Abstract
The ability of wildlife populations to mount rapid responses to novel pathogens will be critical for mitigating the impacts of disease outbreaks in a changing climate. Field studies have documented that amphibians preferring warmer temperatures are less likely to be infected with the fungal pathogen Batrachochytrium dendrobatidis (Bd). However, it is unclear whether this phenomenon is driven by behavioural fever or natural variation in thermal preference. Here, we placed frogs in thermal gradients, tested for temperature preferences and measured Bd growth, prevalence, and the survival of infected animals. Although there was significant individual- and species-level variation in temperature preferences, we found no consistent evidence of behavioural fever across five frog species. Interestingly, for species that preferred warmer temperatures, the preferred temperatures of individuals were negatively correlated with Bd growth on hosts, while the opposite correlation was true for species preferring cooler temperatures. Our results suggest that variation in thermal preference, but not behavioural fever, might shape the outcomes of Bd infections for individuals and populations, potentially resulting in selection for individual hosts and host species whose temperature preferences minimize Bd growth and enhance host survival during epidemics.
Keywords: thermoregulation, behavioural fever, amphibian declines, Batrachochytrium dendrobatidis, disease ecology, thermal biology
1. Introduction
Increases in emerging infectious diseases over the last few decades have caused global declines in biodiversity [1,2]. Anthropogenic global climate change is predicted to influence human and wildlife disease dynamics worldwide, possibly exacerbating these disease-driven declines [3,4]. One reason that climate change might affect disease dynamics is because the infectivity and virulence of pathogens, as well as host resistance and tolerance of infection can vary with climatic conditions [5]. This is especially true for ectothermic hosts, which have only a limited ability to regulate body temperature independent of environmental temperatures and can struggle to combat stressors, such as disease, when exposed to sub-optimal temperatures [6–8]. Additionally, individual ectothermic hosts can vary in their preferred temperatures, which can affect their susceptibility to infections [9]. Hence, epidemics could select for host individuals and species that inherently prefer temperatures that facilitate tolerance and/or resistance to pathogens, a process that would occur across generations [9,10].
Hosts can also cope with pathogens using plasticity, which is a change in host physiology (e.g. acquired immunity), morphology, or behaviour during the life of the host, and thus occurs within rather than across generations. For instance, upon infection, ectothermic hosts could modify their temperature preferences (via behavioural thermoregulation), selecting environmental temperatures that are unfavourable for the parasite, ideal for host defences, or both. Ideally, this plasticity in response to infection should be differentiated from preferred temperatures in the absence of infections. Understanding the extent to which host populations can mount rapid plastic responses to pathogens might be critical for predicting the impacts of continued widespread disease outbreaks in a changing climate.
Many ectothermic hosts exhibit a type of plasticity called behavioural fever, which is when a host increases its temperature preference (Tpref) in response to pathogen exposure [11–13]. Behavioural fever has most commonly been documented in response to bacterial and viral pathogens, which tend to grow well at high temperatures [14]. In these cases, behavioural fever tends to increase host immune responses, which is believed to provide a net benefit to the host despite the increased pathogen growth at the higher temperature [14]. If behavioural fever is effective against thermophilic pathogens, it might be even more effective against psychrophilic (cold-loving) pathogens because the higher temperatures might both stimulate host immunity and be directly detrimental to pathogen growth.
An example of a relatively cold-tolerant pathogen is the fungus Batrachochytrium dendrobatidis (Bd). Bd causes the disease chytridiomycosis, is associated with global amphibian declines [7,15], grows best in culture under cool conditions between 18°C and 22°C, and can be cleared from some hosts when held above 25°C for extended periods of time [16–19]. In fact, field studies have documented little to no Bd in populations associated with hot springs and relatively warm low-elevations, even when surrounding or adjacent high-elevation populations have high prevalence [20–22].
Not surprisingly, several studies suggest that Bd dynamics are influenced by temperature [16,19,23,24], but whether amphibians respond to Bd with behavioural fever in the field and laboratory remains controversial. Multiple field studies correlating amphibian body temperature and Bd infection have shown that individual amphibians with higher body temperatures are less likely to be infected with Bd relative to individuals with lower body temperatures within the same population [9,21,25]. One hypothesis for this pattern is that some but not all individuals preferred microhabitats with temperatures that were unfavourable for Bd, regardless of whether they were infected [9]. By contrast, other researchers have hypothesized that these field patterns were the result of amphibians intentionally moving to warmer microhabitats to resist infection (i.e. behavioural fever) [25]. Two laboratory experiments tested for Bd-induced behavioural fever and reported mixed results. The first experiment found no evidence of Bd-induced behavioural fever in toad tadpoles [26]. The second study claimed to have provided evidence for Bd-induced behavioural fever in adult amphibians, but it had low statistical power and consequently could not conclusively support or rule out a behavioural fever response [27].
These conflicting laboratory and field results might be partly a product of the effectiveness of pathogen defences of some host species not increasing with temperatures. For example, the thermal mismatch hypothesis predicts that host species adapted to warmer temperatures might perform more poorly than the pathogen at cool temperatures, and vice versa, creating a scenario where warm- and cool-adapted hosts most often experience outbreaks at cool and warm temperatures, respectively [24,28]. There is support for this hypothesis in the amphibian-Bd system [24].
Here, we attempt to address the controversy regarding whether anuran amphibians tend to adjust their preferred temperature when infected with Bd. Our goals were to determine if: (i) there was individual-level variation in Tpref within the tested species, (ii) there were correlations between Tpref and Bd growth within and among the tested species of frogs, (iii) there was any support for the thermal mismatch hypothesis, and (iv) any tested amphibian species changed their Tpref in response to Bd exposure. To accomplish these goals, we exposed five species of adult frogs (Cuban tree frogs, Osteopilus septentrionalis, southern toads, Anaxyrus terrestris, Panamanian golden frogs, Atelopus zeteki, northern cricket frogs, Acris crepitans, and American toads, Anaxyrus americanus) to Bd in thermal gradients ranging in temperature from 9°C to 34°C [29] to assess individual Tpref before and after Bd exposure. We also measured Bd growth on individuals over time to assess whether any variation in Tpref affected Bd growth.
2. Methods
(a). Thermoregulation experiments
Experiments were conducted at the three locations: O. septentrionalis and An. terrestris experiments took place in Tampa, FL, An. americanus and Ac. crepitans experiments took place in Champaign, IL, and At. zeteki experiments took place in New Orleans, LA. See the electronic supplementary material, methods for details regarding animal collection and maintenance as well as protocols regarding Bd exposures and measuring Bd growth on hosts. In each experiment, we first measured individual baseline non-infected Tpref in thermal gradient apparatuses. All species except for At. zeteki (thermal gradient range: 19°C to 38°C; see the electronic supplementary material, methods for more details and description) were in thermal gradient apparatuses that were previously shown to provide variation in temperature that is independent of moisture/humidity and which does not confound amphibian and prey temperature preferences (12°C to 33°C see the electronic supplementary material, figure S4 and methods; and Sauer et al. [29] for thermogradient construction and validation details). After measuring non-infected Tpref, individuals were split into three treatment groups with similar mean body masses and non-infected Tpref: (i) a sham-exposed control group that was allowed to thermoregulate, (ii) a Bd-exposed group that was allowed to thermoregulate, and (iii) a Bd-exposed non-regulating group where each individual was held at their individual preferred body temperature (O. septentrionalis), at the population-level temperature preference (Ac. crepitans, An. americanus, An. terrestris), or at acclimation temperature (At. zeteki) by transferring them to temperature-controlled Styrofoam incubators (electronic supplementary material, figure S6) or environmental chambers (see the electronic supplementary material, methods).
Throughout the experiment, temperature measurements were taken each day, every four hours, four times a day, between 08.00 h and 22.00 h using an infrared thermometer [30] (Micro-Epsilon ThermoMeter LS (accuracy: ±0.75%) for At. zeteki and an Extech® High Temperature IR Thermometer (accuracy: ±2% < 932°F) for all other species) from the centre of each animal's dorsum [30] and from the substrate adjacent to the animal, except for during feeding periods (see the electronic supplementary material, methods for details on feeding). Temperature measurements were taken for at least four days before Bd or sham exposure and for at least two weeks after these exposures. Experiments were conducted using multiple temporal blocks to ensure adequate sample sizes (see the electronic supplementary material, table S2 for sample sizes for each temporal block in each experiment).
Osteopilus septentrionalis has previously been shown to acquire immunological resistance to Bd after a previous exposure and clearance [17], so we tested whether this species could acquire the ability to exhibit a behavioural fever response to Bd. We exposed half of the O. septentrionalis to Bd and half to a sham inoculate, held all individuals at 23°C for 10 days, and then shifted all frogs to 30°C for 14 days for heat clearance [16]. After confirming that all individuals were uninfected, we proceeded with the Tpref trials previously described but with six treatments, Bd-naive versus Bd-experienced animals crossed with the three treatment groups previously described (mean n = 6, N = 37).
We were concerned that, by placing frogs into the thermal gradients immediately after Bd inoculations, they could quickly select a high temperature to clear the infection before it successfully established. Consequently, we conducted a separate experiment on An. terrestris, where individuals received Bd or sham exposures. We then held them at 17°C for 7 days to ensure that there was Bd establishment followed by considerable pathogen population growth, and then placed them into the thermogradients to test for behavioural fever as described above.
(b). Data analysis
All statistics were conducted with R 3.4.0 [31]. To test for repeatability within individuals in Tpref and variation in Tpref among individuals before infection, we conducted a one-way repeated measures ANOVA (stats package, aov function). This analysis tested whether temperature preferences of individuals varied significantly across days (main effect of day) and whether temperature preferences varied among individuals (within-individual variance, s2). Additionally, we calculated repeatability (see the electronic supplementary material, methods for formula), the proportion of the variance explained by the individual [32].
We used a weight of evidence approach to test for behavioural fever across species (three-factor: treatment, time and species) and within species (two-factor: treatment and time) we conducted multiple repeated measures ANOVAs with individual treated as a random variable (stats package, aov function, assuming normal error distribution). For each model, we paired all pre-exposure days with each post-exposure day (time; one model for each post-exposure day) and looked for an interaction between treatment and time on ΔTpref (the difference between mean pre-exposure Tpref of each animal and its Tpref at each time point). We then assessed significance using the Benjamini–Hochberg (B-H) procedure [33].
We also tested for an effect of infection intensity (log-transformed Bd load divided by mass of the individual) on ΔTpref (difference between mean pre-exposed Tpref and Tpref during the 24 h after being swabbed) on At. zeteki and An. terrestris by conducting a linear mixed-effects model with individual as a random effect (nlme package, lme function). Individual-level Bd growth rates for An. terrestris were determined by first calculating infection intensity by dividing Bd loads (DNA copies) by individual mass, then log transforming infection intensity, then extracting the slope parameter from a generalized linear model of each individual's infection intensity over time (stats package, glm function; time in days). Bd growth rates for At. zeteki were determined by first calculating log infection intensity using the aforementioned methods then extracting the growth parameter from a logistic growth model of each individual's infection intensity over time (bbmle package, mle2 function; time in weeks; see the electronic supplementary material, methods for model). Growth models for each species were chosen based on a visual examination of the shape of Bd load data over time. To test the influence of individual-level Tpref on Bd growth, we conducted a linear regression with the previously calculated Bd growth rates as the response and an individual's mean Tpref for the 7 days following Bd exposure as the predictor (stats package, glm function). To test for differences in Bd intensity (main effect of treatment) and growth (interaction between treatment and time) between regulating and non-regulating exposed treatments over time, we conducted a two-factor (treatment and time) ANOVA with individual included as a random effect (nlme and stats packages, lme function). We also ensured there was no effect of body mass on Tpref by conducting a one-way repeated measures ANOVA for these two species.
Additionally, we tested for reductions in Bd prevalence over time. To do this, we calculated prevalence for all species using animals from the Bd-exposed treatment and then ran a one-way ANOVA for each species separately to determine if there was a significant change in prevalence from week 1 to week 2. We also ran a two-factor (species and treatment) ANOVA for each of the two weeks followed by Tukey's post hoc multiple comparison tests to assess differences in prevalence between species and treatments (regulating or non-regulating) (stats package, Tukey HSD function). Tukey's post hoc multiple comparisons tests were also used to assess differences when a treatment had more than two levels (multcomp package, glht function). Finally, to test for differences in survival among treatments, we conducted a Cox-proportional hazards model (survival package, coxph function).
3. Results
(a). Temperature preferences across individuals and species
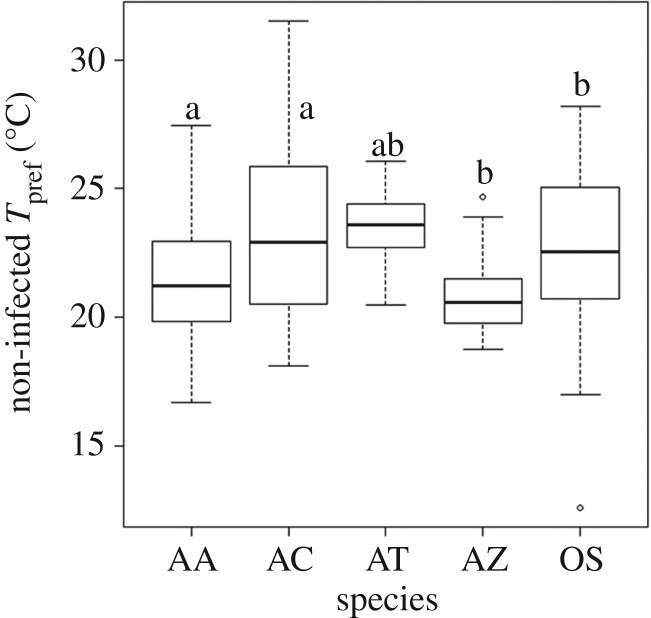
Before Bd exposure, we were able to detect consistency in the Tpref of individuals (repeatability: r > 0.90 for all species; electronic supplementary material, table S1) and variation in temperature preferences among individuals (electronic supplementary material, table S1) and across species (F4,158 = 6.82, p < 1.0 × 10−4). Atelopus zeteki (mean Tpref: 20.8°C ± 0.65 s.e.) and An. americanus (21.3°C ± 0.43) preferred significantly cooler temperatures than Ac. crepitans (23.4°C ± 0.61) and An. terrestris (23.5°C ± 0.65). Osteopilus septentrionalis (22.5°C ± 0.70) preferred moderate temperatures and was not significantly different from any other species (figure 1). To ask whether these Tpref might be an artefact of differences in acclimation temperature, we tested for a correlation between acclimation temperature and species-level Tpref and found no trend (t4 = 0.60, p = 0.59), but the power of this analysis is admittedly low.
Figure 1.
Temperature preferences (Tpref) for Atelopus zeteki (AZ), Anaxyrus americanus (AA), Osteopilus septentrionalis (OS), Acris crepitans (AC), and Anaxyrus terrestris (AT) prior to Batrachochytrium dendrobatidis exposure. Species marked with the same letter do not have significantly different Tpref based on a Tukey's HSD multiple comparison test (p > 0.05). Centre lines represent medians, boxes are first and third quartiles, and whiskers are highest and lowest points.
(b). Behavioural fever
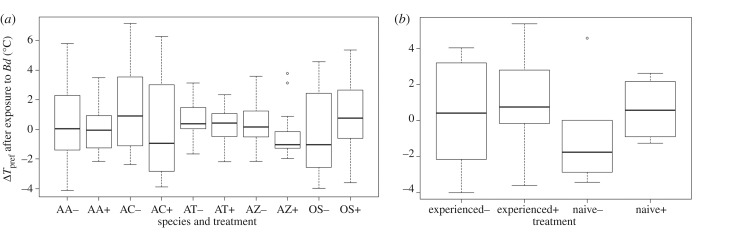
When we adjusted our alpha for multiple comparison tests, we found no evidence of behavioural fever after exposure to Bd for the omnibus test across species (interaction between treatment and time, p < B-H critical value; figure 2a; electronic supplementary material, figure S1 and table S2). If we looked at individual species, we found no evidence of behavioural fever or shifts in Tpref for An. americanus, An. terrestris, or At. zeteki (interaction between treatment and time p > adjusted threshold; figure 2a; electronic supplementary material, figures S1 and S2 and table S2). There were some days with significant interactions between treatment and time for O. septentrionalis (days 3 and 10 for the treatment group were significantly warmer; electronic supplementary material, figure S2 and table S2) and Ac. crepitans. For Ac. crepitans, the control frogs preferred significantly warmer temperatures than the Bd-exposed frogs, (days 6–11, 13, 17; electronic supplementary material, figure S1 and table S2), which is inconsistent with behavioural fever. Additionally, infection intensity had no effect on Tpref in the species where quantitative PCR was conducted (main effect of intensity on Tpref for An. terrestris: β = 0.06, p = 0.38 and At. zeteki: β = 0.03, p = 0.44). Despite evidence that O. septentrionalis can acquire immunological resistance to Bd after previous clearance of infections [18], previous exposure to Bd did not alter the Tpref of O. septentrionalis when infected with Bd a second time (figure 2b; electronic supplementary material, figure S2 and table S3).
Figure 2.
Change in temperature preferences (ΔTpref) after exposure to Bd across all time points for Atelopus zeteki (AZ), Anaxyrus americanus (AA), Osteopilus septentrionalis (OS), Acris crepitans (AC), and Anaxyrus terrestris (AT) after frogs were (+) or were not (−) exposed to Batrachochytrium dendrobatidis: when all frogs were naive to Bd (a) or when half the OS were naive and half were previously exposed and cleared of Bd (b). Centre lines represent medians, boxes are upper and lower quartiles, and whiskers are highest and lowest points.
(c). Batrachochytrium dendrobatidis abundance and disease susceptibility
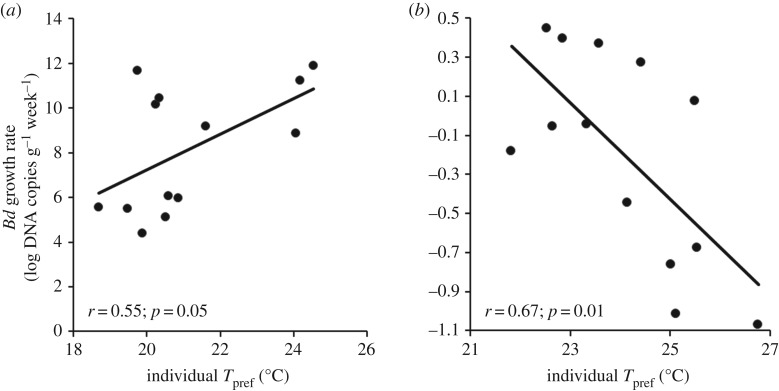
For thermoregulating An. terrestris and At. zeteki, we found that individual Tpref during the first week after Bd exposure had a significant effect on Bd growth rate in the thermal gradients over the course of the three week experiment. Atelopus zeteki, which preferred the coolest temperatures, showed a positive relationship between individual Tpref and Bd growth rate (F1,11 = 4.73, p = 0.05; figure 3a), indicating that Bd grew better on this species at warmer temperatures. Anaxyrus terrestris, which preferred the warmest temperatures, showed a negative relationship between individual Tpref and Bd growth (F1,11 = 8.86, p = 0.01; figure 3b). We also tested for an effect of mass on Tpref for these two species and found no effect (At. zeteki: F1,26 = 1.02, p = 0.32; At. terrestris: F1,29 = 0.05, p = 0.82). We were unable to calculate Bd growth rates for O. septentrionalis owing to low Bd prevalence.
Figure 3.
Relationship between individual-level temperature preference (Tpref) and Batrachochytrium dendrobatidis (Bd) growth on frogs for (a) Atelopus zeteki and (b) Anaxyrus terrestris. Atelopus zeteki, which preferred the coolest temperatures (figure 1), showed a positive relationship between Bd growth rate on individual hosts and host Tpref (F1,11 = 4.73, p = 0.05), indicating that Bd grew better on this species at warmer temperatures. By contrast, Anaxyrus terrestris, which preferred the warmest temperatures (figure 1), showed a negative relationship between Bd growth on individual hosts and host Tpref (F1,11 = 8.86, p = 0.01), indicating that Bd grew better on this species at cooler temperatures.
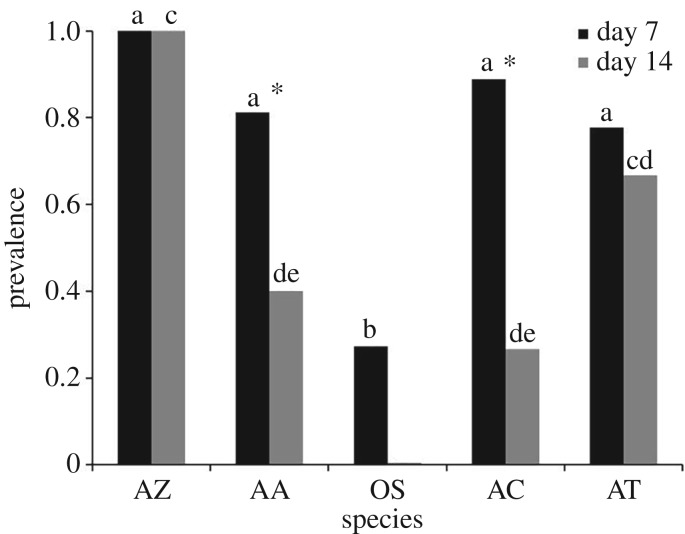
There were no detectable differences in Bd loads or Bd growth rates between regulating and non-regulating Bd-exposed groups (An. terrestris main effect of treatment: β = 0.78, d.f. = 35, p = 0.36; interaction between treatment and time: β = −0.22, d.f. = 63, p = 0.58 and At. zeteki main effect of treatment: β = 0.68, d.f. = 24, p = 0.42; interaction between treatment and time: β = 0.01, d.f. = 24, p = 0.94; see the electronic supplementary material, figure S3). However, there were differences in prevalence across species and within species across weeks (figure 4). Two week prevalences ranged from 100% for At. zeteki to 0% for O. septentrionalis. For At. zeteki, prevalence remained a constant 100% between week 1 and 2 of the experiment, whereas for Ac. crepitans prevalence dropped from 89% to 27% over this time period (figure 4). Atelopus zeteki was the only species with substantial Bd-induced mortality and there was no significant difference in the survival curves between regulating and non-regulating treatment groups (100% and 100% mortality and 25.1 and 20.3 mean days alive, respectively; β = 0.45, p = 0.08; electronic supplementary material, figure S4). The maximum mortality for any of the other species was 15% in the non-regulating An. americanus (electronic supplementary material, figure S4).
Figure 4.
Prevalence of Batrachochytrium dendrobatidis (Bd) infection one and two weeks after pathogen exposure in Atelopus zeteki (AZ), Anaxyrus americanus (AA), Osteopilus septentrionalis (OS), Acris crepitans (AC), and Anaxyrus terrestris (AT) when they were free to roam in a temperature gradient. Within week means with different letters are significantly different from one another based on Tukey's HSD test (p < 0.05). Asterisks denote species that showed significant drops in prevalence from week 1 to week 2 based on an ANOVA (AA: F1,22 = 6.33, p = 0.02; AC: F1,22 = 20.84, p < 0.0001).
4. Discussion
We set out to determine if the tested species of amphibians showed any individual- or species-level variation in Tpref, if variation in Tpref among individuals or species was correlated with Bd growth on frogs, whether relationships between Tpref and Bd growth were consistent with the thermal mismatch hypothesis, and if any of the tested species responded to Bd infections by increasing their Tpref. We were able to detect differences in Tpref among individuals within a species, as well as differences in Tpref across species. Our methods for testing Tpref were identical for all species but At. zeteki and we found no evidence that acclimation temperature impacted species-level Tpref. Moreover, given that Ac. crepitans was acclimated to the lowest temperature and had one of the highest preferred temperatures and At. zeteki was acclimated to one of the higher temperatures and had the lowest preferred temperature, any undetected effect of acclimation temperature was probably small relative to any inherent species-level differences in temperature preference. We demonstrated that individual-level Tpref was correlated with Bd growth on frogs and that differences in species-level Tpref predicted the direction of this correlation. Though there were some effects of treatment on Tpref in two of the five species, we were unable to detect a significant behavioural fever response to Bd exposure across species. Our experimental findings suggest that previously reported field patterns correlating body temperature with Bd infection [9,25,34] were probably owing to standing variation in Tpref, where frogs that preferred warmer temperatures were less likely to be infected because of reduced Bd exposure and/or reduced Bd growth. Our study, with experiments performed across three laboratories and five species, is probably the most comprehensive test for behavioural thermoregulatory responses to Bd exposure in amphibian hosts.
Importantly, for each species, we demonstrated that variation among individuals in Tpref was greater than the variation in Tpref within an individual through time. That is, there was variation among individuals in their Tpref. Individuals often found a suitable thermal microhabitat and continuously chose that preferred temperature, even after being moved to the centre of the gradient each night. This variation among individuals represents the raw material upon which natural selection can act. Assuming that Tpref is heritable [35] via genetic or maternal effects [36], it stands to reason that over time a selective sweep could eliminate some of this variation, resulting in a change in average Tpref and a decrease in Bd prevalence [19]. Other disturbances that select for Tpref or reduce thermal microhabitat availability, such as climate change, deforestation, or disease, might also lead to population-level shifts in thermal microhabitat selection [37,38].
Additionally, we confirmed previous findings by detecting differences in Tpref among species that probably reflect their adaptations to environmental temperatures [24]. For example, At. zeteki was our coolest-preferring species and, not surprisingly, it is native to cool, mid-elevation sites in Central America where daily air temperatures remain in the mid to low-twenties (°C) year round [25]. By contrast, An. terrestris was our warmest preferring species, and it is native to warm, low elevation sites in the southeastern United States where mean temperatures in the summer reach into the high-twenties with average daily highs in the low-thirties (°C) [24]. While this study used slightly different methods to measure Tpref across these two species, we previously published that At. zeteki might prefer even cooler temperatures (Tpref 17.85 ± 0.14°C) [24] when tested using methods identical to those used for An. terrestris in this study. In this previous experiment, much lower minimum temperatures were available for At. zeteki to select (average low of 12°C compared to 19°C) than in the current experiment, which is probably why it had a lower temperature preference.
Although we experimentally tested for behavioural fever in both of the species that have been previously thought to respond to Bd exposure with fever (At. zeteki and An. americanus) [25,27], there was no evidence that those species or, for that matter, any of the five species exhibited a behavioural fever response to Bd. While our experimental results suggest that At. zeteki individuals which prefer warmer temperatures experience more rapid Bd growth, previous field studies showed that warmer At. zeteki were less likely to be infected with Bd than cooler preferring individuals in the population [25]. This inconsistency could be explained by differences in exposure given that Bd is considered saprophytic. In the absence of a host, Bd may persist better at low temperatures. If so, then At. zeteki which prefer warmer temperatures might have lower exposure to Bd. However, once exposed, Bd might grow faster on At. zeteki at higher than at lower temperatures.
We found that one species, Ac. crepitans, appeared to decrease preferred temperature after infection. The change in preferred temperature, however, did not appear to be beneficial to the host or pathogen as there was no difference in prevalence or survival between frogs in the regulating and non-regulating treatments. After prior exposure and heat clearance, individuals of O. septentrionalis, a species known to acquire immunological resistance to Bd [17], did not alter their thermoregulatory behaviour significantly. When we lumped the four treatments into exposed and sham-exposed, we did find that the Bd-exposed animals were warmer than the sham-exposed animals on day 3 and again on day 10. However, the day 3 differences were largely owing to the naive sham-exposed group sharply decreasing in temperature; there was no difference between the experienced sham-exposed and two Bd-exposed groups. Like the drop in temperature preference observed for Ac. crepitans, this change in preferred temperature did not appear to be beneficial to the host or pathogen as there was no difference in prevalence or survival between frogs in the regulating and non-regulating treatments. Hence, both of these changes are possibly spurious and do not appear to be biologically significant. We also found that allowing Bd to grow on hosts for a week before introducing them to the thermal gradients had no effect on the likelihood of exhibiting behavioural fever.
Our results suggest that previous field associations between host temperatures and Bd abundance were probably a result of the pre-existing variation in Tpref, rather than a change in thermoregulatory behaviour in response to infection. That is, frogs which already preferred warmer temperatures were less likely to be infected because their warmer temperatures caused them to either experience reduced Bd growth or avoid Bd exposure altogether. These results do not suggest that amphibians are incapable of behavioural fever, only that the species of anurans we tested did not respond to Bd with a behavioural fever response. In contrast to fungi, viral and bacterial pathogens have been shown to induce behavioural fevers in amphibians [39,40] as well as other ectothermic vertebrate and invertebrate hosts [11,12]. Additionally, our study controlled for moisture to avoid confounding Tpref with moisture preference. Thus, we cannot draw any conclusions about amphibians attempting to resist Bd infection by ‘drying-out’, a strategy that could be as effective at as behavioural fever [41].
We demonstrated that differences in species-level Tpref could predict the direction of the correlation between Tpref and Bd growth. The coolest preferring species (At. zeteki) had high Bd growth rates at relatively warm body temperatures, whereas the warmest preferring species (An. terrestris), had high Bd growth rates at relatively cool body temperatures. This result is consistent with the thermal mismatch hypothesis, which suggests that cool- and warm-adapted hosts might be more susceptible to disease outbreaks at abnormally warm and cool temperatures, respectively. This is hypothesized to occur because pathogens generally have wider thermal tolerances than their hosts [42], allowing them to outperform hosts under thermal mismatch conditions [24]. In addition to documenting temperature-dependent species-level variation in Bd susceptibility, our data also show that variation in Tpref among individuals can drive individual-level variation in disease susceptibility within a species. While field evidence showing variation in susceptibility and prevalence of Bd can be driven by variation in environmental temperature across individuals [9,25] and populations [21,43], there are very few studies that experimentally test how individual Tpref can drive differences in disease susceptibility within a population for this or any host–pathogen system.
In summary, none of the five host species tested exhibited a clear behavioural fever response to Bd infection but there were differences in individual-level Tpref that affected Bd growth. Additionally, we found species-level differences in the direction of the effect of individual-level Tpref on Bd growth that were consistent with the thermal mismatch hypothesis [24]. These results suggest that variation in Tpref within a population might be vital to buffer a species or populations against extirpation when a temperature-sensitive pathogen sweeps through an environment. Variation in Tpref might be more easily maintained in an ectothermic population when there are a wide variety of thermal microhabitats available. Thus, degradation of the thermal environment and microhabitat availability might reduce the ability of a species or population to buffer against temperature sensitive pathogens.
Supplementary Material
Acknowledgements
We thank the Maryland Zoo for providing us with the At. zeteki used in the experiments. We thank G. Addison, L. Brannelly, K. Medina, J. Nguyen, M. Robak, X. Sonn and A. Styf for their assistance in all aspects of the laboratory work and maintenance of experiments. In addition, we thank D. Civitello, J. Cohen, C. Haggerty, D. Jones, K. Nguyen, C. Ramsay, S. Rumschlag, K. Surbaugh for their helpful comments on the manuscript.
Ethics
Atelopus zeteki were obtained and used with permission from the Maryland Zoo, An. terrestris and O. septentrionalis were collected under permit with the Florida Fish and Wildlife Conservation Commission, and An. americanus and Ac. crepitans were collected under permit with the Illinois Department of Natural Resources. Experimental methods were approved by the Tulane, University of South Florida, and University of Illinois International Animal Care and Use Committees (protocols 0430R, 14112, and W IS00000548, respectfully).
Data accessibility
Data available from the Dryad Digital Repository at http://dx.doi.org/10.5061/dryad.643r37b [44].
Authors' contributions
E.L.S., C.L.R.-Z., J.H.S. and J.R.R. conceived ideas and designed experiments, E.L.S. and J.R.R. oversaw experiments in Tampa, FL, C.L.R.-Z. and J.S. oversaw experiments in New Orleans, LA, J.H.S. and R.C.F. oversaw experiments in Champaign, IL, E.L.S. and J.R.R. conducted statistical analyses, and E.L.S. and J.R.R. wrote the paper with comments and edits from R.C.F., C.L.R.-Z. and J.H.S. All authors agreed to submission of the manuscript and accept the responsibility for the accuracy and integrity of the manuscript.
Competing interests
We declare we have no competing interests.
Funding
Funds were provided by grants to J.R.R. from the Department of the Army, South Florida Caribbean Cooperative Ecosystems Studies Unit, National Science Foundation (EF-1241889 and DEB-1518681), the National Institutes of Health (R01GM109499, R01TW010286-01), the US Department of Agriculture (2009-35102-0543) and the US Environmental Protection Agency (CAREER 83518801), and by grants to C.L.R.-Z. from the National Science Foundation (1649443) and Louisiana Board of Regents (LEQSF (2011-14)-RD-A-26).
References
- 1.Daszak P, Cunningham AA, Hyatt AD. 2000. Emerging infectious diseases of wildlife: threats to biodiversity and human health. Science 287, 443–449. ( 10.1126/science.287.5452.443) [DOI] [PubMed] [Google Scholar]
- 2.Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, Gittleman JL, Daszak P. 2008. Global trends in emerging infectious diseases. Nature 451, 990–993. ( 10.1038/nature06536) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patz JA, Campbell-Lendrum D, Holloway T, Foley JA. 2005. Impact of regional climate change on human health. Nature 438, 310–317. ( 10.1038/nature04188) [DOI] [PubMed] [Google Scholar]
- 4.Rohr JR, Raffel TR, Romansic JM, McCallum H, Hudson PJ. 2008. Evaluating the links between climate, disease spread, and amphibian declines. Proc. Natl Acad. Sci. USA 105, 17 436–17 441. ( 10.1073/pnas.0806368105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rohr JR, Dobson AP, Johnson PTJ, Kilpatrick AM, Paull SH, Raffel TR, Ruiz-Moreno D, Thomas MB. 2011. Frontiers in climate change–disease research. Trends Ecol. Evol. 26, 270–277. ( 10.1016/j.tree.2011.03.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raffel TR, Romansic JM, Halstead NT, McMahon TA, Venesky MD, Rohr JR. 2013. Disease and thermal acclimation in a more variable and unpredictable climate. Nat. Clim. Chang. 3, 146–151. ( 10.1038/nclimate1659) [DOI] [Google Scholar]
- 7.Rohr JR, Raffel TR. 2010. Linking global climate and temperature variability to widespread amphibian declines putatively caused by disease. Proc. Natl Acad. Sci. USA 107, 8269–8274. ( 10.1073/pnas.0912883107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sunday JM, Bates AE, Dulvy NK. 2011. Global analysis of thermal tolerance and latitude in ectotherms. Proc. R. Soc. B 278, 1823–1830. ( 10.1098/rspb.2010.1295) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rowley JJL, Alford RA. 2013. Hot bodies protect amphibians against chytrid infection in nature. Sci. Rep. 3, 1515 ( 10.1038/srep01515) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Catenazzi A. 2015. State of the world's amphibians. Annu. Rev. Environ. Resour. 40, 91–119. ( 10.1146/annurev-environ-102014-021358) [DOI] [Google Scholar]
- 11.Burns G, Ramos A, Muchlinski A. 1996. Fever response in North American snakes. J. Herpetol. 30, 133–139. ( 10.2307/1565503) [DOI] [Google Scholar]
- 12.Thomas MB, Blanford S. 2003. Thermal biology in insect-parasite interactions. Trends Ecol. Evol. 18, 344–350. ( 10.1016/S0169-5347(03)00069-7) [DOI] [Google Scholar]
- 13.Reynolds WW, Casterlin ME, Covert JB. 1977. Febrile responses of aquatic ectotherms to bacterial pyrogens. Am. Zool. 17, 903 ( 10.1093/icb/17.1.121) [DOI] [Google Scholar]
- 14.Kluger MJ. 1992. Fever revisited. Pediatrics 90, 846–850. [PubMed] [Google Scholar]
- 15.Skerratt LF, Berger L, Speare R, Cashins S, McDonald KR, Phillott AD, Hines HB, Kenyon N. 2007. Spread of chytridiomycosis has caused the rapid global decline and extinction of frogs. Ecohealth 4, 125–134. ( 10.1007/s10393-007-0093-5) [DOI] [Google Scholar]
- 16.Woodhams DC, Alford RA, Marantelli G. 2003. Emerging disease of amphibians cured by elevated body temperature. Dis. Aquat. Organ. 55, 65–67. ( 10.3354/dao055065) [DOI] [PubMed] [Google Scholar]
- 17.McMahon TA, et al. 2014. Amphibians acquire resistance to live and dead fungus overcoming fungal immunosuppression. Nature 511, 224–227. ( 10.1038/nature13491) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chatfield MWH, Richards-Zawacki CL. 2011. Elevated temperature as a treatment for Batrachochytrium dendrobatidis infection in captive frogs. Dis. Aquat. Organ. 94, 235–238. ( 10.3354/dao02337) [DOI] [PubMed] [Google Scholar]
- 19.Greenspan SE, et al. 2017. Realistic heat pulses protect frogs from disease under simulated rainforest frog thermal regimes. Funct. Ecol. 31, 2274–2286. ( 10.1111/1365-2435.12944) [DOI] [Google Scholar]
- 20.Roznik EA, Alford RA. 2015. Seasonal ecology and behavior of an endangered rainforest frog (Litoria rheocola) threatened by disease. PLoS ONE 10, e0127851 ( 10.1371/journal.pone.0127851) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Forrest MJ, Schlaepfer MA. 2011. Nothing a hot bath won't cure: infection rates of amphibian chytrid fungus correlate negatively with water temperature under natural field settings. PLoS ONE 6, e28444 ( 10.1371/journal.pone.0028444) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schlaepfer MA, Sredl MJ, Rosen PC, Ryan MJ. 2007. High prevalence of Batrachochytrium dendrobatidis in wild populations of lowland leopard frogs Rana yavapaiensis in Arizona. Ecohealth 4, 421 ( 10.1007/s10393-007-0136-y) [DOI] [Google Scholar]
- 23.Venesky MD, Raffel TR, McMahon TA, Rohr JR. 2014. Confronting inconsistencies in the amphibian-chytridiomycosis system: implications for disease management. Biol. Rev. 89, 477–483. ( 10.1111/brv.12064) [DOI] [PubMed] [Google Scholar]
- 24.Cohen JM, Venesky MD, Sauer EL, Civitello DJ, McMahon TA, Roznik EA, Rohr JR. 2017. The thermal mismatch hypothesis explains host susceptibility to an emerging infectious disease. Ecol. Lett. 20, 184–193. ( 10.1111/ele.12720) [DOI] [PubMed] [Google Scholar]
- 25.Richards-Zawacki CL. 2010. Thermoregulatory behaviour affects prevalence of chytrid fungal infection in a wild population of Panamanian golden frogs. Proc. R. Soc. B 277, 519–528. ( 10.1098/rspb.2009.1656) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han BA, Bradley PW, Blaustein AR. 2008. Ancient behaviors of larval amphibians in response to an emerging fungal pathogen, Batrachochytrium dendrobatidis. Behav. Ecol. Sociobiol. 63, 241–250. ( 10.1007/s00265-008-0655-8) [DOI] [Google Scholar]
- 27.Karavlan SA, Venesky MD. 2016. Thermoregulatory behavior of Anaxyrus americanus in response to infection with Batrachochytrium dendrobatidis. Copeia 104, 746–751. ( 10.1643/CH-15-299) [DOI] [Google Scholar]
- 28.Sonn JM, Berman S, Richards-Zawacki CL. 2017. The influence of temperature on chytridiomycosis in vivo. Ecohealth 14, 762–770. ( 10.1007/s10393-017-1269-2) [DOI] [PubMed] [Google Scholar]
- 29.Sauer EL, Sperry JH, Rohr JR. 2016. An efficient and inexpensive method for measuring long-term thermoregulatory behavior. J. Therm. Biol. 60, 231–236. ( 10.1016/j.jtherbio.2016.07.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rowley JJ, Alford RA. 2007. Non-contact infrared thermometers can accurately measure amphibian body temperatures. Herpetol. Rev. 38, 308–316. [Google Scholar]
- 31.R Development Core Team. 2017. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 32.Lessells C, Boag PT. 1987. Unrepeatable repeatabilities: a common mistake. Auk 104, 116–121. ( 10.2307/4087240) [DOI] [Google Scholar]
- 33.Benjamini Y, Hochberg Y. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. B 57, 289–300. [Google Scholar]
- 34.Roznik EA, Sapsford SJ, Pike DA, Schwarzkopf L, Alford RA. 2015. Natural disturbance reduces disease risk in endangered rainforest frog populations. Sci. Rep. 5, 13472 ( 10.1038/srep13472) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paranjpe DA, Bastiaans E, Patten A, Cooper RD, Sinervo B. 2013. Evidence of maternal effects on temperature preference in side-blotched lizards: implications for evolutionary response to climate change. Ecol. Evol. 3, 1977–1991. ( 10.1002/ece3.614) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aubret F, Shine R. 2010. Thermal plasticity in young snakes: how will climate change affect the thermoregulatory tactics of ectotherms? J. Exp. Biol. 213, 242–248. ( 10.1242/jeb.035931) [DOI] [PubMed] [Google Scholar]
- 37.Sinervo B, et al. 2010. Erosion of lizard diversity by climate change and altered thermal niches. Science 328, 894–899. ( 10.1126/science.1184695) [DOI] [PubMed] [Google Scholar]
- 38.Turton SM, Siegenthaler DT. 2004. Immediate impacts of a severe tropical cyclone on the microclimate of a rain-forest canopy in north-east Australia. J. Trop. Ecol. 20, 583–586. ( 10.1017/S0266467404001622) [DOI] [Google Scholar]
- 39.Kluger MJ. 1977. Fever in frog Hyla cinerea. J. Therm. Biol. 2, 79–81. ( 10.1016/0306-4565(77)90042-0) [DOI] [Google Scholar]
- 40.Sherman E, Baldwin L, Fernandez G, Deurell E. 1991. Fever and thermal tolerance in the toad Bufo marinus. J. Therm. Biol. 16, 297–301. ( 10.1016/0306-4565(91)90021-S) [DOI] [Google Scholar]
- 41.Raffel TR, Halstead NT, McMahon TA, Davis AK, Rohr JR. 2015. Temperature variability and moisture synergistically interact to exacerbate an epizootic disease. Proc. R. Soc. B 282, 593–602. ( 10.1098/rspb.2014.2039) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rohr JR, Civitello DJ, Cohen JM, Roznik EA, Sinervo B, Dell AI, Hillebrand H In press. The complex drivers of thermal acclimation and breadth in ectotherms. Ecol. Lett. ( 10.1111/ele.13107) [DOI] [PubMed] [Google Scholar]
- 43.Zumbado-Ulate H, Bolanos F, Gutierrez-Espeleta G, Puschendorf R. 2014. Extremely low prevalence of Batrachochytrium dendrobatidis in frog populations from Neotropical dry forest of Costa Rica supports the existence of a climatic refuge from disease. Ecohealth 11, 593–602. [DOI] [PubMed] [Google Scholar]
- 44.Sauer EL, Fuller RC, Richards-Zawacki CL, Sonn J, Sperry JH, Rohr JR. 2018. Data from: Variation in individual temperature preferences, not behavioural fever, affects susceptibility to chytridiomycosis in amphibians Dryad Digital Repository. ( 10.5061/dryad.643r37b) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Sauer EL, Fuller RC, Richards-Zawacki CL, Sonn J, Sperry JH, Rohr JR. 2018. Data from: Variation in individual temperature preferences, not behavioural fever, affects susceptibility to chytridiomycosis in amphibians Dryad Digital Repository. ( 10.5061/dryad.643r37b) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data available from the Dryad Digital Repository at http://dx.doi.org/10.5061/dryad.643r37b [44].