Abstract
Study Design:
Prospective cohort study.
Objectives:
To evaluate the role of magnetic resonance imaging (MRI) in evaluation of fusion status following anterior lumbar interbody fusion (ALIF) and compare agreement and confidence in assessing fusion or its absence on MRI to the current standard computed tomography (CT).
Methods:
A prospective follow up of patients undergoing surgery by 2 spine surgeons between 2012 and 2015 at a single institution. Fusion was assessed at different time points in these patients by 2 independent musculoskeletal radiologists. Fusion was analyzed in coronal and sagittal planes using both imaging modalities, with confidence being attributed on a scale of 0 to 3. Assessors were blinded to patient data.
Results:
Fourteen patients (25 levels) with mean follow-up of 10.2 months (range 2.4-20.3 years) and age of 41 years (range 20.7-61.5 years) were assessed. MRI within the interbody cage in coronal (κ = .58) and sagittal (κ = .50) planes had the highest interobserver agreement. CT anterior to the cage in coronal (κ = .48) and sagittal (κ = .44) planes, as well as within the cage in coronal (κ = .50) and sagittal planes (κ = .44) showed moderate agreement. Confidence anterior to the interbody cage using MRI scan was reduced when compared with remaining angles and imaging modalities.
Conclusions:
The study demonstrates that MRI may be a useful tool in the assessment of fusion following ALIF with results comparable to CT, and that it may have a useful role in select patients especially considering marked radiation exposure reduction.
Keywords: anterior lumbar interbody fusion (ALIF), magnetic resonance imaging (MRI), fusion, computed tomography (CT)
Introduction
Spinal fusion was first described in the early 21st century by Albee1 and since then several approaches have been undertaken for the purpose of performing fusion. The anterior approach to lumbar interbody fusion (ALIF) was first described in 1932 by Capener2,3 and is associated with reduced operative times and shorter hospital stays compared with alternative approaches to lumbar interbody fusion.2,4,5 The chief advantage of ALIF is excellent exposure of the lower lumbar disc spaces, which permits thorough discectomy and placement of large, wedge-shaped, lordotic devices6 with other advantages, including restoration of disc height, reduced bleeding and reduced damage to the posterior musculoligamentous structures as well as indirect restoration of foraminal height.6
The aim of spinal fusion in the setting of degenerative disc disease is to obtain a stable arthrodesis between 2 or more adjacent vertebrae for the purpose of eliminating painful movements or recurrent prolapse from pathological discs. More broadly, fusion procedures have been used in the management of spinal tumors, infection, fracture, and deformity in addition to degenerative disc disease.7 Historically, the only means of being able to assess for spinal fusion involved surgical intervention with direct visual inspection.8,9 While open assessment of spinal fusion still remains a useful tool in experimental models, its everyday clinical use is now only appropriate during revision surgery. Noninvasive methods of assessing fusion include computed tomography (CT) scan, magnetic resonance imaging (MRI) scan as well as plain radiographic assessment. The current gold standard for assessment of lumbar interbody fusion is CT scan. Although displaying a higher ability to detect fusion in comparison with plain radiographs and the advantage of circumferential assessment,7,10,11 its use comes with exposure to ionizing radiation. There has been an increasing body of evidence that has linked the use of CT scans to an elevated risk of development of neoplasia in later life.12 This increased risk of malignancy has been stated to be as high as 1 in 3300 per CT scan of the lumbar spine.7,13
In contrast to CT scan, MRI scan is not associated with the increased risk of neoplasia and subsequent to this is increasingly being seen as a potential alternative for the assessment of fusion in spinal surgery. MRI scan also has the advantages of being able to better assess the neural elements, posterior elements and adjacent segments.7,14,15 The theoretical disadvantages also need to be considered and include cost, access and the potential for movement, heating or dysfunction of retained metallic implants and fragments. Although routinely used preoperatively prior to surgery, the utility of MRI at assessing fusion is as yet to be determined. In order to address this question, we aimed to compare the agreement and confidence between independent assessors in determining fusion using both MRI and CT postoperatively following ALIF. Although the role of MRI in evaluating lumbar fusion has been studied previously,14 to the authors knowledge this is the first study evaluating MRI for fusion in ALIF and also the first study to compare MRI and CT.
Methods
Cohort Sample
This is a prospective cohort study of consecutive series of patients who underwent ALIF for degenerative disc disease by 2 senior consultant spine surgeons between 2012 and 2015 at 2 hospitals. The cohort consisted of 14 patients—7 male and 7 female—with a mean age at time of surgery of 41 years (range 20.7-61.5 years). ALIF surgery was performed using a standard left retroperitoneal approach using carbon fiber cages (Anterior COUGAR cage, Depuy-Synthes, West Chester, PA, USA) and iliac crest bone graft. Anterior plate stabilization (Aegis Plate, Depuy-Synthes, West Chester, PA, USA) composed of titanium alloy was used to supplement the fixation.
Local ethics approval (St Andrews Hospital, Project 56) was obtained prior to patient enrolment in the study and all participants signed written consent to participate. All patients underwent CT scan (GE Healthcare Optima, 64 slices per rotation, minimum slice acquisition thickness of 0.625 mm) and MRI scan (Siemens Magnetom Aera 1.5 Tesla, 48 Channel System, XQ Gradients) assessment concurrently at postoperative review organized by the treating surgeons. Two independent consultant musculoskeletal radiologists assessed all scans on diagnostic quality monitor. All images were reviewed for fusion in relation to the interbody cages, with fusion anterior to, posterior, lateral, and within the interbody cages in both the coronal and sagittal planes assessed.
Criteria to Assess Fusion
The criteria for the determination of the degree of fusion on CT scan imaging followed the Brantigan-Steffee-Fraser (BSF) classification system specific for fusion in the setting of ALIF,16 in which bone bridges at least half of the fusion area with at least the density originally achieved at surgery. MRI scan criteria for fusion was also based on the same BSF classification.
Fusion was analyzed by 2 independent musculoskeletal radiologists in coronal and sagittal planes using both CT and MRI scans, with a level of confidence being attributed and graded on a scale of 0 to 3. Both specialists were blinded to patient identity of the data and were also blinded to each other’s assessment result for each patients’ imaging.
Confidence in the degree of the assessment was applied by each assessor using a 0 to 3 grading system where 0 was none, 1 minimal, 2 moderate, and 3 complete.
Patients were scheduled for follow-up 3 to 12 months following surgery with imaging then arranged. This time course was chosen as at this point the imaging is likely to demonstrate progression toward union or locked pseudoarthrosis rather than having complete fusion across the intervertebral segment. Indeed, other studies assessing imaging following posterior lumbar interbody fusion (PLIF) have suggested that although fusion can be evaluated using MRI scan and CT scan by the 12-month mark, further progress toward complete union can take up to 24 months or longer.14 The aim of our study was not to determine whether or not fusion had taken place, but whether CT scan and MRI scan had comparable agreement in regard to the level of fusion that had taken place at that time point in order to determine the use of MRI scan as an alternative modality in postoperative follow-up following ALIF.
Statistical Analysis
A statistician modeled adjusted kappa statistics (Table 1) with regard to intra- and interobserver agreement with the different techniques and angles assessed with confidence assessed using a regression analysis. The interrater agreement and 95% confidence intervals were computed using unweighted Fleiss’s kappa statistics for all patients. Fleiss’s kappa coefficient is related to Cohen’s kappa, but is able to compare inter- and intrarater reliability across more than 2 observers.17 A Fleiss’s κ coefficient less than 0 represents “poor agreement,” κ between .01 and .20 represents “light agreement,” κ between .21 and .40 represents “fair agreement,” κ between .41 and .60 represents “moderate agreement,” κ between .61 and .80 represents “substantial agreement,” and κ between .81 and 1.00 represents “almost perfect agreement”18 (Table 1). All statistics were calculated using Stata (StataCorp 2015, Stata Statistical Software: Release 14, College Station, TX, USA) with a P value of <.05 taken as statistically significant.
Table 1.
Fleiss’s Kappa and Interater Reliability Agreement.
| Kappa | Agreement |
|---|---|
| .01-.20 | Slight agreement |
| .21-.40 | Fair agreement |
| .41-.60 | Moderate agreement |
| .61-.80 | Substantial agreement |
| .81-.99 | Almost perfect agreement |
Results
There were 4 single-level ALIF and 10 multilevel ALIF surgeries with L4/5—(12), L5/S1—(11), and L3/4—(2) levels of ALIF. Patients were reviewed at a mean time post operatively of 10.2 months (range 2.4-20.3 months) with CT and MRI scans being performed at the same sitting. Overall, 25 levels of fusion were assessed with some levels having combined imaging on more than one occasion, giving a total of 38 levels reviewed. The combined total fusion rate within the interbody cage was 17.8%, with this being 28.0% on CT scan and 10.0% on MRI. The partial fusion rate was 72.2% overall, 70.1% on CT and 86.5% on MRI. The rate at which no union was identified within the interbody cage was 2.8% overall, 2.0% on CT with 3.6% on MRI.
On comparison of fusion status within the interbody cage in coronal plane, when CT reported partial fusion (Figure 1) there was a high correlation with MRI (mean 95%, range 88%-100%) but when CT reported complete fusion (Figure 2) the correlation with MRI was poor (mean 24%, range 0%-44%). Comparison within the interbody cage in the coronal planes again showed a high correlation with regard to partial fusion (mean 74%, range 61%-91%), but with poor agreement with regard to complete fusion (mean 25%, range 0%-71%).
Figure 1.
Computed tomography (CT) and magnetic resonance imaging (MRI) scans in coronal plane demonstrating complete union. (A, C, E) Coronal CT scans. (B, D, F) Coronal views of the same level and patient on MRI.
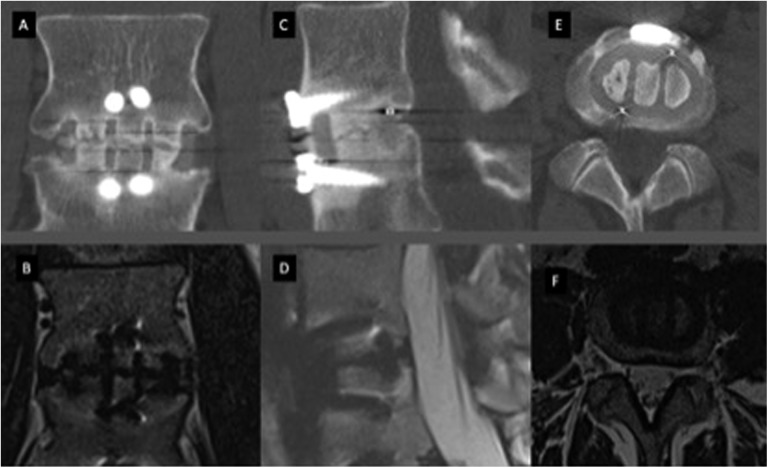
Figure 2.
Computed tomography (CT) and magnetic resonance imaging (MRI) scans of anterior lumbar interbody fusion showing incomplete union. (A, B) CT and MRI scans in coronal plane, respectively. (C, D) CT and MRI scans in sagittal plane, respectively. (E, F) CT and MRI scans in axial plane, respectively.
Overall confidence in reporting fusion status across all angles showed a statistically significant difference (P < .001) with the radiologist feeling more confident in reporting within cage fusion in CT and MRI in coronal and sagittal planes (Table 2). They were less confident in reporting fusion on MRI scan anterior to the interbody cage in both coronal and sagittal planes (Table 2). A similar lack of confidence was also seen when reporting anterior to the cage on CT. Overall mean confidence attributed to MRI anterior to the interbody cage was 0.58 and 0.55 in sagittal and coronal planes, respectively. All other angles had a mean confidence of >1.7, with the lowest value being found in CT scan anterior to the interbody cage in the coronal plane at 1.8 (Table 2).
Table 2.
Evaluation of Fusion: Confidence by Angle.
| Angle | Confidence |
|---|---|
| CT anterior coronal | 1.8 |
| CT anterior sagittal | 2.2 |
| CT within coronal | 2.7 |
| CT within sagittal | 2.7 |
| CT posterior coronal | 2.4 |
| CT posterior sagittal | 2.4 |
| CT lateral coronal | 2.3 |
| CT lateral sagittal | 2.3 |
| MRI anterior coronal | 0.6 |
| MRI anterior sagittal | 0.6 |
| MRI within coronal | 2.2 |
| MRI within sagittal | 2.3 |
| MRI posterior coronal | 2.1 |
| MRI posterior sagittal | 2.1 |
| MRI lateral coronal | 2 |
| MRI lateral sagittal | 2 |
Abbreviations: CT, computed tomography; MRI, magnetic resonance imaging.
Interobserver analysis showed no statistical significant difference across all angles (P = .2). The highest level of agreement was found on MRI scan on assessment of fusion within the interbody cage in coronal (κ = .58) and sagittal planes (κ = .50), showing moderate agreement (Table 3). Moderate agreement was also found on CT scan both anterior to and within the interbody cages in coronal and sagittal planes (Table 3). There was no difference found when directly assessing CT versus MRI with regard to interobserver assessment (P = .05).
Table 3.
Evaluation of Fusion: Kappa Values and Agreement by Angle.
| Angle | Kappa | Agreement |
|---|---|---|
| CT anterior coronal | .48 | Moderate |
| CT anterior sagittal | .44 | Moderate |
| CT within coronal | .50 | Moderate |
| CT within sagittal | .44 | Moderate |
| CT posterior coronal | .05 | Slight |
| CT posterior sagittal | .04 | Slight |
| CT lateral coronal | .02 | Slight |
| CT lateral sagittal | <.01 | None |
| MRI anterior coronal | <.01 | None |
| MRI anterior sagittal | <.01 | None |
| MRI within coronal | .58 | Moderate |
| MRI within sagittal | .50 | Moderate |
| MRI posterior coronal | <.01 | None |
| MRI posterior sagittal | <.01 | None |
| MRI lateral coronal | .02 | Slight |
| MRI lateral sagittal | .02 | Slight |
Abbreviations: CT, computed tomography; MRI, magnetic resonance imaging.
Intraobserver analysis across all planes also failed to show any statistically significant difference (P = .35). However, when comparing CT scan versus MRI scan on fusion status, there was a statistically significant difference when all planes were included (P = .002). As anterior to interbody fusion status determination on MRI was difficult, when results anterior to the interbody cage were removed there was no statistically significant difference (P = .26).
Discussion
Determination of fusion status is an important aspect of any fusion procedure, although its relevance to clinical outcome is contentious in asymptomatic patients. Several radiological parameters are used, including static and dynamic radiographs, CT scan, and MRI scan. Fine cut CT by far has the highest confidence in reporting with almost 89% confidence7,11 and hence was used as the gold standard. Lang et al19 found that interpretation of sagittal and curved coronal multiplanar reconstruction (MPR) was more reliable than any other imaging method applied to the detection of spinal fusion. In a series of 30 patients with posterior lumbar fusion, they were able to identify pseudarthrosis in 4 patients using the above modality, all of which were confirmed at surgery. This comes with an added radiation exposure, which is quite significant.
MRI scan has rarely been used to assess lumbar fusion except in a series by Kroner et al.14 They found successful bony bridging in 49 cases using carbon fiber cages in PLIF. But they did not compare this with CT or radiographic assessment of fusion. Similar work in cervical spine has been performed but not using appropriate sequences, with the coronal plane not assessed or metallic artifact being reduced. Hence our study was aimed at assessing fusion status on MRI and CT with time point being early fusion rather than complete fusion. This study as per the authors view is the first study to compare CT and MRI in ALIF for fusion status.
Our results have demonstrated that when assessed across all angles in relation to the interbody cages in both the sagittal and coronal planes no statistically significant difference was found, either in inter- or intraobserver agreement. However, when grouping all angles assessed using CT scan and comparing them with MRI scan, a statistical significance was noted with regard to intraobserver assessment of fusion (P = .002). In our study the assessment of fusion anterior to the interbody cages with CT scan, but most notably with MRI scan, was the most challenging, with the lowest levels of confidence being attributed to radiological assessment in this region (Table 2). Previous work has suggested that the assessment of fusion in the region anterior to the interbody cage is the least reliable indicator of whether true fusion has taken place.20 Because of the difficult nature of assessment anterior to the interbody cages, exclusion of these results from the data set meant that the statistical significance found in regard to the intraobserver analysis of fusion between MRI scan and CT scan was no longer observed (P = .26). Although interobserver assessment of CT versus MRI scan did not show statistical significance, the P value of .05 could suggest that in a larger sample size may well show statistical significance.
Further to this, we evaluated CT against MRI in determining fusion within the interbody cage. When assessing in both coronal and sagittal planes the P value was now .58, which additionally supports comparability of MRI and CT scan in assessing fusion within the interbody cages. Our results further support the difficulty in assessing fusion anterior to interbody cages. By excluding data for the assessment anterior to the interbody cage and by comparing the evaluation within the interbody cages, our findings suggest that CT scan and MRI scan are comparable in the determination of fusion following ALIF surgery. We believe from these results that MRI assessment of fusion in ALIF is best assessed in coronal plane within the cage.
There were more complete fusions on CT than MRI, 28% and 10%, respectively. This could be due to a partial voluming artifact, with CT overcalling the fusion status. McAfee et al21 felt that at 6 months CT scan would not be able to differentiate between avascular and live bone formation in the fusion mass. Could MRI be more accurate? Further studies are required with comparison at longer follow-up.
The goal of surgery in patients undergoing spinal arthrodesis is the elimination of movement across the spinal segments with the aim in reduction of patient symptoms. Failure to obtain fusion does not necessarily obviate clinical success. Indeed, attaining a good clinical outcome with reduction of axial back pain and leg pain can be achieved without bridging trabecular bone found between these intervertebral segments.22 As has been previously noted by Fraser et al,22 good clinical outcomes in the absence of matching radiologic fusion and conversely poor clinical outcomes despite radiologic evidence of fusion led to the concept of a “locked pseudoarthrosis,” in which patients may not have a full fusion but the segments are behaving as if fused.
One of the long-term sequelae of spinal arthrodesis is adjacent segment disease (ASD) in which there is degeneration of the intervertebral disc directly above or below the fused segment.6,23 The relevant pathology can include foraminal and spinal stenosis as well as disc herniation, which may be associated with subsequent symptomatology. MRI is generally used to further characterize pathology in the adjacent segments. The incidence of ASD is at least 5 to 10 years from the index surgery and selecting MRI as modality of choice for assessing fusion along with ASD is not an indication as such.
Although our study only investigates patients who have undergone ALIF, other approaches to lumbar fusion have been previously described.6,24 Kröner et al14 had previously investigated the use of MRI scan in the assessment of fusion in patients having undergone PLIF. In their study, they found that MRI scan images in the coronal plane best demonstrated bony fusion, which would be consistent with MRI scan results in our own dataset. However, to our knowledge this is the first time that MRI and CT scan have been directly compared in their ability to assess fusion.
Although we do not advocate the routine use of CT scan or indeed MRI scan in the postoperative assessment of all patients who have undergone ALIF, imaging assessment of fusion is important clinically and medicolegally in documenting the progression of fusion. This is particularly relevant in those individuals whom have ongoing symptoms that are potentially attributable to failure of fusion. Previous work has suggested that CT scans had an 89% correspondence for detecting fusion when compared to intraoperative assessment.7,11 The ionizing radiation exposure associated with such assessment however is something that must be considered when further imaging is requested. In comparison with CT imaging, assessment using plain radiographic imaging is associated with significantly reduced radiation exposure; however, this decreased radiation burden is also associated with reduced concordance with regard to intraoperative assessment of spinal fusion.10,22 The reliability, sensitivity, specificity, and positive predictive value of MRI in assessing fusion following lumbar fusion is currently unknown and requires further investigation. Should MRI scan show comparable results to CT scan in assessing fusion then in that instance, it could be recommended for definitive assessment of fusion. In particular, it may be considered in younger patient groups who are likely to have an increased lifetime risk of malignancy from subsequent further investigation using modalities associated with radiation exposure.
Limitations
There are several limitations to the study. The sample size is small and hence further studies with larger sample size are required to validate the study results. Furthermore, the follow-up periods of the included patients were variable, which can also undermine the validity of the presented results. Relative CT and MRI concordance with regard to the time points of fusion progression were not assessed in the present study.
Conclusions
Our data suggests that MRI could potentially be equivalent to CT in assessment of fusion in anterior lumbar interbody fusion. With regard to the reduced radiation burden, as well as the ability to further assess the neural elements, MRI could be considered as an alternative imaging modality in this patient group where possible further intervention may be required. Further studies on MRI in assessing lumbar fusion are required prior to recommending it in the routine review of lumbar fusion.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr Michael Selby has received fellowship support from Depuy Synthes and LifeHealthCare. He has also consulted with Depuy Synthes, K2M, and LifeHealthCare.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Mario Zotti  http://orcid.org/0000-0003-2385-3914
http://orcid.org/0000-0003-2385-3914
References
- 1. Albee FH. Transplantation of a portion of the tibia into the spine for Pott’s disease: a preliminary report 1911. Clin Orthop Relat Res. 2007;460:14–16. [DOI] [PubMed] [Google Scholar]
- 2. Rao PJ, Ghent F, Phan K, Lee K, Reddy R, Mobbs RJ. Stand-alone anterior lumbar interbody fusion for treatment of degenerative spondylolisthesis. J Clin Neurosci. 2015;22:1619–1624. [DOI] [PubMed] [Google Scholar]
- 3. Stender W, Meissner HJ, Thomas W. Ventral interbody spondylodesis using a new plug-shaped implant. Neurosurg Rev. 1990;13:25–34. [DOI] [PubMed] [Google Scholar]
- 4. Burke PJ. Anterior lumbar interbody fusion. Radiol Technol. 2001;72:423–430. [PubMed] [Google Scholar]
- 5. Riouallon G, Lachaniette CHF, Poignard A, Allain J. Outcomes of anterior lumbar interbody fusion in low-grade isthmic spondylolisthesis in adults: a continuous series of 65 cases with an average follow-up of 6.6 years. Orthop Traumatol Surg Res. 2013;99:155–161. [DOI] [PubMed] [Google Scholar]
- 6. Mobbs RJ, Loganathan A, Yeung V, Rao PJ. Indications for anterior lumbar interbody fusion. Orthop Surg. 2013;5:153–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Selby MD, Clark SR, Hall DJ, Freeman BJC. Radiologic assessment of spinal fusion. J Am Acad Orthop Surg. 2012;20:694–703. [DOI] [PubMed] [Google Scholar]
- 8. Cook SD, Patron LP, Christakis PM, Bailey KJ, Banta C, Glazer PA. Comparison of methods for determining the presence and extent of anterior lumbar interbody fusion. Spine (Phila Pa 1976). 2004;29:1118–1123. [DOI] [PubMed] [Google Scholar]
- 9. Buchowski JM, Liu G, Bunmaprasert T, Rose PS, Riew KD. Anterior cervical fusion assessment: surgical exploration versus radiographic evaluation. Spine (Phila Pa 1976). 2008;33:1185–1191. [DOI] [PubMed] [Google Scholar]
- 10. Kant AP, Daum WJ, Dean SM, Uchida T. Evaluation of lumbar spine fusion. Plain radiographs versus direct surgical exploration and observation. Spine (Phila Pa 1976). 1995;20:2313–2317. [PubMed] [Google Scholar]
- 11. Carreon LY, Djurasovic M, Glassman SD, Sailer P. Diagnostic accuracy and reliability of fine-cut CT scans with reconstructions to determine the status of an instrumented posterolateral fusion with surgical exploration as reference standard. Spine (Phila Pa 1976). 2007;32:892–895. [DOI] [PubMed] [Google Scholar]
- 12. Mathews JD, Forsythe AV, Brady Z, et al. Cancer risk in 680,000 people exposed to computed tomography scans in childhood or adolescence: data linkage study of 11 million Australians. BMJ. 2013;346:f2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Biswas D, Bible JE, Bohan M, Simpson AK, Whang PG, Grauer JN. Radiation exposure from musculoskeletal computerized tomographic scans. J Bone Joint Surg Am. 2009;91:1882–1889. [DOI] [PubMed] [Google Scholar]
- 14. Kröner AH, Eyb R, Lange A, Lomoschitz K, Mahdi T, Engel A. Magnetic resonance imaging evaluation of posterior lumbar interbody fusion. Spine (Phila Pa 1976). 2006;31:1365–1371. [DOI] [PubMed] [Google Scholar]
- 15. Lang P, Chafetz N, Genant HK, Morris JM. Lumbar spinal fusion. Assessment of functional stability with magnetic resonance imaging. Spine (Phila Pa 1976). 1990;15:581–588. [DOI] [PubMed] [Google Scholar]
- 16. Fogel GR, Toohey JS, Neidre A, Brantigan JW. Fusion assessment of posterior lumbar interbody fusion using radiolucent cages: X-ray films and helical computed tomography scans compared with surgical exploration of fusion. Spine J. 2008;8:570–577. [DOI] [PubMed] [Google Scholar]
- 17. Fleiss JL. Measuring nominal scale agreement among many raters. Psychol Bull. 1971;76:378–382. [Google Scholar]
- 18. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 19. Lang P, Genant HK, Chafetz N, Steiger P, Morris JM. Three-dimensional computed tomography and multiplanar reformations in the assessment of pseudarthrosis in posterior lumbar fusion patients. Spine (Phila Pa 1976). 1988;13:69–75. [DOI] [PubMed] [Google Scholar]
- 20. Burkus JK, Foley K, Haid RW, LeHuec JC. Surgical Interbody Research Group—radiographic assessment of interbody fusion devices: fusion criteria for anterior lumbar interbody surgery. Neurosurg Focus. 2001;10:E11. [DOI] [PubMed] [Google Scholar]
- 21. McAfee PC, Boden SD, Brantigan JW, et al. Symposium: a critical discrepancy-a criteria of successful arthrodesis following interbody spinal fusions. Spine (Phila Pa 1976). 2001;26:320–334. [DOI] [PubMed] [Google Scholar]
- 22. Santos ERG, Goss DG, Morcom RK, Fraser RD. Radiologic assessment of interbody fusion using carbon fiber cages. Spine (Phila Pa 1976). 2003;28:997–1001. [DOI] [PubMed] [Google Scholar]
- 23. Park P, Garton HJ, Gala VC, Hoff JT, McGillicuddy JE. Adjacent segment disease after lumbar or lumbosacral fusion: review of the literature. Spine (Phila Pa 1976). 2004;29:1938–1944. [DOI] [PubMed] [Google Scholar]
- 24. Macki M, Bydon M, Weingart R, et al. Posterolateral fusion with interbody for lumbar spondylolisthesis is associated with less repeat surgery than posterolateral fusion alone. Clin Neurol Neurosurg. 2015;138:117–123. [DOI] [PubMed] [Google Scholar]