Abstract
Study Design:
In vitro cadaveric biomechanical study.
Objectives:
Despite numerous techniques employed to establish solid lumbosacral fixation, there are little biomechanical data correlating fixation methods at L5/S1 to thoracolumbosacral (TLS) construct length. We aimed to determine the optimal construct with the hypothesis that under physiological loads, lumbosacral constructs can be stabilized by L5/S1 anterior lumbar interbody fusion (ALIF) alone, without iliac screw fixation (ISF), and that TLS constructs would require ISF, with or without ALIF.
Methods:
By using a robot capable of motion in 6 axes, force-moment sensor, motion-tracking camera system and software, we simulated the spinal loading effects in flexion-extension, axial rotation, and lateral bending, and compared torques in different construct groups of T4-S1, T10-S1, and L2-S1. By conducting multidirectional flexibility testing we assessed the effects of constructs of various lengths on the L5/S1 segment.
Results:
L2-S1 constructs may be equivalently stabilized by L5/S1 ALIF alone without ISF. Longer TLS constructs exerted increasing motion at L5/S1, exhibiting trends in favor of ISF when extending to T10 and statistically improved fixation when extending to T4. Lastly, TLS constructs with ISF exhibited a statistically significant reduction in L5-S1 range of motion from the addition of ALIF when extending to T4-pelvis but not T10-pelvis.
Conclusions:
We found that ALIF alone may sufficiently support the L2-S1 construct, reducing L5/S1 range of motion and transmitting loads instead to the sacropelvis. Furthermore, ALIF was found to add significant stability to the T4-pelvis construct when added to ISF. This difference was not significant for the T10-pelvis construct.
Keywords: ALIF, anterior lumbar interbody fusion, biomechanics, iliac screw fixation, lumbosacral fixation, sacroiliac fixation
Introduction
The lumbosacral junction is a susceptible transition point between the mobile lumbar spine and the rigid pelvis. Subject to high rates of failure and instability, this level can experience up to 67% to 69% radiographic adjacent segment degeneration following instrumentation with long constructs terminating at L5.1,2 Studies have shown that up to 22% of adolescent scoliosis constructs terminating in the lower lumbar spine required revision within a 15-year follow up period.3,4 The sacropelvis is often included in cases of deformity correction and/or revision including those requiring osteotomy, high-grade spondylolistheisis, and “long” thoracolumbar constructs.1,5-9 What constitutes a long posterior construct, and when sacropelvic fixation is required are unclear at this time, and the optimal sacropelvic fixation technique for varying construct lengths is yet to be determined.5,10
Biomechanical and clinical evidence supports that iliac screws remain the strongest and the most stable method of sacropelvic fixation.7 However, iliac screws also may increase operative time, blood loss, postoperative sacroiliac (SI) joint pain, and rarely cause neurovascular injury.7 Given the associated morbidity, iliac screws are not considered “the standard of care” for all long lumbar and thoracolumbar constructs. Other options include pedicle screw fixation alone with posterolateral arthrodesis, or in combination with interbody fusion. From a kinematic perspective, we know interbody devices in the lower lumbar spine convey tremendous loads, stiffen posterior constructs, and may obviate the need for iliac fixation in constructs under a certain length. However, interbody devices, specifically anterior lumbar interbody fusion (ALIF), have been shown to have diminishing returns as the length of the lumbosacral construct decreases.6
Despite tremendous advancements in sacropelvic fixation biomechanics and techniques, a number of important clinical questions remain. Herein, we hypothesized that under physiological loads, long lumbosacral constructs (L2-S1) can be equivalently stabilized by L5/S1 ALIF without iliac screw fixation (ISF). Second, we hypothesized that longer thoracolumbosacral (TLS) constructs would exert increasing motion at the L5/S1 interspace, which would require ISF support. Finally, in TLS constructs with ISF, we hypothesized that the addition of ALIF would reduce L5/S1 range of motion (ROM) to a greater extent than ISF alone.
Methods
Seven fresh-frozen human cadaveric specimens were used for the in vitro biomechanical study, and were obtained from Science Care (Phoenix, AZ, USA). Specimens were obtained based on requests for 1 specimen with intact torso (T1-pelvis) and 6 separate specimens with intact musculoskeletal attachments from L1 to the pelvis (1 female, 6 males, age range 34-85 years, mean age 68 years). We conducted multidirectional flexibility testing to assess the effects of increasingly long TLS constructs on the L5/S1 segment. The specimens were sealed in double plastic bags and kept frozen at −20°C with all ligamentous articulations intact. All specimens were screened with computed tomography (CT) scans to exclude those with neoplasm, marked degenerative changes, and congenital anomalies of vertebrae. Soft tissue was carefully removed while keeping supraspinous/interspinous ligaments (SIL), SI joint, and facet capsules intact. Custom-made fixtures were fashioned to affix the rostral vertebra and caudal aspect (pelvis) onto the spine testing apparatus. The study was divided into 2 phases. The first phase used a single specimen (T1-pelvis) to determine the loads to apply to the spine to best replicate the clinical scenario. The second phase used 7 specimens (L2-pelvis) to determine the relative spinal motions that occur under the different surgical and loading conditions. Six of the 7 specimens were new, and 1 was the initial single specimen cut down and remounted (L2-pelvis).
Spinal Loading Technique
An industrial robot (KUKA, GmbH, Augsburg, Germany) capable of motion in 6 axes was used as the spine testing apparatus for implementing in vitro flexibility tests. A 6-axes, force-moment sensor (ATI-Delta, Apex, NC, USA) was used to measure the applied load and provide feedback for the controller software. Motion tracking was performed using an optoelectronic camera system (Optotrak, Northern Digital Inc, Waterloo, Ontario, Canada) with accuracy 0.1 mm and 0.02°. The controller software, simVITRO (Cleveland Clinic, Cleveland, OH, USA), collected and time-synchronized all kinetic and kinematic data, and controlled the robot. The robot was programmed to apply 3 continuous loading and unloading cycles of pure moment (maximum ± 10 N·m) in force/torque control along each of the primary axes of the spine to simulate flexion-extension (FE), axial rotation (AR), and lateral bending (LB) while measuring and minimizing off-axis forces and moments. The relative vertebral motion was determined by creating coordinate systems within simVITRO that use the digitized anatomical landmarks for each level, per International Society of Biomechanics standards, relative to infrared markers that were placed on each vertebral segment.11 Two markers were placed in the pelvis (right, left), and sacroiliac motion was determined by an average of the 2 motions. ROM for each primary axis was determined as the kinematic difference between the positive and negative loading conditions (eg, kinematics at +10 N·m vs −10 N·m). Specimens were regularly sprayed with 0.9% saline solution to prevent dehydration during load testing.
Torque Ratio Determination
In the first phase of the study, a single specimen of full length (T2-pelvis) was used to determine which loading conditions to apply to the 7 specimens of reduced length (L2-pelvis). One technical challenge with the study design was to be able to vary both the construct length (T4-S1, T10-S1, and L2-S1) and surgical technique (with and without ALIF, with and without iliac screws), while ensuring the initial stability prior to any forces applied. To perform a full factorial study design where each surgical condition was configured on each specimen carries with it the risk of introducing other variables that could confound the results, a novel, variable torque, loading technique was developed, based on Panjabi’s hybrid loading protocol.12
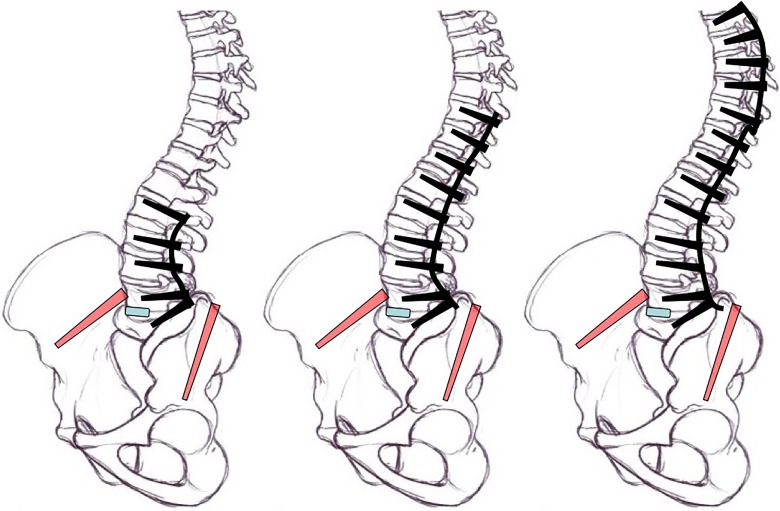
Panjabi’s hybrid loading protocol predicts that longer constructs will increase the torque, and thus, the range of motion at those levels increases.12 He developed the hybrid loading protocol to analyze adjacent level disease where he measured the motion for a desired loading state, and measured the increased loads and changes in relative vertebral motions at each level after fusion.12 The principle was that increasing fusion length increases the applied torque required to bend the spine. Using this concept, we sought to use a long specimen (T1-pelvis), to best replicate the clinical scenario, and determine the relative loading for the following 3 surgical conditions (Figure 1).
Figure 1.
Varying lengths of baseline testing constructs (black) with modifications in blue (TLIF or ALIF interbody device) and red (iliac screws). ALIF, anterior lumbar interbody fusion; TLIF; transforaminal lumbar interbody fusion.
We used the Panjabi protocol, but determined the length/torque relationship in an inverse experimental order. Instead of measuring increases in torques due to fusing more levels, we measured decreases in applied torques as the number of fused levels was decreased. This methodology yielded the length/torque ratios for the different construct lengths, while preventing supraphysiological loads from being applied throughout the test.
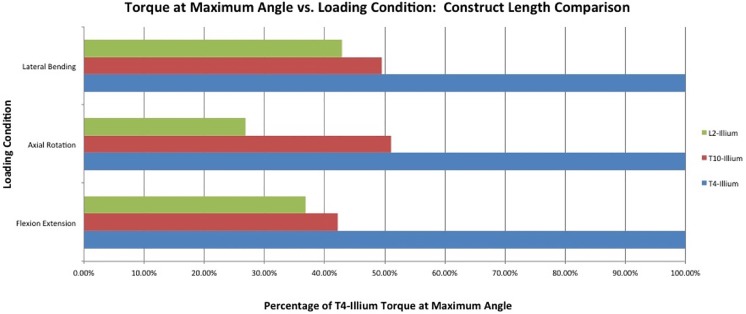
First, the long T4-S1 construct was loaded with ±10 N·m in the FE, LB, and AR axes. The terminal positions at the maximum load were then set as the target positions for subsequent construct lengths. The specimen was then moved to those target positions in the primary axis with a T10-S1 construct and then the L2-S1 construct, while the off-axis degrees of freedom remained in force/torque control in order to minimize loads. The reduction in torque at these target positions was recorded for each construct length. By implementing this inverse Panjabi protocol, we avoided reaching supraphysiological loads and were able to estimate three relative torque values that may simulate the relative loading at the base of the spine due to each of the 3 surgical constructs (Figure 2). Not all loading directions produced the same torque ratios between conditions. Since these ratios were calculated with data from a single specimen, and assumptions in Panjabi’s hybrid protocol subject to uncertainty, the torque ratios for all axes were computed using the maximum load in any of the axes, rounded to the nearest 10%, rather than underestimate the potential loads applied to the base of the spine.
Figure 2.
The percentage of the T4-S1 construct torque at maximum angle during each loading condition.
Test Design
The 3 torque targets acquired (simulated fusion lengths) were then applied to 7 lumbosacropelvic specimens (L2-pelvis) in 4 testing conditions (Table 1). At each testing condition, the 3 torque levels, which were surrogates for fusion length, were applied in a randomized order (10 N·m, 5 N·m, and 4 N·m). In addition, at each torque level three loading directions were applied: FE, LB, and AR. Surgical procedures were performed by neurosurgery residents experienced with this technique and under the direction of the principle investigator. Pedicle and iliac screws were placed via free hand techniques described elsewhere using the “Revere” Thoracolumbar Instrumentation System (Globus Medical, Inc, Audubon, PA, USA).13 The ALIF procedure involved performing a box annulotomy at L5-S1, then removing the cartilaginous endplates with a series of curettes. The ALIF grafts were then sized for each specimen using various trials, then PEEK (polyetherether ketone) interbody spacers were inserted from the “Continental” interbody spacer system (Globus Medical, Inc, Audubon, PA, USA). The spacers were secured in place using an anterior lumbar staple from the same system. Once the screws were placed, they were never removed or replaced after the initial placement, and instead, were simply disconnected from the rods, in order to minimize the weakness that could have been created in the construct from the implantation, removal, and re-placement of the screws.
Table 1.
Four Test Conditions.
| Test Condition | Description |
|---|---|
| 1 | Native |
| 2 | PS + ISF |
| 3 | PS + ISF + ALIF |
| 4 | PS + ALIF |
Abbreviations: PS, pedicle screw fixation; ISF, iliac screw fixation; ALIF, anterior lumbar interbody fixation.
Data Analysis
L5-S1 and sacroiliac ROM were determined in each primary loading axis for each surgical condition and simulated construct length. In addition, a normalized ROM was determined (% of ROM at 10 N·m loading in the native test condition). Predictive models for L5-S1 and sacroiliac ROM were developed using the methods of repeated measures mixed models. Models were first constructed using all the data. The pattern of the model residuals plotted against samples was examined. Samples, whose residual patterns indicated their presence might exert undue influence on model term significance, were removed from the data set, and the analysis was rerun. The models resulting from the full and the reduced data set were compared. While there were numeric differences in prediction error and model term coefficients, none of these differences were statistically significant, nor was there any comparable difference between the residual plots of models based on either the full or the reduced data sets. Therefore, the models based on the full data set were summarized and used for prediction purposes.
Results
Torque Ratio Determination
A torque of 10 N·m in force control was imposed on a T1-pelvis specimen instrumented from T4-S1 (Figure 3). The maximum angle achieved in FE was +14.8° in flexion, −7.4° in extension, producing a range of 22.2°. The T10-S1 and L2-S1 constructs were moved to similar positions in flexion and extension, requiring torques 42% and 37% of initial torque, respectively (Figure 2). In AR, the T4-S1 condition achieved 64° of motion. The T10-S1 and L2-S1 constructs were moved to similar positions in AR, requiring torques 51% and 27% of initial torque, respectively. In LB, the T4-S1 condition achieved 9° of total motion. The T10-S1 and L2-S1 constructs were moved to similar positions in LB, requiring torques 50% and 43% of initial torque, respectively. The torque ratios were established such that (1) T4-S1 fusion was represented by 10 N·m, (2) T10-S1 fusion was represented by 5 N·m, and (3) L2-S1 fusion was represented by 4 N·m (Table 2).
Figure 3.
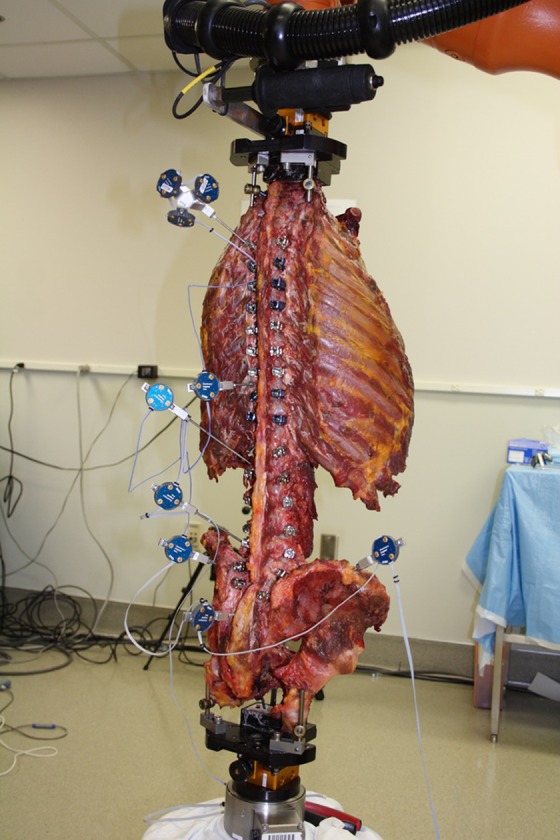
T1-pelvis specimen. This human cadaveric specimen is mounted and configured with appropriate optoelectric sensors. The construct depicted here is T4-S1, to which 10 N·m of torque was applied for each loading condition: FE, AR, LB, and maximum ROM recorded. AR, axial rotation; flexion-extension; LB, lateral bending; ROM, range of motion.
Table 2.
Experimentally Determined Torque Ratios Between Various Construct Lengths.
| Construct Length | Torque (Percent of T4-S1 Construct) |
|---|---|
| T4-S1 | 100 |
| T10-S1 | 50 |
| L2-S1 | 40 |
Native Specimens
When tested at 100% torque (10 N·m), the L5/S1 segment displayed an average ROM of 12.91° (±6.9°), 4.64° (±2.77°), and 7.42° (±1.34°) in FE, AR, and LB respectively. The SI joint ROM was 1.29° (±0.22°), 0.71° (±0.30°), and 0.14° (±0.07°) in FE, AR, and LB respectively.
Statistical Analysis
The models resulting from the full and the reduced data set were compared. Comparisons were made between surgical conditions (with or without iliac screws, and with or without ALIF) at each construct length (L2-S1 vs T10-S1 vs T4-S1). All statistical analysis was performed using SAS 9.4 Software (SAS Institute, Cary, NC, USA), and P < .05 was considered statistically significant.
L2-S1 Constructs
Pedicle screw fixation (PSF) with ISF resulted in statistically significant decreases in ROM, relative to the native specimen, across L5/S1: −95%, −87%, and −97% (P < .0001, P = .0012, P < .0001) in FE, AR, and LB respectively. Motion across the SI joint was also decreased in a statistically significant manner: −57%, −53% (P < .0001, P = .003) in FE and AR, respectively. The addition of ALIF support to the ISF displayed no statistical changes in L5/S1 ROM (P = .66, P = .97, P = .98) or SI joint ROM (P = .14, P = .50, P = .93), in FE, AR, and LB, respectively.
ALIF support alone (no ISF) for the L2-S1 construct resulted in statistically significant decreases in ROM across L5/S1: −81%, −66%, and −78% (P < .0001, P < .011, P < .0001) in FE, AR, and LB, respectively. When comparing ALIF support alone to ISF alone, there was no significant difference in ROM across L5/S1 in FE (14%, P = .127), AR (21%, P = .402), or LB (20%, P = .198). However, SI ROM had statistically increased in FE (59%, P < .0001) and AR (37%, P = .039) in the ALIF construct alone as compared to ISF alone.
T10-S1 Constructs
PSF with ISF resulted in statistically significant decreases in ROM, relative to the native specimen, across L5/S1: −95%, −85%, and −97% (P < .0001, P = .015, P < .0001) in FE, AR, and LB, respectively. The addition of ALIF support to ISF resulted in no statistical change in L5/S1 ROM (P = .74, P = .98, P = .98), or in SI ROM (P = .30, P = .86, P = .88). ALIF support alone for the T10-S1 construct resulted in statistically significant decreases in ROM, relative to the native specimen, across L5/S1: −81%, −61%, and −76% (P < .0001, P = .075, P < .0001) in FE, AR, and LB, respectively. When comparing ALIF support alone to ISF alone, there was no significant difference in FE (14%, P = .186), AR (24%, P = .47), or LB (21%, P = .22). However, SI ROM had statistically increased in FE (59%, P < .0001) and AR (45%, P = .016) in the ALIF construct alone as compared with ISF alone.
T4-S1 Constructs
We found statistically significant reduction of motion across L5/S1 in each surgical condition and in each loading direction as compared with the native condition: −91%, −76%, and −93% (P < .0001, P = .0054, P < .0001) in FE, AR, and LB, respectively. At this construct length, the addition of ALIF support to ISF resulted in no statistically significant changes in ROM at L5/S1 (P = .59, P = .95, P = .97), or in SI joint ROM (P = .75, P = 1.0, P = .93) in FE, AR, and LB respectively. ALIF support alone (no ISF) for the T4-S1 construct resulted in statistically significant decreases in ROM, relative to the native specimen, across L5/S1: −75%, −49%, and −65% (P < .0001, P = .0638, P = .0004,) in FE, AR, and LB respectively. However, when comparing ALIF support alone to ISF alone, there were no statistically significant differences in L5/S1 ROM in the ALIF construct (27% in AR, P = .30) but a trend in both FE (17%, P = .077) and LB (29%, P = .08). In addition, SI ROM had statistically increased in FE (48%, P < .0001) and AR (34%, P = .029), but not in LB (P = .39) in the ALIF construct alone as compared with ISF alone.
Measurement Variability
Across all specimens and conditions, at the distinct quasi-static loading region of interest, the RMS errors between the prescribed and actual loads were 0.5 N, 9.0 N, 0.6 N, 0.10 N·m, 0.04 N·m, and 0.06 N·m in the posterior, compression, lateral, LB, AR, and FE degrees of freedom.
Discussion
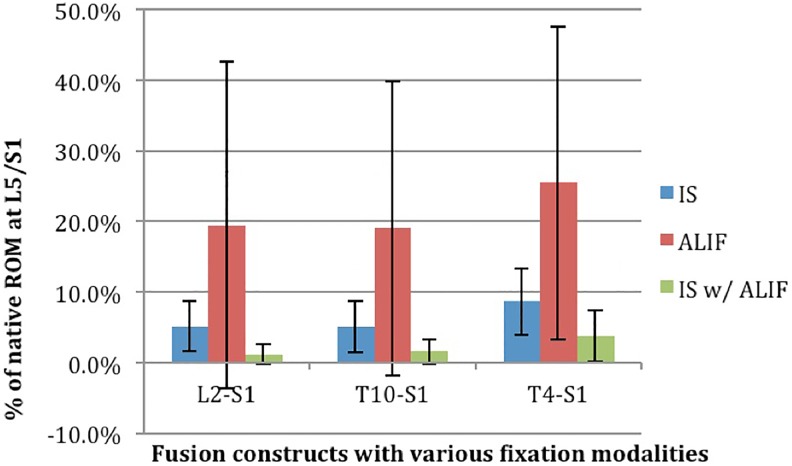
Under physiological loading, the L5/S1 interspace experiences increasing strain as the length of LS and TLS constructs increase, most significantly in flexion-extension.6,14-16 In our biomechanical model, we found that under physiological loads: (1) long LS constructs (L2-S1) may be equivalently stabilized by L5/S1 ALIF alone; however, (2) longer TLS constructs begin exerting increasing motion at L5/S1, exhibiting trends supporting when extending to T10 and statistically improved fixation when extending to T4. Finally, we found that (3) TLS constructs with ISF exhibited significant stability from the addition of ALIF support when extending T4-pelvis but not T10-pelvis (Figure 4, Table 3).
Figure 4.
Percent of native L5-S1 ROM magnitude in FE loading direction with different fusion lengths (L2-S1, T10-S1, and T4-S1), compared among various fusion modalities. IS, iliac screw fixation; ALIF, anterior lumbar interbody fixation; FE, flexion-extension; ROM, range of motion.
Table 3.
Percent of Native L5-S1 ROM Magnitude in FE Loading Direction With Different Fusion Lengths (L2-S1, T10-S1, and T4-S1), Compared Among Various Fusion Modalities.
| L2-S1 | T10-S1 | T4-S1 | ||
|---|---|---|---|---|
| Percent of native ROM at L5/S1 | IS | 5.2 ± 3.6 | 5.1 ± 3.6 | 8.7 ± 4.7 |
| ALIF | 19.4 ± 23.1 | 19 ± 20.8 | 25.4 ± 22.1 | |
| IS with ALIF | 1.2 ± 1.4 | 1.6 ± 1.7 | 3.8 ± 3.6 |
Abbreviations: IS, iliac screw fixation; ALIF, anterior lumbar interbody fixation; ROM range of motion; FE, flexion-extension.
The biomechanics of sacral fixation have been investigated in depth in porcine, calf, and human cadaver models.6,14,17-19 With various techniques employed to off-load these short screws, iliac screws have become the definitive method to support LS fixation, shown to reduce strain more effectively than an ALIF FRA at L5/S1, improve fixation over Galveston rods or S2 screw/hook constructs, and are the most protective against clinically significant instrumentation failure.6,17-19 Our results echo these findings, with ISF statistically reducing L5/S1 LB ROM by 93% as compared with the native condition and by 29% as compared with ALIF alone when considering the torques imposed by long TLS constructs (T4-pelvis).
Although these results are significant, and highlight the strength of iliac fixation, their placement requires extended exposure, increasing operative time, possibly increased infection rates and blood loss, in addition to less common risks, and removal rates as high as 34.3% over a 5-year period.2,20,21 Therefore, under physiologic loading, the addition of anterior column support may also effectively off-load the strain at L5/S1, transmitting the anterior column load to a greater surface area for arthrodesis while off-loading the sacral screws, and increasing construct stiffness.8,10,14,20-22 In human cadaveric specimens, interbody devices are effective in load-sharing and reducing sacral screw strain.6,14 In our biomechanical model, although ISF demonstrated a significant reduction in motion for long TLS constructs (T4-S1), the differences between ISF and ALIF support for T10-S1 and L2-S1 were not statistically significant.
Based on this biomechanical model, we suggest the importance of ALIF interbody support in a few ways. (1) Although not equivalent at higher loads, the use of ALIF without ISF may sufficiently support L2-S1 constructs, reducing L5/S1 ROM and transmitting loads instead to the sacropelvis, represented by statistically increased ROM across the SI joints with the addition of ALIF. (2) ALIF added significant stability to the long TLS construct (T4-pelvis) when added to ISF. This difference was not significant for short TLS constructs (T10-pelvis). (3) Finally, although not separately tested in our study, ALIF is known to improve lordosis, provide direct ventral access for ventral pathology and reduction of translation, ventral release, and wide endplate preparation.23,24 These unmeasured benefits must also be taken into consideration.
Limitations
There are a number of limitations that are intrinsic to any in vitro biomechanical study including specimen preparation and integrity. We have standardized the preparation, carried out by the same individuals under the same conditions to limit these effects on our study.
Second, the clinical relevance of the methodology is important. The in vivo applied load may be different from patient to patient, level by level, and vary across construct types. The biomechanical test method is a slight modification to the well-established Panjabi’s hybrid multidirectional testing method.12 The clinical relevance assumptions in this study are no different from those used to develop Panjabi’s hybrid protocol, which seeks to create a reasoned and repeatable set of loading conditions. The hybrid multidirectional test method is performed by applying a range of motion determined from an intact spine, to the same spine after instrumentation. This results in higher torques and is roughly equivalent to what a patient might experience while performing daily tasks before and after a spine surgery. The Panjabi protocol makes the assumption that patients will achieve the same motions before and after fusion surgery. For long TLS constructs, this is likely not the case, and positions attainable for the intact spine would require supraphysiologic loads to achieve the postfusion status. We therefore utilized a modified protocol as described in the methodology section, which may be useful in future studies but could need further validation moving forward.
Finally, we tested motion across the L5/S1 joint. In the past, biomechanical tests of thoracolumbar constructs were performed by measuring S1 screw strain.18 However, we feel this strain depends on the screw-bone interface, the angle of load application and the load applied. Furthermore, we felt the more relevant clinical question is not the amount of strain on the S1 screw, but the motion allowed at the L5/S1 joint. Quantifying this reduction in motion may better reflect the possibility of achieving fusion across the segment and was determined to be the primary outcome measure of our investigation. Whether this correlates with fusion rate is not clearly elucidated, and other factors such as the use of interbody rhBMP-2 (recombinant human bone morphogenetic protein–2), sagittal alignment, and physiologic factors also play a role that cannot be accounted for in the in vitro setting.
Conclusions
Our findings show that, when considering the torques imposed by long TLS constructs (T4-pelvis), ISF statistically reduces L5/S1 LB ROM by 93% as compared with the native specimen, and by 29% as compared with ALIF alone. Based on this biomechanical model, we find that although not equivalent at higher loads, ALIF may sufficiently support L2-S1 constructs without the need for ISF, reducing L5/S1 ROM and transmitting loads instead to the sacropelvis. Furthermore, ALIF was found to add significant stability to the long TLS construct (T4-pelvis) when added to ISF. This difference was not significant for short TLS constructs (T10-pelvis). The results from our biomechanical study can be clinically applied to design constructs with varying lengths using different interbodies/grafts and techniques, in order to optimize sacropelvic fixation and stabilize lumbosacral junction.
Acknowledgments
The authors would like to acknowledge Dylan Beckler, Tara Bonner, and Callan Gillespie for testing support; Robert Butler and Ben Wesorick for analysis support; and Cleveland Clinic Stanley Zielony Spinal Surgery Research and Education Fund for funding.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study received funding from the Cleveland Clinic Stanley Zielony Spinal Surgery Research and Education Fund, and Regis Haid Award from the Congress of Neurological Surgeons.
ORCID iD: Kevin M. Walsh, MD  http://orcid.org/0000-0002-6595-9461
http://orcid.org/0000-0002-6595-9461
References
- 1. Edwards CC, 2nd, Bridwell KH, Patel A, Rinella AS, Berra A, Lenke LG. Long adult deformity fusions to L5 and the sacrum. A matched cohort analysis. Spine (Phila Pa 1976). 2004;29:1996–2005. [DOI] [PubMed] [Google Scholar]
- 2. Kuhns CA, Bridwell KH, Lenke LG, et al. Thoracolumbar deformity arthrodesis stopping at L5: fate of the L5-S1 disc, minimum 5-year follow-up. Spine (Phila Pa 1976). 2007;32:2771–2776. doi:10.1097/BRS.0b013e31815a7ece. [DOI] [PubMed] [Google Scholar]
- 3. Connolly PJ, Von Schroeder HP, Johnson GE, Kostuik JP. Adolescent idiopathic scoliosis. Long-term effect of instrumentation extending to the lumbar spine. J Bone Joint Surg Am. 1995;77:1210–1216. [DOI] [PubMed] [Google Scholar]
- 4. Kostuik JP, Musha Y. Extension to the sacrum of previous adolescent scoliosis fusions in adult life. Clin Orthop Relat Res. 1999;(364):53–60. [DOI] [PubMed] [Google Scholar]
- 5. Bridwell KH. Utilization of iliac screws and structural interbody grafting for revision spondylolisthesis surgery. Spine (Phila Pa 1976). 2005;30(6 suppl):S88–S96. [DOI] [PubMed] [Google Scholar]
- 6. Cunningham BW, Sefter JC, Hu N, Kim SW, Bridwell KH, McAfee PC. Biomechanical comparison of iliac screws versus interbody femoral ring allograft on lumbosacral kinematics and sacral screw strain. Spine (Phila Pa 1976). 2010;35:E198–E205. doi:10.1097/BRS.0b013e3181c142bf. [DOI] [PubMed] [Google Scholar]
- 7. Kebaish KM. Sacropelvic fixation: techniques and complications. Spine (Phila Pa 1976). 2010;35:2245–2251. doi:10.1097/BRS.0b013e3181f5cfae. [DOI] [PubMed] [Google Scholar]
- 8. Kostuik JP, Hall BB. Spinal fusions to the sacrum in adults with scoliosis. Spine (Phila Pa 1976). 1983;8:489–500. [DOI] [PubMed] [Google Scholar]
- 9. Molinari RW, Bridwell KH, Lenke LG, Ungacta FF, Riew KD. Complications in the surgical treatment of pediatric high-grade, isthmic dysplastic spondylolisthesis. A comparison of three surgical approaches. Spine (Phila Pa 1976). 1999;24:1701–1711. [DOI] [PubMed] [Google Scholar]
- 10. Kostuik JP, Munting E, Valdevit A. Biomechanical analysis of screw load sharing in pedicle fixation of the lumbar spine. J Spinal Disord. 1994;7:394–401. [PubMed] [Google Scholar]
- 11. Wu G, Siegler S, Allard P, et al. ISB recommendation on definitions of joint coordinate system of various joints for the reporting of human joint motion—part I: ankle, hip, and spine. International Society of Biomechanics. J Biomech. 2002;35:543–548. [DOI] [PubMed] [Google Scholar]
- 12. Panjabi MM. Hybrid multidirectional test method to evaluate spinal adjacent-level effects. Clin Biomech (Bristol, Avon). 2007;22:257–265. doi:10.1016/j.clinbiomech.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 13. Peters P, Langlotz F, Nolte LP. Computer assisted screw insertion into real 3D rapid prototyping pelvis models. Clin Biomech (Bristol, Avon). 2002;17:376–382. [DOI] [PubMed] [Google Scholar]
- 14. Fleischer GD, Kim YJ, Ferrara LA, Freeman AL, Boachie-Adjei O. Biomechanical analysis of sacral screw strain and range of motion in long posterior spinal fixation constructs: effects of lumbosacral fixation strategies in reducing sacral screw strains. Spine (Phila Pa 1976). 2012;37:E163–E169. doi:10.1097/BRS.0b013e31822ce9a7. [DOI] [PubMed] [Google Scholar]
- 15. Kuklo TR, Bridwell KH, Lewis SJ, et al. Minimum 2-year analysis of sacropelvic fixation and L5-S1 fusion using S1 and iliac screws. Spine (Phila Pa 1976). 2001;26:1976–1983. [DOI] [PubMed] [Google Scholar]
- 16. Weistroffer JK, Perra JH, Lonstein JE, et al. Complications in long fusions to the sacrum for adult scoliosis: minimum five-year analysis of fifty patients. Spine (Phila Pa 1976). 2008;33:1478–1483. doi:10.1097/BRS.0b013e3181753c53. [DOI] [PubMed] [Google Scholar]
- 17. Cunningham BW, Lewis SJ, Long J, Dmitriev AE, Linville DA, Bridwell KH. Biomechanical evaluation of lumbosacral reconstruction techniques for spondylolisthesis: an in vitro porcine model. Spine (Phila Pa 1976). 2002;27:2321–2327. doi:10.1097/01.BRS.0000030852.79881.F1. [DOI] [PubMed] [Google Scholar]
- 18. Lebwohl NH, Cunningham BW, Dmitriev A, et al. Biomechanical comparison of lumbosacral fixation techniques in a calf spine model. Spine (Phila Pa 1976). 2002;27:2312–2320. doi:10.1097/01.BRS.0000030302.08190.6C. [DOI] [PubMed] [Google Scholar]
- 19. McCord DH, Cunningham BW, Shono Y, Myers JJ, McAfee PC. Biomechanical analysis of lumbosacral fixation. Spine (Phila Pa 1976). 1992;17(8 suppl):S235–S243. [DOI] [PubMed] [Google Scholar]
- 20. Emami A, Deviren V, Berven S, Smith JA, Hu SS, Bradford DS. Outcome and complications of long fusions to the sacrum in adult spine deformity: luque-galveston, combined iliac and sacral screws, and sacral fixation. Spine (Phila Pa 1976). 2002;27:776–786. [DOI] [PubMed] [Google Scholar]
- 21. Moshirfar A, Rand FF, Sponseller PD, et al. Pelvic fixation in spine surgery. Historical overview, indications, biomechanical relevance, and current techniques. J Bone Joint Surg Am. 2005;87(suppl 2):89–106. doi:10.2106/JBJS.E.00453. [DOI] [PubMed] [Google Scholar]
- 22. Polly DW, Jr, Klemme WR, Cunningham BW, Burnette JB, Haggerty CJ, Oda I. The biomechanical significance of anterior column support in a simulated single-level spinal fusion. J Spinal Disord. 2000;13:58–62. [DOI] [PubMed] [Google Scholar]
- 23. Lim JK, Kim SM. Radiographic results of minimally invasive (MIS) lumbar interbody fusion (LIF) compared with conventional lumbar interbody fusion. Korean J Spine. 2013;10:65–71. doi:10.14245/kjs.2013.10.2.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Phan K, Thayaparan GK, Mobbs RJ. Anterior lumbar interbody fusion versus transforaminal lumbar interbody fusion—systematic review and meta-analysis. Br J Neurosurg. 2015;29:705–711. doi:10.3109/02688697.2015.1036838. [DOI] [PubMed] [Google Scholar]