Abstract
Depending on the adequacy of collateral sources of blood flow, arterial stenosis or occlusion may lead to reduced perfusion pressure and ultimately reduced blood flow in the distal territory supplied by that vessel. There are two well-defined compensatory mechanisms to reduced pressure or flow – autoregulatory vasodilation and increased oxygen extraction fraction. Other changes, such as metabolic downregulation, are likely. The positive identification of autoregulatory vasodilation and increased oxygen extraction fraction in humans is an established risk factor for future ischemic stroke in some disease states such as atherosclerotic carotid stenosis and occlusion. The mechanisms by which ischemic stroke may occur are not clear, and may include an increased vulnerability to embolic events. The use of hemodynamic assessment to identify patients with occlusive vasculopathy at an increased risk for stroke is very appealing for several different patient populations, such as those with symptomatic intracranial atherosclerotic disease, moyamoya phenomenon, complete internal carotid artery occlusion, and asymptomatic cervical carotid artery stenosis. While there is very good data for stroke risk prediction in some of these groups, no intervention based on these tools has been proven effective yet. In this manuscript, we will review these topics above and identify areas for future research.
Keywords: Arterial stenosis, cerebrovascular disease, occlusion, oxygen extraction, stroke
Introduction
The use of imaging tools to measure and assess cerebral hemodynamics in patients with chronic large vessel arterial occlusive diseases, such as atherosclerotic carotid stenosis or occlusion, has been the focus of extensive clinical research for decades.1,2 These tools are commonly used for many current clinical applications. Despite the wealth of information we have gained, major questions remain unanswered. These questions include:
What is the clinical relevance of hemodynamic data for different steno-occlusive diseases, and is it different for different methods of assessment?
Do other compensatory mechanisms in addition to classic autoregulation and increased oxygen extraction occur in humans with chronic regional reductions in perfusion pressure?
Why are people with impaired hemodynamics at risk for future stroke? What are the mechanisms?
These questions are challenging to answer, owing to several reasons. First, there is no good animal model for chronic, regional hypoperfusion; we are limited to human studies with more heterogeneity and variability. Second, there are a wide variety of tools to assess hemodynamic impairment and their physiological basis varies. Some are based on vasodilatory capacity after physiologically different challenges, some are based on mean transit time, and some are based on measurement of oxygen extraction. Within each of these categories, the methodology varies as well. Third, the different occlusive diseases that have been studied involve different patient populations, and these diseases and populations may have different stroke etiologies. For example, embolic mechanisms may play more of a synergistic role with hemodynamic factors for stroke pathophysiology in patients with atherosclerosis than in those with moyamoya phenomenon, or vice versa.
The purpose of this opinion is to focus on these three important questions, summarize the current state of our knowledge and identify key areas for future investigation for hemodynamic assessment in human large vessel arterial occlusive disease. Before addressing these questions, we will briefly review the current basis for our understanding of chronic regional hemodynamic impairment secondary to arterial occlusive disease and current clinical methods of assessment.
Responses to reductions in cerebral perfusion pressure
Our understanding of the compensatory mechanisms that occur in the human brain and cerebrovasculature in response to chronic regional reductions in perfusion pressure and blood flow comes largely from studies of acute, and generally global, reductions in mean arterial pressure.3–7 These studies, extending back to the 1930s, established autoregulatory vasodilation and increased oxygen extraction as critical responses that serve to maintain normal brain oxygen metabolism. The advent of human brain imaging technologies in the 1970s and 1980s opened up the opportunity to investigate this physiology in humans with cerebrovascular disease, specifically in those with atherosclerotic internal carotid artery occlusion undergoing surgical bypass procedures.2,8 The information below is a brief review of the topic relevant to the questions we have posed.
An arterial stenosis or occlusion may cause a reduction in cerebral perfusion pressure if collateral sources of flow are not adequate.9 The presence of arterial stenosis or occlusion does not equate with hemodynamic impairment: up to 50% of patients with complete internal carotid artery occlusion and prior ischemic symptoms have normal oxygen extraction.10 The adequacy of collateral sources of flow determines whether an occlusive lesion will cause a reduction in perfusion pressure.
When perfusion pressure falls owing to an occlusive lesion and an inadequate collateral system, the brain and its vasculature will maintain normal oxygen metabolism through two mechanisms, autoregulatory vasodilation to maintain cerebral blood flow (CBF) (the delivery of oxygen) and increased oxygen extraction fraction (OEF) to support normal oxygen metabolism.11 Changes in perfusion pressure have little effect on CBF over a wide range of pressure owing to vascular autoregulation. When perfusion pressure falls, reflex vasodilation will maintain CBF at near normal levels.3,12 Two measurable parameters that indicate autoregulatory vasodilation are mean transit time (MTT) and cerebral blood volume (CBV) (Figure 1). In addition, there is some reduction in CBF through the autoregulatory range, leading to a slight increase in oxygen extraction (Figure 1).11,13
Figure 1.
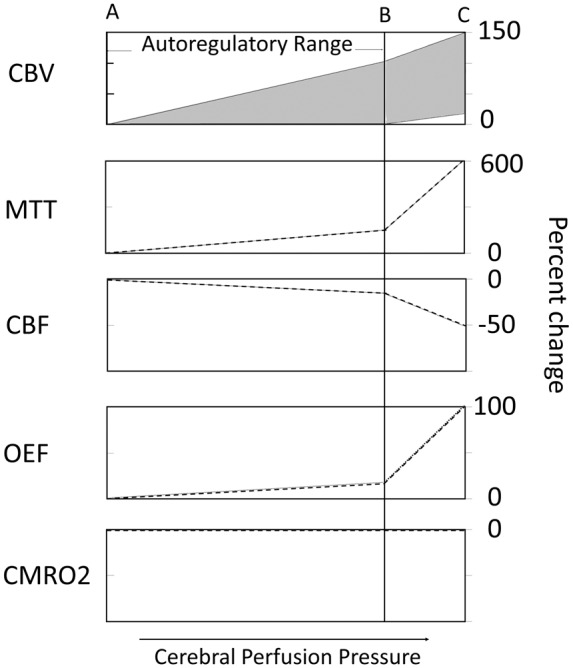
Oligemia – modified schematic of hemodynamic and metabolic responses to reductions in cerebral perfusion pressure after Derdeyn et al.11 The x-axis represents progressive reduction in perfusion pressure through the oligemic range (not true ischemia). The region between points A and B is the autoregulatory range. The region between points B and C is the region of autoregulatory failure where cerebral blood flow (CBF) falls passively as a function of pressure. Point C represents the exhaustion of compensatory mechanisms to maintain normal oxygen metabolism and the onset of true ischemia. CBV: Cerebral blood volume either remains unchanged or increases with autoregulatory vasodilation (crosshatched area), depending largely on the methods used to measure CBV. With autoregulatory failure, most investigators have found further increases in CBV. CBF: Cerebral blood flow falls slightly through the autoregulatory range. Once autoregulatory capacity is exceeded, CBF falls passively as a function of pressure down to 50% of baseline values. OEF: Oxygen extraction fraction (OEF) increases slightly with the reductions in CBF through the autoregulatory range. After autoregulatory capacity is exceeded and flow falls up to 50% of baseline, OEF may increase up to 100% from baseline. CMRO2: The cerebral metabolic rate for oxygen consumption remains unchanged throughout this range of CPP reduction owing to both autoregulatory vasodilation and increased OEF.
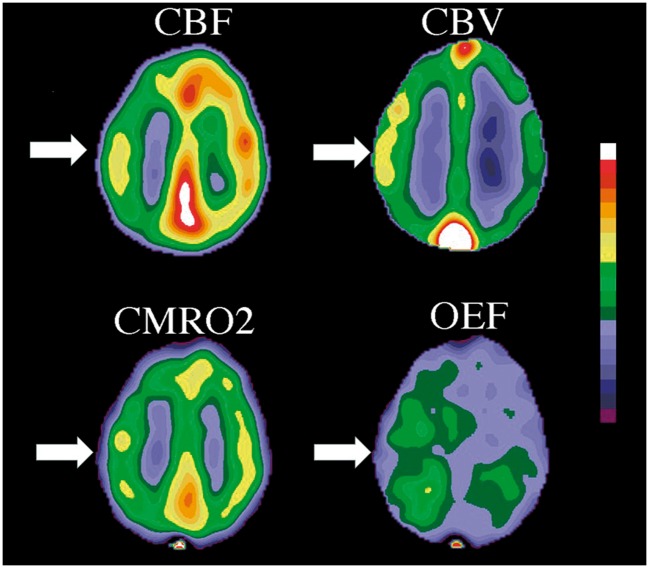
At some point, the capacity for autoregulatory vasodilation can be exceeded. The threshold value for autoregulatory failure is variable between patients and can be shifted higher or lower by prior ischemic injury or long-standing hypertension. Beyond this point, CBF falls passively as a function of pressure. Direct measurements of arteriovenous oxygen differences have demonstrated the brain's capacity to increase OEF and maintain normal cerebral oxygen metabolism (CMRO2) while the oxygen delivery diminishes due to decreasing CBF (Figures 1 and 2).4 Baron et al.2 coined the term “misery perfusion” to describe the condition of reduced CBF and increased oxygen extraction in some patients with complete atherosclerotic internal carotid occlusion and also reported its reversibility with successful surgical extracranial to intracranial arterial bypass. This work, and similar work from other investigators,8 in the late 1970s and 1980s helped advance the study of cerebral hemodynamics in chronic cerebral occlusive disease.
Figure 2.
PET study showing misery perfusion in a patient with a left internal carotid artery occlusion. White arrows indicate the left hemispheric reduction in CBF, and the increased CBV and increased OEF compensating for the low flow. Tissue oxygen metabolism is maintained (CMRO2).
One widely used clinical application of hemodynamic assessment that is outside the scope of this review but related to large artery occlusive disease is for the prediction of hyperperfusion phenomenon after revascularization.14–16 Hypoperfusion syndrome is uncommon and thought to be related to the inability of the distal vasculature to autoregulate, owing to long-standing maximal autoregulatory vasodilation.16 With reperfusion and restoration of normal arterial pressure, this may lead to seizures, edema, and hemorrhage. While the syndrome is uncommon, the phenomenon (increased CBF) is common.14 Patients with maximal hemodynamic impairment pretreatment are at a higher risk of the phenomenon and likely the syndrome.14
Clinical methods of assessment of hemodynamic impairment
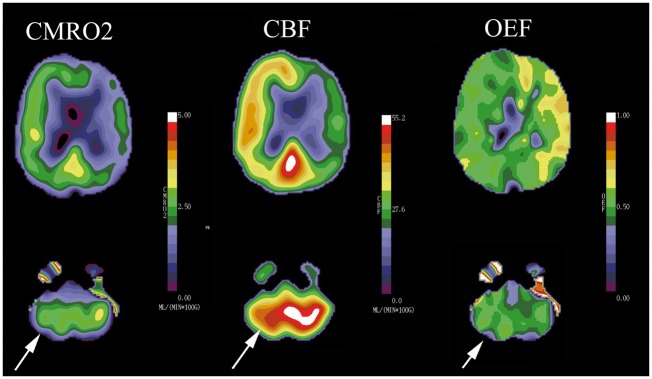
As discussed above, the hemodynamic effect of an arterial stenosis or occlusion depends on the adequacy of collateral circulation as well as the degree of stenosis. An occluded internal carotid artery, for example, often has no measurable effect on the distal cerebral perfusion pressure because the collateral flow through the circle of Willis is adequate.17 In addition, a single measurement of flow is meaningless when investigating the effects of an arterial lesion: normal values of CBF do not exclude the presence of autoregulatory vasodilation and reduced CBF may be present with normal perfusion pressure. This second situation may occur with prior stroke in the region of interest or in a remote area.18–21 Remote reduction in brain metabolism (and consequently blood flow) owing to reduced neuronal activity classically occurs in the contralateral cerebellum (crossed cerebellar diaschisis) after contralateral frontal lobe infarction, and can also be seen with lacunar stroke in the basal ganglia or thalamus and reduced metabolic demand of the overlying cortex and secondary reduction in flow, for example (Figure 3).18
Figure 3.
Diaschisis: This patient has a complete atherosclerotic occlusion of the right internal carotid artery and previous stroke in this territory. Consequently, the CMRO2 image demonstrates reduced oxygen metabolism relative to the contralateral hemisphere (white arrow). The reduced metabolic activity in the right frontal area has caused reduced metabolic activity in the structurally normal left cerebellar hemisphere. This phenomenon is known as diaschisis. The primary reduction in metabolism in the cerebellum leads to a reduction in CBF in both the frontal lobe and the cerebellum (white arrows).
Owing to the issues described above, four general categories for hemodynamic assessment have been developed. The literature describing these methods in detail and their variations is beyond the scope of this review.22 These include (1) paired flow studies, before and after a vasodilatory challenge; (2) measurements of MTT; (3) quantitative flow measurements; and (4) measurements of oxygen extraction.
The paired flow methods rely on measurements of absolute or relative CBF (with MR, CT, PET, single-photon emission computed tomography SPECT), and near-infrared spectroscopy), blood oxygen level dependent (BOLD) signal with MR, or blood flow velocity with TCD or MR at rest and during some form of vasodilatory stimulus, such as with intravenous acetazolamide (Diamox), breathholding, or CO2 inhalation.14,23–26 Muting or absence of the normal increase in CBF, BOLD or blood flow velocity expected in response to these stimuli is taken as evidence of pre-existing autoregulatory vasodilatation. Paired-flow studies have been referred to using a variety of terms including cerebrovascular reactivity or reserve (CVR) or vasodilatory or reserve. In some patients, CBF, BOLD, or blood flow velocity may fall in the affected region after a stimulus and this has been attributed to a steal phenomenon.27 Another important issue related to these techniques is uncertainty regarding the degree to which the vasodilatory response to these stimuli (acetazolamide or CO2 inhalation) reflects autoregulatory vasodilation.28,29 These issues point to the need for empiric validation of these methods as predictors of stroke risk.
The second category involves resting measurements of CBV or MTT. The most common modalities used for these measurements are computed tomography (CT) and magnetic resonance (MR) imaging. PET techniques can also be used to make these measurements. These tools have become used much more frequently recently in the setting of acute ischemic stroke.30,31 When CPP is reduced, autoregulatory vasodilatation will cause an increase in CBV.32,33 Because the increase in blood volume from dilation of resistance arterioles is minimal, most of the measured increase in blood volume likely comes from the capillary or venous components.33 Different imaging tools may be more sensitive to pial or parenchymal components of CBV and this may account for some of the variability seen in different studies of CBV changes with autoregulation.32,33 MTT may be a more sensitive indicator than CBV measurements alone: by the central volume theorem, the CBV/CBF ratio equals MTT and CBF falls slightly through the autoregulatory range and more dramatically beyond.13
The third method, quantitative flow measurement, has been used primarily in the posterior circulation. As it only measures bulk flow in the vessels, the contribution of collateral flow via the circle of Willis must be accounted for.34 This technique does not account for reductions in flow that maybe metabolic, such as prior stroke in the territory or diaschisis, or for pial collateral flow.
The final method involves measurements of oxygen extraction. At present, this is limited to positron emission tomography (PET) using short-lived radiopharmaceuticals (O-15 oxygen) (Figure 2).1,35 Different quantitative and qualitative methods have been used in human studies.36,37 MR methods are being investigated.38–41
Question 1: What is the clinical relevance of hemodynamic data for different steno-occlusive diseases, and is it different for different methods of assessment?
These tools are commonly used to identify patients with hemodynamic impairment secondary to an occlusive lesion. In this section, we will review some of the data linking hemodynamic impairment with the risk for future stroke. At present, there is no good evidence supporting the use of these tools to select patients for revascularization procedures and that is an active area of ongoing research. A major barrier for this final step for proof of therapeutic efficacy is the development of safer techniques for revascularization. The summary of this author’s answers to this question is included in Table 1.
Table 1.
Summary: Clinical relevance of hemodynamic assessment.
| Disease | Methods | Current evidence | Future research | Notes |
|---|---|---|---|---|
| Asymptomatic extracranial carotid stenosis | Paired flow (TCD, SPECT) | Strong association with stroke risk | Trials of revascularization versus medical therapy | |
| Symptomatic extra and intracranial carotid occlusion | Paired flow, MTT and OEF | Strong association with stroke risk | Trials of revascularization versus medical therapy | Safer methods of revascularization needed |
| Symptomatic intracranial stenosis | Paired flow, OEF, quantitative flow | Strong association with stroke risk | Trials of revascularization versus medical therapy | |
| Moyamoya disease | OEF | High prevalence of hemodynamic impairment but no prospective association with future stroke risk | Longitudinal studies of hemodynamics and stroke risk | Hemodynamic impairment may be dynamic, regionally and temporally |
SPECT: single-photon emission computed tomography; MTT: mean transit time; OEF: oxygen extraction fraction.
Atherosclerotic disease
The best evidence for clinical relevance of hemodynamic measurements is prediction of stroke risk for both symptomatic and asymptomatic patients with atherosclerotic extracranial carotid stenosis or internal carotid artery occlusion. Several studies, most using transcranial Doppler methods, have established an strong association of impaired vasodilatory responses and the risk for future stroke in patients with symptomatic and asymptomatic extracranial carotid stenosis and occlusion.42–44
A recent meta-analysis by Reinhard et al.44 identified and evaluated nine prospective studies of stroke risk for symptomatic and asymptomatic patients with carotid atherosclerotic stenosis or occlusion studied with transcranial Doppler CO2 (or breath-holding) reactivity. Patient-level data from nine studies were included in the analysis. Impaired CO2 reactivity (defined as <20% increase) was independently associated with an increased risk of ipsilateral stroke (Hazard ratio 3.69; confidence interval 2.01, 6.77; p < 0.0001). Similar risk was observed for symptomatic and asymptomatic patients, as well as for patients with asymptomatic carotid stenosis as a subgroup. Impaired reactivity was significant as a continuous variable as well.
One of the nine studies included in the analysis was by Silvestrini et al.,45 in which they performed a longitudinal study of 95 asymptomatic patients with greater than 70% extracranial carotid artery stenosis. At study enrollment, measurements of middle cerebral artery mean flow velocity were acquired at baseline and after 30 s of breath-holding. Patients with hemodynamic impairment – defined as a reduced or absent elevation in velocity (as compared to normal controls) – were at much higher risk for future stroke than asymptomatic patients with normal vasodilatory flow responses.
Ogasawara et al.46 used Xenon-133 SPECT to measure CBF before and after acetazolamide in 70 patients with symptomatic middle or internal carotid artery occlusion. Recurrent stroke occurred in 8 of 23 patients with impaired CVR and in 3 of 47 with normal CVR during two years of follow-up.
The presence of increased OEF and MTT as measured by PET has been established as a powerful and independent risk factor for future stroke in symptomatic patients with complete atherosclerotic internal carotid artery occlusion.10,47 The St. Louis Carotid Occlusion Study was a blinded prospective study of stroke risk designed to test the hypothesis that increased OEF in patients with symptomatic atherosclerotic internal carotid occlusion predicted future stroke risk.10 Eighty-one patients with complete carotid occlusion and ipsilateral ischemic symptoms were enrolled. At baseline, 17 clinical, epidemiologic, and laboratory stroke risk factors were recorded. PET measurements of oxygen extraction were obtained.48 Thirty-nine of the eighty-one patients had increased OEF. All 81 patients were followed for a mean duration of 3.1 years. Thirteen ipsilateral ischemic strokes occurred during this period; 11 of the 13 ipsilateral strokes occurred in the 39 patients with increased OEF. Multivariate analysis found only age and OEF as predictors of stroke risk. Log-rank analysis demonstrated increased OEF to be a powerful predictor of subsequent stroke (p = 0.004). Similar results for PET OEF measurements in patients with symptomatic internal carotid occlusion were found by Yamauchi et al.36
Grubb et al.47 repeated the outcome analysis for the St Louis Carotid Occlusion Study data using PET-derived measurements of MTT.47 Increased MTT was associated with an increased risk of ipsilateral stroke. Subjects with an MTT ratio of 1.387 or higher had a 29.3% two-year risk of ipsilateral stroke compared to 4.6% for those without (P < .001).
The recent Vertebrobasilar Flow Evaluation and Risk of Transient Ischemic Attack and Stroke (VERiTAS) study used quantitative flow measurements with MR to identify a high-risk group of patients with intracranial vertebrobasilar disease.49 In this prospective, blindly adjudicated study of 72 subjects with symptomatic vertebrobasilar disease and recent ischemic symptoms, patients with low distal flow status (18 of the 72 participants (25%)) had a much higher risk for subsequent stroke than those with normal flow. Low-flow status was significantly associated with risk for a subsequent VB stroke (P = .04), with 12- and 24-month event-free survival rates of 78% and 70%, respectively, in the low-flow group vs. 96% and 87%, respectively, in the normal-flow group. Distal flow status remained significantly associated with risk even when controlling for the degree of stenosis and location.
There is no good data to date proving that the use of these tools can be used to improve patient outcomes, however. Two randomized trials to date have used hemodynamic assessment to select patients at risk for stroke for surgical revascularization for complete atherosclerotic carotid occlusion: The Carotid Occlusion Surgery Study (COSS)50 and the Japanese EC/IC Trial (JET).51 Both employed superficial temporal artery to middle cerebral artery bypass procedures for revascularization, which has been documented as capable of reversing the OEF abnormality.2,52 The COSS study enrolled patients with complete atherosclerotic carotid artery occlusion and recent (120 days) ipsilateral cerebral ischemic symptoms. A count-based method of estimating OEF was used.53 One hundred and ninety-five patients were randomized to surgery or best medical care.54 The trial was terminated early for futility. Two-year rates for the primary end point (peri-operative stroke and death, and ipsilateral stroke after 30 days) were 21.0% (95% CI, 12.8% to 29.2%; 20 events) for the surgical group and 22.7% (95% CI, 13.9% to 31.6%; 20 events) for the nonsurgical group (P = .78, Z test), a difference of 1.7% (95% CI, −10.4% to 13.8%). Thirty-day rates for ipsilateral ischemic stroke were 14.4% (14/97) in the surgical group and 2.0% (2/98) in the nonsurgical group, a difference of 12.4% (95% CI, 4.9% to 19.9%). It is important to note, however, that the risk of stroke in the surgical arm after the 30-day perioperative period was 6%, suggesting that a revascularization procedure with less morbidity may be effective in reducing stroke risk in this population.
The Japanese EC-IC Bypass Trial (JET) used the combination of reduced resting CBF (<80% compared to contralateral CBF) and reduced CVR after acetazolamide (<10% increase) to identify patients with hemodynamic cerebral ischemia.51 Two hundred and six patients with recently symptomatic (within 120 days) internal or middle cerebral artery stenosis or occlusion were enrolled between 1998 and 2002. An interim analysis of data from 196 patients followed up through January 2002 reported primary end points in 14 of 98 nonsurgically treated patients and 5 of 98 surgically treated patients (P = .046 by Kaplan–Meier analysis). No primary end points occurred within the first month in the surgical group. Thirty day postoperative morbidity and mortality rates were not reported. Final JET results have not been published.
The state of evidence regarding treatment options for intracranial atherosclerotic disease (ICAD) is similar in some respects to carotid occlusion after the EC/IC bypass trial. A recent trial, SAMMPRIS, found no benefit of endovascular revascularization when offered to patients unselected by hemodynamic criteria.55,56 Hemodynamic impairment is a risk factor for future stroke risk in patients with symptomatic ICAD, as discussed above. A trial of angioplasty and stenting in ICAD patients at risk for future stroke owing to hemodynamic factors is needed.57
There is no good evidence that modern medical management reduces stroke risk in patients with atherosclerotic occlusive disease and hemodynamic risk factors. Yamauchi et al.58 reported the outcomes of 165 patients with symptomatic atherosclerotic internal carotid artery or middle cerebral artery occlusive disease over a nine-year period from 1999 to 2008.58 While the risk of stroke with medical management fell over time in patients without reduced CBF and increase OEF, it remained unchanged in those with misery perfusion. The two-year risk of stroke in the medical group in the COSS trial (22.7%) was lower the estimate generated from the COSS-eligible patients in the STLCOS study (40%). However, the sample size from STLCOS was small and 95% confidence limits included the 22.7% rate.59
Moyamoya disease is an obliterative vasculopathy of the anterior circulation at the circle of Willis. The etiology is unknown. In North America and Europe, it most frequently affects women in their third and fourth decades.60,61 In Asia, where it is more common and more commonly associated with family history, the presentation is bimodal with a higher incidence in children.62 Ischemic symptoms of stroke or transient ischemic attacks are the most common presentation for Asian children and North American and European adult cohorts.63 Hemodynamic studies of Asian children, and some North American studies of adults, with symptomatic moyamoya phenomenon consistently show hemodynamic impairment.64–67 In addition, there is an extensive literature showing improvement in hemodynamics after revascularization surgery.67 There is little prospective data on stroke risk and hemodynamic factors, however. Large pediatric case series have reported dramatic reduction in stroke risk after surgical revascularization.68 No prospective randomized surgical trials have been performed for ischemic stroke risk reduction.
We recently completed a study designed to determine whether increased OEF predicts stroke risk in this patient population.69,70 We enrolled 49 patients with idiopathic moyamoya and followed them for median of 3.7 years. One of sixteen patients with increased OEF on enrollment had an ischemic stroke and another had an intraparenchymal hemorrhage. Three of thirty-three patients with normal OEF had an ischemic stroke. Sixteen patients (20 hemispheres), including five with increased OEF at enrollment, were censored at a mean of 5.3 months after enrollment for revascularization surgery. The risk of new or recurrent stroke and the prevalence of increased OEF were lower than expected in this study. These factors, and the potential selection bias introduced by revascularization surgery, limit strong conclusions about the association of increased OEF and future stroke risk in this population. However, it is possible that embolic factors may be more important than currently appreciated.71,72 In addition, hemodynamic impairment maybe more regionally or temporally dynamic in this population than those with atherosclerotic disease.
In summary, impaired hemodynamics is a major risk factor for future stroke in patients with atherosclerotic occlusive disease. Importantly, this has been observed in patient populations that are symptomatic or asymptomatic, and stenotic or occluded. In addition, nearly all methods of assessment have some degree of clinical validation in prospective studies of stroke risk: paired flow measurements with TCD and SPECT, quantitative flow with MR, and MTT and OEF PET methods. The data supporting hemodynamic risk in other patient populations, such as adult moyamoya patients, are lacking. This may be related to other stroke risk factors, such as thrombo-embolism, or a more dynamic nature of hemodynamic stroke risk – regionally or temporally. More investigation of the role of hemodynamic factors in stroke risk in this population is needed.
The critical next step for research in this area is in studies of therapeutic efficacy (Table 1). This hinges on the relative risks of intervention and natural history. For patients with symptomatic extracranial carotid stenosis, this question is moot as revascularization is dramatically effective for all patients.73 Groups for whom the issue may be of paramount importance are those with asymptomatic carotid stenosis, symptomatic carotid occlusion, and symptomatic intracranial stenosis. The benefit of endarterectomy or stenting for patients with asymptomatic carotid artery stenosis has been questioned, owing to the improvement in outcome on medical management.74 A high-risk subgroup, such as those with hemodynamic impairment, may benefit from revascularization. The failure of bypass surgery to provide a benefit in trials to date likely relate to high complication rates, not a lack of benefit from revascularization. A lower risk procedure, either surgical or endovascular needs to be developed and tested.75,76 Finally, angioplasty and stenting may provide a benefit for patients with symptomatic intracranial stenosis and impaired hemodynamics.57
Question 2: Are there other compensatory mechanisms to chronic, regional hemodynamic impairment?
The mechanisms of autoregulatory vasodilation and increased oxygen extraction defined above were largely based on animal and human studies of acute, and generally global, reductions in mean arterial pressure. Chronic regional oligemia may lead to other compensatory mechanisms, in addition to autoregulatory vasodilation and increased OEF. Animal studies of chronic hypoxia have demonstrated increased glucose transport,77,78 but this has not been observed in humans with carotid occlusion and increased oxygen extraction.79
One definite compensatory mechanism that is common in animal models and humans is improvement in collateral flow over time.79–81 This improvement may come from the development or enlargement of extracranial to intracranial carotid anastomoses, the circle of Willis or leptomeningeal collaterals. One reason that there is no good rat model of chronic regional hemodynamic impairment is that healthy rats rapidly improve collateral flow after unilateral carotid occlusion, via enlargement of the anterior communicating artery.81–83 A recent review of rodent models for vascular cognitive impairment that include reduced CBF was published by Yang et al.84
In some patients with atherosclerotic carotid occlusion, hemodynamic impairment can improve over time, as collateral flow increases.79,80,85 Widder et al.80 used transcranial Doppler sonography with CO2 challenge to study 98 patients with carotid occlusion at least twice. They found that CO2 reactivity improved in more than half of their patients with unilateral carotid occlusion (28 of 55 patients) with diminished or exhausted cerebrovascular reserve. These changes generally occurred within the first few months. Hasegawa et al.86 reported improvement in vasoreactivity in 3 of 20 patients studied with single-photon emission CT using 123 I iodoamphetamine and an acetazolamide challenge.86 We repeated PET measurements in 10 patients with complete atherosclerotic carotid artery occlusion with increased OEF by PET and no interval stroke 12 to 59 months after the initial examination.79 As a group, the ratio of ipsilateral to contralateral OEF declined from a mean of 1.16 to 1.08 (P = 0.022). Greater reductions were seen with longer duration of follow-up (P = 0.023, r = 0.707). The CBF ratio improved from 0.81 to 0.85 (P = 0.021). No change in CBV or CMRO2 was observed. CMRGlc was reduced in the ipsilateral hemisphere (P = 0.001 compared with normal), but the CMRO2/CMRGlc ratio was normal.
It is important to note that while hemodynamics may improve in some patients, others may progress.87 Yamauchi et al. followed seven patients with internal carotid artery occlusion and initially normal hemodynamics over two to five years. CBF fell and OEF increased as a group. OEF was increased above normal values in three subjects. This may be related to progression of the arterial occlusive process (e.g. atherosclerosis in this patient population).
Improvement in collateral flow (as a group) is very likely a major factor for the reduction in recurrent stroke risk over time in patients with symptomatic cervical or intracranial atherosclerotic stenosis or occlusion.54,56,88,89 The greatest risk for stroke in medically treated patients in the North American Symptomatic Carotid Stenosis Trial, the EC/IC bypass trial, the European Carotid Stenosis Trial, the Stenting and Aggressive Medical Management for the Prevention of Recurrent Ischemic Stroke (SAMMPRIS) trial as well as the St. Louis Carotid Occlusion Study was in the first two years after stroke.55,88–90
Metabolic downregulation, possibly reversible and accompanied by a reversible cognitive impairment, has been advanced as a response to hemodynamic impairment.91–93 This phenomenon remains an unproven hypothesis.94 The hemodynamic changes that may occur with metabolic downregulation are not defined. It is possible that the reduced oxygen needs may reduce OEF and may allow some autoregulatory capacity to be regained. This may offer some reduction in stroke risk.11 Sette et al.93 have proposed that downregulation of CMRO2, which is hemodynamically mediated and possibly reversible, occurs in response to misery perfusion (reduced CBF and increased OEF).93 Selective ischemic neuronal loss in the absence of infarction of all cellular elements has also been postulated as a cause of reduced CMRO2 in regions of brain that are structurally normal.20,21,52,95
We examined stroke risk as a function of CBV and OEF in the STLCOS patients and found the highest risk in those with both increased CBV and OEF.11 Nearly half the patients with increased OEF had normal or reduced CBV, and their risk for stroke was much lower. We found no evidence for reduced CMRO2 in these patients, however. A recent study by Yamauchi et al.96 reported increased 18F-Fluoroacetate uptake, an indicator of glial metabolism in non-infarcted regions with reduced CBF, increased OEF, CBV, and reduced CMRO2.
The phenomenon of improved neurocognition after revascularization is well-described,91 but no prospective study has yet proven the ability of revascularization to improve cognition. There have been several large case series documenting improvement after revascularization and an association with hemodynamic improvement; however, these studies have suffered from methodological problems, including the lack of a control group or inclusion of patients with recent stroke, in whom improvement would be expected as part of recovery.97 Baseline data from the Randomized Evaluation of Carotid Occlusion and Neurocognition (RECON) trial, a substudy of the COSS trial provided strong evidence of an association of hemodynamic impairment and reduced cognition.98 At baseline, patients presenting with TIA and increased OEF had much poorer performance on neurocognitive batteries than matched patients presenting with TIA and normal OEF. The final results of RECON found no difference in neurocognition at two years between the surgical and medical groups; however, a small difference could have been missed (the sample size was smaller than projected owing to early closure of the COSS trial) and it is also possible that both surgical and medical groups improved over time (owing to improved collateral flow over time in medical group).94
Some intriguing data on changes in cortical thickness were reported by the Toronto group in moyamoya patients before and after surgical revascularization.23,99,100 Brain regions with evidence of reduced cerebrovascular reserve (vasodilatory response to CO2) had significant cortical thinning, relative to regions with normal perfusion. After surgical revascularization, regions that normalized flow also normalized thickness on follow up. Neurocognition and brain metabolism were not measured in this study.
More information on this area will be generated by the recently funded hemodynamic study for the Carotid Revascularization Endarterectomy versus Stenting Trial (CREST) (Marshall PI, NIHReporter – https://projectreporter.nih.gov/reporter.cfm). They plan to study 158 CREST patients over a five-year period to determine the spectrum of cognitive dysfunction at baseline and over a two-year follow-up period. They will investigate the correlation of cognitive dysfunction with four hemodynamic factors: CBF as measured quantitatively with arterial spin labeling (ASL) MRI, cerebral vasomotor reactivity (VMR) and dynamic cerebral autoregulation (DCA) as measured by transcranial Doppler, and neurovascular coupling measured quantitatively with functional ASL imaging of the hemodynamic response to task-related neuronal activity.
In summary, long-term, regional, impaired hemodynamics in humans is associated with autoregulatory vasodilation and increased OEF. Hemodynamic status improves in many patients, likely secondary to improved collateral flow. Other effects that may occur include selective neuronal loss (non-recoverable) and reversible metabolic down-regulation (potentially associated with changes in executive function, and cortical thinning).
Question 3: Why are people with impaired hemodynamics at risk for future stroke? What are the mechanisms?
There are several lines of evidence supporting a synergystic effect of embolic and hemodynamic factors in patients with large arterial occlusive disease and hemodynamic impairment. Primary hemodynamic stroke, even in the arterial borderzones, may be rare or uncommon in patients with large artery occlusive disease. Exactly how these embolic and hemodynamic factors relate is not known.
Patients with symptomatic carotid stenosis, as a group, are much more likely to have impaired vasodilatory responses than asymptomatic patients with similar degrees of atherosclerotic carotid stenosis.42 Low flow increases infarct volume in animal models of embolic stroke and higher baseline flow reduces infarct volume.101,102 The clinical features and pattern of stroke in patients with large arterial occlusive disease and hemodynamic impairment are most consistent with embolic mechanisms.103
Asymptomatic emboli are common in patients with symptomatic carotid disease.104 There is some evidence from animal and human studies suggesting a synergistic effect between pre-existing hemodynamic impairment and embolic injury, creating a larger area of infarction than might occur with embolism alone.
Omae et al.101 ligated the common carotid artery in a group of experimental rats. The external carotid artery was then retrograde cannulated in both the experimental group and in a group of animals with a patent common carotid artery. Microspheres (2000 × 50 µm) were then injected through the external to internal carotid catheter to simulate an embolic shower. The occlusion group had significantly larger infarctions and greater deficits by functional outcome measures. A second line of evidence supporting this hypothesis is the finding that increased basal CBF reduces infarction size in models of temporary middle cerebral artery occlusion.102 Finally, Pullicino et al.105 found that patients with low cardiac ejection fraction (<35%) and severe carotid artery stenosis had larger volumes of cerebral infarction than patients with similar carotid disease and normal ejection fraction.105
We performed a retrospective analysis of the clinical and imaging features of endpoint strokes in STLCOS patients.106,107 Eleven patients experienced a stroke in the hemisphere with increased OEF during the follow-up period of this study. The majority appeared to be thrombo-embolic events, sudden onset clinical events occurring in core middle cerebral artery territory, involving cortex and underlying white matter.
Patients with pre-existing hemodynamic failure may be at greater risk for a clinical stroke after a thrombo-embolic event through several mechanisms. Grubb et al.108 suggested that hemodynamic failure either predisposes to the formation of thrombo-emboli or that thrombo-emboli may cause infarction more readily in the setting of poor collateral circulation. The later theory was further advanced by Caplan and Hennerici,108,109 who suggested that hemodynamic failure may lead to delayed washout (lysis) of micro embolic material. Patients with pre-existing hemodynamic impairment and lower flow states may be less likely to spontaneously lyse an occlusive or partially occlusive thrombus.
Acute reductions in perfusion pressure can cause ischemic infarction of the cortex and adjacent subcortical white matter located at the borderzones between major cerebral arterial territories, such as the middle and anterior cerebral arteries.110,111 Severe systemic hypotension is a well-recognized cause of multiple bilateral discrete or confluent cortical borderzone infarctions.110 While the mechanism of cortical borderzone infarction in most patients with carotid atherosclerotic disease is likely embolic,112–115 stroke in the white matter borderzones is likely to have hemodynamic or embolic factors involved or both in synergy.116,117 The strongest indicator of a hemodynamic mechanism on anatomic imaging studies is white matter infarctions in the corona radiata or centrum semiovale.113,118–122 These areas are served by penetrating arterioles from the most distal middle and anterior cerebral arteries and have no potential for collateral supply.123,124 This pattern of infarction is strongly associated with underlying hemodynamic impairment.113,118 It is also observed in situations where thrombo-embolism may be less likely, such as in patients with cardiac arrest and global hypotension, or after therapeutic carotid sacrifice. However, embolism may also lead to infarction in these brain regions, making this pattern a poor predictor of stroke mechanism for any given patient.114,116,125
While impaired hemodynamics is strongly associated with this pattern of infarction, the exact pathophysiologic factors responsible in patients with chronic large artery occlusion are unclear. Evidence for a selective increase in OEF in normal-appearing white matter of the centrum semiovale in patients with carotid occlusion is lacking.126 Selective white matter ischemia may be an acute phenomenon, occurring at the time of occlusion, that either improves or results in infarction.
In summary, most clinical stroke in patients with hemodynamic impairment is likely related to synergistic embolic and hemodynamic factors, not primary hemodynamic infarction. This may be secondary to delayed washout or lysis of emboli or an increased tissue vulnerability to embolic events.
Conclusions
Hemodynamic impairment is a validated risk factor for subsequent stroke in many, but maybe not all, patient populations with chronic occlusive cerebrovascular disease. Multiple high-quality studies have been done using a variety of hemodynamic assessment tools, including CVR, MTT, and OEF methods. To date, no revascularization procedure, other than carotid endarterectomy, has been proven effective to reduce stroke risk, and no procedure has been proven specifically to benefit those with hemodynamic impairment. There is a great opportunity, and clinical need, to prove the therapeutic efficacy of hemodynamic assessment in patients with atherosclerotic asymptomatic extracranial carotid stenosis, and symptomatic internal carotid occlusion and intracranial stenosis (Table 1). Finally, a better understanding of the long-term metabolic and physiologic impact of chronic regional hemodynamic impairment and the mechanism of hemodynamic stroke is needed.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Jones T, Chesler DA, Ter-Pogossian MM. The continuous inhalation of Oxygen-15 for assessing regional oxygen extraction in the brain of man. Br J Radiol 1976; 49: 339–343. [DOI] [PubMed] [Google Scholar]
- 2.Baron JC, Bousser MG, Rey A, et al. Reversal of focal “misery perfusion syndrome” by extra-intracranial artery bypass in hemodynamic cerebral ischemia. A case study with 0-15 positron emission tomography. Stroke 1981; 12: 454–459. [DOI] [PubMed] [Google Scholar]
- 3.Fog M. Cerebral circulation. The reaction of the pial arteries to a fall in blood pressure. Arch Neurol Psychiatry 1937; 24: 351–364. [Google Scholar]
- 4.McHenry LC, Jr, Fazekas JF, Sullivan JF. Cerebral hemodynamics of syncope. Am J Med Sci 1961; 80: 173–178. [DOI] [PubMed] [Google Scholar]
- 5.Dirnagl U, Pulsinelli W. Autoregulation of cerebral blood flow in experimental focal brain ischemia. J Cereb Blood Flow Metab 1990; 10: 327–336. [DOI] [PubMed] [Google Scholar]
- 6.Harper AM, Glass HI. Effect of alterations in the arterial carbon dioxide tension on the blood flow through the cerebral cortex at normal and low arterial blood pressures. J Neurol Neurosurg Psychiatry 1965; 28: 449–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grubb RL, Jr, Phelps ME, Raichle ME, et al. The effects of arterial blood pressure on the regional cerebral blood volume by X-ray fluorescence. Stroke 1973; 4: 390–399. [DOI] [PubMed] [Google Scholar]
- 8.Grubb RL, Jr., Ratcheson RA, Raichle ME, et al. Regional cerebral blood flow and oxygen utilization in superficial temporal-middle cerebral artery anastomosis patients: an exploratory definition of clinical problems. J Neurosurg 1979; 50: 733–741. [DOI] [PubMed] [Google Scholar]
- 9.Powers WJ, Tempel LW, Grubb RL, Jr, et al. Clinical correlates of cerebral hemodynamics. Stroke 1987; 18: 284. [Google Scholar]
- 10.Grubb RL, Jr, Derdeyn CP, Fritsch SM, et al. Cerebral hemodynamics and stroke risk. In: Choi D, Powers WJ. (eds). 21st annual research (Princeton) conference, St. Louis, MO: Raven Press, 1998. [Google Scholar]
- 11.Derdeyn CP, Videen TO, Yundt KD, et al. Variability of cerebral blood volume and oxygen extraction: stages of cerebral haemodynamic impairment revisited. Brain 2002; 125: 595–607. [DOI] [PubMed] [Google Scholar]
- 12.Rapela CE, Green HD. Autoregulation of canine cerebral blood flow. Circ Res 1964; 15: I205–I211. [PubMed] [Google Scholar]
- 13.Schumann P, Touzani O, Young AR, et al. Evaluation of the ratio of cerebral blood flow to cerebral blood volume as an index of local cerebral perfusion pressure. Brain 1998; 121: 1369–1379. [DOI] [PubMed] [Google Scholar]
- 14.Nakagawa I, Park HS, Yokoyama S, et al. Indocyanine green kinetics with near-infrared spectroscopy predicts cerebral hyperperfusion syndrome after carotid artery stenting. PLoS One 2017; 12: e0180684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buczek J, Karliński M, Kobayashi A, et al. Hyperperfusion syndrome after carotid endarterectomy and carotid stenting. Cerebrovasc Dis 2013; 35: 531–537. [DOI] [PubMed] [Google Scholar]
- 16.Farooq MU, Goshgarian C, Min J, et al. Pathophysiology and management of reperfusion injury and hyperperfusion syndrome after carotid endarterectomy and carotid artery stenting. Exp Transl Stroke Med 2016; 8: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grubb RL, Jr, Derdeyn CP, Fritsch SM, et al. Importance of hemodynamic factors in the prognosis of symptomatic carotid occlusion. JAMA 1998; 280: 1055–1060. [DOI] [PubMed] [Google Scholar]
- 18.Feeney DM, Baron JC. Diaschisis. Stroke 1986; 17: 817–830. [DOI] [PubMed] [Google Scholar]
- 19.Yamauchi H, Fukuyama H, Kimura J. Hemodynamic and metabolic changes in crossed cerebellar diaschisis. Stroke 1992; 23: 855–860. [DOI] [PubMed] [Google Scholar]
- 20.Yamauchi H, Nishii R, Higashi T, et al. Silent cortical neuronal damage in atherosclerotic disease of the major cerebral arteries. J Cereb Blood Flow Metab 2011; 31: 953–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baron J-C, Yamauchi H, Fujioka M, et al. Selective neuronal loss in ischemic stroke and cerebrovascular disease. J Cereb Blood Flow Metab 2014; 34: 2–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Derdeyn CP, Grubb RL, Jr., Powers WJ. Cerebral hemodynamic impairment: methods of measurement and association with stroke risk. Neurology 1999; 53: 251–259. [DOI] [PubMed] [Google Scholar]
- 23.Mikulis DJ, Krolczyk G, Desal H, et al. Preoperative and postoperative mapping of cerebrovascular reactivity in moyamoya disease by using blood oxygen level-dependent magnetic resonance imaging. J Neurosurg 2005; 103: 347–355. [DOI] [PubMed] [Google Scholar]
- 24.Widder B, Paulat K, Hackspacher J, et al. Transcranial Doppler CO2 test for the detection of hemodynamically significant carotid artery stenoses and occlusions. Eur Arch Psychiatry Neurol Sci 1986; 236: 162–168. [DOI] [PubMed] [Google Scholar]
- 25.Silvestrini M, Troisi E, Cupini LM, et al. Transcranial Doppler assessment of the functional effects of symptomatic carotid stenosis. Neurology 1994; 44: 1910–1914. [DOI] [PubMed] [Google Scholar]
- 26.Donahue MJ, Dethrage LM, Faraco CC, et al. Routine clinical evaluation of cerebrovascular reserve capacity using carbogen in patients with intracranial stenosis. Stroke 2014; 45: 2335–2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith HA, Thompson-Dobkin J, Yonas H, et al. Correlation of Xenon-enhanced computed tomography-defined cerebral blood flow reactivity and collateral flow patterns. Stroke 1994; 25: 1784–1787. [DOI] [PubMed] [Google Scholar]
- 28.Inao S, Tadokoro M, Nishino M, et al. Neural activation of the brain with hemodynamic insufficiency. J Cereb Blood Flow and Metab 1998; 18: 960–967. [DOI] [PubMed] [Google Scholar]
- 29.Kazumata K, Tanaka N, Ishikawa T, et al. Dissociation of vasoreactivity to acetazolamide and hypercapnia. Comparative study in patients with chronic occlusive major cerebral artery disease. Stroke 1996; 27: 2052–2058. [DOI] [PubMed] [Google Scholar]
- 30.Albers GW, Thijs VN, Wechsler L, et al. Magnetic resonance imaging profiles predict clinical response to early reperfusion: the diffusion and perfusion imaging evaluation for understanding stroke evolution (DEFUSE) study. Ann Neurol 2006; 60: 508–517. [DOI] [PubMed] [Google Scholar]
- 31.Wintermark M, Reichhart M, Cuisenaire O, et al. Comparison of admission perfusion computed tomography and qualitative diffusion- and perfusion-weighted magnetic resonance imaging in acute stroke patients. Stroke 2002; 33: 2025–2031. [DOI] [PubMed] [Google Scholar]
- 32.Zaharchuk G, Mandeville JB, Bogdanov AA, et al. Cerebrovascular dynamics of autoregulation and hypoperfusion: an MRI study of CBF and changes in total and microvascular cerebral blood volume during hemorrhagic hypotension. Stroke 1999; 30: 2197–2205. [DOI] [PubMed] [Google Scholar]
- 33.Tomita. Significance of cerebral blood volume. In: Tomita M, Sawada T, Naritomi H, et al. (eds) Cerebral hyperemia and ischemia: from the standpoint of cerebral blood volume. Amsterdam: Elsevier Science Publishers BV, 1988, pp.3–30.
- 34.Amin-Hanjani S, Du X, Zhao M, et al. Use of quantitative magnetic resonance angiography to stratify stroke risk in symptomatic vertebrobasilar disease. Stroke 2005; 36: 1140–1145. [DOI] [PubMed] [Google Scholar]
- 35.Mintun MA, Raichle ME, Martin WRW, et al. Brain oxygen utilization measured with O-15 radiotracers and positron emission tomography. J Nucl Med 1984; 25: 177–187. [PubMed] [Google Scholar]
- 36.Yamauchi H, Fukuyama H, Nagahama Y, et al. Significance of increased oxygen extraction fraction in five-year prognosis of major cerebral arterial occlusive disease. J Nucl Med 1999; 40: 1992–1998. [PubMed] [Google Scholar]
- 37.Derdeyn CP, Videen TO, Simmons NR, et al. Count-based PET method for predicting ischemic stroke in patients with symptomatic carotid arterial occlusion. Radiology 1999; 212: 499–506. [DOI] [PubMed] [Google Scholar]
- 38.Lin W, Paczynski RP, Celik A, et al. Experimental hypoxemic hypoxia: changes in R2* of brain parenchyma accurately reflect the combined effects of changes in arterial and cerebral venous oxygen saturation. Magn Reson Med 1998; 39: 474–481. [DOI] [PubMed] [Google Scholar]
- 39.An H, Ford AL, Chen Y, et al. Defining the ischemic penumbra using magnetic resonance oxygen metabolic index. Stroke 2015; 46: 982–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Uwano I, Kudo K, Sato R, et al. Noninvasive assessment of oxygen extraction fraction in chronic ischemia using quantitative susceptibility mapping at 7 Tesla. Stroke 2017; 48: 2136–2141. [DOI] [PubMed]
- 41.Kudo K, Liu T, Murakami T, et al. Oxygen extraction fraction measurement using quantitative susceptibility mapping: comparison with positron emission tomography. J Cereb Blood Flow Metab 2016; 36: 1424–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Silvestrini M, Troisi E, Matteis M, et al. Transcranial Doppler assessment of cerebrovascular reactivity in symptomatic and asymptomatic severe carotid stenosis. Stroke 1996; 27: 1970–1973. [DOI] [PubMed] [Google Scholar]
- 43.Vernieri F, Pasqualetti P, Matteis M, et al. Effect of collateral blood flow and cerebral vasomotor reactivity on the outcome of carotid artery occlusion. Stroke 2001; 32: 1552–1558. [DOI] [PubMed] [Google Scholar]
- 44.Reinhard M, Schwarzer G, Briel M, et al. Cerebrovascular reactivity predicts stroke in high-grade carotid artery disease. Neurology 2014; 83: 1424–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Silvestrini M, Vernieri F, Pasqualetti P, et al. Impaired cerebral vasoreactivity and risk of stroke in patients with asymptomatic carotid artery stenosis. JAMA 2000; 283: 2122–2127. [DOI] [PubMed] [Google Scholar]
- 46.Ogasawara K, Ogawa A, Yoshimoto T. Cerebrovascular reactivity to acetazolamide and outcome in patients with symptomatic internal carotid or middle cerebral artery occlusion: a xenon-133 single-photon emission computed tomography study. Stroke 2002; 33: 1857–1862. [DOI] [PubMed] [Google Scholar]
- 47.Grubb RL, Jr., Derdeyn CP, Videen TO, et al. Relative mean transit time predicts subsequent stroke in symptomatic carotid occlusion. J Stroke Cerebrovasc Dis 2016; 25: 1421–1424. [DOI] [PubMed] [Google Scholar]
- 48.Derdeyn CP, Yundt KD, Videen TO, et al. Increased oxygen extraction fraction is associated with prior ischemic events in patients with carotid occlusion. Stroke 1998; 29: 754–758. [DOI] [PubMed] [Google Scholar]
- 49.Amin-Hanjani S, Pandey DK, Rose-Finnell L, et al. Effect of hemodynamics on stroke risk in symptomatic atherosclerotic vertebrobasilar occlusive disease. JAMA Neurol 2016; 73: 178–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grubb RL, Jr., Powers WJ, Derdeyn CP, et al. The carotid occlusion surgery study. Neurosurg Focus 2003; 14: e9. [DOI] [PubMed] [Google Scholar]
- 51.JET Study Group. Japanese EC-IC Bypass Trial (JET Study): the second interim analysis. Surg Cereb Stroke 2002; 30: 434–437. [Google Scholar]
- 52.Powers WJ, Martin WR, Herscovitch P, et al. Extracranial-intracranial bypass surgery: hemodynamic and metabolic effects. Neurology 1984; 34: 1168–1174. [DOI] [PubMed] [Google Scholar]
- 53.Derdeyn CP, Videen TO, Grubb RL, Jr., et al. Comparison of PET oxygen extraction fraction methods for the prediction of stroke risk. J Nucl Med 2001; 42: 1195–1197. [PubMed] [Google Scholar]
- 54.Powers WJ, Clarke WR, Grubb RL, Jr, et al. Extracranial-intracranial bypass surgery for stroke prevention in hemodynamic cerebral ischemia. JAMA 2011; 306: 1983–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Derdeyn CP, Chimowitz MI, Lynn MJ, et al. Aggressive medical treatment with or without stenting in high-risk patients with intracranial artery stenosis (SAMMPRIS): the final results of a randomised trial. Lancet 2014; 383: 333–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chimowitz MI, Lynn MJ, Derdeyn CP, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med 2011; 365: 993–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Amin-Hanjani S, Siddiqui AH, Turan TN, et al. Vertebrobasilar atherosclerotic disease: is it time to revisit angioplasty? J Neurointerv Surg. Epub ahead of print 31 August 2016. DOI: 10.1136/neurintsurg-2016-012624. [DOI] [PubMed]
- 58.Yamauchi H, Higashi T, Kagawa S, et al. Is misery perfusion still a predictor of stroke in symptomatic major cerebral artery disease? Brain 2012; 135: 2515–2526. [DOI] [PubMed] [Google Scholar]
- 59.Powers WJ, Clarke WR, Adams JHP, et al. Commentary: extracranial-intracranial bypass for stroke in 2012 response to the critique of the carotid occlusion surgery study “It was déjà vu all over again”. Neurosurgery 2012; 71: E772–E776. [DOI] [PubMed] [Google Scholar]
- 60.Kraemer M, Heienbrok W, Berlit P. Moyamoya disease in Europeans. Stroke 2008; 39: 3193–3200. [DOI] [PubMed] [Google Scholar]
- 61.Hallemeier CL, Rich KM, Grubb RL, Jr., et al. Clinical features and outcome in North American adults with moyamoya phenomenon. Stroke 2006; 37: 1490–1496. [DOI] [PubMed] [Google Scholar]
- 62.Ikezaki K, Inamura T, Kawano T, et al. Clinical features of probable moyamoya disease in Japan. Clin Neurol Neurosurg 1997; (99 Suppl 2): S173–S177. [DOI] [PubMed] [Google Scholar]
- 63.Chiu D, Shedden P, Bratina P, et al. Clinical features of Moyamoya disease in the United States. Stroke 1998; 29: 1347–1351. [DOI] [PubMed] [Google Scholar]
- 64.Horowitz M, Yonas H, Albright AL. Evaluation of cerebral blood flow and hemodynamic reserve in symptomatic moyamoya disease using stable Xenon-CT blood flow. Surg Neurol 1995; 44: 251–261. discussion 62. [DOI] [PubMed] [Google Scholar]
- 65.Kuroda S, Houkin K. Moyamoya disease: current concepts and future perspectives. Lancet Neurol 2008; 7: 1056–1066. [DOI] [PubMed] [Google Scholar]
- 66.Kato H, Shimosegawa E, Oku N, et al. Cerebral hemodynamics and oxygen metabolism in patients with moyamoya syndrome associated with atherosclerotic steno-occlusive arterial lesions. Cerebrovasc Dis 2008; 26: 9–15. [DOI] [PubMed] [Google Scholar]
- 67.Lee M, Zaharchuk G, Guzman R, et al. Quantitative hemodynamic studies in moyamoya disease: a review. Neurosurg Focus 2009; 26: E5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Scott RM, Smith JL, Robertson RL, et al. Long-term outcome in children with moyamoya syndrome after cranial revascularization by pial synangiosis. J Neurosurg 2004; 100: 142–149. [DOI] [PubMed] [Google Scholar]
- 69.Zipfel GJ, Sagar J, Miller JP, et al. Cerebral hemodynamics as a predictor of stroke in adult patients with moyamoya disease: a prospective observational study. Neurosurg Focus 2009; 26: E6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Derdeyn CP, Zipfel GJ, Zazulia AR, et al. Baseline hemodynamic impairment and future stroke risk in adult idiopathic moyamoya phenomenon: results of a prospective natural history study. Stroke 2017; 48: 894–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Horn P, Lanczik O, Vajkoczy P, et al. Hemodynamic reserve and high-intensity transient signals in moyamoya disease. Cerebrovasc Dis 2005; 19: 141–146. [DOI] [PubMed] [Google Scholar]
- 72.Chen J, Duan L, Xu WH, et al. Microembolic signals predict cerebral ischaemic events in patients with moyamoya disease. Eur J Neurol 2014; 21: 785–790. [DOI] [PubMed] [Google Scholar]
- 73.Rothwell PM, Eliasziw M, Gutnikov SA, et al. Analysis of pooled data from the randomised controlled trials of endarterectomy for symptomatic carotid stenosis. Lancet 2003; 361: 107–116. [DOI] [PubMed] [Google Scholar]
- 74.Naylor AR, Gaines PA, Rothwell PM. Who benefits most from intervention for asymptomatic carotid stenosis: patients or professionals? Eur J Vasc Endovasc Surg 2009; 37: 625–632. [DOI] [PubMed] [Google Scholar]
- 75.Lin MS, Lin LC, Li HY, et al. Procedural safety and potential vascular complication of endovascular recanalization for chronic cervical internal carotid artery occlusion. Circ Cardiovasc Intervent 2008; 1: 119–125. [DOI] [PubMed] [Google Scholar]
- 76.Tulleken CA, van Dieren A, Verdaasdonk RM, et al. End-to-side anastomosis of small vessels using an Nd: YAG laser with a hemispherical contact probe. J Neurosurg 1992; 76: 546–549. [DOI] [PubMed] [Google Scholar]
- 77.Harik SI, Behmand RA, LaManna JC. Hypoxia increases glucose transport at blood-brain barrier in rats. J Appl Physiol 1994; 77: 896–901. [DOI] [PubMed] [Google Scholar]
- 78.Harik SI, Lust DW, Jones SC, et al. Brain glucose metabolism in hypobaric hypoxia. J Appl Physiol 1995; 79: 136–140. [DOI] [PubMed] [Google Scholar]
- 79.Derdeyn CP, Videen TO, Fritsch SM, et al. Compensatory mechanisms for chronic cerebral hypoperfusion in patients with carotid occlusion. Stroke 1999; 30: 1019–1024. [DOI] [PubMed] [Google Scholar]
- 80.Widder B, Kleiser B, Krapf H. Course of cerebrovascular reactivity in patients with carotid artery occlusions. Stroke 1994; 25: 1963–1967. [DOI] [PubMed] [Google Scholar]
- 81.Tajima Y, Takuwa H, Kokuryo D, et al. Changes in cortical microvasculature during misery perfusion measured by two-photon laser scanning microscopy. J Cereb Blood Flow Metab 2014; 34: 1363–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Coyle P, Panzenbeck MJ. Collateral development after carotid artery occlusion in Fischer 344 rats. Stroke 1990; 21: 316–321. [DOI] [PubMed] [Google Scholar]
- 83.De Ley G, Nshimyumuremyi JB, Leusen J. Hemispheric blood flow in the rat after unilateral carotid common carotid occlusion: evaluation with time. Stroke 1985; 16: 69–73. [DOI] [PubMed] [Google Scholar]
- 84.Yang Y, Kimura-Ohba S, Thompson J, et al. Rodent models of vascular cognitive impairment. Transl Stroke Res 2016; 7: 407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kleiser B, Widder N. Course of carotid artery occlusions with impaired cerebrovascular reactivity. Stroke 1992; 23: 171–174. [DOI] [PubMed] [Google Scholar]
- 86.Hasegawa Y, Yamaguchi T, Tsuchiya T, et al. Sequential change of hemodynamic reserve in patients with major cerebral artery occlusions or severe stenosis. Neuroradiology 1992; 34: 15–21. [DOI] [PubMed] [Google Scholar]
- 87.Yamauchi H, Fukuyama H, Nagahama Y, et al. Long-term changes of hemodynamics and metabolism after carotid artery occlusion. Neurology 2000; 54: 2095–2102. [DOI] [PubMed] [Google Scholar]
- 88.North American Symptomatic Carotid Endarterectomy Trial (NASCET) Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med 1991; 325: 445–453. [DOI] [PubMed] [Google Scholar]
- 89.The EC/IC Bypass Study Group. Failure of extracranial-intracranial arterial bypass to reduce the risk of ischemic stroke: results of an international randomized trial. N Engl J Med 1985; 313: 1191–2000. [DOI] [PubMed] [Google Scholar]
- 90.European Carotid Surgery Trialists’ Collaborative Group. MRC European Carotid Surgery Trial: interim results for symptomatic patients with severe (70-99%) or with mild (0-29%) carotid stenosis. Lancet 1991; 337: 1235–1243. [PubMed] [Google Scholar]
- 91.Sasoh M, Ogasawara K, Kuroda K, et al. Effects of EC-IC bypass surgery on cognitive impairment in patients with hemodynamic cerebral ischemia. Surg Neurol 2003; 59: 455–460. discussion: 60–63. [DOI] [PubMed] [Google Scholar]
- 92.Chmayssani M, Festa JR, Marshall RS. Chronic ischemia and neurocognition. Neuroimag Clin N Am 2007; 17: 313–324. [DOI] [PubMed] [Google Scholar]
- 93.Sette G, Baron JC, Mazoyer B, et al. Local brain haemodynamics and oxygen metabolism in cerebrovascular disease. Brain 1989; 113: 931–951. [DOI] [PubMed] [Google Scholar]
- 94.Marshall RS, Festa JR, Cheung YK, et al. Randomized evaluation of carotid occlusion and neurocognition (RECON) trial: main results. Neurology 2014; 82: 744–751. [DOI] [PMC free article] [PubMed]
- 95.Lassen NA, Olsen TS, Hojgaard K, et al. Incomplete infarction: a CT-negative irreversible ischemic brain lesion. J Cereb Blood Flow and Metab 1983; 3(3suppl): S602–S603.
- 96.Yamauchi H, Kagawa S, Kishibe Y, et al. Increase in [18F]-Fluoroacetate uptake in patients with chronic hemodynamic cerebral ischemia. Stroke 2015; 46: 2669–2672. [DOI] [PubMed] [Google Scholar]
- 97.Turk AS, Chaudry I, Haughton VM, et al. Effect of carotid artery stenting on cognitive function in patients with carotid artery stenosis: preliminary results. Am J Neuroradiol 2008; 29: 265–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Marshall RS, Festa JR, Cheung YK, et al. Cerebral hemodynamics and cognitive impairment: baseline data from the RECON trial. Neurology 2012; 78: 250–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fierstra J, Maclean DB, Fisher JA, et al. Surgical revascularization reverses cerebral cortical thinning in patients with severe cerebrovascular steno-occlusive disease. Stroke 2011; 42: 1631–1637. [DOI] [PubMed] [Google Scholar]
- 100.Fierstra J, Poublanc J, Han JS, et al. Steal physiology is spatially associated with cortical thinning. J Neurol Neurosurg Psychiatry 2010; 81: 290–293. [DOI] [PubMed] [Google Scholar]
- 101.Omae T, Mayzel-Oreg O, Li F, et al. Inapparent hemodynamic insufficiency exacerbates ischemic damage in a rat microembolic stroke model. Stroke 2000; 31: 2494–2499. [DOI] [PubMed] [Google Scholar]
- 102.Endres M, Laufs U, Huang Z, et al. Stroke protection by 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase inhibitors mediated by endothelial nitric oxide synthase. Proc Natl Acad Sci U S A 1998; 95: 8880–8885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kang DW, Chu K, Ko SB, et al. Lesion patterns and mechanism of ischemia in internal carotid artery disease: a diffusion-weighted imaging study. Arch Neurol 2002; 59: 1577–1582. [DOI] [PubMed] [Google Scholar]
- 104.Molloy J, Markus HS. Asymptomatic embolization predicts stroke and TIA risk in patients with carotid artery stenosis. Stroke 1999; 30: 1440–1443. [DOI] [PubMed] [Google Scholar]
- 105.Pullicino P, Mifsud V, Wong E, et al. Hypoperfusion-related cerebral ischemia and cardiac left ventricular systolic dysfunction. J Stroke Cerebrovasc Dis 2001; 10: 178–182. [DOI] [PubMed] [Google Scholar]
- 106.Derdeyn CP. Mechanisms of ischemic stroke secondary to large artery atherosclerotic disease. Neuroimaging Clin N Am 2007; 17: 303–311, vii–viii. [DOI] [PubMed] [Google Scholar]
- 107.Derdeyn CP, Carpenter DA, Videen TO, et al. Patterns of infarction in hemodynamic failure. Cerebrovasc Dis 2007; 24: 11–19. [DOI] [PubMed] [Google Scholar]
- 108.Grubb RL, Jr., Derdeyn CP, Fritsch SM, et al. Importance of hemodynamic factors in the prognosis of symptomatic carotid occlusion. JAMA 1998; 280: 1055–1060. [DOI] [PubMed] [Google Scholar]
- 109.Caplan LR, Hennerici M. Impaired clearance of emboli (Washout) is an important link between hypoperfusion, embolism, and ischemic stroke. Arch Neurol 1998; 55: 1475–1482. [DOI] [PubMed] [Google Scholar]
- 110.Adams J, Brierley J, Connor R, et al. The effects of systemic hypotension upon the human brain. Clinical and neuropathological observations in 11 cases. Brain 1966; 89: 2350268. [DOI] [PubMed] [Google Scholar]
- 111.Brierley JB, Excell BJ. The effects of profound systemic hypotension upon the brain of M. Rhesus: physiological and pathological observations. Brain 1966; 89: 269–298. [DOI] [PubMed] [Google Scholar]
- 112.De Reuck JL. Pathophysiology of carotid artery disease and related clinical syndromes. Acta Chir Belg 2004; 104: 30–34. [DOI] [PubMed] [Google Scholar]
- 113.Derdeyn CP, Khosla A, Videen TO, et al. Severe hemodynamic impairment and border zone – region infarction. Radiology 2001; 220: 195–201. [DOI] [PubMed] [Google Scholar]
- 114.Torvik A, Skellerud K. Wastershed infarcts in the brain caused by microemboli. Clin Neuropath 1982; 1: 99–105. [PubMed] [Google Scholar]
- 115.Torvik A. The pathogenesis of watershed infarctions in the brain. Stroke 1984; 15: 221–223. [DOI] [PubMed] [Google Scholar]
- 116.Moustafa RR, Izquierdo-Garcia D, Jones PS, et al. Watershed infarcts in transient ischemic attack/minor stroke with ≥50% carotid stenosis. Hemodynamic or embolic? Stroke 2010; 41: 1410–1416. [DOI] [PubMed] [Google Scholar]
- 117.Moustafa RR, Momjian-Mayor I, Jones PS, et al. Microembolism versus hemodynamic impairment in rosary-like deep watershed infarcts. A combined positron emission tomography and transcranial Doppler study. Stroke 2011; 42: 3138–3143. [DOI] [PubMed] [Google Scholar]
- 118.Waterston JA, Brown MM, Butler P, et al. Small deep cerebral infarcts associated with occlusive internal carotid artery disease. A hemodynamic phenomenon? Arch Neurol 1990; 47: 953–957. [DOI] [PubMed] [Google Scholar]
- 119.Weiller C, Ringelstein EB, Reiche W, et al. Clinical and hemodynamic aspects of low-flow infarcts. Stroke 1991; 22: 1117–1123. [DOI] [PubMed] [Google Scholar]
- 120.Yamauchi H, Fukuyama H, Yamaguchi S, et al. High-intensity area in the deep white matter indicating hemodynamic compromise in internal carotid artery occlusive disorders. Arch Neurol 1991; 48: 1067–1071. [DOI] [PubMed] [Google Scholar]
- 121.Krapf H, Widder B, Skalej M. Small rosary-like infarctions in the centrum semiovale suggest hemodynamic failure. Am J Neuroradiol 1998; 19: 1479–1484. [PMC free article] [PubMed] [Google Scholar]
- 122.Isaka Y, Nagano K, Narita M, et al. High signal intensity on T2-weighted magnetic resonance imaging and cerebral hemodynamic reserve in carotid occlusive disease. Stroke 1997; 28: 354–357. [DOI] [PubMed] [Google Scholar]
- 123.Moody DM, Bell MA, Challa VR. Features of the cerebral vascular pattern that predict vulnerability to perfusion or oxygen deficiency: an anatomic study. Am J Neuroradiol 1990; 11: 431–439. [PMC free article] [PubMed] [Google Scholar]
- 124.Zulch KJ. Uber die entstehung und lokalization der hirn-infarkte. Zentralbl Neurochir 1961; 21: 158–178. [PubMed] [Google Scholar]
- 125.Pollanen MS, Deck JH. Directed embolization is an alternate cause of cerebral watershed infarction. Arch Pathol Lab Med 1989; 113: 1139–1141. [PubMed] [Google Scholar]
- 126.Derdeyn CP, Simmons NR, Videen TO, et al. Absence of selective deep white matter ischemia in chronic carotid disease: a positron emission tomographic study of regional oxygen extraction. Am J Neuroradiol 2000; 21: 631–638. [PMC free article] [PubMed] [Google Scholar]