Abstract
Prey naiveté is a failure to recognize novel predators and thought to cause exaggerated impacts of alien predators on native wildlife. Yet there is equivocal evidence in the literature for native prey naiveté towards aliens. To address this, we conducted a meta-analysis of Australian mammal responses to native and alien predators. Australia has the world's worst record of extinction and declines of native mammals, largely owing to two alien predators introduced more than 150 years ago: the feral cat, Felis catus, and European red fox, Vulpes vulpes. Analysis of 94 responses to predator cues shows that Australian mammals consistently recognize alien foxes as a predation threat, possibly because of thousands of years of experience with another canid predator, the dingo, Canis lupus dingo. We also found recognition responses towards cats; however, in four of the seven studies available, these responses were of risk-taking behaviour rather than antipredator behaviour. Our results suggest that a simple failure to recognize alien predators is not behind the ongoing exaggerated impacts of alien predators in Australia. Instead, our results highlight an urgent need to better understand the appropriateness of antipredator responses in prey towards alien predators in order to understand native prey vulnerability.
Keywords: prey naiveté, alien species, predator recognition, meta-analysis, feral cat, red fox
1. Introduction
Alien species are a major threat to biodiversity worldwide [1–3], primarily because they have novel and exaggerated impacts on local species. For example, a review of predator removal experiments showed that alien predators have twice the impact on native mammal and bird populations as compared to native predators [4]. The primary reason for the exaggerated impact of alien predators was attributed to naiveté in native prey, i.e. a failure to recognize or respond appropriately to the threat posed by a novel predator [1,4]. However, evidence for native prey naiveté as a driver of alien impacts remains equivocal for two key reasons; firstly, because naiveté can take many forms, and secondly, because the extent of naiveté of prey towards any given alien predator cannot be easily detected. At its most extreme and simplistic, prey naiveté is the failure to recognize any risk of predation, such as that seen in some island endemic fauna that have evolved without predation [5]—such animals become easy prey for novel predators [6] (so-called island syndrome). But where prey have had exposure to predators over an evolutionary or ecological time frame, the nature of prey naiveté towards new or novel predators is more complex.
There are multiple general hypotheses that predict the nature of prey naiveté outside of insular systems. The predator archetype hypothesis [1] predicts that prey are likely to be naive towards novel predator ‘archetypes’—i.e. alien predators with morphological and behavioural characteristics distinct to those of any native predators that prey share evolutionary history. The extent that alien predators must differ from native predators to be considered a different archetype is not well understood; however, similarities at the taxonomic level of Family have been proposed to represent the same archetype [1], and there is some experimental support for this [7].
The length of time since an alien predator's introduction should also influence prey naiveté. Prey cannot remain eternally naive to an alien predator as predation pressure from alien species means that naive prey populations will either go extinct or overcome their naiveté with increasing experience [8–10]. The multiple levels of naiveté framework proposed by Banks & Dickman [11] suggests that naiveté consists of three levels, each related to the amount of experience that prey have with a particular predator. Level 1 naiveté is considered to be a failure to recognize the predation risk posed by a predator. Level 2 naiveté involves the recognition of a predator, but a failure to respond in an appropriate manner, for example freezing rather than fleeing. Finally, level 3 naiveté occurs when prey recognize the predator and respond appropriately, but are simply ‘out-gunned’ by the predators’ superior defence-mitigating strategies (e.g. prey are out-run). Progress towards predator wariness will be influenced by the eco-evolutionary experience of prey [12] and thus may occur over longer time frames via evolution, or over shorter time frames through ontogenetic experience and learning [7]. There are examples of both learning and evolution in response to novel predators in a wide range of taxa, including fishes, birds and mammals [13–18]. Mammals, in particular, can exhibit strong predator neophobia (fear of novel stimuli perceived to impart predation threat [19]), which may aid ontogenetic learning about new predators by increasing the chances of surviving an initial predation attempt. The local familiarity of a predator (i.e. whether or not it is sympatric with prey) is likely to influence prey ontogenetic experience. If prey use experience gained through interactions to develop antipredator responses, then the local familiarity of a predator could affect prey naiveté.
Here, we examine these three scenarios (island syndrome, predator archetypes and eco-evolutionary exposure to predation) of naiveté, using mammalian native prey in Australia as a test case. Alien predators have been notoriously devastating for Australian wildlife. Australia has the world's worst record of mammal extinctions, having lost 29 species since European settlement, representing more than 10% of Australia's endemic terrestrial mammal fauna and nearly 30% of the world's mammal extinctions over the last 400 years [20,21]. Unlike the rest of the world, where the primary causes of extinction are habitat loss and hunting, in Australia recent mammal extinctions are thought primarily driven by predation from the feral cat (Felis catus) and European red fox (Vulpes vulpes) [22]. Feral cats and foxes are listed as threats for 82% and 50% of threatened native mammal species in Australia, respectively [22]. These two predators were introduced to Australia some 150–200 years ago [23,24], and their subsequent spread coincided with numerous extinctions and declines of small- to medium-sized (critical weight range (CWR); 35 g–5.5 kg) native mammals [25–27]. Cat and fox predation also drives the failure of many threatened species reintroduction and recovery programmes in Australia [28,29]. A larger mammalian predator, the dingo (Canis lupus dingo), arrived in Australia some 4000 years ago [27,30]. Dingoes readily interbreed with domestic dogs (Canis lupus familiaris), which are a very closely related subspecies of canid, and were introduced approximately 150–200 years ago [31]. Dingoes appear to have extirpated two native marsupial predators (the thylacine, Thylacinus cynocephalus and Tasmanian devil, Sarcophilus harrisii) from the mainland soon after their arrival [32–35].
We expect that these alien placental predators in Australia are of a different archetype to native marsupial predators [1]. Placental and marsupial predators diverged evolutionarily more than 160 million years ago [36]. Although remarkably convergent in many ways, these medium-sized placental and marsupial predators use different hunting techniques to find and kill prey (e.g. in running speed, prey capture mode and arboreality [8,37]), and produce distinctly different olfactory information in their urine, scats and body odours, which is important because mammalian prey use olfaction to detect and avoid predators [38]. As a result, the antipredator tactics used by native mammals to recognize and respond to their historical native predators may be inappropriate or ineffective for dogs, foxes and cats, leading to exaggerated predation impacts [1]. On the other hand, a long evolutionary history of predation risk from native marsupials (devils, S. harrisii and quolls, Dasyurus sp.), reptiles (goannas, Varanus sp.) and raptors suggests that native wildlife should have a wide array of antipredator detection and defence capabilities. At the same time, thousands of years of coexistence with dingoes suggests that native prey will be wary of this predator (see [39,40]). Dogs and foxes, like dingoes, are canids. Previous research [41,42] has shown that some native wildlife recognize domestic dogs as predators, possibly because of prey having thousands of years of experience with the very closely related dingo. While foxes are more distantly related to dingoes than are domestic dogs, it is also possible that native prey would generalize antipredator behaviour for dingoes towards foxes, if membership of the Canidae is an appropriate proxy for a predator archetype [1]. There is no native predator archetype at the Family level for introduced cats.
Australia is an ideal location for studying prey naiveté, as there are a mixture of different mammalian predator archetypes (canids, felids and marsupials) with known introduction timelines for alien species, and a diversity of other native predators for comparison including raptors and reptiles. Over recent years, there has amassed a large body of research into naiveté in Australian native mammals, with equivocal and often conflicting evidence for their recognition of, and responses towards dogs, foxes and cats. There are, of course, a wide variety of methodologies used and species studied, and we have only recently begun unravelling the complex nature of prey naiveté (e.g. [1,8,11,43]). Yet, we lack a definitive picture of native prey naiveté towards alien predators in Australia.
To address this gap, we performed a meta-analysis of research into prey naiveté in Australia, to determine the nature of naiveté in Australian native mammals towards their alien predators. Specifically, we look for evidence of predator recognition in prey, representing the most fundamental form of potential naiveté in prey (level 1 naiveté or ‘island syndrome’) and the nature of any responses to novel predators (needed to understand other levels of potential naiveté). In doing so, we test predictions of three hypotheses about the processes driving Australian native prey naiveté:
(i) if placental predators are a different archetype to native marsupial predators (cf. the archetypes hypothesis [1]), then Australian mammals will show level 1 naiveté towards alien placental predators (dogs, foxes and cats);
(ii) however, if thousands of years of experience with dingoes mean that wildlife recognize them as predators, and taxonomic differences at the Family level translate to archetypes, then native mammals will respond to dogs and foxes (both canids), but not cats (felids); or
(iii) if predator recognition has developed through eco-evolutionary experience, then Australian mammals will show recognition-type response to locally familiar (sympatric) predators, but not to locally novel (allopatric) predators.
2. Material and methods
We conducted a literature search using the online databases Web of Science, Scopus, Ovid, Zoological Record and Google Scholar, searching combinations of the following search terms: predat*, risk, Australia*, mammal*, naive* and recogni*, which generated 233 papers. We also checked the reference lists of these papers for additional candidate papers. We only retained papers describing manipulation experiments investigating the behavioural responses of native Australian mammals to predators or their cues. We excluded papers with the following conditions: were unreplicated or lacked a control; used humans as a predator surrogate; used unconfirmed predator presence; were on taste aversion, or antipredator training without measures of response prior to training; or had a sample size of less than five individuals. Our final dataset included 94 tests of prey responses within 28 papers published between 1996 and 2016 (see the electronic supplementary material for reference list). Where experiments involved tests of multiple predators or multiple prey species, each test of predator recognition/response was entered as a separate row in the dataset. Where experiments involved multiple tests of prey responses to predators (e.g. time spent vigilant and time spent foraging), only one test was used in our analyses to maintain independence. As any response to a predator cue may suggest that an animal is not naive, the test with the largest effect size was included to identify predator recognition: for multiple positive or negative effect sizes the greatest response (largest distance from zero) was used, a positive effect size was employed over a negative effect size, and a negative effect size was chosen over an effect size of zero (no response).
We calculated effect size (Hedges' d) and sampling variance in MetaWin v.2.1 [44] (see the electronic supplementary material for further information), using the mean values, standard deviations and sample sizes (n) in the predator and the control treatment from each test. Where confidence intervals around Hedge's d overlapped zero, we interpreted no significant difference between prey responses to predators or to the controls. A positive Hedge's d was interpreted as a prey response to predators that was greater than that to controls, and vice versa for a negative Hedge's d.
For every test in the dataset, we recorded additional information on potential explanatory variables of prey responses (table 1). Dingoes and dogs were combined as one predator type given that they readily interbreed, dingoes cannot be reliably distinguished from hybrids without genetic testing [45,46] and they produce similar olfactory cues used by prey [38]. There was no evidence of publication bias (normal quantile plot, see the electronic supplementary material).
Table 1.
Potential explanatory variables of prey responses.
| variable | description |
|---|---|
| prey species | |
| prey infraclassa | placental or marsupial |
| prey Family or Order | e.g. macropod or rodent |
| prey size | CWR, below or above |
| predator typea | cat, fox, dingo/dog, native marsupial, raptor or reptile |
| predator local familiaritya | whether the target prey had had prior exposure to the predator, e.g. owing to range overlaps as determined from the original manuscript or the Atlas of Living Australia; familiar or novel |
| study environmenta | field or enclosure |
| predator cuea | olfactory, visual, acoustic or whole animal |
| prey behavioural response categorya | vigilance, foraging, spatial response or other |
aModel analyses were performed for that variable.
Our final dataset comprised 30 tests (31.91%) that reported prey responses to foxes, seven (7.45%) to cats, 18 (19.15%) to dingoes or dogs, and 37 (39.36%) to native predators (19 to marsupial mammals, 12 to raptors and six to reptiles), while two tests (2.13%) reported prey responses to simultaneous exposure to multiple predators (these tests were excluded from analyses on predator type but were included in all other analyses). Predators were locally familiar to prey in 59 tests and locally novel in 31 tests, while three tests reported on prey responses to both familiar and novel predators, and predator familiarity could not be determined for one test. Most experiments used an olfactory cue (64 tests, 68%; predominantly faeces, 46 tests), 15 tests used a visual cue (e.g. taxidermic mount), 12 tests used acoustic cues, and three tests used a live predator. For the control treatment in calculating effect sizes, we used the control as used in each study. We used the blank (no treatment) control in cases where additional procedural controls (e.g. non-predator odour or acoustic call) were used because such ‘controls’ also carry unique information that prey can respond to (see [47]).
Of the potential prey species included in the dataset, 91% were within the CWR (35 g–5.5 kg) [25] which are the subset of Australian native mammals that have declined most since European settlement, largely owing to predation by alien predators [26]. Fifty-four tests (57%) involved marsupial prey, of which the majority were conducted on macropods (29 tests—primarily tammar wallabies (Macropus eugenii)), brushtail possums (Trichosurus vulpecula—10 tests) and dasyurids (antechinus and quolls—nine tests). The only placental prey tested were rodents (40 tests), predominantly bush rats (Rattus fuscipes, 20 tests). Most experiments reported on the spatial response of prey to predator cues (46 tests; e.g. visitation to predator cue and trap success), while 25 reported on foraging, 20 on vigilance and three on ‘other’ behavioural responses (two on comfort (feeding, grooming and resting) and one on sniffing). Tests lasted from 15 s to 6 days with the majority of the tests conducted in the field (60% versus 40% in enclosures). Each response (where different from the matching control) represents a form of predator recognition and hence an absence of level 1 naivete. Each response is also potentially appropriate (cf. level 2 naivete) and effective (level 3 naivete) in reducing predation risk, but the extent of their effectiveness against alien predators was not reported in any study.
We performed categorical random-effects model analyses using the homogeneity statistic (Q) (MetaWin v.2.1. [44]) to compare prey responses between (i) predator type, (ii) local predator familiarity, and (iii) other potential explanatory variables (table 1). Total heterogeneity (QT) was partitioned (similar to analysis of variance (ANOVA)) to give a value for variance in effect sizes explained by the categorical variable in the model (QM) and the residual error variance (QE). Within- and between-group heterogeneity was tested against a χ2 distribution. We conducted resampling tests with 4999 iterations [48]. All tests were two-tailed, and bias-corrected confidence intervals were applied to evaluate the probability at 0.05. Significant differences were investigated with Tukey's honestly significant difference (HSD) post hoc test. Given that simultaneous analysis of multiple factors is not possible in MetaWin, potential interactions of the explanatory variables predator type and predator local familiarity to prey were explored using ANOVA in JMP® Pro 11.2.0 [4].
3. Results
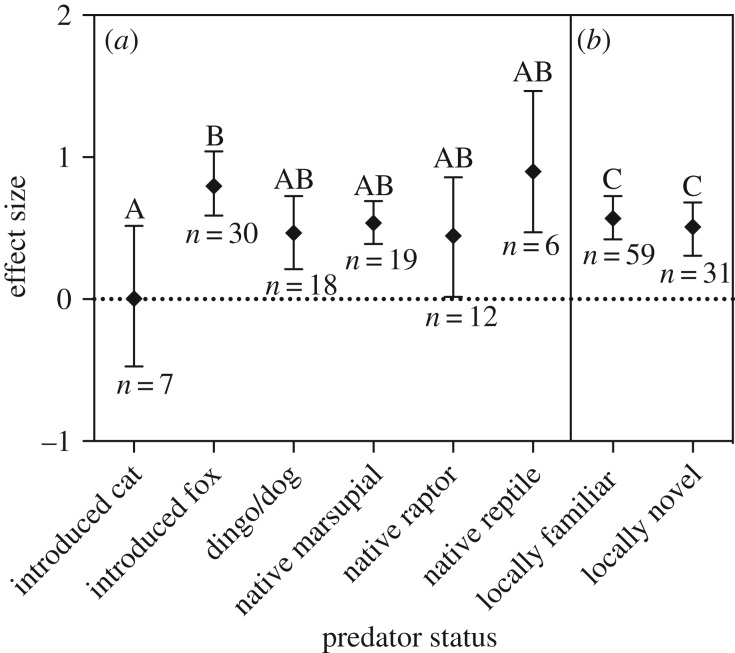
Overall, Australian mammals show antipredator responses indicative of predator recognition towards native predators, dogs and foxes—all mean effect sizes were significantly above zero (table 2 and figure 1a). More specifically, individual tests indicated that Australian mammals responded to all native marsupial and reptilian predators (positive effect sizes). For raptors however, prey species differed in their response, and while the majority of tests (75%, prey species included M. eugenii, Macrotis lagotis, T. vulpecula and Macropus rufogriseus banksianus) had positive effect sizes, prey species in three tests had a greater response in the control compared to the predator treatment (negative effect size, prey species included Dasyurus viverrinus, Perameles bougainville and Thylogale thetis).
Table 2.
Homogeneity test results for the meta-analysis. (Effect size is calculated as Hedges' d. 95% Confidence intervals (CIs) are bias-corrected. An asterisk (*) indicates significance.)
| variable | level | mean effect size d | lower 95% CI | upper 95% CI | n | QM | d.f. | p-value |
|---|---|---|---|---|---|---|---|---|
| predator type | 13.19 | 5 | 0.02* | |||||
| introduced cat | 0.00 | −0.47 | 0.52 | 7 | ||||
| introduced fox | 0.80 | 0.59 | 1.04 | 30 | ||||
| dingo/dog | 0.47 | 0.21 | 0.73 | 18 | ||||
| native marsupial | 0.54 | 0.39 | 0.69 | 19 | ||||
| native raptor | 0.45 | 0.02 | 0.86 | 12 | ||||
| native reptile | 0.90 | 0.47 | 1.47 | 6 | ||||
| local predator familiarity | 0.22 | 1 | 0.64 | |||||
| familiar | 0.57 | 0.42 | 0.73 | 59 | ||||
| novel | 0.51 | 0.31 | 0.68 | 31 | ||||
| prey infraclass | 0.30 | 1 | 0.58 | |||||
| marsupial | 0.61 | 0.42 | 0.81 | 54 | ||||
| placental | 0.54 | 0.41 | 0.68 | 40 | ||||
| behavioural response category | 1.14 | 3 | 0.77 | |||||
| vigilance | 0.55 | 0.24 | 0.81 | 20 | ||||
| foraging | 0.48 | 0.26 | 0.72 | 25 | ||||
| spatial response | 0.64 | 0.48 | 0.83 | 46 | ||||
| other | 0.66 | 0.08 | 1.05 | 3 | ||||
| predator cue | 1.85 | 3 | 0.60 | |||||
| olfactory | 0.58 | 0.44 | 0.74 | 64 | ||||
| visual | 0.56 | 0.27 | 0.83 | 15 | ||||
| acoustic | 0.50 | −0.02 | 0.85 | 12 | ||||
| whole animal | 1.03 | 0.65 | 2.01 | 3 | ||||
| study environment | 1.29 | 1 | 0.26 | |||||
| field | 0.63 | 0.48 | 0.81 | 52 | ||||
| enclosure | 0.48 | 0.29 | 0.67 | 40 |
Figure 1.
Mean effect sizes of prey towards (a) predator type and (b) predator familiarity. Effect size is calculated as Hedges' d; values greater than zero indicate antipredator response performed. Bars represent 95% bias-corrected confidence intervals. Letters indicate significant differences between treatments (Tukey's HSD p < 0.05). Two tests are excluded in part (a) owing to multi-species predators, and three tests are excluded in part (b) owing to predator familiarity being combined or undetermined.
For dogs as predators, most (83%) individual tests reported prey responses with positive effect sizes. The three tests with negative effect sizes had no variables related to study design or methodology that consistently differed to those with positive effect sizes. For foxes as predators, all 30 of the tests assessing mammal responses had positive effect sizes, indicating consistent recognition of foxes as a potential predator. Half of the mammals in the fox tests were native rodents (six species; the majority bush rats R. fuscipes, n = 8) and the other half were a range of marsupial prey including macropods (n = 6, five species), dasyurids (n = 4, two species) and diprotodonts (n = 5, three species), indicating that a diversity of prey taxa are responding to foxes.
By contrast, cats did not induce consistent recognition responses in Australian mammals. Although the mean effect size was not different from zero, there was large variation in responses (table 2 and figure 1a). Of the seven individual cat tests available, three tests showed an antipredator response to cats (positive effect size), whereas the other four tests showed the opposite response (negative effect size—prey response was lower in the predator treatment compared to the control). The studies reporting negative effect sizes each used a different prey species; red-necked pademelon (T. thetis), bush rat (R. fuscipes), eastern quoll (D. viverrinus) and spinifex hopping-mouse (Notomys alexis): prey species in the three tests that recorded an antipredator response to cats included two on tammar wallabies (M. eugenii) and one on bilbies (M. lagotis).
The local familiarity of predators (i.e. familiar or novel) showed no association with effect sizes (table 2 and figure 1b). Similarly, there was no effect of predator familiarity within each predator type (electronic supplementary material, table S1), nor any interaction between predator type and local familiarity (F = 0.25, d.f. = 5, 76, p = 0.94). There was also no effect of prey infraclass, behavioural response category, predator cue or study environment (whether field or enclosure based) (table 2).
4. Discussion
Overall, we found that Australian mammals perform responses that are consistent with their recognition of introduced foxes and dogs as a predation threat. However, our results also suggest while some Australian mammals may not be naive to the predation threat posed by cats, other native mammals appear to show lowered risk aversion to cats. These results reject the notion that Australian mammals fail to recognize introduced predators as a predation threat (level 1 naiveté) and suggests that there are more complex reasons for the impacts of alien predators. Instead, our results support the predictions of our second hypothesis that prior exposure to a similar predator archetype influences response towards novel predators.
We found antipredator responses towards dingoes/dogs, at least for prey on mainland Australia. Although dogs/dingoes, like cats, are placental mammals and hence a novel predator archetype over very long evolutionary time frames, Australian mammals have had thousands of years of experience with canid predation from dingoes. Canids would have been as novel as cats upon first introduction to Australia some 4000 years ago [27,30], but our meta-analysis shows that native mammals now recognize dogs and dingoes as predators. Notably, parallel studies using identical methodology in Tasmania and mainland Australia [39,40] showed that bandicoots on mainland Australia avoid areas with dogs, whereas bandicoots in Tasmania, where dingoes never reached, show no avoidance of dogs, even though bandicoots were locally familiar with dogs in both studies. These patterns of recognition are probably owing to thousands of years of selective pressure from predation by dingoes on the Australian mainland [39].
We found that Australian mammals recognize and respond to the risks posed by foxes. This result supports our second hypothesis—that predator archetypes may correspond to family taxonomic levels [1] (as foxes and dingoes are both canids) and that thousands of years of experience with dingoes may have facilitated antipredator behaviour towards foxes. Nevertheless, this result was somewhat unexpected given the historical and ongoing impacts of foxes on native wildlife [25]. We had only four tests from where foxes do not occur (Tasmania and far north Queensland) and prey in these tests appeared to respond to foxes (positive Hedges' d). Another Tasmanian test which could not be included (incompatible data: Hedges’ d could not be calculated) reports no responses to foxes. It is nonetheless possible that some species did not initially recognize foxes when they were first encountered; however, our meta-analysis shows that for all species tested so far, this is no longer the case.
Given the available published data, our meta-analysis could only assess level 1 naiveté (predator recognition). However, the ongoing exaggerated impacts of foxes on wildlife [22,49,50] support the notion that Australian mammals still experience level 2 or 3 naiveté (inappropriate or inadequate responses to foxes). Thus, wildlife may continue to suffer heavy mortality and other impacts, especially in altered environments that reduce the effectiveness of antipredator tactics (e.g. loss of shelter; see review by Woinarski et al. [22]) and where fox numbers are supported by other introduced species [51]. It is also important to note that it is possible that prey species which suffered severe naiveté towards foxes have already gone extinct and hence no experiments on these species were available to be included in this meta-analysis. The earliest fox test included here was published in 1998.
Cats are the most novel alien predator archetype in Australia, and we found substantial variation in the reported responses to cats by Australian mammals. Prior to the introduction of domestic cats more than 150 years ago with the arrival of Europeans [23,52], there had been no native felids or close relatives of felids on the continent. The cues emitted by cats (via scats, urine and body odours) are chemically very different to those of native marsupial predators (as is the case for dogs and foxes also) [53], most likely owing to their very distant evolutionary relationship. Given that mammals rely strongly on olfactory cues to detect and avoid predation [54], it is likely that naiveté in Australian mammals towards this novel predator archetype would be influenced by the novel odour cues cats produce [53]. Cats probably also differ in other important ways from native marsupials, such as hunting style and visual appearance, which are also likely to influence predator recognition by prey [8].
However, we found a mixture of effect sizes in response to cats, with some prey (three out of seven) showing recognition and avoidance responses consistent with those towards foxes and dogs, but others (four out of seven) showing the opposite response (negative effect sizes) rather than a lack of recognition. Despite the small sample size of studies available, the diversity of prey species showing positive and negative effects suggests that taxonomic bias in study species was not driving this difference. Instead, it seems that whereas some Australian native mammals have begun to recognize cats as a predation threat and respond accordingly, others have recognition but are showing the wrong type of response, which accords with level 2 naiveté. However, the responses shown (three showing increases in foraging and one showing reduction in vigilance in the presence of cat cues) seem unlikely to be tactics effective against any predator. Instead, it is possible that prey populations in the cat tests with negative effect sizes could potentially be infected with Toxoplasma gondii, an introduced protozoan parasite common in Australia that can occur in native wildlife [55] and reverse antipredator responses in affected animals. Infection by T. gondii causes attraction to cat odour and lowered risk aversion in laboratory rats (Rattus norvegicus), presumably as a way for the parasite to complete its life cycle after ingestion of cysts by cats, which are the obligate host [56]. If the parasite causes these same behavioural changes in Australian mammals, then infection by T. gondii could, in part, explain the lowered antipredator behaviour towards cats seen in these tests. The prevalence of infection with T. gondii in Australian mammals can be locally high, especially where cats are abundant (reviewed in [57]). However, infection with T. gondii oocytes can also be rapidly fatal for some Australian mammals, leading to underestimates of infection rates in free-living wildlife, which complicates attempts to link infection with T. gondii and risk-taking behaviour. Instead, testing the responses of Australian mammals towards cat odour after experimental infection with T. gondii would provide the strongest test of this hypothesis.
There is compelling evidence that predation by feral cats is a principal cause of the extinction of 22 Australian endemic mammal species and threatens a further 75 endangered, vulnerable and near threatened native mammal species [22]. Cats appear to pose different risks compared to other alien predators in Australia. For example, compared to foxes, they predate smaller mammals (although there is considerable diet overlap), have a less restricted distribution (foxes are absent across much of northern Australia) and their management has been less successful [58], partly owing to their preference for live prey [59]. Furthermore, a manipulation experiment revealed that when foxes were controlled but cats were left uncontrolled, small mammal captures declined by 80% [60]. Cats are known surplus killers [61] and can have large impacts even at low densities, making it particularly difficult for small mammal populations to recover [62–65]. For example, in a controlled experiment in northern Australia, reintroduced native rodent (Rattus villosissimus) populations were hunted to extinction soon after release by only one or two individual cats, while they persisted at paired sites where cats were excluded [63]. Recognition of a predator is not enough to reduce its impact if prey responses are not effective and can be potentially disastrous if parasite infections make this response a form of predator attraction. Our results point to more complex issues of naiveté towards cats at play, and more work on the role of naiveté to cats is urgently needed.
Interestingly, we found no support for the role of ontogenetic experience or learning in antipredator responses to predators (hypothesis (iii)), as the local familiarity of a predator did not affect native Australian mammal naiveté. We had comparatively few tests from places without alien predators in Australia, in part owing to their widespread distribution. These predators also spread rapidly to their current distributions more than 120 years ago, which constrained options to compare native mammal responses to predator cues with the duration of sympatry at the study site. The lack of influence of predator familiarity supports the idea that prey may use innate recognition templates rather than experiences gained through interactions [8,66]. However, after recognizing a predator, prey must respond appropriately and effectively to avoid predation. It is possible that ontogenetic learning may play a larger role in developing appropriate and effective antipredator responses (levels 2 and 3 naiveté (sensu [11])), rather than in recognizing the predator on initial encounter. Unfortunately, the type of data that were available for this meta-analysis do not permit us to investigate this idea further.
To better understand the reasons for the exaggerated impacts of alien predators, more nuanced tests for naiveté beyond level 1 have begun to include the processes by which antipredator behaviour develops over time [67]. However, there is still a dearth of research into level 2 and level 3 naiveté, where prey responses to alien predators are inappropriate or ineffective. Incorporating the nature of any mismatch between a predator's hunting modality and behaviour and the prey's antipredator behaviour and ecology will be essential to improve our mechanistic understanding of other forms of prey naiveté. For example, the benefits for prey that run and hide after detecting a predator depend on the hunting modality of the predator, the nesting or burrowing system of the prey, as well as the relative speed and agility of both the predator and prey (reviewed in [8]).
Despite great conservation concern about the impacts of alien predators on native prey via the mechanism of naivete (both globally and in Australia) [1,8,11,43], unequivocal evidence for such a phenomenon has remained lacking. While there is inarguably a case to be made that native prey naiveté towards alien predators in the immediate period after introduction may contribute to rapid decimation and extinctions (e.g. [25]), few have recognized the important distinction between these worst-case scenarios and the ongoing interactions between native prey and their alien predators (but see [39,68]). Prey that do not go quickly extinct may persist with their alien predators for many generations, enabling learning and evolutionary mechanisms to operate and improve their antipredator behaviour. Similarly, some alien predators may be functionally quite similar to native predators, in which case prey are expected to use their eco-evolutionary experience with certain predator archetypes [1] to generalize their antipredator responses from native to alien predators [8,12]. Our results suggest that a combination of these processes are operating for native Australian mammal prey that have survived the acute phase of alien predator invasion. Our pool of studies was biased towards non-threatened species (87% of studies), possibly owing to the difficulties and ethical challenges in working with rare, threatened wildlife. Even though the effect size did not differ for threatened and non-threatened species (QM = 1.49, d.f. = 1, p = 0.23), more studies are needed on threatened mammals to better understand how naiveté towards alien predators is a factor in their status. Nevertheless, ongoing impacts by foxes on native prey [22,49,50] indicate that recognition is not enough to overcome vulnerability, suggesting that higher levels of naiveté may be occurring.
Our results support the growing recognition that prey naiveté is not fixed in time [11,12,43]. In turn, we propose that the alienness of introduced predators must also change with time, and that the exaggerated impacts of alien predators will necessarily decrease as prey transition away from naiveté and evolve with their new predators to achieve effective wariness [11]. Our results suggest that this process may be underway for prey responses to alien predators in Australia, but more research is needed into the efficacy of such antipredator responses after predator recognition. Such an understanding of prey naiveté could then be used to better prioritize management approaches towards alien predators to which native prey have the least defences.
Supplementary Material
Data accessibility
The supporting data for this paper have been deposited in the Dryad Digital Repository at: http://dx.doi.org/10.5061/dryad.d317663 [69].
Authors' contributions
P.B.B. conceived and coordinated the study. J.P.B. and P.B.B. designed the study. J.P.B. performed the meta-analysis and analysed the data. All authors wrote the manuscript. All authors gave final approval for publication.
Competing interests
We have no competing interests.
Funding
This study was funded by Australia Research Council Discovery Grant DP140104413 awarded to P.B.B.
References
- 1.Cox JG, Lima SL. 2006. Naiveté and an aquatic–terrestrial dichotomy in the effects of introduced predators. Trends Ecol. Evol. 21, 674–680. ( 10.1016/j.tree.2006.07.011) [DOI] [PubMed] [Google Scholar]
- 2.Gurevitch J, Padilla DK. 2004. Are invasive species a major cause of extinctions? Trends Ecol. Evol. 19, 470–474. [DOI] [PubMed] [Google Scholar]
- 3.Mack RN, Simberloff D, Lonsdale MW, Evans H, Clout M, Bazzaz FA. 2000. Biotic invasions: causes, epidemiology, global consequences, and control. Ecol. Appl. 10, 689–710. ( 10.1890/1051-0761(2000)010%5B0689:BICEGC%5D2.0.CO;2) [DOI] [Google Scholar]
- 4.Salo P, Korpimaki E, Banks PB, Nordstrom M, Dickman CR. 2007. Alien predators are more dangerous than native predators to prey populations. Proc. R. Soc. B 274, 1237–1243. ( 10.1098/rspb.2006.0444) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Darwin C. 1839. Narrative of the surveying voyages of His Majesty's Ships Adventure and Beagle between the years 1826 and 1836, describing their examination of the southern shores of South America, and the Beagle's circumnavigation of the globe. Journal and remarks. 1832–1836. London, UK: Henry Colburn.
- 6.Diamond J, Case TJ. 1986. Overview: introductions, extinctions, exterminations and invasions. In Community ecology (eds Diamond J, Case TJ), pp. 65–79. New York: NY: Harper & Row. [Google Scholar]
- 7.Ferrari MC, Gonzalo A, Messier F, Chivers DP. 2007. Generalization of learned predator recognition: an experimental test and framework for future studies. Proc. R. Soc. B 274, 1853–1859. ( 10.1098/rspb.2007.0297) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carthey AJ, Banks PB. 2014. Naivete in novel ecological interactions: lessons from theory and experimental evidence. Biol. Rev. 89, 932–949. ( 10.1111/brv.12087) [DOI] [PubMed] [Google Scholar]
- 9.Cox GW. 2004. Alien species and evolution: the evolutionary ecology of exotic plants, animals, microbes, and interacting native species. Washington, DC: Island Press. [Google Scholar]
- 10.Strauss SY, Lau JA, Carroll SP. 2006. Evolutionary responses of natives to introduced species: what do introductions tell us about natural communities? Ecol. Lett. 9, 357–374. [DOI] [PubMed] [Google Scholar]
- 11.Banks PB, Dickman CR. 2007. Alien predation and the effects of multiple levels of prey naivete. Trends Ecol. Evol. 22, 229–230. ( 10.1016/j.tree.2007.02.006) [DOI] [PubMed] [Google Scholar]
- 12.Saul WC, Jeschke JM. 2015. Eco-evolutionary experience in novel species interactions. Ecol. Lett. 18, 236–245. ( 10.1111/ele.12408) [DOI] [PubMed] [Google Scholar]
- 13.Berejikian BA, Tezak E, LaRae AL. 2003. Innate and enhanced predator recognition in hatchery-reared chinook salmon. Environ. Biol. Fish. 67, 241–251. ( 10.1023/A:1025887015436) [DOI] [Google Scholar]
- 14.Chivers D, Smith R. 1994. The role of experience and chemical alarm signalling in predator recognition by fathead minnows, Pimephales promelas. J. Fish Biol. 44, 273–285. ( 10.1111/j.1095-8649.1994.tb01205.x) [DOI] [Google Scholar]
- 15.Fendt M. 2006. Exposure to urine of canids and felids, but not of herbivores, induces defensive behavior in laboratory rats. J. Chem. Ecol. 32, 2617 ( 10.1007/s10886-006-9186-9) [DOI] [PubMed] [Google Scholar]
- 16.McLean IG, Hölzer C, Studholme BJ. 1999. Teaching predator-recognition to a naive bird: implications for management. Biol. Conserv. 87, 123–130. ( 10.1016/S0006-3207(98)00024-X) [DOI] [Google Scholar]
- 17.Miller B, Biggins D, Wemmer C, Powell R, Calvo L, Hanebury L, Wharton T. 1990. Development of survival skills in captive-raised Siberian polecats (Mustela eversmanni) II: predator avoidance. J. Ethol. 8, 95–104. ( 10.1007/BF02350280) [DOI] [Google Scholar]
- 18.Wiebe KL. 2004. Innate and learned components of defence by flickers against a novel nest competitor, the European starling. Ethology 110, 779–791. ( 10.1111/j.1439-0310.2004.01016.x) [DOI] [Google Scholar]
- 19.Crane AL, Ferrari MC. 2017. Patterns of predator neophobia: a meta-analytic review. Proc. R. Soc. B 284, 20170583 ( 10.1098/rspb.2017.0583) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McKenzie N, et al. 2007. Analysis of factors implicated in the recent decline of Australia's mammal fauna. J. Biogeogr. 34, 597–611. ( 10.1111/j.1365-2699.2006.01639.x) [DOI] [Google Scholar]
- 21.Woinarski J, Burbidge A, Harrison P. 2014. The action plan for Australian mammals 2012. Collingwood, Australia: CSIRO Publishing. [Google Scholar]
- 22.Woinarski JC, Burbidge AA, Harrison PL. 2015. Ongoing unraveling of a continental fauna: decline and extinction of Australian mammals since European settlement. Proc. Natl Acad. Sci. USA 112, 4531–4540. ( 10.1073/pnas.1417301112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abbott I. 2002. Origin and spread of the cat, Felis catus, on mainland Australia, with a discussion of the magnitude of its early impact on native fauna. Wildl. Res. 29, 51–74. ( 10.1071/WR01011) [DOI] [Google Scholar]
- 24.Rolls EC. 1969. They all ran wild, the animals and plants that plague Australia. Sydney, Australia: Angus and Robertson. [Google Scholar]
- 25.Burbidge AA, McKenzie N. 1989. Patterns in the modern decline of Western Australia's vertebrate fauna: causes and conservation implications. Biol. Conserv. 50, 143–198. ( 10.1016/0006-3207(89)90009-8) [DOI] [Google Scholar]
- 26.Johnson CN, Isaac JL. 2009. Body mass and extinction risk in Australian marsupials: the 'Critical Weight Range' revisited. Austral. Ecol. 34, 35–40. ( 10.1111/j.1442-9993.2008.01878.x) [DOI] [Google Scholar]
- 27.Dickman CR. 1996. Impact of exotic generalist predators on the native fauna of Australia. Wildl. Biol. 2, 185–195. ( 10.2981/wlb.1996.018) [DOI] [Google Scholar]
- 28.Moseby KE, Carthey AJ, Schroeder T. 2015. The influence of predators and prey naivety on reintroduction success: current and future directions. In Advances in reintroduction biology of Australian and New Zealand Fauna (eds Armstrong D, Hayward M, Moro D, Seddon PJ), pp. 29–42. Clayton South, Australia: CSIRO publishing. [Google Scholar]
- 29.Short J, Bradshaw S, Giles J, Prince R, Wilson GR. 1992. Reintroduction of macropods (Marsupialia: Macropodoidea) in Australia: a review. Biol. Conserv. 62, 189–204. ( 10.1016/0006-3207(92)91047-V) [DOI] [Google Scholar]
- 30.Corbett LK. 1995. The dingo in Australia and Asia. Sydney, Australia: UNSW Press Ltd. [Google Scholar]
- 31.Corbett LK. 2008. Dingo Canis lupus. In The mammals of Australia (eds Van Dyck S, Strahan R), pp. 737–739, 3rd edn Sydney, Australia: Reed New Holland. [Google Scholar]
- 32.Gould R, O'Connor S, Veth P. 2002. Bones of contention: reply to Walshe. Archaeol. Ocean. 37, 96–101. ( 10.1002/j.1834-4453.2002.tb00511.x) [DOI] [Google Scholar]
- 33.Guiler E. 1970. Obsevations on the tasmanian devil, Sarcophilus harrisii (Marsupialia: Dasyuridae). I. Numbers, home, range, movements and food in two populations. Aust. J. Zool. 18, 49–62. ( 10.1071/ZO9700049) [DOI] [Google Scholar]
- 34.Jones ME, Stoddart DM. 1998. Reconstruction of the predatory behaviour of the extinct marsupial thylacine (Thylacinus cynocephalus). J. Zool. 246, 239–246. ( 10.1111/j.1469-7998.1998.tb00152.x) [DOI] [Google Scholar]
- 35.Paddle R. 2002. The last Tasmanian tiger: the history and extinction of the thylacine. Melbourne, Australia: Cambridge University Press. [Google Scholar]
- 36.Luo Z-X, Yuan C-X, Meng Q-J, Ji Q. 2011. A Jurassic eutherian mammal and divergence of marsupials and placentals. Nature 476, 442–445. ( 10.1038/nature10291) [DOI] [PubMed] [Google Scholar]
- 37.Jones ME, Oakwood M, Belcher CA, Morris K, Murray AJ, Woolley PA, Firestone KB, Johnson B, Burnett S. 2003. Carnivore concerns: problems, issues and solutions for conserving Australasia's marsupial carnivores. In Predators with pouches: the biology of carnivorous marsupials (eds Jones ME, Dickman C, Archer M), pp. 422–443. Collingwood, Australia: CSIRO Publishing. [Google Scholar]
- 38.Carthey AJ, Wierucka K, Bucknall MP, Banks PB. 2017. Novel predators emit novel cues: a mechanism for prey naivety towards alien predators. Sci. Rep. 7, 16377 ( 10.1038/s41598-017-16656-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carthey AJ, Banks PB. 2012. When does an alien become a native species? A vulnerable native mammal recognizes and responds to its long-term alien predator. PLoS ONE 7, e31804 ( 10.1371/journal.pone.0031804) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frank AS, Carthey AJ, Banks PB. 2016. Does historical coexistence with dingoes explain current avoidance of domestic dogs? Island bandicoots are naïve to dogs, unlike their mainland counterparts. PLoS ONE 11, e0161447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bytheway JP, Carthey AJ, Banks PB. 2013. Risk vs. reward: how predators and prey respond to aging olfactory cues. Behav. Ecol. Sociobiol. 67, 715–725. ( 10.1007/s00265-013-1494-9) [DOI] [Google Scholar]
- 42.Heise-Pavlov SR. 2016. Evolutionary aspects of the use of predator odors in antipredator behaviors of Lumholtz's tree-kangaroos (Dendrolagus lumholtzi). In Chemical signals in vertebrates 13 (eds Schulte BA, Goodwin TE, Ferkin MH), pp. 261–280. Basel, Switzerland: Springer International Publishing. [Google Scholar]
- 43.Carthey AJ, Blumstein DT. 2017. Predicting predator recognition in a changing world. Trends Ecol. Evol. 33, 106–115. ( 10.1016/j.tree.2017.10.009) [DOI] [PubMed] [Google Scholar]
- 44.Rosenberg MS, Adams DC, Gurevitch J. 2000. Metawin: statistical software for meta-analysis. Version 2.1 Sunderland, MA: Sinauer Associates. [Google Scholar]
- 45.Corbett L. 2001. The conservation status of the dingo Canis lupus dingo in Australia, with particular reference to New South Wales: threats to pure dingoes and potential solutions. In A symposium on the dingo (eds Dickman CR, Lunney D), pp. 10–19. Sydney, Australia: Royal Zoological Society of New South Wales. [Google Scholar]
- 46.Elledge AE, Leung LKP, Allen LR, Firestone K, Wilton AN. 2006. Assessing the taxonomic status of dingoes Canis familiaris dingo for conservation. Mammal Rev. 36, 142–156. ( 10.1111/j.1365-2907.2006.00086.x) [DOI] [Google Scholar]
- 47.Hughes NK, Kelley JL, Banks PB. 2012. Dangerous liaisons: the predation risks of receiving social signals. Ecol. Lett. 15, 1326–1339. ( 10.1111/j.1461-0248.2012.01856.x) [DOI] [PubMed] [Google Scholar]
- 48.Adams DC, Anthony CD. 1996. Using randomization techniques to analyse behavioural data. Anim. Behav. 51, 733–738. ( 10.1006/anbe.1996.0077) [DOI] [Google Scholar]
- 49.Dexter N, Murray A. 2009. The impact of fox control on the relative abundance of forest mammals in East Gippsland, Victoria. Wildl. Res. 36, 252–261. ( 10.1071/wr08135) [DOI] [Google Scholar]
- 50.Saunders GR, Gentle MN, Dickman CR. 2010. The impacts and management of foxes Vulpes vulpes in Australia. Mammal Rev. 40, 181–211. ( 10.1111/j.1365-2907.2010.00159.x) [DOI] [Google Scholar]
- 51.Pech RP, Hood G. 1998. Foxes, rabbits, alternative prey and rabbit calicivirus disease: consequences of a new biological control agent for an outbreaking species in Australia. J. Appl. Ecol. 35, 434–453. ( 10.1046/j.1365-2664.1998.00318.x) [DOI] [Google Scholar]
- 52.Dickman CR. 1996. Overview of the impacts of feral cats on Australian native fauna. Canberra, Australia: Australian Nature Conservation Agency. [Google Scholar]
- 53.Carthey AJ. 2013. Naivete, novelty and native status: mismatched ecological interactions in the Australian environment. PhD thesis, University of Sydney, Sydney, Australia. [Google Scholar]
- 54.Apfelbach R, Blanchard CD, Blanchard RJ, Hayes RA, McGregor IS. 2005. The effects of predator odors in mammalian prey species: a review of field and laboratory studies. Neurosci. Biobehav. Rev. 29, 1123–1144. ( 10.1016/j.neubiorev.2005.05.005) [DOI] [PubMed] [Google Scholar]
- 55.Parameswaran N. 2008. Toxoplasma gondii in Australian marsupials. PhD thesis, Murdoch University, Perth, Western Australia. [Google Scholar]
- 56.Vyas A, Kim S-K, Giacomini N, Boothroyd JC, Sapolsky RM. 2007. Behavioral changes induced by Toxoplasma infection of rodents are highly specific to aversion of cat odors. Proc. Natl Acad. Sci. USA 104, 6442–6447. ( 10.1073/pnas.0608310104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hillman AE, Lymbery AJ, Thompson RCA. 2016. Is Toxoplasma gondii a threat to the conservation of free-ranging Australian marsupial populations? Int. J. Parasitol. Parasites Wildl. 5, 17–27. ( 10.1016/j.ijppaw.2015.12.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fisher DO, et al. 2014. The current decline of tropical marsupials in Australia: is history repeating? Global Ecol. Biogeogr. 23, 181–190. ( 10.1111/geb.12088) [DOI] [Google Scholar]
- 59.Algar D, Burrows N. 2004. Feral cat control research: Western Shield review-February 2003. Conserv. Sci. West. Aust. 5, 131. [Google Scholar]
- 60.Risbey DA, Calver MC, Short J, Bradley JS, Wright IW. 2000. The impact of cats and foxes on the small vertebrate fauna of Heirisson Prong, Western Australia. II. A field experiment. Wildl. Res. 27, 223–235. ( 10.1071/WR98092) [DOI] [Google Scholar]
- 61.McGregor H, Legge S, Jones ME, Johnson CN. 2015. Feral cats are better killers in open habitats, revealed by animal-borne video. PLoS ONE 10, e0133915 ( 10.1371/journal.pone.0133915) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gibson D, Lundie-Jenkins G, Langford D, Cole J, Clarke D, Johnson K. 1994. Predation by feral cats, Felis catus, on the rufous hare-wallaby, Lagorchestes hirsutus, in the Tanami Desert. Aust. Mammal. 17, 103–107. [Google Scholar]
- 63.Frank AS, et al. 2014. Experimental evidence that feral cats cause local extirpation of small mammals in Australia's tropical savannas. J. Appl. Ecol. 51, 1486–1493. ( 10.1111/1365-2664.12323) [DOI] [Google Scholar]
- 64.Moseby KE, Cameron A, Crisp HA. 2012. Can predator avoidance training improve reintroduction outcomes for the greater bilby in arid Australia? Anim. Behav. 83, 1011–1021. ( 10.1016/j.anbehav.2012.01.023) [DOI] [Google Scholar]
- 65.Priddel D, Wheeler R. 2004. An experimental translocation of brush-tailed bettongs (Bettongia penicillata) to western New South Wales. Wildl. Res. 31, 421–432. ( 10.1071/WR03050) [DOI] [Google Scholar]
- 66.Payne CM, Tillberg CV, Suarez AV. 2004. Recognition systems and biological invasions. Ann. Zool. Fennici. 41, 843–858. [Google Scholar]
- 67.Mencía A, Ortega Z, Pérez-Mellado V. 2017. From tameness to wariness: chemical recognition of snake predators by lizards in a Mediterranean island. PeerJ 5, e2828 ( 10.7717/peerj.2828) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Davis MA, et al. 2011. Don't judge species on their origins. Nature 474, 153–154. ( 10.1038/474153a) [DOI] [PubMed] [Google Scholar]
- 69.Banks PB, Carthey AJR, Bytheway JP. 2018. Data from: Australian native mammals recognize and respond to alien predators: a meta-analysis Dryad Digital Repository. ( 10.5061/dryad.d317663) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Banks PB, Carthey AJR, Bytheway JP. 2018. Data from: Australian native mammals recognize and respond to alien predators: a meta-analysis Dryad Digital Repository. ( 10.5061/dryad.d317663) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
The supporting data for this paper have been deposited in the Dryad Digital Repository at: http://dx.doi.org/10.5061/dryad.d317663 [69].