Abstract
Individuals increase lifetime reproductive output through a trade-off between investment in future survival and immediate reproductive success. This pattern may be obscured in certain higher quality individuals that possess greater reproductive potential. The Cassin's auklet (Ptychoramphus aleuticus) is a long-lived species where some individuals exhibit greater reproductive ability through a behaviour called double brooding. Here, we analyse 32 years of breeding histories from marked known-age auklets to test whether double brooding increases lifetime fitness despite the increased mortality and reduced lifespan higher reproductive effort would be expected to incur. Multistate mark–recapture modelling revealed that double brooding was strongly positively associated with higher annual survival and longevity. The mean (95% confidence interval) apparent survival was 0.69 (0.21, 0.91) for individuals that executed a single brood and 0.96 (0.84, 0.99) for those that double-brooded. Generalized linear mixed models indicated individuals that attempted multiple double broods over their lifetime were able to produce on average seven times as many chicks and live nearly 6 years longer than birds that never attempted a double brood. We found that high-quality individuals exhibited both increased reproductive effort and longevity, where heterogeneity in individual quality masked expected life-history trade-offs.
Keywords: double brooding, Cassin's auklet, mark–recapture, individual quality, survival, heterogeneity
1. Introduction
A central tenet of life-history theory predicts individuals are faced with a trade-off between self-maintenance and investment in offspring [1,2]. Those that devote more energy into offspring development early in life are expected to experience a penalty as they age, in the form of earlier senescence and a higher risk of mortality [3–5]. Conversely, individuals that devote more energy into somatic growth and maintenance may increase their odds of surviving into older age, accumulating offspring over a longer period of time. These are sometimes referred to as ‘fast-living’ and ‘slow-living’ strategies, respectively [6,7]. This model of an expected trade-off is challenged in long-lived species inhabiting highly variable environments, where resources are periodically and unpredictably limited. Such environments grant individuals the flexibility in deciding how resources are allocated in a given year, as a means of balancing the costs of reproduction against the odds of future survival.
An approach used by some birds to maximize fecundity during periods of favourable conditions is to double brood, where the same pair attempts two broods in a single season. Double brooding differs from a re-lay or replacement clutch in that the second brood follows the successful fledging of the chick from the first brood, as opposed to following the loss of the initial egg or chick. Species with shorter lifespans (LSs) have been shown to improve overall fitness at the cost of future survival by double brooding in years when resources are plentiful, a strategy used by many passerine species [8–12]. Double brooding is less common among long-lived species that tend to invest in future potential rather than immediate reproductive success [3,7]. Stochastic fluctuations in environmental conditions reduce the odds of producing offspring in some years and increase it in others [13], so individuals with the capacity to adopt double brooding as a life-long breeding strategy when conditions permit should achieve higher overall fitness, provided the costs are not too great.
Cassin's auklets (Ptychoramphus aleuticus) are the only member of the Alcidae and the only seabird in the Northern Hemisphere known to double brood [14–17]. This planktivorous diving seabird breeds on offshore islands from Baja California, Mexico, to the western Aleutian Islands of Alaska. Double brooding has only been reported in the southern portion of its range, with records from San Benito Island in Mexico [14], the Channel Islands off southern California [15] and the Farallon Islands off central California [16–18]. This behaviour has been well documented on the Farallon Islands, where on average 32% of an intensely studied population attempt double brooding in a given year [18]. Double brooding is most prevalent in pairs containing females that are between 8 and 10 years of age [18], particularly during years where upwelling favourable winds lead to enhanced local marine production and improved foraging conditions [18]. Some older females, however, will attempt double brooding even when upwelling is weak and resources limited. This suggests that some combination of higher individual quality and greater breeding experience provides an advantage for certain females, enabling them to successfully invest in reproduction when it would be disadvantageous for a lower quality or less experienced female to do so.
Double brooding possibly leads to an energetic deficit; a major assumption explaining why attempting such a behaviour is uncommon for long-lived species and limited to populations with access to abundant resources [19,20] and to individuals in good physical condition [8]. For Cassin's auklets, both sexes share incubation and chick-rearing duties [21], and pairs that attempt a double brood must extend parental efforts. This prolonged breeding season overlaps with the energetically costly autumn moult, potentially leading to a physiological strain [9,22]. Further compounding this matter is the added burden of greater foraging distances and extended search times during the chick provisioning period [23], as late-season prey becomes depleted by weakening upwelling in the autumn. Potentially of most importance, double brooding may impact future reproductive potential through a deterioration of the physical condition caused by molecular damage or biological ageing [24]. In experimental studies on other avian species, higher reproductive investment through increased brood sizes resulted in molecular damage and lower survival for females, especially for those with low body condition [25,26]. Given these potential costs, it is questionable whether double brooding would maximize lifetime fitness for individuals that can live more than 20 years.
Differences in individual quality probably contribute to observed variability in life-history strategies used by individuals within a population. Long-lived species like most seabirds should favour the ‘slow-living’ strategy of investing more in self-maintenance, particularly when faced with periodic environmental stressors [27]. Yet double brooding, a form of increased immediate reproductive effort is a common ‘fast-living’ approach attempted by some Cassin's auklets. Here, we use 32 years of breeding histories from uniquely marked, known-age auklets monitored on the Farallon Islands to determine whether individuals that double-brooded multiple times during their lives differed from those that had never double-brooded in terms of (i) annual survival, (ii) age-specific survival and overall longevity, and (iii) lifetime reproductive output. We hypothesized that while birds that double brood probably represent a group of higher quality individuals that should exhibit greater annual survival and productivity rates, the added physiological strain of double brooding multiple times over a lifetime would lead to lower long-term survival rates compared to birds that never attempt a double brood.
2. Methods
(a). Data collection
Southeast Farallon Island (SEFI), the southern most island of the Farallon Islands National Wildlife Refuge, is located 30 miles west of San Francisco, California (37°42′ N, 123°00′ W). This small island supports an estimated 20 000 Cassin's auklets (P. Warzybok, M. E. Johns, R. Bradley 2014, unpublished data), a population that has been continuously monitored since 1972 [17,21]. Beginning in 1983, 446 wooden nest-boxes were installed in suitable nesting habitat throughout the island [28]. Auklets using these boxes were recaptured at the start of the breeding season in mid-March every year to record band information and bill depth measurements to determine sex [29]. Boxes containing an active breeding pair were revisited every 5–15 days to note breeding activity, including egg present or lost, chick present or lost, and chick fledged. Although a second brood attempt in a different site is rare for this population (less than 0.01%; M. Johns 2018, unpublished data), all boxes regardless of previous occupation were checked for double brooding activity. Chicks that reached 35 days of age or fully feathered status were considered fledged and were marked with a uniquely numbered United States Geological Survey (USGS) stainless steel leg band. Only breeding histories of known-aged birds banded as chicks that recruited into monitored nest-boxes were used for the analyses presented here. Few pairs contained two known-aged individuals, and not all could be sexed, leading to an unequal sample of males and females.
(b). Lifetime reproductive success
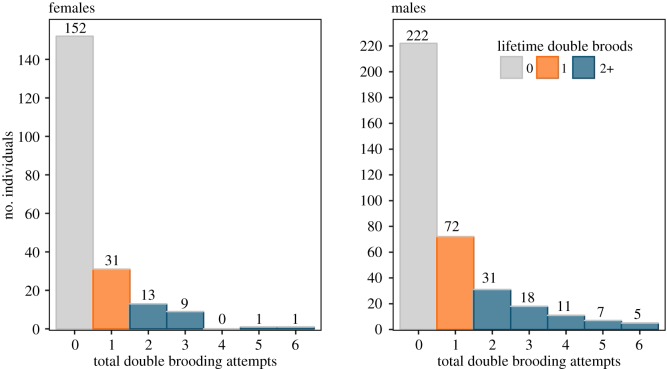
To assess the effects of double brooding on lifetime fitness, several key reproductive parameters were first identified. Lifetime reproductive success (LRS) was calculated by summing the total number of chicks fledged for each known-age parent from 1983 to 2015, including birds that were still alive in 2015. Cassin's auklets lay a single egg per clutch, with the potential for fledging two chicks in a season if a second brood is successful. LS was calculated as the difference between an individual's hatch year and the last year it was seen breeding in a nest-box, because Cassin's auklets are extremely philopatric and have high site fidelity after recruitment [30,31]. Within the LS of each bird, the total number of breeding years (nBY) was calculated as a proxy for total reproductive effort. Regardless of the number of broods attempted, each year where a pair laid at least one egg was counted as a single breeding event. Finally, the total number of double brooding attempts (nDB) observed for each individual across its LS was calculated and binned into three categories: no double brooding attempts (0), one double brooding attempt (1) and two or more double brooding attempts (2+) in a lifetime, the latter referred to as ‘repeat double brooders'. We chose to use a categorical variable over a continuous variable to explain double brooding patterns based on the distribution of lifetime double brooding attempts for all individuals (figure 1), with birds that double-brooded two or more times grouped together to create an adequate sample of repeat double brooders.
Figure 1.
Distribution of total lifetime double brooding attempts (nDB) for individual Cassin's auklets that only attempted single broods (grey), had double-brooded once (orange) or double-brooded two or more times (blue) in their lifetime. Data are not censored for LRS of birds that were still alive in 2015.
Reproductive parameters explaining the variation in LRS were tested using generalized linear mixed-effects models with a Poisson family and log link, and compared with Akaike's information criterion corrected for sample size AICc [32]. Models included the main and pairwise interactive effects of LS, nBY and nDB as predictor variables, all of which were linearly related to the response. Predictors nBY and LS were collinear, both explaining similar variation in LRS, and were not included in the same models. Males and females were compared independently to test whether the mechanisms contributing to an increase in LRS differed between sexes, and to avoid pseudo-replication, given a small proportion of pairs contained two known-age mates. As conditions experienced during the first years of life have been shown to influence future reproductive potential in many seabird species [33,34], parental hatch year was included as a random intercept term to account for heterogeneity among cohorts. Assumptions of normality and homogeneity of variances were confirmed visually with a Q–Q plot of the residuals and a plot of the residuals against the fitted values, respectively, and all predictors within individual models were uncorrelated.
(c). State-specific survival and transition probability
Capture occasions for known-age individuals were assigned to three observable annual states: pre-breeding (A), single brood (B) and double brood (C). Auklets were only observable when found breeding in followed nest-boxes, thus pre-breeders were unobservable from the time they fledged until the time they recruited into a nest-box during their first breeding attempt. For this study, breeding attempt is defined as a pair that succeeded in laying at least one egg. Birds in a single-brooded state only attempted one brood in the given year, while birds in a double-brooded state successfully fledged the first chick and attempted a second brood. A covariate describing the double brooding history of each individual was added to the capture histories, with ‘0’ for birds that never double-brooded, ‘1’ for birds that double-brooded once over a lifetime, and ‘2’ for repeat double brooders. An index of mean upwelling strength (UI) during the months of April through to August for the region surrounding the Farallon Islands, identified as an important environmental driver of double brooding in Cassin's auklets [18], was downloaded from the National Oceanographic and Atmospheric Administration website (www.pfeg.noaa.gov) and included as a covariate to the capture histories. Estimates made from previous studies indicate little difference in survival between male and female Cassin's auklets [35,36]. Additionally, both sexes equally share the energetic burden of incubation and chick-rearing responsibilities [21]. Thus, males, females and unknown sexes were pooled for the multistate analyses to improve model fit and performance by increasing sample sizes of annual recapture events.
Multistate models with the addition of double-brooding history as a grouping variable were fit in the program MARK [37] to generate estimates of apparent survival (ϕ), encounter (p) and transition probabilities (ψ). Parameters of interest were apparent survival probability at time t for both single (B) and double-brooded (C) states (ϕtB,C), and transition probabilities between observable states (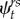 ), where the probability that an individual in state r at time t will be in state s at time t + 1, with r = s indicating that an individual remains in the same state. An unobservable state for birds that skipped a breeding year, had bred in an unmonitored natural burrow, emigrated from the colony, transitioned into a post-breeding state, or died was coded as ‘0’. To account for possible differences in breeding propensity owing to parental age or environmental conditions, encounter probability was allowed to vary by individual age and year to reduce the risk of wrongly attributing these potential sources of variation to apparent survival [38]. Encounter probability was also allowed to vary by state, to insure that variation in apparent survival was not confounded by possible differences in detection between single- and double-brooded pairs. We can assume there is little competition for access to nest-boxes, given there is presumably no difference in chick success between artificial nest-boxes and natural crevices [28], few natural crevices are present within clusters of nest-boxes, and nest-box occupancy generally ranges between 0.2 and 0.8 and rarely reaches full capacity [31]. Additionally, site fidelity is relatively high for established breeders in followed nest-boxes ([39]; electronic supplementary material, figure S4), and there is evidence that these auklets favour mate selection and retention within and between years over site selection [31].
), where the probability that an individual in state r at time t will be in state s at time t + 1, with r = s indicating that an individual remains in the same state. An unobservable state for birds that skipped a breeding year, had bred in an unmonitored natural burrow, emigrated from the colony, transitioned into a post-breeding state, or died was coded as ‘0’. To account for possible differences in breeding propensity owing to parental age or environmental conditions, encounter probability was allowed to vary by individual age and year to reduce the risk of wrongly attributing these potential sources of variation to apparent survival [38]. Encounter probability was also allowed to vary by state, to insure that variation in apparent survival was not confounded by possible differences in detection between single- and double-brooded pairs. We can assume there is little competition for access to nest-boxes, given there is presumably no difference in chick success between artificial nest-boxes and natural crevices [28], few natural crevices are present within clusters of nest-boxes, and nest-box occupancy generally ranges between 0.2 and 0.8 and rarely reaches full capacity [31]. Additionally, site fidelity is relatively high for established breeders in followed nest-boxes ([39]; electronic supplementary material, figure S4), and there is evidence that these auklets favour mate selection and retention within and between years over site selection [31].
A set of candidate models was developed in which apparent survival varied by biologically relevant combinations of breeding state, time, age, double brooding history and mean seasonal upwelling strength along with any meaningful interaction terms. All birds in this study survived to recruitment and were observed breeding in nest-boxes on at least one occasion, thus apparent survival for birds in the pre-breeding state = 1. The oldest recorded Cassin's auklet in this population was 22 years of age, thus apparent and encounter probability beyond age 23 was set to 0, because these sparse data created challenges for model fit. Goodness-of-fit of the fully parameterized model was assessed using median -test, and fits of different models compared with AICc or QAICc depending on the degree of estimated overdispersion. All statistical analyses were carried out in the program R [39]. Mixed-effects models were fitted with the package lme4 [40], and multistate models using the package RMark [41]. Figures were created with the packages ggplot2 [42] and ggridges [43].
-test, and fits of different models compared with AICc or QAICc depending on the degree of estimated overdispersion. All statistical analyses were carried out in the program R [39]. Mixed-effects models were fitted with the package lme4 [40], and multistate models using the package RMark [41]. Figures were created with the packages ggplot2 [42] and ggridges [43].
3. Results
(a). Lifetime reproductive success
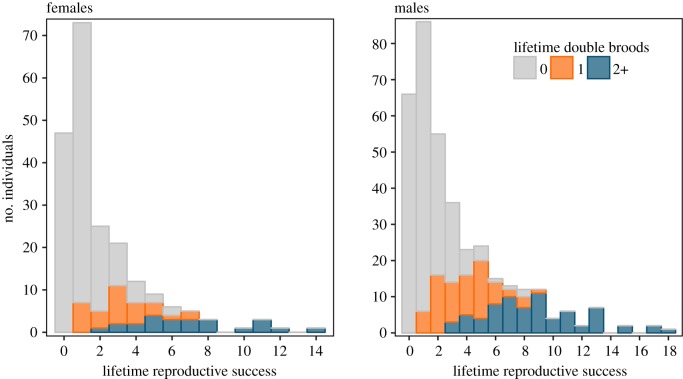
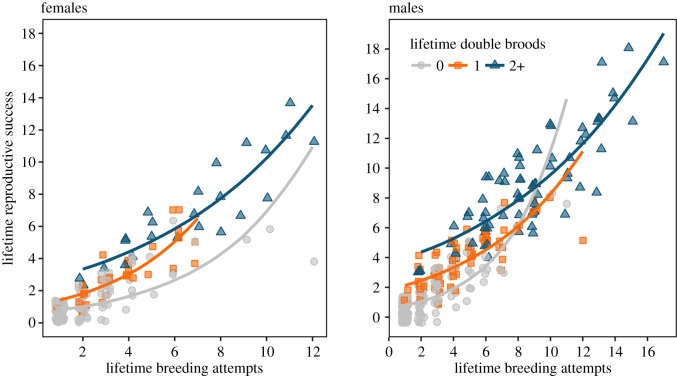
Generalized linear mixed modelling results indicated double brooding was associated with longer LSs and greater LRS. On average, 65% of all followed individuals never double-brooded (n = 152 and 222; females and males, respectively, here and below), with a subset of 18% that double-brooded once (n = 31 and 72), and 17% that double-brooded two or more times (n = 24 and 72; figure 1). Birds that double-brooded multiple times greatly increased the number of offspring produced over a lifetime compared to those that never double-brooded (electronic supplementary material, figure S1). Individuals that double-brooded once produced on average 2.58 (females (F)) to 2.78 (males (M)) times as many chicks and lived 2.3 (F) to 3 (M) years longer than birds that never double-brooded, while repeat double brooders produced on average 5.8 (F) to 7.4 (M) times as many chicks and lived 5.4 (F) to 7.4 (M) years longer (electronic supplementary material, table S1; figure 2). The model explaining LRS as the interaction between nBY and number of lifetime double brooding attempts (nDB) received the most support (ωi = 0.99, n = 366 for males; ωi = 0.86, n = 207 for females; table 1). The importance of the interaction between total breeding attempts and double brooding history appears to be driven by the influence of birds that only double-brooded once in a lifetime, which increased their total fledging success at a higher rate than single brooders (figure 3).
Figure 2.
Distribution of LRS for individual Cassin's auklets that only attempted single broods (grey), had double-brooded once (orange), or double-brooded two or more times (blue) in their lifetime. Data shown are not censored for birds that were still alive in 2015.
Table 1.
Selection results for generalized linear mixed models to determine the effect of total breeding attempts (nBY), breeding LS and lifetime double brooding covariate (nDB; 0, 1 and 2 or more lifetime double broods) on LRS. (All models contained a random intercept for cohort hatch year. Models ranked by lowest ΔAICc, with corresponding number of parameters (K) and ΔAICc weights (ωi).)
| females |
males |
|||||
|---|---|---|---|---|---|---|
| model | ΔAICc | K | ωi | ΔAICc | K | ωi |
| ∼ nDB × nBY | 0 | 7 | 0.86 | 0 | 7 | 1 |
| ∼ nDB + nBY | 3.6 | 5 | 0.14 | 56.5 | 5 | 0 |
| ∼ nDB | 34.8 | 3 | 0 | 260.1 | 3 | 0 |
| ∼ nDB × LS | 48.7 | 7 | 0 | 73.2 | 7 | 0 |
| ∼ nDB + LS | 48.5 | 5 | 0 | 98.1 | 5 | 0 |
| ∼ LS | 100.1 | 3 | 0 | 195.9 | 3 | 0 |
| ∼ nBY | 106.7 | 4 | 0 | 136.6 | 4 | 0 |
Figure 3.
Response curves of the top generalized linear mixed model for LRS as a function of nBY and double brooding attempts (0, 1 and 2 or more lifetime double broods) for male and female Cassin's auklets. Raw data are plotted with a jitter to show clustered points.
(b). State-specific survival and transition probability
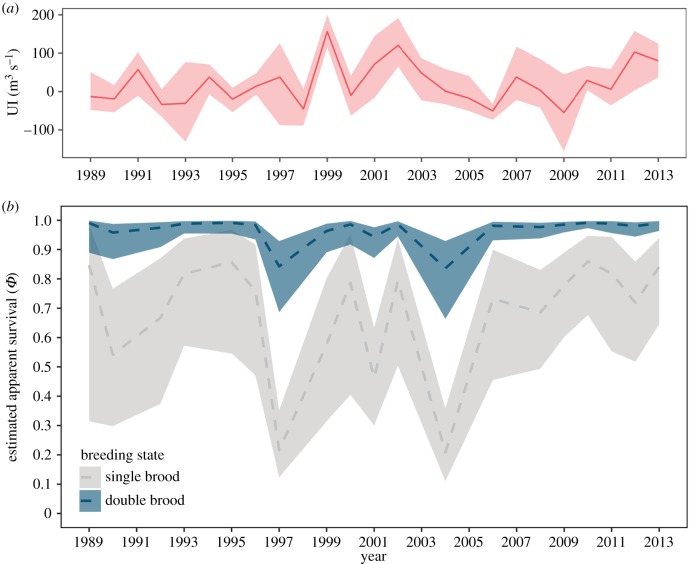
Overdispersion in the fully parameterized model ( = 1.49) was accounted for by comparing model fits with QAICc. Modelling apparent survival as a function of state, time and an interaction between age and double brooding history received the most support of the models tested (table 2). Following years in which individuals attempted only a single brood, repeat double brooders had higher age-specific survival than birds that never double-brooded and had a greater chance of living into old age (figure 4). Birds that double-brooded once in their lifetime had slightly higher survival early in life than single brooders; however, survival later in life matched that of birds that never double-brooded. Survival estimates across all age groups were slightly reduced following years in which they attempted a double brood, just below the lower 95% confidence interval (CI) for estimates when in a single-brooded state (figure 4). Estimated annual survival was lower and more variable for birds following a single-brooded season with a mean (95% CI) of 0.69 (0.21, 0.91), compared to higher less variable estimates of survival for birds following a double-brooded season with a mean of 0.96 (0.84, 0.99) (figure 5b). Individuals were more likely to remain in their respective breeding state the following year rather than transition to a different state, with a probability for staying a single brooder (± s.e.) of 0.58 (± 0.04), and probability of staying a double brooder of 0.64 (± 0.03; electronic supplementary material, figure S2). Estimated encounter probabilities for all ages across all years for both breeding states were close to 1, confirming that detection in nest-boxes was equally high for both single and double-brooded pairs except during years characterized by extremely poor environmental conditions.
= 1.49) was accounted for by comparing model fits with QAICc. Modelling apparent survival as a function of state, time and an interaction between age and double brooding history received the most support of the models tested (table 2). Following years in which individuals attempted only a single brood, repeat double brooders had higher age-specific survival than birds that never double-brooded and had a greater chance of living into old age (figure 4). Birds that double-brooded once in their lifetime had slightly higher survival early in life than single brooders; however, survival later in life matched that of birds that never double-brooded. Survival estimates across all age groups were slightly reduced following years in which they attempted a double brood, just below the lower 95% confidence interval (CI) for estimates when in a single-brooded state (figure 4). Estimated annual survival was lower and more variable for birds following a single-brooded season with a mean (95% CI) of 0.69 (0.21, 0.91), compared to higher less variable estimates of survival for birds following a double-brooded season with a mean of 0.96 (0.84, 0.99) (figure 5b). Individuals were more likely to remain in their respective breeding state the following year rather than transition to a different state, with a probability for staying a single brooder (± s.e.) of 0.58 (± 0.04), and probability of staying a double brooder of 0.64 (± 0.03; electronic supplementary material, figure S2). Estimated encounter probabilities for all ages across all years for both breeding states were close to 1, confirming that detection in nest-boxes was equally high for both single and double-brooded pairs except during years characterized by extremely poor environmental conditions.
Table 2.
Selection results for top multistate models for the effect of reproductive state (single- or double-brooded), lifetime double brooding covariate (nDB; 0, 1 and 2 or more lifetime double broods), time (t), age of parent (age) and upwelling strength index (UI), on apparent survival. (Models ranked by lowest ΔQAICc, with corresponding number of estimable parameters (K) and ΔQAICc weights (ωi). Only the top nine and null models are shown. In all models, encounter probability was parametrized as p(state + t + age) and transition probability as (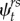 ); the probability an individual in state r at time t will be in state s at time t
+ 1.)
); the probability an individual in state r at time t will be in state s at time t
+ 1.)
| multistate model | ΔQAICc | K | ωi |
|---|---|---|---|
| ϕ (state + t + age × nDB) | 0 | 100 | 0.63 |
| ϕ (state + t + UI + age × nDB) | 2.2 | 101 | 0.21 |
| ϕ (state + t + age + nDB) | 3.5 | 98 | 0.11 |
| ϕ (state + t + UI + age + nDB) | 5.7 | 99 | 0.04 |
| ϕ (state + age + nDB) | 8.3 | 68 | 0.01 |
| ϕ (state + age × nDB) | 9.2 | 70 | 0.01 |
| ϕ (state + t + age × UI + age × nDB) | 90.5 | 103 | 0 |
| ϕ (state + t + nDB) | 95.6 | 97 | 0 |
| ϕ (state + t + UI + nDB) | 97.3 | 98 | 0 |
| ϕ (constant) | 496.8 | 63 | 0 |
Figure 4.
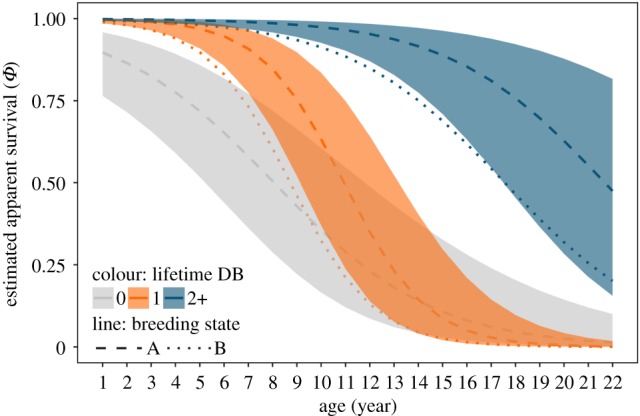
Response curves for modelling apparent survival as a function of the interaction between lifetime double brooding attempts (0, 1 and 2 or more) and age for pooled and unknown sexes, while holding time constant at 2008 (an average year). Shaded ribbons indicated 95% confidence intervals. Dashed lines represent survival of birds following a year where they executed a single brood (state A), dotted lines for survival of birds following double broods (state B). Confidence intervals for estimates of a double-brooded state overlap with those in a single-brooded state, and are not shown.
Figure 5.
Annual survival estimates for Cassin's auklets from SEFI in relation to annual upwelling conditions. (a) Mean (red line) spring upwelling strength (UI) anomalies for the waters surrounding the Farallon Islands. Shaded ribbon indicates annual maximum and minimum values for the months of April–August. (b) Response curves for modelling apparent survival as a function of time, for birds that had never double-brooded in a single-brooded state (grey), and repeat double brooders in a double-brooded state (blue), while holding age constant at 5 years. Shaded ribbons indicated 95% confidence intervals.
4. Discussion
We used an extensive 32 year dataset on the breeding history of Cassin's auklets to describe variation in individual heterogeneity for a long-lived vertebrate species, identifying an uncommon behaviour in seabirds as evidence of higher quality. Individuals that executed double broods multiple times during their lives were able to maximize reproductive output by producing more offspring than did single-brooded birds, while also showing higher survival rates even in the oldest individuals; contradicting the expected ‘fast-living’ model of early mortality predicted for repeat double brooders. For both males and females, the average age at first reproduction was 3 years for single and repeat double brooders (electronic supplementary material, figure S3), so assumed higher quality individuals were not simply delaying the costs of reproduction by recruiting later in life. While the likelihood of double brooding multiple times in a lifetime does increase with age, it is important to note that only roughly 60% of these older auklets will attempt double brooding in a given year [18], suggesting the accumulation of total lifetime double brooding attempts is probably a measure of experience and quality rather than simply a correlate with LS. Birds that survived the first few years of life, lived long enough to develop the skills and experience necessary to be successful breeders, and retained the ability to cope with the added energetic demands of double brooding, were able to produce a greater number of offspring during the same nBY than birds that never attempted a double brood. If these repeat double brooders survived beyond 10 years of age and accumulated enough successful double brooding attempts, they were able to achieve substantially higher lifetime reproductive output than single brooders (electronic supplementary material, figure S1).
Capture mark–recapture results were based on data from known-aged birds banded as chicks that successfully recruited into followed nest-boxes, and did not include unbanded birds or those that bred in non-followed natural burrows. These data were strongly male biased, as female Cassin's auklets tend to disperse further from their natal site than males [28]. The focus for the multistate analysis was to compare survival among birds in known breeding states, thus including all birds would not have been appropriate. We suspect that a higher proportion of low-quality individuals died before recruiting into the breeding population, making our estimates of apparent survival for assumed low- and high-quality breeders conservative. We could not separate true mortality from permanent emigration or movement to non-followed natural burrows with our model, and there is no way of knowing how many birds moved out of nest-boxes. There is, however, no basis for assuming that repeat double brooders switch nest sites more or less than lifetime single brooders (electronic supplementary material, figure S4), and we argue the pattern of higher survival for repeat double brooders is not driven by differences in detection or differential emigration.
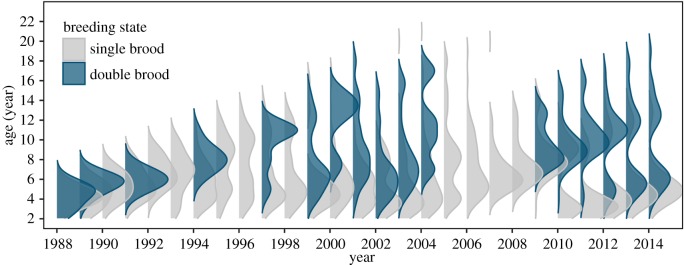
Our estimates of apparent survival for Cassin's auklets on SEFI match those of previous mark–recapture work [35]; however, these studies did not include estimates for specific breeding states. Modelling results presented here suggest apparent annual survival between and within breeding states is partially a product of year effects related to environmental conditions and the age structure of the breeding population. While a positive relationship between adult survival and prey availability driven by favourable upwelling conditions has been described for Cassin's auklets in British Columbia [36], upwelling was not selected as a relevant term in our top model. This effect instead was likely captured by the year term, with major reductions in the survival of single-brooded birds generally during years characterized by weak upwelling (figure 5a), particularly in the years 1997–1998 and 2005. Notably, repeat double brooders still showed a reduction in survival following years in which they attempted a second brood (figure 4), indicating such behaviour does in fact incur a small immediate cost. Estimates of apparent survival were still higher, however, for repeat double brooders following a year in which they double-brooded than were for birds that never double-brooded, even when conditions were less favourable. This shows that repeat double brooders were better able to recover physiologically from the increased stress incurred by producing two broods in a single season, suggesting some degree of unobserved individual heterogeneity [44]. In addition to reduced survival in poor years, the absence of potential new recruits following a complete reproductive failure in 2005 moved as a demographic gap in the age structure of the population in subsequent years, resulting in no individuals of prime breeding age between 2009 and 2012 (figure 6).
Figure 6.
Age structure of the single-brooded (grey) and double-brooded (blue) known-age breeding population in followed nest-boxes, where the width of each density curve indicates the relative number of individuals (n) of a specific age group in a given year.
Auklets that attempt double brooding appear to be physiologically superior to those that never adopt such a behaviour. Some individuals enter the breeding season with greater energy reserves than others, allowing them to both lay earlier and retain the physiological capacity to produce a late season second brood. The fact that some birds attempt multiple double broods throughout their lives with no increase in long-term mortality risk suggests these higher quality individuals are able to delay the effects of biological ageing; either through heritable or derived traits. Given the irregular pattern in the annual proportion of double brooding attempts (figure 6), it may be that early development plays a role in determining which individuals become repeat double brooders and which do not. Auklets exposed to good environmental conditions and thus, lower stress levels during the first few years of life may have a higher capacity to incur biological damage as they age [3,33], allowing for more ‘risky’ high-effort behavioural decisions like double brooding later in life.
The underlying behavioural mechanisms that allow repeat double brooders to achieve both greater reproductive output and longevity are unclear, and remain an open area of investigation. It has been suggested there is a large proportion of prospecting Cassin's auklets on the Farallon Islands [45], at least during periods when the population was estimated to be much larger, providing an evolutionary incentive for pairs to defend their burrows from prospecting individuals by extending occupation of their sites through double brooding [17]. This would be particularly beneficial if late season marine productivity was high enough to allow for an additional breeding attempt. Monogamy is another common behavioural feature in seabirds that has been linked to breeding success for many species [46,47], including the Cassin's auklet [31]. While pair bond duration was not directly addressed in this study, it appears there is no difference in mate fidelity between single and repeat double brooders (electronic supplementary material, figure S4). Ultimately, females bear the physiological burden of producing two eggs in a single season, so it is probably more important that a pair contain an experienced and, as suggested here, higher quality female.
Regardless of the specific causal mechanisms, it is logical to assume that double brooding is an important behaviour at the population level. Going forward, a more applied assessment of how chicks from second broods contribute to the resiliency of this population to the future impacts of climate change, by buffering against years of poor environmental conditions, may prove valuable. The success rate of chicks from double broods can be fairly high, particularly for older females during years of strong upwelling [18]. Most single-brooded birds appear to drop out of the breeding population early, and only produce a single offspring in their lifetime. Future survival and population analyses of Cassin's auklets should not only take into account the age structure of known breeders, but also an estimate of the proportion of repeat double brooders present, in order to provide a more accurate assessment of the trajectory of this species in the face of the threats associated with a warming ocean.
This observational study adds to the broader theoretical discussion of disentangling individual quality from life-history trade-offs using long-term breeding records of marked individuals. Mounting evidence from a robust body of work spanning a range of vertebrate taxa, including birds [3,48,49], mammals [50–52] and reptiles [53], have confirmed individuals that intensify their reproductive effort early in life show a steeper decline in survival and overall shorter LSs compared to individuals that moderate the allocation of reproductive effort over a longer time period. Female Cassin's auklets that attempt double brooding on the Farallon Islands are typically between 8 and 10 years of age [18], middle-aged for birds that can live to at least 22 years. Yet double brooding, a clear example of intensifying immediate reproductive effort, is also associated with much higher LRS, greater annual survival and longer LSs. This correlation between repeat double brooding and longevity can only be explained by some degree of higher individual quality. Our results show that variation in individual quality can translate into a positive relationship between intense reproductive investment and future survival in a long-lived bird species, aligning with studies that have demonstrated heterogeneity among individuals can mask the underlying trade-off between offspring investment and self-maintenance that exists within individuals [54,55].
Supplementary Material
Acknowledgments
We thank Point Blue Conservation Science biologists and volunteers who collected these data, and the US Fish and Wildlife Service for granting permission and providing resources to conduct research on the Farallon Islands National Wildlife Refuge.
Ethics
Work was conducted on the Farallon National Wildlife Refuge with permission and approval of the US Fish and Wildlife Service. This study was conducted under the terms of Cooperative Agreement no. F14AC00237 (2014-2019). Banding procedures were carried out under Point Blue Conservation Science's Federal Banding Permit (no. 09316).
Data accessibility
Data and R code supporting the results of this manuscript are stored on Dryad: http://dx.doi.org/10.5061/dryad.7cp7088 [56].
Authors' contributions
J.J., P.W., R.W.B., M.L. and G.A.B. assisted in formulating objectives, M.L. and G.B. supported analyses, P.W., R.W.B., M.L. and G.A.B. provided feedback on writing, M.E.J. developed objectives, performed analyses and wrote the paper.
Competing interests
We have no competing interests.
Funding
Funders for Point Blue's Farallon Research Program include the Bently Foundation, Elinor Patterson Baker Trust, Marisla Foundation, Giles W. and Elise G. Mead Foundation, Frank A. Campini Foundation, Bernice Barbour Foundation, Kimball Foundation, RHE Charitable Foundation, Volgenau Foundation and individual donors. Additional funding provided by the Betty A. Anderson Memorial for Avian Studies scholarship and Calvin J. Lensink graduate fellowship. This is Point Blue Contribution number 2145.
References
- 1.Williams GC. 1957. Pleiotropy, natural selection, and the evolution of senescence. Evolution 11, 398–411. ( 10.1111/j.1558-5646.1957.tb02911.x) [DOI] [Google Scholar]
- 2.Clutton-Brock TH. 1988. Reproductive success: studies of individual variation in contrasting breeding systems. Chicago, IL: University of Chicago Press. [Google Scholar]
- 3.Reed TE, Kruuk LEB, Wanless S, Frederiksen M, Cunningham EJA, Harris MP. 2008. Reproductive senescence in a long-lived seabird: rates of decline in late-life performance are associated with varying costs of early reproduction. Am. Nat. 171, E89–E101. ( 10.1086/524957) [DOI] [PubMed] [Google Scholar]
- 4.Schaffer WM. 1974. Optimal reproductive effort in fluctuating environments. Am. Nat. 108, 783–790. ( 10.1086/282954) [DOI] [Google Scholar]
- 5.Aubry LM, Cam E, Koons DN, Monnat J, Pavard S. 2011. Drivers of age-specific survival in a long-lived seabird: contributions of observed and hidden sources of heterogeneity. J. Anim. Ecol. 80, 375–383. ( 10.1111/j.1365-2656.2010.01784.x) [DOI] [PubMed] [Google Scholar]
- 6.Promislow D, Harvey P. 1990. Living fast and dying young: a comparative analysis of life-history variation among mammals. J. Zool. Lond. 220, 417–437. ( 10.1111/j.1469-7998.1990.tb04316.x) [DOI] [Google Scholar]
- 7.Stearns SC. 1992. The evolution of life histories. Oxford, UK: Oxford University Press. [Google Scholar]
- 8.O'Brien EL, Dawson RD. 2013. Experimental dissociation of individual quality, food and timing of breeding effects on double-brooding in a migratory songbird. Oecologia 172, 689–699. ( 10.1007/s00442-012-2544-0) [DOI] [PubMed] [Google Scholar]
- 9.Ogden LJE, Stutchbury BJM. 1996. Constraints on double brooding in a neotropical migrant, the hooded warbler. Condor 98, 736–744. ( 10.2307/1369855) [DOI] [Google Scholar]
- 10.Norris K. 1993. Seasonal variation in the reproductive success of blue tits: an experimental study. J. Anim. Ecol. 62, 287–294. ( 10.2307/5360) [DOI] [Google Scholar]
- 11.Nagy LR, Holmes RT. 2005. To double-brood or not? Individual variation in the reproductive effort in black-throated blue warblers (Dendroica caerulescens). Auk 122, 902–914. ( 10.1642/0004-8038(2005)122%5B0902:TDONIV%5D2.0.CO;2) [DOI] [Google Scholar]
- 12.Hoffmann J, Postma E, Schaub M. 2015. Factors influencing double brooding in Eurasian hoopoes (Upupa epops). Ibis (Lond. 1859). 157, 17–30. ( 10.1111/ibi.12188) [DOI] [Google Scholar]
- 13.Newton I. 1989. Lifetime reproduction in birds. London, UK: Academic Press. [Google Scholar]
- 14.Wolf SG, Sydeman WJ, Hipfner JM, Abraham CL, Tershy BR, Croll DA. 2009. Range-wide reproductive consequences of ocean climate variability for the seabird Cassin's auklet. Ecology 90, 742–753. ( 10.1890/07-1267.1) [DOI] [PubMed] [Google Scholar]
- 15.Adams J, Mazurkiewicz D, Harvey AL. 2014. Population monitoring and habitat restoration for Cassin's auklets at Scorpion Rock and Prince Island, Channel Islands National Park, California: 2009–2011. Interim data summary report. US Geological Survey, Western Ecological Research Center, Santa Cruz Field Station, Pacific Coastal Marine Science Center, Santa Cruz, CA and Channel Islands National Park, Ventura, CA. Interim data summary report to Montrose Settlement Restoration Project Trustee Council.
- 16.Manuwal DA. 1979. Reproductive commitment and success of Cassin's auklet. Condor 81, 111–121. ( 10.2307/1367275) [DOI] [Google Scholar]
- 17.Ainley DG, Boekelheide RJ. 1990. Seabirds of the Farallon Islands. Ecology, dynamics, and structure of an upwelling-system community. Stanford, CA: Stanford University Press. [Google Scholar]
- 18.Johns ME, Warzybok P, Bradley RW, Jahncke J, Lindberg M, Breed GA. 2017. Age, timing, and a variable environment affect double brooding of a long-lived seabird. Mar. Ecol. Prog. Ser. 564, 187–197. ( 10.3354/meps11988) [DOI] [Google Scholar]
- 19.Moore DJ, Morris RD. 2005. The production of second clutches in the common tern: proximate effects of timing and food supply. Waterbirds 28, 458–467. ( 10.1675/1524-4695(2005)28%5B458:TPOSCI%5D2.0.CO;2) [DOI] [Google Scholar]
- 20.Husby A, Kruuk LEB, Visser ME. 2009. Decline in the frequency and benefits of multiple brooding in great tits as a consequence of a changing environment. Proc. R. Soc. B 276, 1845–1854. ( 10.1098/rspb.2008.1937) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manuwal DA. 1974. The natural history of Cassin's auklet (Ptychoramphus aleuticus). Condor 76, 421–431. ( 10.2307/1365815) [DOI] [Google Scholar]
- 22.Emslie SD, Henderson RP, Ainley DG. 1990. Annual variation of primary molt with age and sex in Cassin's auklet. Auk 107, 689–695. ( 10.2307/4087999) [DOI] [Google Scholar]
- 23.Brown CR, Roche EA, Brien VAO. 2015. Costs and benefits of late nesting in cliff swallows. Oecologia 177, 413–421. ( 10.1007/s00442-014-3095-3) [DOI] [PubMed] [Google Scholar]
- 24.Selman C, Blount JD, Nussey DH, Speakman JR. 2012. Oxidative damage, ageing, and life-history evolution: where now? Trends Ecol. Evol. 27, 570–577. ( 10.1016/j.tree.2012.06.006) [DOI] [PubMed] [Google Scholar]
- 25.Reichert S, Stier A, Zahn S, Arrivé M, Bize P. 2014. Increased brood size leads to persistent eroded telomeres. Front. Ecol. Evol. 2, 1–11. ( 10.3389/fevo.2014.00009) [DOI] [Google Scholar]
- 26.Cichon M, Olejniczak P, Gustafsson L. 1998. The effect of body condition on the cost of reproduction in female collared flycatchers Ficedula albicollis. Ibis (Lond. 1859) 140, 128–130. ( 10.1111/j.1474-919X.1998.tb04549.x) [DOI] [Google Scholar]
- 27.Schultner J, Kitaysky AS, Gabrielsen GW, Hatch SA, Bech C. 2013. Differential reproductive responses to stress reveal the role of life-history strategies within a species. Proc. R. Soc. B 280, 8–11. ( 10.1098/rspb.2013.2090) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pyle P. 2001. Age at first breeding and datal dispersal in a declining population of Cassin's auklet. Auk 118, 996–1007. ( 10.1642/0004-8038(2001)118) [DOI] [Google Scholar]
- 29.Nelson DA. 1981. Sexual differences in measurements of Cassin's auklet. J. F. Ornithol. 52, 233–234. [Google Scholar]
- 30.Lee DE, Warzybok PM, Bradley RW. 2012. Recruitment of Cassin's auklet (Ptychoramphus aleuticus): individual age and parental age effects. Auk 129, 124–132. ( 10.1525/auk.2012.10224) [DOI] [Google Scholar]
- 31.Pyle P, Sydeman WJ, Hester M. 2001. Effects of age, breeding experience, mate fidelity and site fidelity on breeding performance in a declining population of Cassin's auklets. J. Anim. Ecol. 70, 1088–1097. [Google Scholar]
- 32.Anderson DR, Burnham KP. 2002. Model selection and multimodel inference: a practical information-theoretic approach, 2nd edn New York, NY: Springer. [Google Scholar]
- 33.Lindström J. 1999. Early development and fitness in birds and mammals. Trends Ecol. Evol. 14, 343–348. ( 10.1016/S0169-5347(99)01639-0) [DOI] [PubMed] [Google Scholar]
- 34.Metcalfe NB, Monaghan P, Metcalfe N. 2001. Compensation for a bad start: grow now, pay later? Trends Ecol. Evol. 16, 254–260. ( 10.1016/S0169-5347(01)02124-3) [DOI] [PubMed] [Google Scholar]
- 35.Lee DE, Nur N, Sydeman WJ. 2007. Climate and demography of the planktivorous Cassin's auklet (Ptychoramphus aleuticus) off northern California: Implications for population change. J. Anim. Ecol. 76, 337–347. ( 10.1111/j.1365-2656.2007.01198.x) [DOI] [PubMed] [Google Scholar]
- 36.Bertram DF, Harfenist A, Smith BD. 2005. Ocean climate and El Niño impacts on survival of Cassin's auklets from upwelling and downwelling domains of British Columbia. Can. J. Fish. Aquat. Sci. 62, 2841–2853. ( 10.1139/F05-190) [DOI] [Google Scholar]
- 37.White G, Burnham KP.1999. Program MARK: survival estimation from populations of marked animals. Bird Study 46 , S120–S139. ( ) [DOI]
- 38.Townsend HM, Anderson DJ. 2007. Assessment of costs of reproduction in a pelagic seabird using multistate mark-recapture models. Evolution 61, 1956–1968. ( 10.1111/j.1558-5646.2007.00169.x) [DOI] [PubMed] [Google Scholar]
- 39.R Core Team. 2015. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 40.Bates D, Maechler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. (doi:10.18637/jss.v067.i01) [Google Scholar]
- 41.Laake J. 2013. RMark: an R interface for analysis of capture-recapture data with MARK . AFSC Processed Rep 2013-01. 25 p. Alaska Fisheries.
- 42.Wickman H. 2016. Elegant graphics for data analysis. New York, NY: Springer Verlag. [Google Scholar]
- 43.Wilke CO. 2017. ggridges: ridgeline plots in ‘ggplot2’. R package, v.0.4.1. See https://CRAN.R-project.org/packages=ggridges.
- 44.Vaupel JW, Yashin AI. 1985. Heterogeneity's ruses: some surprising effects of selection on population dynamics. Am. Stat. 39, 176–185. [PubMed] [Google Scholar]
- 45.Manuwal DA. 1974. Effects of territoriality on breeding in a population of Cassin's auklets. Ecology 55, 1399–1406. ( 10.2307/1935468) [DOI] [Google Scholar]
- 46.Lack D. 1968. Ecological adaptations for breeding in birds. London, UK: Methuen and Co, Price. [Google Scholar]
- 47.Bried L, Pontier D, Jouventin P. 2003. Mate fidelity in monogamous birds: a re-examination of the Procellariiformes. Anim. Behav. 65, 235–246. ( 10.1006/anbe.2002.2045) [DOI] [Google Scholar]
- 48.Hanssen SA, Hasselquist D, Folstad I, Erikstad KE. 2005. Cost of reproduction in a long-lived bird: incubation effort reduces immune function and future reproduction. Proc. R. Soc. B 272, 1039–1046. ( 10.1098/rspb.2005.3057) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jacobsen K-O, Erikstad KE. 1995. An experimental study of the costs of reproduction in the kittiwake Rissa tridactyla. Ecology 76, 1636–1642. ( 10.2307/1938164) [DOI] [Google Scholar]
- 50.Berube CH, Festa-Bianchet M, Jorgenson JT. 1999. Individual differences, longevity, and reproductive senescence in bighorn ewes. Ecology 80, 2555–2565. ( 10.1890/0012-9658(1999)080%5B2555:IDLARS%5D2.0.CO;2) [DOI] [Google Scholar]
- 51.Bowen WD, Iverson SJ, McMillan JI, Boness DJ. 2006. Reproductive performance in grey seals: age-related improvement and senescence in a capital breeder. J. Anim. Ecol. 75, 1340–1351. ( 10.1111/j.1365-2656.2006.01157) [DOI] [PubMed] [Google Scholar]
- 52.Descamps S, Boutin S, McAdam AG, Berteauxm D, Gaillard J-M. 2009. Survival costs of reproduction vary with age in North American red squirrels. Proc. R. Soc. B 276, 1129–1135. ( 10.1098/rspb.2008.1401) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Warner DA, Miller DAW, Bronikowski AM, Janzen FJ. 2016. Decades of field data reveal that turtles senesce in the wild. Proc. Natl Acad. Sci. USA 113, 6502–6507. ( 10.1073/pnas.1600035113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reznick D, Nunney L, Tessier A. 2000. Big houses, big cars, superfleas and the costs of reproduction. Trends Ecol. Evol. 15, 421–425. ( 10.1016/S0169-5347(00)01941-8) [DOI] [PubMed] [Google Scholar]
- 55.Hamel S, Cote S, Gaillard J-M, Bianchet-Festa M. 2009. Individual variation in reproductive costs of reproduction: high-quality females always do better. J. Anim. Ecol. 78, 143–151. ( 10.1111/j.1365-2656.2007.0) [DOI] [PubMed] [Google Scholar]
- 56.Johns ME, Warzybok P, Bradley RW, Jahncke J, Lindberg M, Breed GA. 2018. Data from: Increased reproductive investment associated with greater survival and longevity in Cassin's auklets Dryad Digital Respository. ( 10.5061/dryad.7cp7088) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Johns ME, Warzybok P, Bradley RW, Jahncke J, Lindberg M, Breed GA. 2018. Data from: Increased reproductive investment associated with greater survival and longevity in Cassin's auklets Dryad Digital Respository. ( 10.5061/dryad.7cp7088) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data and R code supporting the results of this manuscript are stored on Dryad: http://dx.doi.org/10.5061/dryad.7cp7088 [56].