephotus bourkii). J. Comp. Physiol. A 198, 69–77. ( 10.1007/s00359-011-0689-7) [DOI] [PubMed] [Google Scholar]
- 35.Hart NS. 2004. Microspectrophotometry of visual pigments and oil droplets in a marine bird, the wedge-tailed shearwater Puffinus pacificus: topographic variations in photoreceptor spectral characteristics. J. Exp. Biol. 207, 1229–1240. ( 10.1242/jeb.00857) [DOI] [PubMed] [Google Scholar]
- 36.Del Hoyo J, Elliot A, Sargatal J (eds). 1994. Handbook of the birds of the world. vol. 2: New world vultures to Guineafowl. Barcelona, Spain: Lynx Editions. [Google Scholar]
- 37.Haller NK, Lind O, Steinlechner S, Kelber A. 2014. Stimulus motion improves spatial contrast sensitivity in budgerigars (Melopsittacus undulatus). Vision Res. 102, 19–25. ( 10.1016/j.visres.2014.07.007) [DOI] [PubMed] [Google Scholar]
- 38.Sarno R, Gubanich A. 1995. Prey selection by Wild American Kestrels-the influence of prey size and activity. J. Rapt. Res. 29, 123–126. [Google Scholar]
- 39.Land MF, Nilsson D-E. 2012. Animal eyes. Oxford, UK: Oxford University Press. [Google Scholar]
- 40.Martin GR. 2017. The sensory ecology of birds. Oxford, UK: Oxford University Press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article have been uploaded electronic supplementary material.