Abstract
Moyamoya is a bilateral, complex cerebrovascular condition characterized by progressive non-atherosclerotic intracranial stenosis and collateral vessel formation. Moyamoya treatment focuses on restoring cerebral blood flow (CBF) through surgical revascularization, however stratifying patients for revascularization requires abilities to quantify how well parenchyma is compensating for arterial steno-occlusion. Globally elevated oxygen extraction fraction (OEF) secondary to CBF reduction may serve as a biomarker for tissue health in moyamoya patients, as suggested in patients with sickle cell anemia (SCA) and reduced oxygen carrying capacity. Here, OEF was measured (TRUST-MRI) to test the hypothesis that OEF is globally elevated in patients with moyamoya (n = 18) and SCA (n = 18) relative to age-matched controls (n = 43). Mechanisms underlying the hypothesized OEF increases were evaluated by performing sequential CBF-weighted, cerebrovascular reactivity (CVR)-weighted, and structural MRI. Patients were stratified by treatment and non-parametric tests applied to compare study variables (significance: two-sided P < 0.05). OEF was significantly elevated in moyamoya participants (interquartile range = 0.38–0.45) compared to controls (interquartile range = 0.29–0.38), similar to participants with SCA (interquartile range = 0.37–0.45). CBF was inversely correlated with OEF in moyamoya participants. Elevated OEF was only weakly related to reductions in CVR, consistent with basal CBF level, rather than vascular reserve capacity, being most closely associated with OEF.
Keywords: Oxygen extraction fraction, stroke, moyamoya, MRI, neurophysiology
Introduction
Moyamoya is a cerebrovascular condition characterized by progressive stenosis of the supraclinoid internal carotid arteries (ICAs) and their proximal branches and the corresponding development of collateral blood vessels.1 Moyamoya can be idiopathic (i.e. disease) or secondary (i.e. syndrome) to more common cerebrovascular conditions such as atherosclerosis and fibromuscular dysplasia, and places patients at more than a seven-fold risk increase for stroke.2
Stroke screening procedures for patients with moyamoya, which can be used to prescribe prophylactic revascularization procedures,3 focus on characterizing large vessel steno-occlusion using angiographic techniques at the spatial resolution of large arteries, as well as evaluating prior infarct presence and extent. While helpful, these methods are not conclusive for prognosis, and, due to the invasive nature of many of these methods, are not used routinely for surveillance owing to safety concerns. Therefore, there is a need to better understand the pathophysiology of moyamoya and to develop associated stroke screening protocols.
Due to the highly variable way in which brain parenchyma may compensate for the array of arterial steno-occlusions in moyamoya, as well as inabilities of angiographic techniques to identify all microvascular stenoses at available spatial resolutions, tissue-level imaging is of great interest and required for characterizing how parenchyma compensates for large vessel steno-occlusion. More specifically, brain parenchyma downstream of arterial stenosis may compensate for reduced perfusion pressure through the formation of collateral vessels4,5 and/or by increasing arteriolar cerebral blood volume (CBV, ml blood/ml parenchyma) through autoregulatory vasodilation.6–8 While collateral vessels can be evaluated with gold-standard catheter angiography, it is also important to understand the function of these collaterals specifically and whether these collaterals adequately maintain oxygen delivery to tissue. This issue is fundamental, as patients with nearly identical degrees and extents of measureable stenosis may carry contradistinct stroke risks due to different compensation mechanisms and extents of collateralization.4,9 When the extent of collateralization and/or autoregulation is insufficient to maintain adequate oxygen delivery to tissue, the oxygen extraction fraction (OEF) will increase as described by:
| (1) |
where CMRO2 is the cerebral metabolic rate of oxygen consumption (ml O2/100 g tissue/min), CBF is the cerebral blood flow (ml blood/100 g tissue/min), and Ca is the oxygen carrying capacity of arterial blood, which depends on the arterial oxygen saturation and total hemoglobin. For constant CMRO2, which is believed to be preserved up until very advanced stage disease,7 OEF may increase (a) for normal CBF and reduced oxygen carrying capacity,10 or (b) for normal oxygen carrying capacity but reduced CBF.7
Indeed, based on 15O PET studies in individuals with extracranial and intracranial steno-occlusion, it has been shown that OEF increases regionally when CBV is inadequate to maintain CBF over a normal range, and importantly that regionally elevated OEF and CBV may be prognostic for recurrent stroke risk.7,11 It has also been shown that in patients with symptomatic carotid occlusion due to atherosclerosis, increased OEF is associated with prior stroke or transient ischemic attack (TIA).12 Iwama et al. evaluated a cohort of patients with moyamoya using 15O PET and elevated OEF was identified in middle cerebral artery flow territories in patients with moyamoya configuration compared to adults without moyamoya disease.13 This work has motivated the potential use of OEF as a biomarker of stroke risk in patients with moyamoya, however difficulty performing 15O PET routinely in non-specialized centers has limited translation of these promising findings, as well as mechanisms underlying these elevated OEF measurements in patients with moyamoya have not been thoroughly clarified.
More recently using non-invasive MRI, it has been shown that OEF increases globally in patients with reduced oxygen carrying capacity secondary to sickle cell anemia (SCA) and elevated OEF correlates with increasing levels of clinical impairment defined as vasculopathy or prior stroke.10 Such MRI methods for determining global OEF have additionally been applied across the lifespan of healthy individuals,14 as well as in end-stage renal disease,15 anorexia nervosa,16 and neonates,17 all populations in which cerebrovascular oxygen metabolism is thought to be abnormal.
The purpose of this work is to extend these promising OEF studies to patients with moyamoya to test the hypothesis that owing to the often bilateral nature of the disease and associated hypoperfusion, globally elevated OEF is detectable using an MRI-based approach. Two supplemental objectives are also of interest: (a) To confirm the mechanistic origin of the hypothesized OEF elevation by performing OEF and CBF measurements in sequence in patients with steno-occlusive disease due to moyamoya and in patients without steno-occlusive disease but reduced oxygen carrying capacity secondary to SCA and (b) to evaluate the relationship between cerebrovascular reactivity (CVR) and the hypothesized globally elevated OEF. CVR studies have been performed in patients with arterial steno-occlusion due to moyamoya and atherosclerosis using computed tomography,18 single-photon emission computed tomography,19 PET,20,21 and MRI;22,23 however, to our knowledge, no study has evaluated the relationship between basal CBF, CVR, and global elevations in OEF in patients with moyamoya.
Results of this work are intended to improve our understanding of the mechanism by which brain parenchyma compensates for multiple arterial steno-occlusions in moyamoya, and to determine if OEF is globally elevated in moyamoya, which is logical but not confirmed. If confirmed, this work could provide a foundation for future investigations that may evaluate the utility of using fast and non-invasive measures of OEF as an additional biomarker of tissue impairment and stroke risk in patients with moyamoya.
Materials and methods
Ethical considerations and participant groups
All participants (n = 79) provided informed, written consent for this prospective IRB-approved study. Participants were grouped into four categories: (a) moyamoya participants, (b) age-matched controls for moyamoya, (c) SCA participants, (d) age-matched controls for SCA. Inclusion criteria for the SCA arm included adult participants homozygous for hemoglobin-S on oral hydroxyurea therapy, not receiving routine blood transfusions, and without significant (>70%) steno-occlusion of any major intracranial (first segment of ACA, PCA, and MCA) or cervical artery (to control for heterogeneity). All SCA participants were recruited between 2014 and 2016 from a comprehensive SCA clinic at our institution. Inclusion criteria for the moyamoya arm was clinical diagnosis of moyamoya and prior TIA or stroke; exclusion criteria included extracranial carotid stenosis >50% and anemia. Moyamoya participants were recruited between 2014 and 2016 from the adult neurological surgery service at our institution either during clinic appointments or during a follow-up call after the patient was seen in clinic. Revascularization surgery and location was recorded in patients and sub-analyses were performed to understand how revascularization was associated with the findings. Importantly, a heterogeneous group of moyamoya patients was included, with varying degrees of steno-occlusion and time since diagnosis. This enables a large range of hemo-metabolic parameters to be contrasted to evaluate the study hypotheses, and also increases generalizability beyond a limited and unrepresentative cohort (e.g. short disease duration without revascularization). No control participants for the moyamoya or SCA arm had a history of anemia, significant vasculopathy defined as >70% extracranial or intracranial stenosis, or sickle trait. All components of this study were performed in compliance with the Declaration of Helsinki, Health Insurance Portability and Accountability Act, and all protocols were approved by the Vanderbilt University Institutional Review Board (IRB Study 111116—control participants; IRB Study 110468—moyamoya participants; IRB Study 140915—SCA participants).
Image acquisition
All MR imaging and angiography was performed on a 3T scanner (Philips Medical Systems, Best, The Netherlands) using body coil radiofrequency transmission and SENSE-array reception.
Structural imaging and angiography
Fluid-attenuated-inversion-recovery (FLAIR; TR/TE = 11,000/120 ms) and T1-weighted (TR/TE = 9.0/4.6 ms) MRI were performed in the same scan session as hemodynamic and metabolic imaging. All participants with moyamoya underwent digital subtraction angiography (DSA) of the neck and intracranial arteries within 60 days of MRI for stenosis assessment. Participants with SCA and control participants underwent time-of-flight magnetic resonance angiography (TOF-MRA) of the neck and intracranial arteries either in the same session, or within 30 days of MRI for stenosis assessment.
CBF imaging
CBF-weighted images were obtained from pseudo-continuous arterial spin labeling (pCASL) data. pCASL data from 13 control-label pairs were pair-wise subtracted and used to generate the mean difference magnetization, which was then normalized by a proton-density-weighted (TR = 15,000 ms) equilibrium magnetization image. While pCASL labeling schemes with similarly long post labeling delay (PLD) times were used in all subjects, the PLDs did vary over a small range between some controls and patient volunteers. The PLD range was 1.53–1.9s and label duration range was 1–1.65 s (Supplemental Table I). These parameters have been investigated in more detail previously,10,24 and variation in quantified CBF due to variation in these parameters25 is anticipated to be much smaller than differences in CBF due to reduced oxygen carrying capacity or steno-occlusion. Phase contrast angiography has been previously used to determine labeling efficiency in SCA participants who have known elevated cervical blood velocity; consistent with these results, a labeling efficiency of 0.60 was used for SCA participants, and a labeling efficiency of 0.85 was used for all other volunteers.26
OEF imaging
Whole brain OEF values were quantified using the non-invasive MRI approach T2-relaxation-under-spin-tagging (TRUST).27,28 To perform TRUST, data from an imaging slice containing the inferior portion of the superior sagittal sinus were motion-corrected and pair-wise subtracted at four effective echo times (eTEs) of 0, 40, 80, and 160 ms (Figure 1). The imaging plane (3.4 × 3.4 × 5 mm3) was placed 20 mm superior to the confluence of the sinuses for both the control and label images. TRUST duration was 1 min 12 s.
Figure 1.
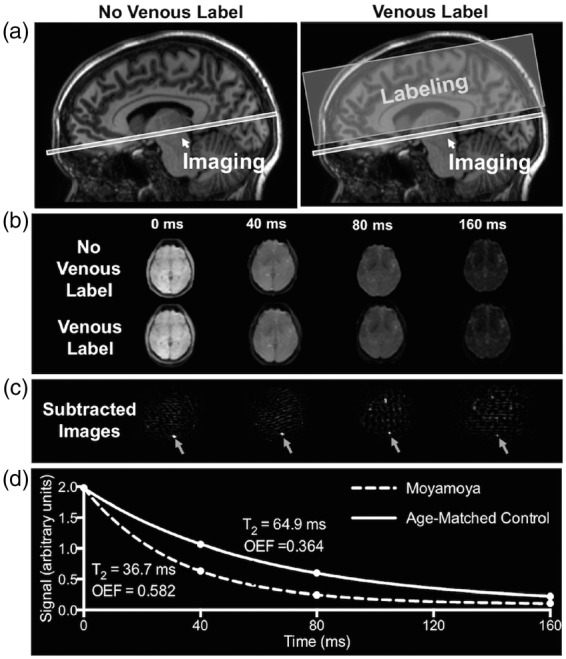
T2-relaxation-under-spin-tagging (TRUST) MRI for non-invasive quantification of whole-brain oxygen extraction fraction (OEF). TRUST-MRI is used to determine the Carr–Purcell–Meiboom–Gill (CPMG) T2 in the superior sagittal sinus, which has a known relationship with venous oxygenation (Yv). Arterial oxygenation (Ya) is measured by pulse oximetry. By taking the fractional difference in oxygenation (Ya-Yv)/Ya, OEF is calculated. A schematic of the venous blood imaging is shown in panel (a). Volume-selective venous labeling pulses are applied to invert inflowing venous blood water magnetization; these labeling acquisitions are interleaved with an image with no labeling (control image). Panel (b) shows representative control and label images at four different effective echo times (eTEs). Panel (c) shows the subtraction of the label image from control image, which allows for isolation of signal in the superior sagittal sinus. Signal intensities from the subtraction image (control-label) at the four eTEs are fitted to a known model (d) and venous blood water T2 is determined. Panel (d) shows T2 decay for a control with a normal OEF (T2 = 64.9 ms; Yv = 0.62; OEF = 0.364) and a participant with moyamoya (T2 = 36.7 ms; Yv = 0.41; OEF = 0.582).
CVR imaging
CVR was assessed in moyamoya participants. All moyamoya participants were fitted with a nasal cannula (Salter Labs, Ref 400F) for end tidal CO2 (EtCO2) monitoring and a non-rebreathing mask (Salter Labs, ref 8130) for administration of medical grade room air (21% O2, 79% N2) and hypercapnic hyperoxia (i.e. carbogen: 5% CO2, 95% O2). Medical grade carbogen, rather than hypercapnic normoxia, was required in high-risk patients at our center by our ethical review board, and this stimulus has been shown to correlate with hypercapnic normoxic stimuli and to provide expected sensitivity to lateralizing stenosis in patients with intracranial stenosis.23 The CVR was assessed using a paradigm consisting of two blocks of 3-min carbogen administration interleaved with 3-min blocks of medical grade room air. Blood pressure, EtCO2, and oxygen saturation were monitored by a respiratory therapist. Gradient echo BOLD images were acquired with a spatial resolution of 3 × 3 ×3.5 mm3 with TE/TR of 35/2000 ms.
Analysis
Structural imaging and angiography evaluation
Stenosis and infarct grading was performed by a board-certified neuroradiologist (LTD) to ensure inclusion criteria and appropriate group assignment. Moyamoya classification was assigned based on clinical criteria and DSA data that were separately acquired as part of the clinical work-up for these patients.1 FLAIR images were evaluated for presence and size of infarct. In addition, for moyamoya participants, a modified Suzuki score (mSS) was calculated for each hemisphere.29 The range is 0 to 4, with 0 representing no steno-occlusive changes and 4 representing complete occlusion of both proximal anterior and middle cerebral arteries.
CBF determination
Voxel-wise CBF analysis was performed according to the ISMRM Perfusion Study Group guidelines.30 CBF was quantified in native space for each voxel and then transformed into a 2 mm T1 -weighted Montreal Neurological Institute atlas using the FMRIB Software Library.31,32 Due to the dependence of hematocrit on T1,blood,33 the T1,blood was determined individually for the SCA participants, and the mean arterial T1,blood for this group was 1900 ± 53 ms. For the non-anemic control and moyamoya participants, the T1,blood for males and females was assumed to be 1671 ms and 1700 ms, respectively, which accounts for males having a higher mean hematocrit than females. Mean gray matter CBF (GM CBF) was calculated and reported as region-of-interest CBF, and a partial volume calculation was performed based on structural determination of white and gray matter contribution to the imaging voxel; these values are reported as partial volume corrected, GM CBF. Gray matter was the focus of the analysis due to the approximately two-fold longer arterial blood arrival times in white matter, which are on the order or longer than arterial blood water T1. Measured CBF values were scaled by a common factor of 1.22, to convert the voxel CBF measurement to a gray matter CBF measurement. This factor was calculated by measuring the fraction of gray and white matter in the common mask and using voxel partial volume estimates from FSL FAST and the T1-weighted structural images, which were determined to be 70% gray matter and 30% white matter, and did not vary significantly between groups or patients. We also applied the relationship that white matter CBF is 2.5-fold lower than gray matter CBF.34 It should be noted that this procedure slightly increases the reported CBF for all volunteers, but does not change any trends between volunteers or groups.
OEF determination
The quantification procedure has been outlined in detail in the literature27,28; in brief, Carr–Purcell–Meiboom–Gill (CPMG) T2 was calculated in the sagittal sinus for each participant by performing pair-wise subtraction of the label from the control image at the four effective echo times (eTE). Four voxels within the sagittal sinus were analyzed for each subject at each eTE to determine the pure-blood (CPMG)-T2. CPMG-T2 is then converted to venous oxygenation (Yv) using knowledge of blood water T2, and hematocrit.28 The standard error of the Yv measurement, from which OEF was calculated, was calculated for each participant to determine if the error in fitting differed between groups. Arterial oxygenation (Ya), which was recorded for each participant using pulse oximetry at time of the TRUST scan, was incorporated to calculate OEF as (Ya-Yv)/Ya. This equation assumes that oxygen dissolved in blood plasma contributes minimally to OEF, as has been described in more detail previously.10
CVR determination
FSL software31 was used for motion correction, baseline drift correction and z-statistic calculation. In addition, the relative signal change (ΔS/S0) was determined as the mean difference in the signal during the final 30 s of the carbogen block divided by the baseline signal, similar to as previously described.23 Data were registered to 4 mm T1 -weighted Montreal Neurological Institute and a gray matter region of interest was used to determine mean gray matter CVR. Data were normalized by change in EtCO2 in units of mmHg.
Statistical concerns and hypothesis testing
The primary objective of this study was to quantify OEF in participants with clinical diagnoses of moyamoya, and to compare these measurements to participants with SCA and to healthy age-matched controls. The primary hypothesis was that participants with moyamoya will have increased OEF and decreased CBF compared to age-matched controls, whereas SCA volunteers will have increased OEF and increased CBF (but reduced oxygen carrying capacity). To test this hypothesis, a two-sided Wilcoxon rank-sum test was performed. A supplemental objective was to confirm the mechanistic origin of OEF elevation by performing OEF and CBF measurements to test the hypothesis that moyamoya participants with hypoperfusion (decreased CBF), have increased OEF, and a Spearman’s rank coefficient was calculated. To evaluate the relationship between CVR and globally elevated OEF, a Spearman’s rank coefficient was also calculated. In all comparisons, two-tailed P-value < 0.05 was required for significance. Additionally, for the primary and supplemental objectives, a sub-analysis of the moyamoya participants was performed whereby non-revascularized participants were evaluated separately to determine whether the findings were preserved in those with vs. without surgical revascularization.
Results
Demographics
Table 1 summarizes participant demographics and calculated OEF and CBF values. Eighteen participants with primary (n = 16) or secondary moyamoya (n = 2) (mean age = 47 years; range = 26–79 years) were included. Nine of the moyamoya participants had prior indirect revascularization, which consisted of either encephalo-duro-arterio-synangiosis (EDAS; n = 8) or encephalo-duro-arterio-myo-synangiosis (EDAMS; n = 1) operations. Of the participants who underwent revascularization, neuroimaging was performed at least 5 months post-operation and these participants were also considered separately to understand if revascularization affected the study hypothesis. Eighteen participants with SCA were included (mean age = 27 years; range = 19–32 years). Control participants were enrolled to obtain age-matched data sets for the moyamoya (mean age = 44 years; range = 21–75 years) and SCA cohorts (mean age = 26 years; range = 21–39 years). A larger cohort of age-matched control volunteers for the moyamoya arm of the study were included due to the larger age-range of moyamoya participants (range =26–79 years). Hematocrit was measured for most SCA participants on the day of imaging, and all within 7 days of imaging. The mean (%) hematocrit in SCA participants was 28.6 ± 2.5. For moyamoya participants, the mean mSS for the right and left hemispheres were 2.4 and 1.9, respectively. No participants had moyamoya secondary to SCA. Prior stroke or TIA was an inclusion criterion for the moyamoya arm of the study and 15/18 of the participants had remote infarcts (defined as T1 hypointense, FLAIR hyperintense lesions > 3 mm). Of the SCA participants, 8/18 had remote infarcts.
Table 1.
Summary of study participants (mean ± standard deviation).
| Moyamoya arm |
Sickle cell anemia (SCA) arm |
|||
|---|---|---|---|---|
| Parameters | Controls (n = 43) | Moyamoya (n = 18) | Controls (n = 11) | SCA (n = 18) |
| Age (years) | 44.8 ± 21.7 | 47.2 ± 14.9 | 26.5 ± 5.1 | 27.0 ± 5.1 |
| Hemoglobin S (%) | 0 | 0 | 0 | 75.6 ± 10.3 |
| Gender (% male) | 71 | 5 a | 45 | 55 |
| Arterial oxygen saturation (%) | 97.1 ± 1.2 | 97.2 ± 1.3 | 97.3 ± 1.2 | 95.6 ± 1.8 |
| Venous oxygen saturation (%) | 63.7 ± 6.0 | 56.3 ± 7.7 | 62.4 ± 5.1 | 56.5 ± 5.7 |
| Standard error of Yv (%) | 1.62 | 1.50 | 1.41 | 0.83 |
| TRUST-measured T2 (ms) | 68.0 ± 12.2 | 56.9 ± 11.8 | 65.7 ± 10.2 | 78.0 ± 11.4 |
| Oxygen extraction fraction | 0.343 ± 0.061 | 0.419 ± 0.083 | 0.359 ± 0.052 | 0.410 ± 0.056 |
| Region-of-interest cerebral blood flow (ml blood/100 g tissue/min) | 40.2 ± 10.3 | 36.4 ± 8.8 | 42.2 ± 7.1 | 69.3 ± 15.2 |
| Gray matter cerebral blood flow, partial volume corrected (ml blood/100 g tissue/min) | 49.2 ± 12.5 | 44.4 ± 10.7 | 51.4 ± 8.6 | 85.3 ± 19.4 |
| Modified Suzuki score, right hemisphere | NA | 2.44 ± 0.86 | NA | NA |
| Modified Suzuki score, left hemisphere | NA | 1.89 ± 0.96 | NA | NA |
| Infarct on FLAIR imaging > 3 mm (%) | 0 | 83 | 0 | 44 |
An additional sub-analysis was performed in which only females were included in the moyamoya arm of the study wherein trends were identical.
OEF and CBF
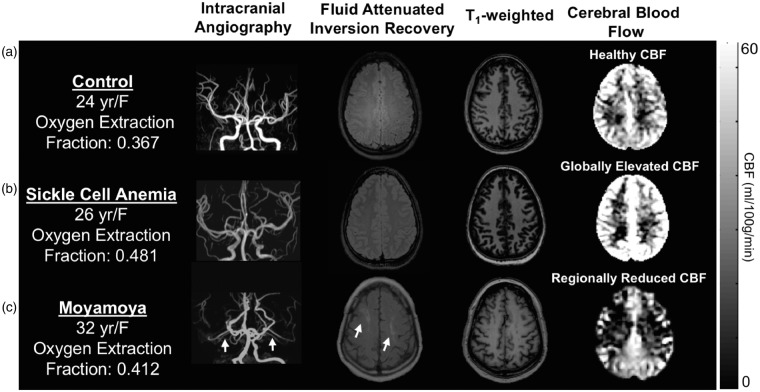
Figure 2 summarizes case examples of structural and functional imaging data from a representative control participant, participant with SCA, and a participant with moyamoya. In Figure 2(a), a control participant with a normal OEF and normal findings on structural and CBF imaging is shown. This is contrasted with a participant with SCA (Figure 2(b)) and moyamoya (Figure 2(c)), both of whom have elevated OEF of 0.481 and 0.412, respectively. The participant with SCA has a venous oxygen saturation of 48% and the participant with moyamoya has venous oxygen saturation of 57%, both lower than the venous oxygen saturation of the control (62%) and consistent with elevated OEF for the measured range of arterial blood oxygen saturation. The participant with SCA demonstrates elevated CBF in the setting of decreased oxygen carrying capacity (i.e. reduced hematocrit). The participant with bilateral moyamoya demonstrates right and left anterior/middle cerebral artery territory infarcts, bilateral hypoperfusion on CBF imaging, and elevated OEF.
Figure 2.
Individual cases of structural, cerebral blood flow (CBF), and oxygen extraction fraction (OEF) imaging. (a) A healthy control participant with a normal OEF, and normal findings on structural and CBF imaging. (b) A participant with sickle cell anemia (SCA) and (c) moyamoya. The participant with SCA demonstrates elevated CBF in the setting of decreased oxygen carrying capacity of hemoglobin and increased OEF (OEF = 0.481). The participant with bilateral moyamoya with lenticulostriate collaterals (white arrows on intracranial angiography) demonstrates right and left anterior and middle cerebral artery territory infarcts seen on FLAIR, bilateral anterior-territory hypoperfusion, and globally elevated OEF (OEF = 0.412) relative to control data.
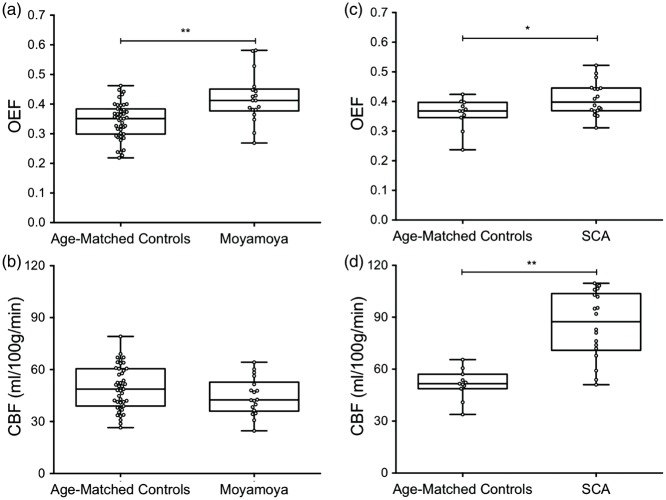
Group-analysis of data in SCA and moyamoya participants is shown in Figure 3. OEF values (mean ± s.d.) of moyamoya participants (OEF = 0.419 ± 0.083) compared to age-matched controls (OEF = 0.343 ± 0.061) are shown in Figure 3(a). These data are consistent with the primary hypothesis of the study that OEF is elevated in participants with moyamoya (P < 0.001). GM CBF values (mean ± s.d.) of age-matched controls (CBF = 49.2 ± 12.5 ml/100 g/min) and moyamoya participants (CBF = 44.4 ± 10.7 ml/100 g/min) are summarized in Figure 3(b). A trend for a reduction in mean GM CBF in moyamoya participants compared to control participants was observed, however this did not meet criteria for significance (see Discussion). To better understand OEF in the context of moyamoya patients who had not undergone revascularization procedure (n = 9) vs. those that had undergone revascularization (n = 9), we considered the groups separately in a sub-analysis. OEF was similarly and significantly elevated in participants who had not undergone revascularization (P = 0.0023) and participants who had undergone revascularization surgeries (P = 0.026), compared to age-matched controls, even with these smaller sample sizes. There was no significant difference in OEF values between participants who had undergone revascularization and participants who had not undergone revascularization, however the study may not be powered sufficiently to investigate this effect rigorously. When considering the SCA arm of the study, OEF was significantly elevated in participants with SCA (OEF = 0.410 ± 0.056) compared to age-matched control participants (OEF = 0.359 ±0.052) and summarized in Figure 3(c). Additionally, GM CBF values in SCA participants (CBF = 85.3 ±19.4 ml/100 g/min) were significantly elevated compared to age-matched controls (CBF = 51.4 ± 8.6 ml/100 g/min) and summarized in Figure 3(d).
Figure 3.
Oxygen extraction fraction (OEF) and cerebral blood flow (CBF) in moyamoya, sickle cell anemia (SCA), and age-matched control participants. A two-sided Wilcoxon rank-sum test was performed to test the primary hypothesis that OEF was elevated in moyamoya and SCA participants compared to age-matched controls, and that CBF was decreased in moyamoya participants compared to controls. (a) OEF is elevated in moyamoya participants compared to age-matched controls (P < 0.001). (b) Reduced mean gray matter CBF in moyamoya participants relative to controls, but this reduction was not significant, likely due to preserved posterior territory CBF in many patients and regions of high ASL intravascular signal. (c) OEF is elevated in SCA participants compared to age-matched controls (P = 0.045). (d) CBF is elevated in SCA participants compared to age-matched controls (P < 0.001). *P < 0.05, **P < 0.01. In the moyamoya arm of the study, a sub-analysis was performed that evaluated only females in both the control (n = 15) and moyamoya (n = 17) groups. Trends were identical, and the OEF was elevated in the moyamoya participants compared to the controls (P = 0.06), and CBF was decreased in the moyamoya participants compared to controls (P = 0.02).
The standard error of the Yv measurement across all participant groups was less than 2%, and there was no difference in confidence intervals or standard error measurements of the T2-decay curve-fitting calculation from the TRUST imaging of OEF (Supplemental Table II). We observed no significant relationship between OEF and mean mSS (Supplemental Figure I).
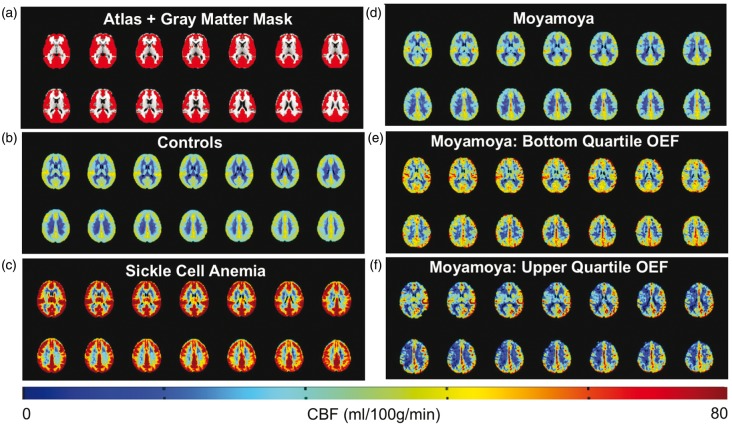
Mean CBF maps for each participant group are shown in Figure 4. Central slices of the T1-weighted atlas with the gray matter mask used for quantification overlaid are shown in Figure 4(a). Mean CBF maps from controls and SCA participants are shown in Figure 4(b) and (c), respectively. Figure 4(d) shows mean CBF maps from moyamoya participants (n = 18). Mean CBF maps from moyamoya participants with bottom quartile OEF values (OEF < 0.38), and moyamoya participants with upper quartile OEF values (OEF > 0.45) are shown in Figure 4(e) and (f), respectively.
Figure 4.
Non-invasive cerebral blood flow (CBF) imaging using pseudo-continuous arterial spin labeling. Central slices of a T1-weighted atlas with gray matter mask (red) overlaid are shown in (a). Mean CBF maps in controls (n = 43) and participants with sickle cell anemia (n = 18) are shown in (b,c). (d) shows mean CBF maps from moyamoya participants (n = 18). Mean CBF maps from moyamoya participants with bottom quartile OEF values (OEF < 0.38), and moyamoya participants with upper quartile OEF values (OEF > 0.45) are shown in (e) and (f), respectively.
Hemo-metabolic relationships in moyamoya between CBF, CVR, and OEF
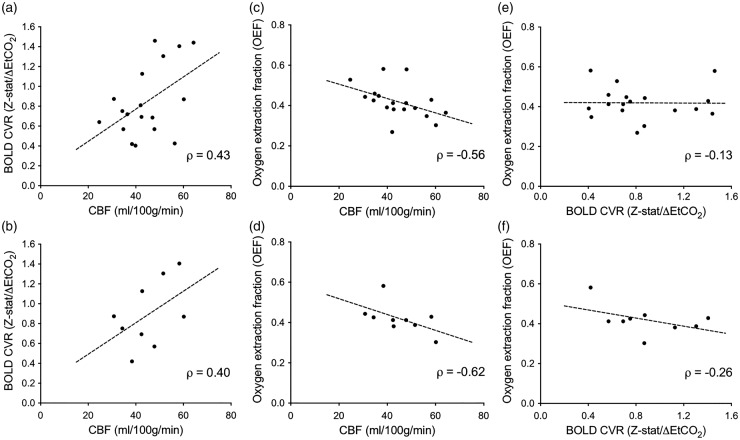
To evaluate our supplemental objectives, we analyzed the relationship between the hemo-metabolic parameters of CBF, CVR, and OEF in participants with moyamoya. In a sub-analysis, we included only patients without prior surgical revascularization. The (mean ±s.d.) change in EtCO2 during the hypercapnic CVR study in moyamoya participants was 4.82 ±2.15 mmHg and the BOLD signal change during hypercapnia normalized by baseline signal strongly correlated with z-statistic values (ρ = 0.80, P < 0.0001). BOLD-weighted CVR positively trended with CBF when considering all moyamoya participants (Figure 5(a); ρ = 0.43, P = 0.073) and only moyamoya participants undergoing surgical revascularization (Figure 5(b); ρ = 0.40, P = 0.29); however, these findings did not meet criteria for significance. The rank-sum coefficient was nearly identical in both cases, but the P-values differed owing to differences in the sample sizes. Next, to understand the mechanistic origin of the hypothesized OEF elevation due to decreased CBF in moyamoya participants, we evaluated the relationship between OEF and CBF and found a significant inverse relationship between CBF and OEF when considering all moyamoya participants (Figure 5(c); ρ = −0.56, P = 0.016) and a trend in moyamoya participants who had not undergone surgical revascularization (Figure 5(d); ρ = −0.62, P = 0.082); however, this latter relationship did not meet criteria for statistical significance. There was only a weak and non-significant inverse relationship between CVR and OEF when considering all moyamoya patients (Figure 5(e); ρ = −0.13, P = 0.082) and moyamoya participants who had not undergone surgical revascularization (Figure 5(f); ρ = −0.26, P = 0.50).
Figure 5.
Relationship between cerebral blood flow (CBF), cerebrovascular reactivity (CVR), and oxygen extraction fraction (OEF) in participants with moyamoya. A Spearman’s rho (ρ) was calculated to test the relationships between CBF, CVR, and OEF. A sub-analysis was performed in only participants who had not undergone surgical revascularization (n = 9; lower). CBF trended positively with blood oxygenation level-dependent (BOLD) hypercapnia-induced cerebrovascular reactivity measured as mean gray-matter z-statistic divided by mean change in end-tidal CO2 (ΔEtCO2) in mmHg (Z-stat/ΔEtCO2) when considering all moyamoya participants (a; ρ = 0.43, P = 0.073) and moyamoya participants who had not undergone surgical revascularization (b; ρ = 0.40, P = 0.29). The larger range in CBF was attributable to known, regional hyperintensity artifacts in ASL due to residual intravascular signal at the time of imaging. CBF was inversely correlated with OEF when considering all moyamoya participants (c; ρ = −0.56, P = 0.016) and moyamoya participants who had not undergone surgical revascularization (d; ρ = −0.62, P = 0.082). There was a non-significant inverse relationship between BOLD reactivity and OEF when considering all moyamoya patients (e; ρ = −0.13, P = 0.61) and moyamoya participants who had not undergone surgical revascularization (f; ρ = −0.26, P = 0.50). The mean change in EtCO2 during the hyercapnic CVR study was 4.82 ± 2.15 mmHg. Additionally, the BOLD signal change during hypercapnia normalized by baseline signal strongly correlated with z-statistic values (ρ = 0.80, P ≤ 0.0001).
Discussion
We applied non-invasive CBF-weighted imaging using pCASL and whole-brain OEF imaging using TRUST in participants with moyamoya and compared results to SCA and age-matched controls. The major findings from this study are (a) OEF is globally elevated in participants with moyamoya compared to controls, a finding that was observed both in patients with and without prior surgical revascularization, and (b) an inverse relationship between whole-brain OEF and CBF in the moyamoya participant population is robustly detectable. We additionally evaluated the relationship between CVR and OEF in moyamoya participants and found no significant relationship between our measures of CVR and OEF.
To our knowledge, this is the first time elevated whole-brain OEF has been reported in participants with moyamoya using TRUST, which can be performed in under 2 min and without exogenous contrast agents. Inter-subject variation in this parameter may provide a biomarker of hemodynamic compensation and possibly stroke risk, which is a logical extension of these initial findings. In our data set, there is a range of OEF values in the age-matched, moyamoya and SCA cohorts, which is consistent with large ranges of previously reported OEF values in healthy populations and patient populations with perturbed cerebrovascular hemodynamics.11,13,35 In 15O PET studies, OEF values are reported as high as 0.54 ± 0.0613 and 0.56 ± 0.117 in diseased hemispheres of patients with intracranial steno-occlusion, and using global OEF studies with TRUST, values in SCA adults are reported as 0.45 ± 0.08,10 and in patients with end-stage renal disease, values are reported as high as 0.472 ± 0.10.15 In healthy populations using MRI based methods, OEF measurements are reported within range of our age-matched mean.36 These ranges across different populations highlight the possibility of using OEF as a biomarker of impairment and stroke risk, as considerable inter-subject variation exists.
Elevated whole-brain OEF in participants with moyamoya is an expected finding given that moyamoya most often affects both cerebral hemispheres, and is a progressive condition that results in widespread, albeit variable, hypoperfusion. In participants with moyamoya, compared to participants with SCA, the oxygen carrying capacity of blood is normal, and the OEF is elevated due to decreased overall delivery of oxygenated blood to tissue (e.g. CBF).
While the moyamoya participants as a group exhibited a strong inverse relationship between CBF and OEF, group-level CBF was only marginally reduced in moyamoya subjects relative to control volunteers. There are several explanations for this finding, which should be considered. First, while regional variation in CBF as visualized by pCASL, consistent with previous reports,18,37 was observed and is expected given the underlying pathology of moyamoya, intravascular signal from delayed blood arrival time to collateral vessels is likely a cause of a non-statistically significant decrease in GM CBF on average compared to age-matched controls. ASL in the setting of very delayed bolus arrival times may lead to focal hyperintensities in ASL signal. Multiple groups have studied patients with moyamoya and reported the presence of arrival time artifacts seen in cortical gray matter using ASL in this population.38,39 Zaharchuk et al. proposed a scoring mechanism for grading collateral presence based on ASL transit time artifacts, and concluded that non-invasive ASL can even predict the presence and intensity of collateral flow on gold-standard catheter angiography. The presence of arrival time artifacts in ASL in such patients however also motivates the need for improved methods for quantifying hemodynamic and metabolic compensation as ASL may not be a quantitative marker of CBF itself in moyamoya patients with significantly delayed arrival times. An additional reason for this finding is that posterior circulation CBF in patients with moyamoya is frequently preserved and the current study focused on global hemodynamics. Therefore, while it is anticipated that ASL provides a good indicator of CBF, it additionally suffers from these contributions, which may bias some of the group level findings in this study.
In addition to OEF and CBF measurements, we examined the relationship between CBF, CVR, and OEF in moyamoya participants. Impaired CVR in moyamoya patients has been reported previously and is well-known.23,40–42 The impaired CVR could be attributable to flow-limiting stenosis at the level of large vessels, exhausted vascular reserve capacity, or collateral vessels not capable of responding to vasoactive stimuli owing to fundamental differences in the structure of these neo-angiogenic vessels relative to healthy vessels. The ability of neoangiogenic collateral vessels to respond to vasoactive stimuli is not well known. In one report, a positive trend between CVR and extent of collateral vessels was found; however, the CVR was still reduced in hemispheres affected by steno-occlusion, compared to individuals with normal cerebral vasculature.39 In our cohort, we found that reduced CVR was not correlated with elevated OEF. As the focus of our study was on moyamoya, CVR data were not available from the participants with SCA. Autoregulatory capacity in patients with impaired cerebrovascular hemodynamics due to SCA is an area of active investigation and preliminary studies support the finding that patients with SCA have reduced CVR and that patients with the highest CBF have correspondently lowest CVR, which is likely due to exhausted autoregulatory capacity.43
While we only provide cross-sectional results to provide motivation for increased OEF as a possible biomarker of interest in moyamoya patients, the extension of this method to longitudinally monitor individuals at risk for new or recurrent stroke could provide a simple, reproducible method for stratifying stoke risk in a diverse patient population and/or evaluating treatment success of therapies.
Beyond regional CBF measurements from ASL, OEF values from TRUST provide discrete values reflective of energy homeostasis of the brain across a large dynamic range. This range of values potentially provides a gradient of disease severity, and while mean CBF could be within normal limits, elevated OEF could be a sensitive marker of disease.
Limitations of this study should also be considered. First, there is heterogeneity in the moyamoya cohort owing to the varied course of the disease, as patients have variable degrees of stenosis and time since disease onset. Therefore, extent of collateralization either by way of spontaneous formation of collaterals or surgical revascularization may affect study findings. However, the focus of this study was alternatively to understand whether elevated OEF was present at the tissue level and related to CBF and CVR. The range of patients included therefore is ideally suited to provide a range of values to investigate these relationships. Additional efforts in larger samples are required to better define normal ranges of OEF values for patients with cerebrovascular disease of varying severity and stroke history. To provide motivation for this future work, we evaluated whether patients with prior TIAs vs. overt strokes had differences in the degree of OEF elevation (Supplemental Figure II) and found evidence for higher OEF in patients with a history of overt stroke compared to a history of TIA only, however additional patients are required to confirm this finding. Second, there were more females than males in the moyamoya participant arm, as this disease predominantly affects females. It is known that across the lifespan females have slightly elevated CBF values compared to males, however it is unlikely that this is a cause of significant bias, and we performed a sub-analysis of only female participants in the moyamoya arm and found identical trends. Third, slightly different pCASL parameters were employed during the course of this study (Supplementary Table II). Importantly, in pCASL, CBF quantification error due to post-labeling delay (PLD) variation is low when data are sampled on the decaying portion of the kinetic curve, which occurs when the labeling duration + PLD ≫ bolus arrival time. This time ranged from approximately 2.9–3.4 s in all data sets, and therefore this requirement is expected to be met and these errors small. A recent study compared GM CBF measures utilizing 1.5 and 2.5 s PLDs with pCASL, and found no difference in GM CBF values between the two conditions (46.67 vs. 46.86 ml/100 g/min for 1.5 s and 2.5 PLDs, respectively, in eight volunteers),25 and our variation in PLD is approximately 1/3 of this range. Overall, the PLD and label duration variation was relatively small and likely to contribute much less to CBF variation than factors related to steno-occlusion and anemia.25 Nonetheless, this was a limitation of the current study. Last, while we find it encouraging that TRUST is sensitive to detect whole-brain elevations of OEF in moyamoya, regional information, as is acquired using 15O PET based approaches, has the potential to provide important additional information in the context of hemispheric interval stroke risk and surgical planning for revascularization. A future direction is to evaluate regional OEF using MRI in this population, as has been recently described,44 as well as integrating new methods for high-resolution mapping of OEF.45
Conclusions
We applied a novel MRI-based approach to quantify OEF in patients with moyamoya, and compared these findings to data from SCA patients, who have known elevated OEF, and to age-matched controls. OEF was significantly elevated in moyamoya participants compared to controls, similar to participants with SCA and CBF was inversely correlated with OEF in moyamoya participants. Elevated OEF was only weakly related to reductions in CVR, consistent with basal CBF level, rather than vascular reserve capacity, being most closely associated with OEF. These results (a) are consistent with non-invasive MRI being able to quickly detect elevated OEF in multiple cases of reduced oxygen delivery to tissue, (b) provide insights regarding differing pathological hemodynamic compensation mechanisms, and (c) suggest that globally elevated OEF in moyamoya is present and inter-subject variation in this parameter could be indicative of tissue-level impairment and elevated stroke risk.
Supplementary Material
Acknowledgments
We are grateful to Charles Nockowski, Christopher Thompson, Clair Jones, Kristen George-Durrett, Leslie McIntosh, and Kimberly Pechman for experimental support.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: American Heart Association (14CSA20380466), NIH/NINDS (6R01NS078828-05 1R01NS097763-01), NIH/NIA (R01-AG034962; K24-AG046373), NIH/NIBIB (5T32EB014841-03), and Vanderbilt Clinical Translational Science Award from the National Center for Research Resources and National Institutes of Health (UL1-TR000445).
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
Jennifer M Watchmaker was involved in experimental design, data acquisition, data analysis and interpretation, and manuscript drafting. Meher R Juttukonda was involved in data acquisition, data analysis and interpretation, and manuscript revision. Larry T Davis was involved in interpretation and scoring of clinical images and manuscript revision. Allison O Scott was involved in data interpretation and manuscript revision. Melissa C Gindville, Lori C Jordan, Carlos C Faraco, Petrice M Cogswell, and Angela L Jefferson were involved in experimental design, data acquisition, data analysis and interpretation, and manuscript editing. Howard S Kirsher was involved in interpretation of clinical findings and manuscript revision. Manus J Donahue was involved in experimental design, data acquisition, data analysis and interpretation, and manuscript revision.
Supplementary material
Supplementary material for this paper can be found at http://journals.sagepub.com/doi/suppl/10.1177/0271678X16682509
References
- 1.Scott RM, Smith ER. Moyamoya disease and moyamoya syndrome. New Engl J Med 2009; 360: 1226–1237. [DOI] [PubMed] [Google Scholar]
- 2.Hallemeier CL, Rich KM, Grubb RL, Jr., et al. Clinical features and outcome in North American adults with moyamoya phenomenon. Stroke 2006; 37: 1490–1496. [DOI] [PubMed] [Google Scholar]
- 3.Arias EJ, Derdeyn CP, Dacey RG, Jr., et al. Advances and surgical considerations in the treatment of moyamoya disease. Neurosurgery 2014; 74(Suppl 1): S116–S125. [DOI] [PubMed] [Google Scholar]
- 4.Liebeskind DS, Cotsonis GA, Saver JL, et al. Collaterals dramatically alter stroke risk in intracranial atherosclerosis. Ann Neurol 2011; 69: 963–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strother MK, Anderson MD, Singer RJ, et al. Cerebrovascular collaterals correlate with disease severity in adult North American patients with Moyamoya disease. AJNR Am J Neuroradiol 2014; 35: 1318–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ostergaard L, Smith DF, Vestergaard-Poulsen P, et al. Absolute cerebral blood flow and blood volume measured by magnetic resonance imaging bolus tracking: comparison with positron emission tomography values. J Cereb Blood Flow Metab 1998; 18: 425–432. [DOI] [PubMed] [Google Scholar]
- 7.Derdeyn CP, Videen TO, Yundt KD, et al. Variability of cerebral blood volume and oxygen extraction: stages of cerebral haemodynamic impairment revisited. Brain: J Neurol 2002; 125: 595–607. [DOI] [PubMed] [Google Scholar]
- 8.Donahue MJ, Sideso E, MacIntosh BJ, et al. Absolute arterial cerebral blood volume quantification using inflow vascular-space-occupancy with dynamic subtraction magnetic resonance imaging. J Cereb Blood Flow Metab 2010; 30: 1329–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leng X, Wong KS, Liebeskind DS. Evaluating intracranial atherosclerosis rather than intracranial stenosis. Stroke 2014; 45: 645–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jordan LC, Gindville MC, Scott AO, et al. Non-invasive imaging of oxygen extraction fraction in adults with sickle cell anaemia. Brain 2016; 139: 738–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamauchi H, Fukuyama H, Nagahama Y, et al. Evidence of misery perfusion and risk for recurrent stroke in major cerebral arterial occlusive diseases from PET. J Neurol Neurosurg Psychiatry 1996; 61: 18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Derdeyn CP, Yundt KD, Videen TO, et al. Increased oxygen extraction fraction is associated with prior ischemic events in patients with carotid occlusion. Stroke 1998; 29: 754–758. [DOI] [PubMed] [Google Scholar]
- 13.Iwama T, Akiyama Y, Morimoto M, et al. Comparison of positron emission tomography study results of cerebral hemodynamics in patients with bleeding- and ischemic-type moyamoya disease. Neurosurg Focus 1998; 5: e3. [DOI] [PubMed] [Google Scholar]
- 14.Lu H, Xu F, Rodrigue KM, et al. Alterations in cerebral metabolic rate and blood supply across the adult lifespan. Cereb Cortex 2011; 21: 1426–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zheng G, Wen J, Lu H, et al. Elevated global cerebral blood flow, oxygen extraction fraction and unchanged metabolic rate of oxygen in young adults with end-stage renal disease: an MRI study. Eur Radiol 2015; 26: 1732–1741. [DOI] [PubMed] [Google Scholar]
- 16.Sheng M, Lu H, Liu P, et al. Cerebral perfusion differences in women currently with and recovered from anorexia nervosa. Psychiatry Res 2015; 232: 175–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu P, Huang H, Rollins N, et al. Quantitative assessment of global cerebral metabolic rate of oxygen (CMRO2) in neonates using MRI. NMR Biomed 2014; 27: 332–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schubert GA, Czabanka M, Seiz M, et al. Perfusion characteristics of Moyamoya disease: an anatomically and clinically oriented analysis and comparison. Stroke 2014; 45: 101–106. [DOI] [PubMed] [Google Scholar]
- 19.Ogasawara K, Ogawa A, Yoshimoto T. Cerebrovascular reactivity to acetazolamide and outcome in patients with symptomatic internal carotid or middle cerebral artery occlusion: a xenon-133 single-photon emission computed tomography study. Stroke 2002; 33: 1857–1862. [DOI] [PubMed] [Google Scholar]
- 20.Ikezaki K, Matsushima T, Kuwabara Y, et al. Cerebral circulation and oxygen metabolism in childhood moyamoya disease: a perioperative positron emission tomography study. J Neurosurg 1994; 81: 843–850. [DOI] [PubMed] [Google Scholar]
- 21.Yamauchi H, Okazawa H, Kishibe Y, et al. Oxygen extraction fraction and acetazolamide reactivity in symptomatic carotid artery disease. J Neurol Neurosurg Psychiatry 2004; 75: 33–37. [PMC free article] [PubMed] [Google Scholar]
- 22.Donahue MJ, Ayad M, Moore R, et al. Relationships between hypercarbic reactivity, cerebral blood flow, and arterial circulation times in patients with moyamoya disease. J Magn Reson Imag 2013; 38: 1129–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Donahue MJ, Dethrage LM, Faraco CC, et al. Routine clinical evaluation of cerebrovascular reserve capacity using carbogen in patients with intracranial stenosis. Stroke 2014; 45: 2335–2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Donahue MJ, Faraco CC, Strother MK, et al. Bolus arrival time and cerebral blood flow responses to hypercarbia. J Cerebral Blood Flow Metab 2014; 34: 1243–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu B, Lou X, Wu X, et al. Intra- and interscanner reliability and reproducibility of 3D whole-brain pseudo-continuous arterial spin-labeling MR perfusion at 3T. J Magn Reson Imag 2014; 39: 402–409. [DOI] [PubMed] [Google Scholar]
- 26.Meher R, Juttukonda MR, Jordan LC, Gindville MC, et al. Cerebral hemodynamics and pseudo-continuous arterial spin labeling considerations in adults with sickle cell anemia. NMR in Biomed. Epub ahead of print 12 November 2016. [DOI] [PMC free article] [PubMed]
- 27.Lu H, Ge Y. Quantitative evaluation of oxygenation in venous vessels using T2-Relaxation-Under-Spin-Tagging MRI. Magn Reson Med 2008; 60: 357–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu H, Xu F, Grgac K, et al. Calibration and validation of TRUST MRI for the estimation of cerebral blood oxygenation. Magn Reson Med 2012; 67: 42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mugikura S, Takahashi S, Higano S, et al. Predominant involvement of ipsilateral anterior and posterior circulations in moyamoya disease. Stroke 2002; 33: 1497–1500. [DOI] [PubMed] [Google Scholar]
- 30.Alsop DC, Detre JA, Golay X, et al. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson Med 2015; 73: spcone. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004; 23(Suppl 1): S208–S219. [DOI] [PubMed] [Google Scholar]
- 32.Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal 2001; 5: 143–156. [DOI] [PubMed] [Google Scholar]
- 33.Lu H, Clingman C, Golay X, et al. Determining the longitudinal relaxation time (T1) of blood at 3.0 Tesla. Magn Reson Med 2004; 52: 679–682. [DOI] [PubMed] [Google Scholar]
- 34.Leenders KL, Perani D, Lammertsma AA, et al. Cerebral blood flow, blood volume and oxygen utilization. Normal values and effect of age. Brain 1990; 113(Pt 1): 27–47. [DOI] [PubMed] [Google Scholar]
- 35.Herold S, Brozovic M, Gibbs J, et al. Measurement of regional cerebral blood flow, blood volume and oxygen metabolism in patients with sickle cell disease using positron emission tomography. Stroke 1986; 17: 692–698. [DOI] [PubMed] [Google Scholar]
- 36.Bulte DP, Kelly M, Germuska M, et al. Quantitative measurement of cerebral physiology using respiratory-calibrated MRI. Neuroimage 2012; 60: 582–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yun TJ, Paeng JC, Sohn CH, et al. Monitoring cerebrovascular reactivity through the use of arterial spin labeling in patients with moyamoya disease. Radiology 2016; 278: 205–213. [DOI] [PubMed] [Google Scholar]
- 38.Zaharchuk G, Do HM, Marks MP, et al. Arterial spin-labeling MRI can identify the presence and intensity of collateral perfusion in patients with moyamoya disease. Stroke 2011; 42: 2485–2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roach BA, Donahue MJ, Davis LT, et al. Interrogating the functional correlates of collateralization in patients with intracranial stenosis using multimodal hemodynamic imaging. AJNR Am J Neuroradiol 2016; 37: 1132–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ni WW, Christen T, Rosenberg J, et al. Imaging of cerebrovascular reserve and oxygenation in Moyamoya disease. J Cereb Blood Flow Metab. Epub ahead of print 20 May 2016. DOI: 10.1177/0271678X16651088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heyn C, Poublanc J, Crawley A, et al. Quantification of cerebrovascular reactivity by blood oxygen level-dependent MR imaging and correlation with conventional angiography in patients with Moyamoya disease. AJNR Am J Neuroradiol 2010; 31: 862–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Han JS, Mikulis DJ, Mardimae A, et al. Measurement of cerebrovascular reactivity in pediatric patients with cerebral vasculopathy using blood oxygen level-dependent MRI. Stroke 2011; 42: 1261–1269. [DOI] [PubMed] [Google Scholar]
- 43.Vaclavu L, Mutsaerts H, Ooij PV, et al. Arterial spin labeling MRI evaluation of cerebrovascular reserve with acetazolamide in patients with sickle cell disease. In: ISMRM 24th annual meeting & exhibition, Singapore, 2016.
- 44.Krishnamurthy LC, Liu P, Ge Y, et al. Vessel-specific quantification of blood oxygenation with T2-relaxation-under-phase-contrast MRI. Magn Reson Med 2014; 71: 978–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wise RG, Harris AD, Stone AJ, et al. Measurement of OEF and absolute CMRO2: MRI-based methods using interleaved and combined hypercapnia and hyperoxia. Neuroimage 2013; 83: 135–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.