Abstract
Acute ischemic stroke represents a major cause of long-term adult disability. Accurate prognostication of post-stroke functional outcomes is invaluable in guiding patient care, targeting early rehabilitation efforts, selecting patients for clinical research, and conveying realistic expectations to families. The involvement of specific brain regions by acute ischemia can alter post-stroke recovery potential. Understanding the influences of infarct topography on neurologic outcomes holds significant promise in prognosis of functional recovery. In this review, we discuss the recent evidence of the contribution of infarct location to patient management decisions and functional outcomes after acute ischemic stroke.
Keywords: Acute ischemic stroke, neuroimaging, outcomes, topography, voxel-based lesion symptom mapping
Introduction
Acute ischemic stroke (AIS) is a significant cause of long-term disability. Two out of three AIS survivors will have residual neurologic deficits and up to half will require assistance with activities of daily living.1,2 Understanding the variables influencing AIS outcomes, therefore, has widespread clinical utility. Effective tools for the prediction of recovery after AIS can potentially guide patient selection for individualized therapies, end-of-life care, and early rehabilitation strategies.3,4 Moreover, accurate prognostic information offers value in clinical trial design because of the potential to enhance patient selection, reduce sample size, and improve the choice of clinical end points.5–7 However, the precise contributions of individual patient traits to post-stroke recovery are not well known, and improving our understanding of these influential factors on functional outcomes offers great clinical opportunity.
Infarct volume, as assessed on either CT or MRI, has been demonstrated to only moderately correlate with clinical outcomes.8–10 Analyses of multiple large data sets, however, have shown that age and stroke severity, measured by the National Institutes of Health Stroke Scale (NIHSS) score, have significant impact on post-stroke outcomes.11 The inclusion of age and NIHSS, and other clinical variables, into models that also incorporate infarct volume improves the prediction of the likelihood of survival or functional recovery after AIS.12–14 More recently, an additional radiographic feature, namely the infarct location, has shown enhanced capabilities, compared to infarct volume alone, in predicting stroke and rehabilitation outcomes.15 The importance of ischemic stroke location for functional outcomes has also been reported in delayed cerebral infarction after subarachnoid hemorrhage16 and strokes involving specifically the cerebellum.17,18 Because of these complexities, long-term functional outcome of patients remains the primary end point in the majority of stroke trials and is the accepted outcome measure by regulatory agencies in trials of new drugs.
In this review, we will first discuss the more common scales employed in evaluating ischemic stroke outcomes. It is important to understand the advantages and disadvantages of instruments for evaluating stroke severity, post-stroke disability and recovery before exploring how infarct topography influences stroke recovery as assessed by these scales. We will then examine the role of stroke laterality on global outcome measures and proceed to investigate the effects of lesion topography on acute patient management and functional outcomes, such as motor, language and cognition. Finally, research on the role of acute infarct topography on acute treatment decisions and outcomes will be summarized.
Outcome measurement in stroke
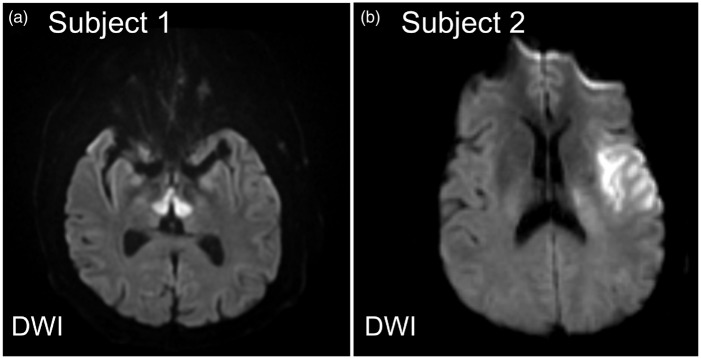
Understanding the strengths and weaknesses of the commonly used stroke scales is critical for evaluating the individual impact of acute ischemic stroke injury on stroke recovery and functional outcomes as well as clinicians’ interpretation of stroke research findings.19 The use of composite clinical rating scales, such as the NIHSS, has prompted many to stress the importance of using modality-specific outcome measures as primary end points in clinical trials of stroke.20 This issue is of special importance in understanding the role of infarct location in functional outcomes. In short, the region of brain injury will manifest specific clinical symptoms that, depending on the outcomes scale, will exert different contributions to the score. For example, small ischemic strokes positioned in eloquent areas such as the corticospinal tracts or brainstem can induce severe deficits comparable to those attributed to large hemispheric strokes (Figure 1).
Figure 1.
Stroke severity is dependent on location of ischemic stroke. DWI images of two patients admitted with AIS with identical admission NIHSS but different functional outcomes. (a) 56-year-old male found unresponsive. Neurologic exam notable for fixed pupils, present corneal and gag reflexes, absent oculocephalic reflexes, and flaccid paralysis of all extremities. MRI brain showed restricted diffusion in the medial thalami bilaterally and dorsal midbrain. Magnetic resonance angiography (MRA) of the head and neck (not shown) showed occlusive thrombus at the top of the basilar artery extending into the P1 segments of the posterior cerebral arteries bilaterally. Admission NIHSS 24; 90-day mRS 6. (b) 48-year-old male with atrial fibrillation presented with left middle cerebral artery (MCA) syndrome. Neurologic exam notable for a global aphasia and weakness of his right face and arm. MRI of the brain showed restricted diffusion in the left frontal operculum and anterior temporal lobe. Admission NIHSS 24; 90 day mRS 1. Images are shown in radiographic orientation.
A number of different measures of stroke outcomes have been employed in clinical trials of ischemic stroke and post-stroke rehabilitation. These metrics each emphasize different aspects of stroke recovery and an understanding of the weights given to various factors of commonly utilized scales is important for interpreting stroke severity and functional recovery. In 2010, in an effort to standardize clinical research for stroke, the National Institutes of Neurological Disorders and Stroke (NINDS) set of Common Data Elements (CDE) was developed.21,22 These included frequently used scales to quantify neurologic outcomes such as the modified Rankin Scale (mRS), and NIHSS.19,23 The mRS is a global outcomes disability scale that has been widely employed in the evaluation of functional outcomes in clinical stroke trials.24,25 The mRS is an ordinal scale between 0 and 6 that assesses the degree of disability of an individual after stroke, with higher values representing greater morbidity (0 = no symptoms, 1 = some symptoms but can carry out all activities, 2 = slight disability but independent, 3 = moderate disability, requires assistance with affairs, 4 = unable to walk unassisted, 5 = bedridden, 6 = death). It is important to note that the mRS accentuates the level of functional independence and, as such, there is significant weight on motor performance (e.g. ambulatory status) as opposed to other areas of stroke recovery such as language. In fact, the mRS scale has been criticized as a disability assessment tool for being too dominated by motor recovery.20
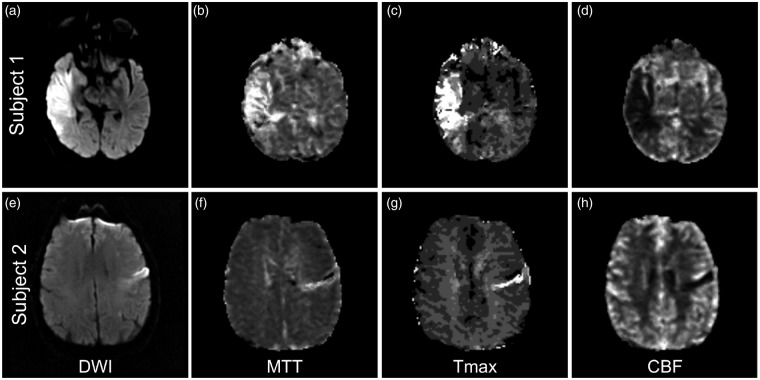
The NIHSS has been used for quantifying initial stroke severity and serial assessments as well as functional outcomes. The NIHSS is an ordinal scale (range 0 to 42) consisting of 11 items to measure degree of dysfunction across various domains including language, ataxia, motor strength, sensation, and neglect, where increasing values represent greater stroke severity. Multiple studies have exemplified that the NIHSS is a powerful, validated tool for assessing clinical stroke severity with high inter-rater reliability that correlates strongly with clinical outcomes.26–28 An important limitation of the NIHSS, however, is that the variables of its scoring system place greater emphasis on deficits associated with left-hemisphere function (e.g. language) rather than right hemisphere or posterior circulation (Figure 2).29,30 This observation is directly related to the inherent composition of the NIHSS: dominant (left) hemisphere dysfunction in the form of aphasia can influence multiple items as opposed to hemispatial neglect from non-dominant (right) hemisphere dysfunction being scored with only one question. Consistent with these points, analysis of hemispheric stroke patients has shown a lower NIHSS in right versus left hemisphere infarcts when controlled for stroke volume.30 Overall, the NIHSS is a powerful tool for evaluating ischemic stroke; however, one of its limitations is the need for either an in person or video-conference examination.
Figure 2.
The NIHSS emphasize dominant/left hemisphere functions. Representative MR images of two patients admitted with right and left hemisphere ischemic strokes. Each patient had the same admission NIHSS and 90-day mRS; however, the ischemic stroke volume was greater in the right hemisphere lesion. (a–d) 53-year-old man with atrial fibrillation and hypertension presents with left facial droop and arm weakness, dysarthria, and decreased sensation in his left arm. NIHSS 11 for right MCA syndrome including left hemineglect. MRI of the brain shows ischemic stroke involving the right insula, frontal operculum, and superior and middle temporal gyri on DWI sequences (a) without evidence of perfusion mismatch on Mean Transit Time (MTT) (b), time to maximum value of the deconvolved residue function (Tmax) (c), and cerebral blood flow (CBF) (d) maps. (e–h) 79-year-old male with coronary artery disease and hyperlipidemia that developed a non-fluent aphasia and right arm weakness. NIHSS 11 for left MCA syndrome involving language output and mild right face and arm weakness. MRI shows a subacute infarct involving the left inferior frontal gyrus on DWI sequences (e) with a matched focal perfusion abnormality on MTT (f), Tmax (g), and CBF (h) to suggest no additional territory at risk.
In addition to these assessments of global functional status, there is a number of modality-specific scales, for example looking specifically at motor function (e.g. Fugl-Meyer Assessment and Functional Independence Measure (FIM) motor subscale), used more frequently in the rehabilitation setting for gauging progress with physical therapy.31,32 FIM is a proprietary 18-item scale that requires assessment by speech, physical, and occupational therapists to assess cognitive and language domains.33 Other NINDS CDE recommended outcome metrics measure emotional and cognitive status (e.g. Montreal Cognitive Assessment (MoCA), center for epidemiologic studies depression scale, trail making test parts A&B), self-reported quality of life (EuroQoL-5, PROMIS-10), and performance measures (walking speed). Each of these scales offers advantages and disadvantages in quantifying post-stroke disability and, as a result, emphasizes slightly different aspects of stroke recovery.
Effect of hemispheric involvement on functional outcomes
Ischemic strokes involving the dominant hemisphere, which in the majority of the population is the left hemisphere, versus the non-dominant or right hemisphere can cause distinctive clinical syndromes and deficits depending on the regions involved.
Studies of the effects of AIS hemispheric lateralization on outcomes have shown mixed results.34–42 A number of investigations have demonstrated that right hemispheric involvement portends worse functional outcomes in patients with AIS.34,37,38,43,44 When ambulatory status was assessed in 183 patients with small or medium-sized infarcts, right hemisphere AIS was associated with significantly worse locomotion: 64.9% of right hemisphere versus 82.1% of left hemisphere AIS patients were walking independently at the completion of rehabilitation.36 On the other hand, there have been reports of hemispheric lateralization having no impact on functional outcomes.39–41 In a study of 70 patients with proximal large vessel occlusion (LVO) that underwent intra-arterial intervention, there was no difference in the rates of good outcomes (mRS≤ 2) at three months between right and left hemisphere AIS patients.42 In an analysis of 1644 patients from the Virtual International Stroke Trials Archive, while baseline NIHSS was higher for left hemisphere strokes, hemispheric lateralization showed no influence on 90-day mRS and mortality, suggesting no relationship with AIS lateralization and functional outcomes.35
The interpretation of these findings bears caution for two reasons. First, as mentioned earlier, the NIHSS is weighted for left hemisphere deficits and so it is not surprising that left hemispheric stroke patients might have higher baseline NIHSS scores. Secondly, the mRS as a marker of global functional status is largely predicated on ambulatory status and lacks sensitivity for deficits in other domains that certainly impact functional outcomes, such as language or neglect. Because of these inherent limitations of the mRS, alternative approaches to evaluating functional outcomes and the impact of AIS lateralization are necessary.
One approach to elucidate the contribution of infarct lateralization to post-stroke outcomes is to examine the relationship of the admission NIHSS with acute lesion volume and the affected hemisphere lateralization. As mentioned above, left hemisphere ischemic stroke patients will typically score higher on the NIHSS than patients with similar infarct volumes involving right hemisphere strokes. In an analysis of 153 patients with acute ischemic stroke, NIHSS scores of 0 to 5 points in right hemisphere strokes were associated with a two-fold increase in diffusion-weighted MRI (DWI) volume (DWIv; 8.8 vs. 3.2 cm3).30 Furthermore another study of patients with similar NIHSS scores up to 20 points found that the median volume of infarct in the right hemisphere was approximately double than the left.29 These findings emphasize the point that there is frequently a mismatch between the clinical presentation and the acute DWI volume depending on the afflicted hemisphere. Using clinical-DWI mismatch (CDM) defined as the NIHSS score exceeding 8 points when the DWIv is less than 25 cm3, the frequency of mismatch was higher in patients with left versus right hemispheric infarcts (65% vs. 38%, p = 0.001). This observation implies that small left hemispheric infarcts (DWIv< 25 cm3) are more likely to manifest with higher stroke severity (NIHSS> 8 points) as compared to an equivalent right hemisphere lesion. Interestingly, patients with CDM were more likely to experience early neurologic deterioration (defined as an increase in NIHSS of 4 or more points in first 72 hours of the admission) and infarct growth at 30 days.45 As left hemisphere infarcts were more frequently associated with CDM, one could interpret these observations as if left hemisphere strokes could be more prone to infarct growth; however, there is a potential bias related to the infarct laterality and the NIHSS use that needs to be considered, given that worsening of clinical symptoms related to the dominant hemisphere is more likely to be detected.
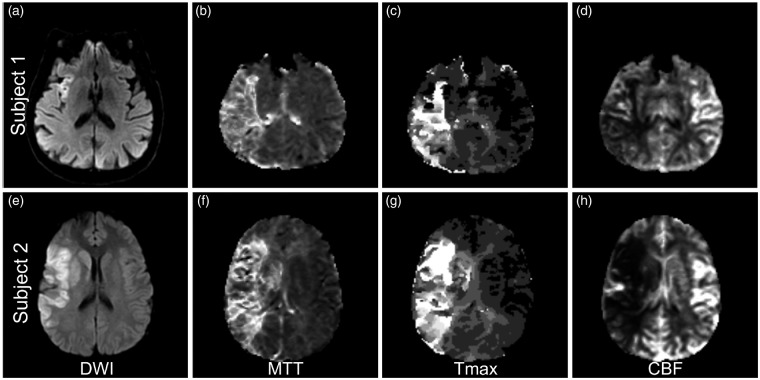
Another interpretation of these findings is that CDM may be the result of a larger area of dysfunctional tissue extending beyond the infarct core visualized on acute DWI sequences. As discussed earlier, acute DWI volume has been shown to moderately correlate with functional outcomes and post-stroke disability.8–10,46,47 For this reason, using perfusion-weighted MRI (PWI) volume (PWIv) may hold value in accurately correlating stroke severity with final infarct volume and stroke outcomes (Figure 3). The initial PWIv has been demonstrated to be a strong predictor of final infarct volume48 and clinical outcome.9,49,50 In support of this hypothesis, PWIv of non-dominant hemisphere and dominant hemisphere stroke patients correlated significantly better than NIHSS with tests of neglect performance and aphasia, respectively.51 When PWIv was compared to DWIv in 40 subjects with acute hemisphere strokes, the performance on cognitive testing strongly correlated with the volume of hypoperfused tissue rather than DWIv.52 These findings suggest that the regional location of hypoperfusion in AIS offers insight into understanding causes of stroke severity and could improve the neuroimaging prognostication of functional outcomes.
Figure 3.
Does perfusion-weighted imaging provide a more accurate correlation with stroke severity? (a–d) DWI and PWI images for a 60-year-old male with hypertension that presented with a right MCA syndrome. NIHSS 14 for left facial droop and homonymous hemianopsia, weakness of the left arm and leg, dysarthria, and left hemineglect. MRI shows a small area of restricted diffusion in the right insula on DWI (a) with PWI evidence of mismatch and a potentially large territory at risk on MTT (b), Tmax (c), and CBF (d). (e–h) DWI and PWI images for a 18-year-old male that presented with sudden onset left sided hemiplegia. NIHSS 14 for right MCA syndrome including right gaze deviation and left hemineglect. MRI brain shows a large area of restricted diffusion in the right basal ganglia, frontal operculum, insula, and inferior parietal lobe on DWI (e), with PWI showing additional territory at risk on MTT (f), Tmax (g), and CBF (h).
An alternative hypothesis for the possible discrepancy between right versus left hemisphere infarcts and stroke outcomes is delays in hospital presentation in non-dominant hemisphere strokes.53 While language dysfunction from left hemisphere injury is more immediately apparent and easily recognized, the subtleties of hemispatial neglect with possible overlay of anosognosia may delay recognition of stroke onset. Further supporting this point, stroke severity, as assessed through scales such as the NIHSS, inversely correlates with time to hospital arrival and thus eligibility for intravenous thrombolysis.54–56 In addition, patients with severe neglect from a right hemisphere stroke or a fluent/receptive aphasia from a left hemisphere stroke may have difficulties participating in certain aspects of rehabilitation therapies, which will impact functional outcomes.
The hemisphere sustaining ischemic injury likely has multifactorial influences on post-stroke recovery through its affects on delay in hospital presentation and thrombolysis eligibility as well as impairing rehabilitation efforts. To further our understanding of the influence of acute stroke topography on functional recovery, we will discuss the role of injury to specific brain regions in various outcome domains.
Infarct topography and modality-specific recovery
The ability to accurately predict recovery of specific neurologic deficits following AIS is challenging but represents a critical question for guiding rehabilitation therapies and counseling the patient and/or family members. While there is evidence to suggest the extent and time course of recovery vary with severity of injury and the specific deficit,57–59 there is a growing body of evidence suggesting a role for infarct topography in modality-specific recovery. We will now review some of the key evidence on the role of infarct location in the recovery of several different neurologic deficits.
Motor recovery
The characterization of motor recovery in AIS is well studied, which is likely a reflection of the number of quantitative assessment tools for measuring motor performance. As a result, our understanding of the role of infarct topography in motor outcomes is more advanced than other areas of stroke recovery. The involvement of specific brain regions in AIS clearly and logically has a strong influence on motor recovery (Figure 1). Comparing cortical versus mixed or subcortical lesions, there is a strong correlation with upper limb motor recovery after AIS. In a study of stroke rehabilitation patients with pure cortical compared to subcortical strokes, 3 out of 4 patients in the cortical group versus 1 out of 17 in the subcortical group had recovery of upper limb motor function of it.60 Importantly, only 1 of 28 patients with radiographic evidence of involvement of the posterior limb of the internal capsule had recovery of upper limb movement compared to 5 out of 13 patients with sparing of it.60 One explanation for these findings is that the lesion load of the corticospinal tract portends motor recovery potential.61,62
In addition to injury of the corticospinal tract, infarction of specific regions of the cortex may also strongly influence motor recovery. Acute involvement of the somatosensory cortex, intraparietal sulcus, and primary motor cortex, evidenced by restricted diffusion on DWI, are associated with worse motor recovery and functional outcomes after AIS.63,64 Another study showed that CT perfusion within 9 h of stroke onset identified multiple brain regions including the insular cortex, superior temporal gyrus, postcentral gyrus, putamen, caudate, and internal capsule that were all independent predictors of motor recovery.65 Involvement of the putamen has also been shown to increase the likelihood of a residual gait disorder following AIS.66
Voxel-based lesion-symptom mapping (VLSM) approaches have also shown value in understanding the role of infarct topography on motor recovery. VLSM compares neurobehavioral scores between patients with and without lesions on a voxel-wise basis.67 VLSM analysis showed that the areas most associated with worsening motor performance are situated at the junction of the corona radiata and corticospinal tract.68 In a study of 50 patients with recent ischemic or hemorrhagic stroke, while corticospinal tract lesion load volume on FLAIR MRI sequences performed three to eight weeks post-event was predictive of performance in two functional mobility scales, using VLSM, walk speed response to gait rehabilitation was predicted by damage to the putamen, external capsule, and insula.69
Not surprisingly, the extent of ischemic injury to the components of the motor pathway will affect motor recovery. These regions include the eloquent portions of the cortex involved in motor function as well as the corticospinal tract as it converges within the corona radiata and descends in the internal capsule. Using clinical variables alone to prognosticate motor recovery post-stroke is only moderately effective.70 Incorporating region-specific or tract-specific involvement, however, is likely to improve predictions of motor recovery.
Recovery of language
In AIS, aphasia is frequently the result of ischemic injury to the language centers of the dominant hemisphere. Post-stroke aphasia is exceedingly common, as 20–40% of AIS patients will present with aphasia as an initial symptom.71–73 While the potential for motor recovery is heavily weighted by the extent of ischemic injury to the corticospinal tracts, the potential for recovery of language after AIS is dependent on the cortical involvement of the language centers. AIS infarct burden in language regions is an important predictor of aphasia recovery potential.74
Multiple studies have highlighted the importance of the superior temporal gyrus (STG) and, in particular, the posterior portion, in aphasia recovery.75–78 In one study, the severity of auditory comprehension deficits was strongly correlated with the extent of injury to the posterior STG: patients with damage to less than half of the STG, detected on CT scans acquired six months post-stroke, were more likely to have good comprehension at six months.78 Lesions of the dominant hemisphere STG also appear more likely to cause a persistent global aphasia as opposed to involvement of the inferior frontal gyrus or pre- and postcentral gyrus.76 These studies suggest that the degree of sparing of the STG is critical for functional aphasia recovery. In addition, in a study of 97 patients with aphasia secondary to left hemisphere AIS, patients with exclusively subcortical involvement (e.g. basal ganglia) had less severe aphasias than those patients with cortical lesions involving Broca’s and Wernicke’s areas.79
Perfusion imaging and, in particular, perfusion-diffusion mismatch (PDM) has also been shown to have prognostic utility in aphasia recovery. The presence of PDM in Brodmann area 37 (left posterior inferior temporal cortex) on MRI 24 h post-stroke, for example, independently predicted the degree of acute improvement in naming performance in a population of patients with acute left hemisphere ischemic strokes.51 In another population of 58 patients with aphasia secondary to AIS, CT perfusion imaging within 9 h of symptom onset demonstrated near-normal to hyperemic relative cerebral blood flow values in the left angular gyrus and insular cortex were independent predictors of aphasia improvement by hospital discharge.80 Moreover, when these variables were incorporated into a model also containing the admission NIHSS aphasia score and presence of a proximal cerebral artery occlusion, there was 91% accuracy in the prediction of aphasia outcomes. These findings would suggest that preserved or recovered perfusion in specific brain regions inform on the presence of salvageable tissue and as a result, could be useful in predicting functional aphasia recovery.
In summary, the potential for language recovery after AIS is influenced by the involvement of different cortical regions including the STG as well as potentially the angular gyrus and insular cortex of the dominant hemisphere. Incorporating the presence or absence of injury of these brain regions into prediction models with standard clinical variables could offer improved prognostication for post-stroke recovery of language deficits.
Cognitive outcomes
Following an ischemic stroke, 10% of patients will develop secondary dementia.81 Understanding the factors that influence the development of post-stroke dementia is of major value in counseling patients and families on recovery expectations. The clinical determinants of post-stroke cognitive dysfunction seem to be heavily influenced by cortical injury or hemispheric involvement.82,83 In one study of 190 patients with first stroke, 74% of patients with cortical stroke compared to 46% of patients with subcortical stroke had evidence of cognitive impairment.84 Another study of patients with AIS performed neuropsychiatric testing and observed that cognitive dysfunction was associated with the specific arterial territory involved and presence of white matter hyperintensity, but not the laterality of the ischemic stroke.85
VLSM have also been used to identify region-specific predictors of cognitive outcomes. One group used VLSM analysis to identify eloquent voxels in patients with AIS using DWI obtained between 24 and 72 h after onset, which were found predominantly in the left hemisphere prefrontal, cingulate, peri-insular, middle, superior temporal cortex, amygdala, hippocampus, and deep nuclei.86 Furthermore, they demonstrated that infarct location was the strongest predictor of good cognitive outcomes (MoCA> 25).86
White matter tract integrity may also be informative in prognosticating cognitive outcomes after ischemic stroke. In a study of 14 right hemispheric stroke patients and 18 healthy controls, diffusion tensor imaging maps were compared between patients with good and poor cognitive recovery, where good recovery was defined as improvement in neuropsychological test results administered at three months post-stroke compared to results from 72 h.87 The poor cognitive recovery group showed decreased contralesional axonal integrity at three months post-stroke in several left hemisphere regions when compared to healthy controls.87 This data highlight the contributions of infarct topography as well as white matter tract integrity on post-stroke recovery of cognitive function. Moving forward, the use of standardized advanced imaging techniques to characterize infarct topography combined with outcome measures representative of the NINDS CDEs could improve the accuracy of prognostication.
To further understand the specific effects of infarct topography on post-stroke outcomes, it is important to consider the potential downstream effects of the infarct on brain functional network activity. A novel approach that has recently been used to explore these points is lesion-based functional connectivity network analysis.88 By evaluating the blood-oxygen-level-dependent time course in regions of the brain exhibiting lesions in resting state functional MRI from healthy controls, common networks of brain regions were found to be involved in specific clinical syndromes such as peduncular hallucinosis, post-stroke pain and subcortical aphasias despite heterogeneous location of lesions.88 The promise of this approach is that the inclusion of network analysis into infarct topography analysis could augment prediction models of more sophisticated outcome measures.
Acute tissue infarction location and prediction of long-term outcomes
In the acute setting, visualizing the infarct location intrinsically impacts the clinical decision-making process including the triage of the patient, the work up pursued for stroke mechanism, and additional therapies. Arguably more so than infarct volume, which is often dichotomized into large versus small, infarct topography influences patient management. For example ischemic strokes involving the posterior fossa will often require close monitoring for cerebral edema and potential prophylactic sub-occipital craniectomy. Alternatively, in strokes damaging the corticospinal tracts there is an expectation for significant hemiparesis and dysphagia that can influence enteral nutrition strategies and discharge destination.
The ability to accurately prognosticate clinical and ischemic tissue outcomes in the acute setting holds significant clinical implications for guiding acute management and accurately informing the patient/family to guide their decision-making. There have been several analytical and imaging approaches used to evaluate infarct topography with respect to AIS outcomes.
The current role of imaging in acute patient triage for clinical trials is predominantly volumetric, most likely due to ease of calculation. We will first discuss volumetric approaches before addressing the potential role of infarct location in patient selection for clinical trials. The current evidence on the effect of infarct volume and topography on functional outcomes following AIS has employed both CT- and MRI-based approaches. In clinical practice, CT-based imaging for AIS is much more widespread due to the speed of acquisition; however, MRI-based approaches afford a more sensitive and specific evaluation of acute ischemic brain parenchyma.89,90 The current data suggest that both approaches are informative in predicting outcomes.
Volumetric analyses
For intravenous (IV) recombinant tissue plasminogen activator (tPA) trials, acute lesion volumes on CT or MRI greater than 1/3 MCA territory are often used as exclusion criteria.91–96 Other MRI approaches proposed for trials investigating IV tPA in extended windows are PDM, LVO site and FLAIR-diffusion mismatches.97 Additional details can be found in another review article.97 Although studies have shown that CDM and PDM are closely related,98 PDM was found to be more accurate for selecting patients likely to benefit from reperfusion therapy in the 3–6-h window,99 with CDM patients showing no increased benefit from thrombolysis.99,100 Because of these findings and known limitations of the clinical score (i.e. NIHSS) from its weighting of left hemispheric infarcts and lesions involving the motor pathways as described above, selection of patients for extended time window treatment based on CDM, although easier to execute, is currently not clinically indicated. However, there is a clinical thrombectomy trial underway investigating the utility of CDM in patients with known MCA LVO.101
In recent thrombectomy trials, infarct volume had a large effect on likelihood of good outcome (mRS< 3). In one study of 107 patients with anterior circulation LVO that underwent endovascular thrombectomy (EVT), follow-up infarct volume of 40 to 50 cm3 (at median time of 41.8 h from symptom onset and assessed with CT in 58.9% of the population) had the highest accuracy for predicting good outcome (sensitivity 74.1–81.5%; specificity 77.5–85.0%).102 Aside from infarct volume, the early clinical response to EVT appears to be highly predictive of functional outcome. In one study, the authors looked at markers of early stroke severity, including infarct volume and baseline NIHSS, and showed that the trajectory of the two-day longitudinal NIHSS revealed subgroups of patients with large, minimal, and no improvement following EVT.103 Not surprisingly, the subgroup with large improvement was more likely to have good outcomes at 90 days and had an accuracy of 84.5% in predicting 90-day mRS.103 Infarct volume continued to have a role, as the “large improvement” subgroup was represented by younger patients with larger regions of hypodensity on acute CT. In a single center MRI study,104 patients classified as likely to benefit from EVT (DWI volume< 70 cm3, age< 80 and pre-stroke mRS< 2), were also found to have more favorable outcomes after treatment. In this study, DWI lesion volume was estimated at the MRI console using measurements from three perpendicular axes and an assumed ellipsoid geometry (ABC/2).105
In the EVT trials, ischemic core volume was one criteria; however, perfusion mismatch106 and collateral status107 were incorporated into the patient selection algorithm for several trials. In ESCAPE, delayed-phase CTA was employed to characterize collateral status and infarct core size as part of the trials inclusion criteria for potential EVT.107 EXTEND-IA and SWIFT PRIME used CT perfusion (CTP) for CT sites or PDM for MRI sites for patient selection. CTP was used to determine the presence of a target mismatch profile by defining the ischemic core as tissue with relative CBF less than 30% of normal tissue and the “penumbra” as tissue with Tmax greater than 6 s. 106,108 The use of CTP to represent the ischemic core in these and other studies, however, is somewhat controversial as it is unclear whether reductions in relative perfusion metrics represent tissue infarction or mere hypoperfusion that can be reversed with revascularization.109 Nonetheless, in patients with a target mismatch profile, reperfusion therapy was strongly associated with a favorable clinical response defined as NIHSS score of 0 to 1 or a ≥ 8-point improvement on NIHSS at 90 days.110,111 DEFUSE-3112 is an ongoing prospective randomized Phase III multicenter controlled trial addressing whether a target mismatch profile can be used to select patients likely to benefit from endovascular treatment in an extended time window.
Aside from using anterior circulation LVO as an inclusion criterion, infarct topography was not utilized in the decision process of patient eligibility for EVT trials. Instead patient selection was based on lesion volumes facilitated by the use by the use of either automated software113 or visual assessment (ABC/2).105 Future studies are needed to compare automated with manual approaches for patient selection for revascularization therapies.
Region-of-interest analyses
The Alberta Stroke Program Early CT Score (ASPECTS),114 which was originally created to assess early acute ischemic injury and is incorporated into many decision models for pursuing endovascular therapy, is one approach that is used to link specific acute infarct locations to long-term stroke outcomes.115–117 Multiple studies have shown that integrating ASPECTS score into prediction models might be clinically useful. In one study, ASPECTS score was evaluated in data from the original NINDS tPA study and lesions involving the primary motor cortex/parietal lobe (M6) and lentiform nucleus increased the likelihood of disability (mRS> 2).116 A simplified ASPECTS (sASPECTS) score, which scored only the caudate, lentiform nucleus, insula, and M5 region lesions, produced similar results to the ASPECTS score, and was found to be an independent predictor of three-month mRS> 2 in patients with anterior circulation AIS.118
Although ASPECTS is traditionally performed on non-contrast CT scans, ASPECTS has also been applied to DWI. One study compared the performance of CT-ASPECTS and DWI-ASPECTS in a large EVT cohort.119 In 74 patients, the inter-rater agreement for CT-ASPECTS and DWI-ASPECTS was 0.58 and 0.87, respectively. DWI-ASPECTS correlated with functional outcome while CT-ASPECTS did not. Both CT- and MRI-based ASPECTS, however, correlated with DWI volume but DWI-ASPECTS was superior.119 In a cohort of patients with AIS treated with intravenous tPA that underwent CT and MRI in the hyperacute stage <3 h), the accuracy (with 1 being the highest accuracy) for CT-ASPECTS was 0.62 and DWI-ASPECTS 0.64 for predicting mRS < 3 at 90 days.120 Comparing CT- to DWI-ASPECTS in AIS patients within 3 h of onset, the detection rate was significantly higher for DWI than CT (76.9% vs. 30.0%; p < 0.01).121 These results suggest the sensitivity for detecting early ischemia is greater with MRI. However, this may be due to later acquisition of DWI than CT, since typically MRI is performed later than CT scans.
One group showed that in 213 patients with intracranial internal carotid artery, M1 or M2 middle cerebral artery occlusions who underwent EVT, on multivariable logistic regression, only M4 and M6 involvement on DWI ASPECTS, obtained < 72 h from symptom onset, were associated with poor outcomes (3 month mRS > 2).117 Interestingly, in right hemispheric strokes, M6 involvement independently predicted poor outcome (odds ratio 5.8; 95% confidence interval 1.9–20.3), whereas in left hemispheric strokes, M4 involvement was a predictor of poor outcomes (odds ratio 4.3; 95% confidence interval 1.3–15.0).117
The advantage of ASPECTS is that in trained professionals, it can quickly be calculated on CT or DWI performed in the acute stages and, appears to be associated with long-term functional outcomes. There are also efforts to automate ASPECTS calculation,122 which will simplify the approach further. The ASPECTS score, either manual or automatically determined, has not yet been found to be able to select patients likely to benefit from endovascular therapy.123 The role of ASPECTS in most current trials is predominantly to exclude patients who present with large strokes (e.g. ASPECTS < 7) as opposed to focusing on particular regions that are involved (e.g. lentiform nuclei).
Voxel-based analyses
Because of the poor sensitivity of CT in the hyperacute stage, voxel-based approaches using CT have been rarely used and therefore discussion will be limited to MRI. As mentioned earlier, acute infarct volume only correlates moderately with AIS outcomes.8–10 The integration of lesion location into models predicting functional outcomes after AIS offers promise for improving the accuracy of long-term prognostication models. An early example is a hazard atlas, which utilized discharge or chronic imaging lesion location combined with an “expert” atlas to predict NIHSS.15 Another study used penalized logistic regression based on chronic imaging lesion location and extent to predict NIHSS.124 These preliminary proof-of-concept studies linking lesion location with degree of disability has motivated the development of new techniques aimed at characterizing the specific role of acute infarct topography on global functional outcome metrics beyond laterality or specific functional domains.
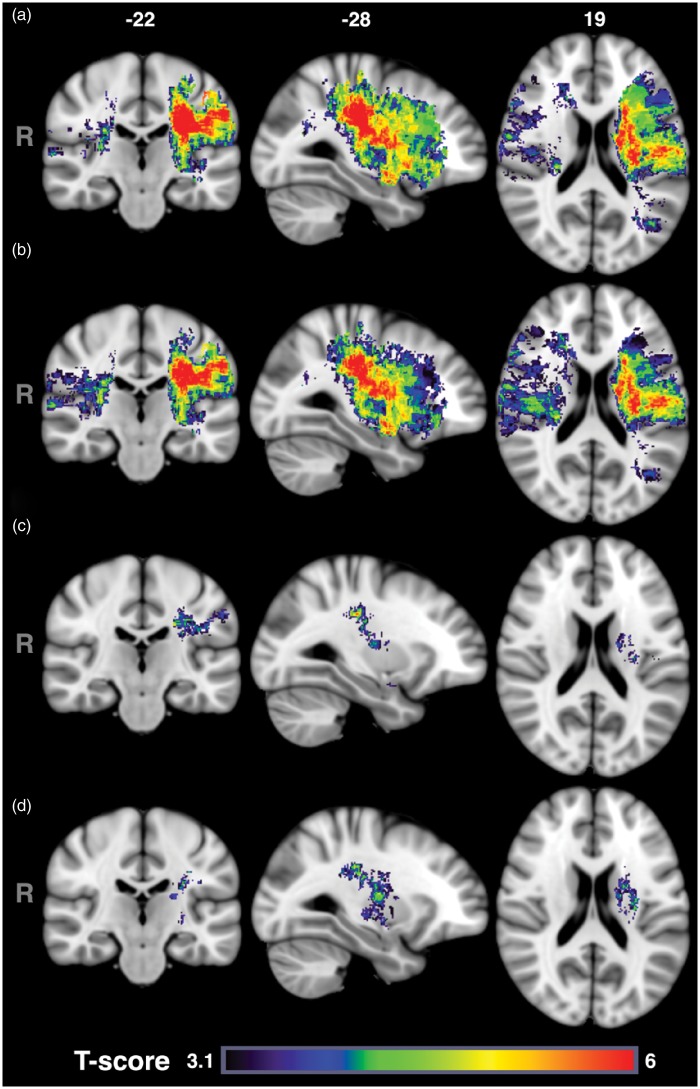
VLSM has also been used to assess long-term global functional outcomes using acute imaging. In 101 patients with middle cerebral artery infarcts and two to three day DWI, greater disability as measured on the one-month mRS were associated with acute injury to the corona radiata, internal capsule, and insula, with asymmetric impact patterns found with respect to injury to the right angular gyrus and left superior temporal gyrus.125 In this study, however, lesion volumes were not taken into consideration. Another study involving 490 AIS subjects who had DWI obtained within 48 h of last known well included lesion volume in VLSM analysis to investigate the role of acute lesion topography in AIS severity and long-term functional outcome.126 Figure 4 shows the T-score results, on a voxel-by-voxel basis, of the effect of injury to each voxel on three to six month mRS. Adjusting for age and gender (Figure 4(b)), larger regions of tissue in the right hemisphere were implicated with worse mRS, while the opposite was true for the left hemisphere compared to unadjusted results (Figure 4(a)). When also adjusting for lesion volume (Figure 4(c)), injury to smaller regions within only the left hemisphere (e.g. corona radiata, internal capsule, postcentral gyrus, putamen, and operculum) was found to be independently associated with poor mRS. That is, patients with acute infarcts in these regions (independent of age, sex and lesion volume) were likely to have more disability at three to six months, as measured by mRS, than patients who did not have lesions affecting those regions. Similar results were observed when the analysis was limited to only patients who were still alive at six months post-stroke (Figure 4(d)). Moreover, the authors showed that left hemisphere injury, especially to the posterior limb of the internal capsule and white matter tracts was also associated with higher admission NIHSS.126 VLSM has also been used to identify critical regions in functional outcomes after cerebellar ischemic strokes by examining injury at two weeks on 3D T1-weighted image17 or within 72 h.18 Both studies showed that in patients with impaired motor performance after cerebellar stroke lesions were more common in the paravermal lobules IV/V, deep cerebellar nuclei, and the middle cerebellar peduncle.17,18
Figure 4.
Voxel-based lesion symptom mapping results for acute ischemic stroke functional outcomes (mRS). T-score maps with voxel-wise threshold of p < 0.001 and permutation method for follow-up mRS scores without covariates (a), using sex and age (b), or sex, age, and lesion volume as covariates (c). Subset analysis for patients alive at 6 months post AIS (d). A voxel with a high T-score (red) indicates that patients with lesions involving the individual voxel had worse mRS scores than patients who did not have a lesion at that voxel. Conversely, a voxel with a low T-score reflects no statistically significant difference (p > 0.001) in mRS scores between patients with and without a lesion at that voxel. From Ona Wu, Lisa Cloonan, Steven JT Mocking, Mark JRJ Bouts, William A Copen, Pedro T Cougo-Pinto, Kaitlin Fitzpatrick, Allison Kanakis, Pamela W Schaefer, Jonathan Rosand, Karen L Furie, Natalia S Rost. Role of acute lesion topography in initial ischemic stroke severity and long-term functional outcomes. Stroke 2015; 46: 2438–2444 and reproduced with permission from Wolters Kluwer Health.
The use of the ASPECTS score and VLSM represents two approaches to incorporate acute lesion location into predictions of long-term outcomes at the acute stage. Early identification of patients at risk of poor recovery can potentially be used for selection of patients for clinical trials, aggressive intervention and focused post-stroke rehabilitation programs. Although ASPECTS on CT or MRI is becoming frequently used for selection of patients for thrombectomy in clinical trials,101 anatomical territories identified by VLSM analyses as regions linked with poor long-term outcomes if infarcted are not currently being used. Indeed, these VLSM identified regions overlap the “clinically relevant penumbra” found by others to determine functional outcome after thrombolysis more so than volume of salvaged tissue.127,128 Future prospective studies are needed to validate both techniques for their utility in patient selection for revascularization therapy.
Conclusions and future directions
Understanding ischemic stroke functional topography holds major potential value in the acute management of patients with AIS as well as in targeting individualized early rehabilitation strategies. The outstanding questions are twofold: first, what is the additive value of considering infarct topography in the acute and chronic setting; and secondly, what neuroimaging approaches are most practical in the acute setting?
At present, the incorporation of infarct topography into hyperacute prognostication models holds significant promise of improving predictive accuracy of individualized recovery potential but with the current evidence, clinical implementation would be premature. Future studies evaluating the utility of infarct topography in patient selection trials are warranted.
The current data suggest that infarct topography has significant influence on post-stroke functional recovery and, as a result, including infarct location into post-stroke outcome prediction models holds major promise. In addition, for novel stroke trials targeting specific cognitive domains and brain regions,129,130 knowledge of the role of infarct topography on behavioral outcomes will be critical. Lesion topography has a role in improving our understanding of post-stroke disability and treatment planning for the rehabilitation setting.
Search strategy and selection criteria
We searched PubMed from January 2000 to October 2016, using the terms and synonyms “ischemic stroke,” “outcomes,” “topography,” “cognitive dysfunction,” “functional recovery,” “speech,” “aphasia,” “cognitive,” and “motor,” in combination with the key terms “infarct location” and “ischemic stroke”. We only searched for papers published in English. Of identified original research articles and relevant reviews, reference lists were also searched to identify additional relevant papers. Subsequently, we selected mainly original research articles or systematic reviews reported in core clinical journals during the past 16 years. Our final selection of references was made on basis of the relevance to the topics covered in this review.
Supplementary Material
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institutes of Health NINDS R01NS059775; R01NS082285; R01NS086905, P50-NS051343 and NIBIB P41EB015896.
Declaration of conflicting interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Ona Wu is the co-inventor of a patent on “Delay-compensated calculation of tissue blood flow,” US Patent 7,512,435. 31 March 2009, and the patent has been licensed to General Electric, Siemens, Imaging Biometrics and Olea Medical.
Supplementary material
Supplementary material for this paper can be found at the journal website: http://journals.sagepub.com/doi/suppl/10.1177/0271678X17700666
References
- 1.Feigin VL, Forouzanfar MH, Krishnamurthi R, et al. Global and regional burden of stroke during 1990-2010: Findings from the Global Burden of Disease Study 2010. Lancet 2014; 383: 245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller EL, Murray L, Richards L, et al. Comprehensive overview of nursing and interdisciplinary rehabilitation care of the stroke patient: A scientific statement from the American Heart Association. Stroke 2010; 41: 2402–2448. [DOI] [PubMed] [Google Scholar]
- 3.Veerbeek JM, Kwakkel G, van Wegen EE, et al. Early prediction of outcome of activities of daily living after stroke: A systematic review. Stroke 2011; 42: 1482–1488. [DOI] [PubMed] [Google Scholar]
- 4.Heiss WD, Kidwell CS. Imaging for prediction of functional outcome and assessment of recovery in ischemic stroke. Stroke 2014; 45: 1195–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weimar C, Ho TW, Katsarava Z, et al. Improving patient selection for clinical acute stroke trials. Cerebrovasc Dis 2006; 21: 386–392. [DOI] [PubMed] [Google Scholar]
- 6.Muir KW. Heterogeneity of stroke pathophysiology and neuroprotective clinical trial design. Stroke 2002; 33: 1545–1550. [DOI] [PubMed] [Google Scholar]
- 7.Young FB, Lees KR, Weir CJ, et al. Improving trial power through use of prognosis-adjusted end points. Stroke 2005; 36: 597–601. [DOI] [PubMed] [Google Scholar]
- 8.Farr TD, Wegener S. Use of magnetic resonance imaging to predict outcome after stroke: A review of experimental and clinical evidence. J Cereb Blood Flow Metab 2010; 30: 703–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lev MH, Segal AZ, Farkas J, et al. Utility of perfusion-weighted CT imaging in acute middle cerebral artery stroke treated with intra-arterial thrombolysis: Prediction of final infarct volume and clinical outcome. Stroke 2001; 32: 2021–2028. [DOI] [PubMed] [Google Scholar]
- 10.Saver JL, Johnston KC, Homer D, et al. Infarct volume as a surrogate or auxiliary outcome measure in ischemic stroke clinical trials. The RANTTAS Investigators. Stroke 1999; 30: 293–298. [DOI] [PubMed] [Google Scholar]
- 11.Weimar C, Konig IR, Kraywinkel K, et al. Age and National Institutes of Health Stroke Scale Score within 6 hours after onset are accurate predictors of outcome after cerebral ischemia: Development and external validation of prognostic models. Stroke 2004; 35: 158–162. [DOI] [PubMed] [Google Scholar]
- 12.Johnston KC, Connors AF, Jr., Wagner DP, et al. Predicting outcome in ischemic stroke: External validation of predictive risk models. Stroke 2003; 34: 200–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnston KC, Wagner DP, Haley EC, Jr., et al. Combined clinical and imaging information as an early stroke outcome measure. Stroke 2002; 33: 466–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Konig IR, Ziegler A, Bluhmki E, et al. Predicting long-term outcome after acute ischemic stroke: A simple index works in patients from controlled clinical trials. Stroke 2008; 39: 1821–1826. [DOI] [PubMed] [Google Scholar]
- 15.Menezes NM, Ay H, Wang Zhu M, et al. The real estate factor: Quantifying the impact of infarct location on stroke severity. Stroke 2007; 38: 194–197. [DOI] [PubMed] [Google Scholar]
- 16.Wong GK, Nung RC, Sitt JC, et al. Location, infarct load, and 3-month outcomes of delayed cerebral infarction after aneurysmal subarachnoid hemorrhage. Stroke 2015; 46: 3099–3104. [DOI] [PubMed] [Google Scholar]
- 17.Konczak J, Pierscianek D, Hirsiger S, et al. Recovery of upper limb function after cerebellar stroke: Lesion symptom mapping and arm kinematics. Stroke 2010; 41: 2191–2200. [DOI] [PubMed] [Google Scholar]
- 18.Picelli A, Zuccher P, Tomelleri G, et al. Prognostic importance of lesion location on functional outcome in patients with cerebellar ischemic stroke: A prospective pilot study. Cerebellum 2017; 16: 257–261. [DOI] [PubMed] [Google Scholar]
- 19.Kasner SE. Clinical interpretation and use of stroke scales. Lancet Neurol 2006; 5: 603–612. [DOI] [PubMed] [Google Scholar]
- 20.Cramer SC, Koroshetz WJ, Finklestein SP. The case for modality-specific outcome measures in clinical trials of stroke recovery-promoting agents. Stroke 2007; 38: 1393–1395. [DOI] [PubMed] [Google Scholar]
- 21.Liebeskind DS, Albers GW, Crawford K, et al. Imaging in strokenet: Realizing the potential of big data. Stroke 2015; 46: 2000–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saver JL, Warach S, Janis S, et al. Standardizing the structure of stroke clinical and epidemiologic research data: The National Institute of Neurological Disorders and Stroke (NINDS) Stroke Common Data Element (CDE) project. Stroke 2012; 43: 967–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sulter G, Steen C, De Keyser J. Use of the Barthel index and modified Rankin scale in acute stroke trials. Stroke 1999; 30: 1538–1541. [DOI] [PubMed] [Google Scholar]
- 24.Banks JL, Marotta CA. Outcomes validity and reliability of the modified Rankin scale: Implications for stroke clinical trials: A literature review and synthesis. Stroke 2007; 38: 1091–1096. [DOI] [PubMed] [Google Scholar]
- 25.van Swieten JC, Koudstaal PJ, Visser MC, et al. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988; 19: 604–607. [DOI] [PubMed] [Google Scholar]
- 26.Adams HP, Jr., Davis PH, Leira EC, et al. Baseline NIH Stroke Scale score strongly predicts outcome after stroke: A report of the Trial of Org 10172 in Acute Stroke Treatment (TOAST). Neurology 1999; 53: 126–131. [DOI] [PubMed] [Google Scholar]
- 27.Frankel MR, Morgenstern LB, Kwiatkowski T, et al. Predicting prognosis after stroke: A placebo group analysis from the National Institute of Neurological Disorders and Stroke rt-PA Stroke Trial. Neurology 2000; 55: 952–959. [DOI] [PubMed] [Google Scholar]
- 28.Goldstein LB, Bertels C, Davis JN. Interrater reliability of the NIH stroke scale. Arch Neurol 1989; 46: 660–662. [DOI] [PubMed] [Google Scholar]
- 29.Woo D, Broderick JP, Kothari RU, et al. Does the National Institutes of Health Stroke Scale favor left hemisphere strokes? NINDS t-PA stroke study group. Stroke 1999; 30: 2355–2359. [DOI] [PubMed] [Google Scholar]
- 30.Fink JN, Selim MH, Kumar S, et al. Is the association of National Institutes of Health Stroke Scale scores and acute magnetic resonance imaging stroke volume equal for patients with right- and left-hemisphere ischemic stroke? Stroke 2002; 33: 954–958. [DOI] [PubMed] [Google Scholar]
- 31.Alt Murphy M, Resteghini C, Feys P, et al. An overview of systematic reviews on upper extremity outcome measures after stroke. BMC Neurol 2015; 15: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gladstone DJ, Danells CJ, Black SE. The fugl-meyer assessment of motor recovery after stroke: A critical review of its measurement properties. Neurorehabil Neural Repair 2002; 16: 232–240. [DOI] [PubMed] [Google Scholar]
- 33.Keith RA, Granger CV, Hamilton BB, et al. The functional independence measure: A new tool for rehabilitation. Adv Clin Rehabil 1987; 1: 6–18. [PubMed] [Google Scholar]
- 34.Denes G, Semenza C, Stoppa E, et al. Unilateral spatial neglect and recovery from hemiplegia: A follow-up study. Brain 1982; 105(Pt 3): 543–552. [DOI] [PubMed] [Google Scholar]
- 35.Fink JN, Frampton CM, Lyden P, et al. Does hemispheric lateralization influence functional and cardiovascular outcomes after stroke? An analysis of placebo-treated patients from prospective acute stroke trials. Stroke 2008; 39: 3335–3340. [DOI] [PubMed] [Google Scholar]
- 36.Goto A, Okuda S, Ito S, et al. Locomotion outcome in hemiplegic patients with middle cerebral artery infarction: The difference between right- and left-sided lesions. J Stroke Cerebrovasc Dis 2009; 18: 60–67. [DOI] [PubMed] [Google Scholar]
- 37.Johansson BB, Jadback G, Norrving B, et al. Evaluation of long-term functional status in first-ever stroke patients in a defined population. Scand J Rehabil Med Suppl 1992; 26: 105–114. [PubMed] [Google Scholar]
- 38.Laufer Y, Sivan D, Schwarzmann R, et al. Standing balance and functional recovery of patients with right and left hemiparesis in the early stages of rehabilitation. Neurorehabil Neural Repair 2003; 17: 207–213. [DOI] [PubMed] [Google Scholar]
- 39.Paolucci S, Antonucci G, Pratesi L, et al. Functional outcome in stroke inpatient rehabilitation: Predicting no, low and high response patients. Cerebrovasc Dis 1998; 8: 228–234. [DOI] [PubMed] [Google Scholar]
- 40.Ring H, Feder M, Schwartz J, et al. Functional measures of first-stroke rehabilitation inpatients: Usefulness of the Functional Independence Measure total score with a clinical rationale. Arch Phys Med Rehabil 1997; 78: 630–635. [DOI] [PubMed] [Google Scholar]
- 41.Wade DT, Hewer RL, Wood VA. Stroke: influence of patient's sex and side of weakness on outcome. Arch Phys Med Rehabil 1984; 65: 513–516. [PubMed] [Google Scholar]
- 42.Yoo AJ, Romero J, Hakimelahi R, et al. Predictors of functional outcome vary by the hemisphere of involvement in major ischemic stroke treated with intra-arterial therapy: A retrospective cohort study. BMC Neurol 2010; 10: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kalra L, Smith DH, Crome P. Stroke in patients aged over 75 years: Outcome and predictors. Postgrad Med J 1993; 69: 33–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ween JE, Alexander MP, D'Esposito M, et al. Factors predictive of stroke outcome in a rehabilitation setting. Neurology 1996; 47: 388–392. [DOI] [PubMed] [Google Scholar]
- 45.Davalos A, Blanco M, Pedraza S, et al. The clinical-DWI mismatch: A new diagnostic approach to the brain tissue at risk of infarction. Neurology 2004; 62: 2187–2192. [DOI] [PubMed] [Google Scholar]
- 46.Schiemanck SK, Post MW, Kwakkel G, et al. Ischemic lesion volume correlates with long-term functional outcome and quality of life of middle cerebral artery stroke survivors. Restor Neurol Neurosci 2005; 23: 257–263. [PubMed] [Google Scholar]
- 47.Vogt G, Laage R, Shuaib A, et al. Initial lesion volume is an independent predictor of clinical stroke outcome at day 90: An analysis of the Virtual International Stroke Trials Archive (VISTA) database. Stroke 2012; 43: 1266–1272. [DOI] [PubMed] [Google Scholar]
- 48.Wu O, Koroshetz WJ, Ostergaard L, et al. Predicting tissue outcome in acute human cerebral ischemia using combined diffusion- and perfusion-weighted MR imaging. Stroke 2001; 32: 933–942. [DOI] [PubMed] [Google Scholar]
- 49.Barber PA, Davis SM, Infeld B, et al. Spontaneous reperfusion after ischemic stroke is associated with improved outcome. Stroke 1998; 29: 2522–2528. [DOI] [PubMed] [Google Scholar]
- 50.Schellinger PD, Latour LL, Wu CS, et al. The association between neurological deficit in acute ischemic stroke and mean transit time: Comparison of four different perfusion MRI algorithms. Neuroradiology 2006; 48: 69–77. [DOI] [PubMed] [Google Scholar]
- 51.Hillis AE, Gold L, Kannan V, et al. Site of the ischemic penumbra as a predictor of potential for recovery of functions. Neurology 2008; 71: 184–189. [DOI] [PubMed] [Google Scholar]
- 52.Hillis AE, Barker PB, Beauchamp NJ, et al. MR perfusion imaging reveals regions of hypoperfusion associated with aphasia and neglect. Neurology 2000; 55: 782–788. [DOI] [PubMed] [Google Scholar]
- 53.Foerch C, Misselwitz B, Sitzer M, et al. Difference in recognition of right and left hemispheric stroke. Lancet 2005; 366: 392–393. [DOI] [PubMed] [Google Scholar]
- 54.Di Legge S, Fang J, Saposnik G, et al. The impact of lesion side on acute stroke treatment. Neurology 2005; 65: 81–86. [DOI] [PubMed] [Google Scholar]
- 55.Jorgensen HS, Nakayama H, Reith J, et al. Factors delaying hospital admission in acute stroke: The Copenhagen Stroke Study. Neurology 1996; 47: 383–387. [DOI] [PubMed] [Google Scholar]
- 56.Agyeman O, Nedeltchev K, Arnold M, et al. Time to admission in acute ischemic stroke and transient ischemic attack. Stroke 2006; 37: 963–966. [DOI] [PubMed] [Google Scholar]
- 57.Jorgensen HS, Nakayama H, Raaschou HO, et al. Outcome and time course of recovery in stroke. Part I: Outcome. The Copenhagen Stroke Study. Arch Phys Med Rehabil 1995; 76: 399–405. [DOI] [PubMed] [Google Scholar]
- 58.Jorgensen HS, Nakayama H, Raaschou HO, et al. Outcome and time course of recovery in stroke. Part II: Time course of recovery. The Copenhagen Stroke Study. Arch Phys Med Rehabil 1995; 76: 406–412. [DOI] [PubMed] [Google Scholar]
- 59.Cramer SC. Repairing the human brain after stroke: I. Mechanisms of spontaneous recovery. Ann Neurol 2008; 63: 272–287. [DOI] [PubMed] [Google Scholar]
- 60.Shelton FN, Reding MJ. Effect of lesion location on upper limb motor recovery after stroke. Stroke 2001; 32: 107–112. [DOI] [PubMed] [Google Scholar]
- 61.Lindenberg R, Renga V, Zhu LL, et al. Structural integrity of corticospinal motor fibers predicts motor impairment in chronic stroke. Neurology 2010; 74: 280–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhu LL, Lindenberg R, Alexander MP, et al. Lesion load of the corticospinal tract predicts motor impairment in chronic stroke. Stroke 2010; 41: 910–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Abela E, Missimer J, Wiest R, et al. Lesions to primary sensory and posterior parietal cortices impair recovery from hand paresis after stroke. PLoS One 2012; 7: e31275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kaya D, Dincer A, Arman F, et al. Ischemic involvement of the primary motor cortex is a prognostic factor in acute stroke. Int J Stroke 2015; 10: 1277–1283. [DOI] [PubMed] [Google Scholar]
- 65.Payabvash S, Souza LC, Kamalian S, et al. Location-weighted CTP analysis predicts early motor improvement in stroke: A preliminary study. Neurology 2012; 78: 1853–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Alexander LD, Black SE, Patterson KK, et al. Association between gait asymmetry and brain lesion location in stroke patients. Stroke 2009; 40: 537–544. [DOI] [PubMed] [Google Scholar]
- 67.Bates E, Wilson SM, Saygin AP, et al. Voxel-based lesion-symptom mapping. Nat Neurosci 2003; 6: 448–450. [DOI] [PubMed] [Google Scholar]
- 68.Lo R, Gitelman D, Levy R, et al. Identification of critical areas for motor function recovery in chronic stroke subjects using voxel-based lesion symptom mapping. Neuroimage 2010; 49: 9–18. [DOI] [PubMed] [Google Scholar]
- 69.Jones PS, Pomeroy VM, Wang J, et al. Does stroke location predict walk speed response to gait rehabilitation? Hum Brain Mapp 2016; 37: 689–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Prabhakaran S, Zarahn E, Riley C, et al. Inter-individual variability in the capacity for motor recovery after ischemic stroke. Neurorehabil Neural Repair 2008; 22: 64–71. [DOI] [PubMed] [Google Scholar]
- 71.El Hachioui H, Lingsma HF, van de Sandt-Koenderman ME, et al. Recovery of aphasia after stroke: A 1-year follow-up study. J Neurol 2013; 260: 166–171. [DOI] [PubMed] [Google Scholar]
- 72.Pedersen PM, Vinter K, Olsen TS. Aphasia after stroke: Type, severity and prognosis. The Copenhagen aphasia study. Cerebrovasc Dis 2004; 17: 35–43. [DOI] [PubMed] [Google Scholar]
- 73.M MW, S AB. Factors predicting post-stroke aphasia recovery. J Neurol Sci 2015; 352: 12–18. [DOI] [PubMed] [Google Scholar]
- 74.Goldenberg G, Spatt J. Influence of size and site of cerebral lesions on spontaneous recovery of aphasia and on success of language therapy. Brain Lang 1994; 47: 684–698. [DOI] [PubMed] [Google Scholar]
- 75.Selnes OA, Knopman DS, Niccum N, et al. Computed tomographic scan correlates of auditory comprehension deficits in aphasia: A prospective recovery study. Ann Neurol 1983; 13: 558–566. [DOI] [PubMed] [Google Scholar]
- 76.Hanlon RE, Lux WE, Dromerick AW. Global aphasia without hemiparesis: Language profiles and lesion distribution. J Neurol Neurosurg Psychiatry 1999; 66: 365–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kertesz A, Lau WK, Polk M. The structural determinants of recovery in Wernicke's aphasia. Brain Lang 1993; 44: 153–164. [DOI] [PubMed] [Google Scholar]
- 78.Naeser MA, Helm-Estabrooks N, Haas G, et al. Relationship between lesion extent in ‘Wernicke's area' on computed tomographic scan and predicting recovery of comprehension in Wernicke's aphasia. Arch Neurol 1987; 44: 73–82. [DOI] [PubMed] [Google Scholar]
- 79.Kang EK, Sohn HM, Han MK, et al. Severity of post-stroke aphasia according to aphasia type and lesion location in Koreans. J Korean Med Sci 2010; 25: 123–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Payabvash S, Kamalian S, Fung S, et al. Predicting language improvement in acute stroke patients presenting with aphasia: A multivariate logistic model using location-weighted atlas-based analysis of admission CT perfusion scans. AJNR Am J Neuroradiol 2010; 31: 1661–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pendlebury ST, Rothwell PM. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: A systematic review and meta-analysis. Lancet Neurol 2009; 8: 1006–1018. [DOI] [PubMed] [Google Scholar]
- 82.Desmond DW, Moroney JT, Paik MC, et al. Frequency and clinical determinants of dementia after ischemic stroke. Neurology 2000; 54: 1124–1131. [DOI] [PubMed] [Google Scholar]
- 83.Desmond DW, Moroney JT, Sano M, et al. Recovery of cognitive function after stroke. Stroke 1996; 27: 1798–1803. [DOI] [PubMed] [Google Scholar]
- 84.Nys GM, van Zandvoort MJ, de Kort PL, et al. Cognitive disorders in acute stroke: Prevalence and clinical determinants. Cerebrovasc Dis 2007; 23: 408–416. [DOI] [PubMed] [Google Scholar]
- 85.Jaillard A, Grand S, Le Bas JF, et al. Predicting cognitive dysfunctioning in nondemented patients early after stroke. Cerebrovasc Dis 2010; 29: 415–423. [DOI] [PubMed] [Google Scholar]
- 86.Munsch F, Sagnier S, Asselineau J, et al. Stroke location is an independent predictor of cognitive outcome. Stroke 2016; 47: 66–73. [DOI] [PubMed] [Google Scholar]
- 87.Dacosta-Aguayo R, Grana M, Fernandez-Andujar M, et al. Structural integrity of the contralesional hemisphere predicts cognitive impairment in ischemic stroke at three months. PLoS One 2014; 9: e86119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Boes AD, Prasad S, Liu H, et al. Network localization of neurological symptoms from focal brain lesions. Brain 2015; 138: 3061–3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gonzalez RG, Schaefer PW, Buonanno FS, et al. Diffusion-weighted MR imaging: Diagnostic accuracy in patients imaged within 6 hours of stroke symptom onset. Radiology 1999; 210: 155–162. [DOI] [PubMed] [Google Scholar]
- 90.Mullins ME, Schaefer PW, Sorensen AG, et al. CT and conventional and diffusion-weighted MR imaging in acute stroke: Study in 691 patients at presentation to the emergency department. Radiology 2002; 224: 353–360. [DOI] [PubMed] [Google Scholar]
- 91.Hacke W, Kaste M, Fieschi C, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European Cooperative Acute Stroke Study (ECASS). JAMA 1995; 274: 1017–1025. [PubMed] [Google Scholar]
- 92.Hacke W, Kaste M, Fieschi C, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian Acute Stroke Study Investigators. Lancet 1998; 352: 1245–1251. [DOI] [PubMed] [Google Scholar]
- 93.Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 2008; 359: 1317–1329. [DOI] [PubMed] [Google Scholar]
- 94.Davis SM, Donnan GA, Parsons MW, et al. Effects of alteplase beyond 3 h after stroke in the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET): A placebo-controlled randomised trial. Lancet Neurol 2008; 7: 299–309. [DOI] [PubMed] [Google Scholar]
- 95.Clark WM, Wissman S, Albers GW, et al. Recombinant tissue-type plasminogen activator (Alteplase) for ischemic stroke 3 to 5 hours after symptom onset. The ATLANTIS Study: A randomized controlled trial. Alteplase thrombolysis for acute noninterventional therapy in ischemic stroke. JAMA 1999; 282: 2019–2026. [DOI] [PubMed] [Google Scholar]
- 96.Ma H, Parsons MW, Christensen S, et al. A multicentre, randomized, double-blinded, placebo-controlled Phase III study to investigate EXtending the time for Thrombolysis in Emergency Neurological Deficits (EXTEND). Int J Stroke 2012; 7: 74–80. [DOI] [PubMed] [Google Scholar]
- 97.Wu O, Schwamm LH, Sorensen AG. Imaging stroke patients with unclear onset times. Neuroimaging Clin N Am 2011; 21: 327–344. xi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Prosser J, Butcher K, Allport L, et al. Clinical-diffusion mismatch predicts the putative penumbra with high specificity. Stroke 2005; 36: 1700–1704. [DOI] [PubMed] [Google Scholar]
- 99.Lansberg MG, Thijs VN, Hamilton S, et al. Evaluation of the clinical-diffusion and perfusion-diffusion mismatch models in DEFUSE. Stroke 2007; 38: 1826–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ebinger M, Iwanaga T, Prosser JF, et al. Clinical-diffusion mismatch and benefit from thrombolysis 3 to 6 hours after acute stroke. Stroke 2009; 40: 2572–2574. [DOI] [PubMed] [Google Scholar]
- 101.Stryker N. Trevo and Medical Management Versus Medical Management Alone in Wake Up and Late Presenting Strokes (DAWN). Bethesda (MD): National Library of Medicine (US). NLM Identifier: NCT02142283. Available at: http://clinicaltrials.gov/show/NCT02142283 (2000, accessed 12 February 2017).
- 102.Yoo AJ, Chaudhry ZA, Nogueira RG, et al. Infarct volume is a pivotal biomarker after intra-arterial stroke therapy. Stroke 2012; 43: 1323–1330. [DOI] [PubMed] [Google Scholar]
- 103.Sajobi TT, Menon BK, Wang M, et al. Early trajectory of stroke severity predicts long-term functional outcomes in ischemic stroke subjects: Results from the ESCAPE trial (endovascular treatment for small core and anterior circulation proximal occlusion with emphasis on minimizing ct to recanalization times). Stroke 2017; 48: 105–110. [DOI] [PubMed] [Google Scholar]
- 104.Leslie-Mazwi TM, Hirsch JA, Falcone GJ, et al. Endovascular stroke treatment outcomes after patient selection based on magnetic resonance imaging and clinical criteria. JAMA Neurol 2016; 73: 43–49. [DOI] [PubMed] [Google Scholar]
- 105.Sims JR, Gharai LR, Schaefer PW, et al. ABC/2 for rapid clinical estimate of infarct, perfusion, and mismatch volumes. Neurology 2009; 72: 2104–2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Campbell BC, Mitchell PJ, Kleinig TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 2015; 372: 1009–1018. [DOI] [PubMed] [Google Scholar]
- 107.Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 2015; 372: 1019–1030. [DOI] [PubMed] [Google Scholar]
- 108.Saver JL, Goyal M, Bonafe A, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med 2015; 372: 2285–2295. [DOI] [PubMed] [Google Scholar]
- 109.Copen WA, Morais LT, Wu O, et al. In acute stroke, can CT perfusion-derived cerebral blood volume maps substitute for diffusion-weighted imaging in identifying the ischemic core? PLoS One 2015; 10: e0133566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lansberg MG, Lee J, Christensen S, et al. RAPID automated patient selection for reperfusion therapy: A pooled analysis of the echoplanar imaging thrombolytic evaluation trial (EPITHET) and the diffusion and perfusion imaging evaluation for understanding stroke evolution (DEFUSE) study. Stroke 2011; 42: 1608–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lansberg MG, Straka M, Kemp S, et al. MRI profile and response to endovascular reperfusion after stroke (DEFUSE 2): A prospective cohort study. Lancet Neurol 2012; 11: 860–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Albers GW. Endovascular Therapy Following Imaging Evaluation for Ischemic Stroke 3 (DEFUSE 3). Bethesda (MD): National Library of Medicine (US). NLM Identifier: NCT02586415. Available at: http://clinicaltrials.gov/show/NCT02586415 (2000, accessed 12 February 2017).
- 113.Campbell BC, Yassi N, Ma H, et al. Imaging selection in ischemic stroke: Feasibility of automated CT-perfusion analysis. Int J Stroke 2015; 10: 51–54. [DOI] [PubMed] [Google Scholar]
- 114.Barber PA, Demchuk AM, Zhang J, et al. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. ASPECTS Study Group. Alberta Stroke Programme Early CT Score. Lancet 2000; 355: 1670–1674. [DOI] [PubMed] [Google Scholar]
- 115.Beare R, Chen J, Phan TG, et al. Googling stroke ASPECTS to determine disability: Exploratory analysis from VISTA-acute collaboration. PLoS One 2015; 10: e0125687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Phan TG, Demchuk A, Srikanth V, et al. Proof of concept study: Relating infarct location to stroke disability in the NINDS rt-PA trial. Cerebrovasc Dis 2013; 35: 560–565. [DOI] [PubMed] [Google Scholar]
- 117.Rangaraju S, Streib C, Aghaebrahim A, et al. Relationship between lesion topology and clinical outcome in anterior circulation large vessel occlusions. Stroke 2015; 46: 1787–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Payabvash S, Noorbaloochi S and Qureshi AI. Topographic assessment of acute ischemic changes for prognostication of anterior circulation stroke. J Neuroimaging Epub ahead of print 4 September 2016. DOI: 10.1111/jon.12383. [DOI] [PubMed]
- 119.McTaggart RA, Jovin TG, Lansberg MG, et al. Alberta stroke program early computed tomographic scoring performance in a series of patients undergoing computed tomography and MRI: Reader agreement, modality agreement, and outcome prediction. Stroke 2015; 46: 407–412. [DOI] [PubMed] [Google Scholar]
- 120.Nezu T, Koga M, Nakagawara J, et al. Early ischemic change on CT versus diffusion-weighted imaging for patients with stroke receiving intravenous recombinant tissue-type plasminogen activator therapy: Stroke acute management with urgent risk-factor assessment and improvement (SAMURAI) rt-PA registry. Stroke 2011; 42: 2196–2200. [DOI] [PubMed] [Google Scholar]
- 121.Mitomi M, Kimura K, Aoki J, et al. Comparison of CT and DWI findings in ischemic stroke patients within 3 hours of onset. J Stroke Cerebrovasc Dis 2014; 23: 37–42. [DOI] [PubMed] [Google Scholar]
- 122.Nagel S, Sinha D, Day D, et al. e-ASPECTS software is non-inferior to neuroradiologists in applying the ASPECT score to computed tomography scans of acute ischemic stroke patients. Int J Stroke. Epub ahead of print 29 November 2016. DOI: 10.1177/1747493016681020. [DOI] [PubMed]
- 123.Hill MD, Demchuk AM, Goyal M, et al. Alberta Stroke Program early computed tomography score to select patients for endovascular treatment: Interventional Management of Stroke (IMS)-III Trial. Stroke 2014; 45: 444–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Phan TG, Chen J, Donnan G, et al. Development of a new tool to correlate stroke outcome with infarct topography: A proof-of-concept study. Neuroimage 2010; 49: 127–133. [DOI] [PubMed] [Google Scholar]
- 125.Cheng B, Forkert ND, Zavaglia M, et al. Influence of stroke infarct location on functional outcome measured by the modified rankin scale. Stroke 2014; 45: 1695–1702. [DOI] [PubMed] [Google Scholar]
- 126.Wu O, Cloonan L, Mocking SJ, et al. Role of acute lesion topography in initial ischemic stroke severity and long-term functional outcomes. Stroke 2015; 46: 2438–2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Seitz RJ, Sondermann V, Wittsack HJ, et al. Lesion patterns in successful and failed thrombolysis in middle cerebral artery stroke. Neuroradiology 2009; 51: 865–871. [DOI] [PubMed] [Google Scholar]
- 128.Rosso C, Samson Y. The ischemic penumbra: The location rather than the volume of recovery determines outcome. Curr Opin Neurol 2014; 27: 35–41. [DOI] [PubMed] [Google Scholar]
- 129.Campana S, Caltagirone C, Marangolo P. Combining voxel-based lesion-symptom mapping (VLSM) with A-tDCS language treatment: Predicting outcome of recovery in nonfluent chronic aphasia. Brain Stimul 2015; 8: 769–776. [DOI] [PubMed] [Google Scholar]
- 130.Hayward KS, Schmidt J, Lohse KR, et al. Are we armed with the right data? Pooled individual data review of biomarkers in people with severe upper limb impairment after stroke. Neuroimage Clin 2017; 13: 310–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.