Abstract
Children and adults with sickle cell anemia (SCA) have a higher risk of strokes compared to age- and race-matched peers. Velocity in the middle cerebral or distal internal carotid artery as measured by transcranial Doppler ultrasound is a recognized method to identify children but not adults with SCA at high-risk for first stroke. For both children and adults with SCA that have had a stroke, no methods clearly identify individuals at highest risk of recurrent strokes or an initial silent stroke, the most common neurological injury. Methods to assess cerebral hemodynamics in SCA have been utilized for decades but often required radiotracers making them not feasible for screening and longitudinal follow-up. MRI approaches that do not require exogenous contrast have been introduced and are appealing in both clinical and research scenarios. Improved neuroimaging strategies hold promise for identifying individuals with SCA at increased risk of initial and recurrent infarcts, justifying more aggressive risk-based therapy. We review the epidemiology of stroke in SCA, the impact of strokes, stroke mechanisms, and potential imaging strategies including regional and global oxygen extraction fraction, cerebral blood flow, and vessel wall imaging to identify individuals at high-risk of stroke.
Keywords: Arterial spin labelling, brain imaging, cerebral hemodynamics, cognitive impairment, hematology, lacunar infarcts, stroke
Epidemiology and neurological complications of sickle cell disease in children and adults
Sickle cell disease (SCD) is a group of recessively inherited hemoglobinopathies. The most common and severe form, sickle cell anemia (SCA), including genotype HbSS and HbSβthalassemia zero, affects approximately 66,000 individuals in the United States.1 The prevalence of SCA is highest in Africa, India and the Mediterranean; globally in 2010, there were an estimated 305,800 newborns with SCA.2 Among children with SCA, the incidence of stroke is approximately 2/1000/year and in adults with SCA, the incidence is even higher at approximately 9/1000/year.3 The majority of adults with SCA will suffer a cerebral infarct.4 African-American adults with SCA have a three-fold higher risk of overt strokes than African-Americans of similar age (35–64 years) without the condition.5
Silent strokes (also referred to as silent cerebral infarcts) can only be detected with MRI of the brain and are defined as infarcts that are at least 3 mm in one dimension, are visible in two planes on fluid-attenuated inversion recovery (FLAIR) T2-weighted images, and are accompanied by a neurological examination that reveals no abnormalities referable to the location of the brain lesion,6 Figure 1. Untreated silent strokes increase with age with a prevalence of approximately 39% at age 18 years and 50% at 30 years.4,7,8 Children and adults with untreated silent strokes have a high-risk of infarct recurrence or progression.8–11 Silent strokes may go undetected, but have life-altering consequences because they are associated with a decrease in global intelligence quotient (IQ),12 as well as poor academic performance.13 The sequelae of both overt and silent strokes include decreased educational attainment and cognitive performance, lower rates of employment, increased health care costs,14 and premature death.5,15
Figure 1.
Silent and overt strokes. A child with sickle cell anemia (SCA) with no history of stroke-like symptoms had a screening MRI performed. On fluid-attenuated inversion recovery (FLAIR) T2-weighted images, a silent stroke is a lesion that is 3 mm or larger in one dimension and is visible in two imaging planes, typically axial (a) and coronal (b). A normal neurological examination with no symptoms that localize to this lesion confirms this is a silent stroke. Notably, the infarct volume is quite small. In contrast, in a child with neurological symptoms, MRI can confirm an overt stroke. Panel (c) shows a diffusion weighted image of a child with SCA and restricted diffusion confirming overt stroke in the posterior limb of the right internal capsule and putamen at the time of acute presentation (awoke with left sided weakness and numbness). Panel (d) shows the same lesion on FLAIR at two-year follow-up. Note that the mature infarct volume is much smaller than at the time of acute symptoms.
Hemorrhagic stroke is another frequent neurological complication associated with SCA, particularly in adults. In a large prospective cohort study of adults with SCA, the incidence of hemorrhagic stroke in individuals with SCA was highest among patients aged 20 to 29 years.3 Overall, hemorrhagic stroke accounts for about 25% of strokes.16 Some of the increased rate of hemorrhagic stroke is attributable to the higher prevalence of aneurysms in SCA; in a hospital-based cohort, adults with SCA had a prevalence of aneurysms of 10.8%.17
Cognitive impact of strokes in children and adults with sickle cell anemia
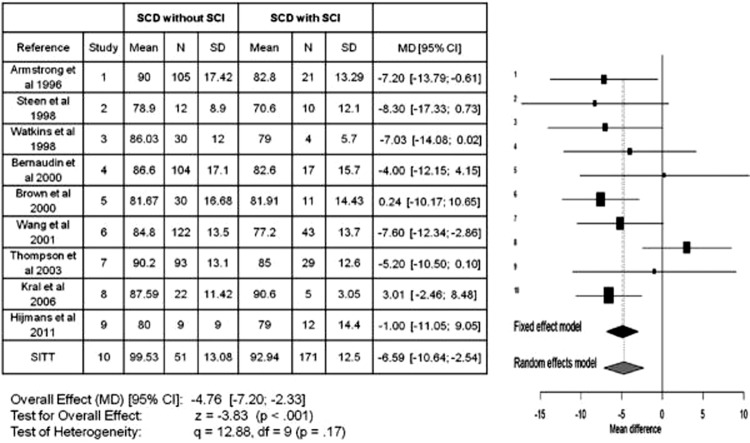
Children with SCA with either overt or silent strokes often have a significant drop in their Full Scale Intelligence Quotient (FSIQ). In the most comprehensive study of children with strokes, there was approximately a 20 point drop in the FSIQ after an overt stroke.18 In a pooled analysis of 10 studies comparing the FSIQ in children with SCA with and without silent strokes, silent strokes were associated with approximately a 5 point drop in FSIQ (Figure 2).12 Lesion burden appears to be important for cognitive function such that increasing numbers of silent strokes leads to poorer intellectual function.19
Figure 2.
Meta-analysis of studies in children with sickle cell anemia that included full scale intelligence quotient (FSIQ) for those with and without silent cerebral infarcts (silent strokes). The meta-analysis includes a total of 10 published studies and compares the mean difference in FSIQ between those children with sickle cell anemia with and without a silent stroke. The Forrest plot x-axis reflects the mean FSIQ difference between those with and without silent stroke. The horizontal lines represent the upper and lower boundaries of the 95% confidence interval. If the 95% confidence interval overlaps zero or crosses the zero threshold, then no statistical differences were observed in that study. The black and gray diamonds represent the results of the fixed and random effect models. The edges of the diamonds represent the 95% confidence interval of the meta-analyses for the fixed and random effect models.12 The Forrest plot graphically shows the study size, the effect size, and the direction of change in the FSIQ. The figure summarizes the strong evidence that silent strokes lead to a reduction in FSIQ in children with SCA. Used with permission.
The natural history of adults with SCA with either strokes or silent strokes is poorly defined, and the impact of low FSIQ is less established in adults versus children with SCA. In the most comprehensive study to date, Vichinsky et al.20 demonstrated in 149 adults with SCA without neurological findings that mean FSIQ was significantly lower than FSIQ of age- and race-matched controls, 90.5 and 95.7, respectively (mean difference, −5.2; 95% confidence interval (CI), −9.2, −1.13). The largest difference between adults with SCA, and controls was in processing speed (86.5 vs. 97.9 (mean difference, −11; 95% CI, −15.51, 7.40); P < .001). Silent strokes, referred to as lacunae in this study, were more frequent in adults with SCA, but were not independently related to neurocognitive function.20
In a single center retrospective cohort study of adults with SCA, Sanger et al.21 described the important relationship between poor cognition and unemployment. The mean age of the sample was 30.7 years (range =19–59) and 44% of the participants were unemployed. In a multivariable logistic regression model, lower FSIQ scores were associated with unemployment (odds ratio = 0.88; P = .002, 95% CI, 0.82, 0.96]). In this sample of 50 adults with SCA, the mean FSIQ was 88.0, and the presence of silent strokes was not associated with cognitive deficits.
In both the Vichinsky et al.20 and Sanger et al.21 studies, no relationship between the presence of silent strokes and low FSIQ was found.20,21 The absence of an association between silent strokes and a reduction in FSIQ in adults with SCA may be related to co-morbid conditions frequently occurring in adults, such as hypertension or diabetes. Alternatively, adults with SCA may have chronic injury to the brain without evidence for infarcts seen on MRI at current magnet strengths. Evidence supporting injury to the brain without detection of cerebral infarcts includes two studies that show a lower than average mean FSIQ in adults with SCA, 90.520 and 88.0.21
Mechanisms underlying acute stroke and chronic ischemia in sickle cell anemia
Cerebral hemodynamics in SCA are complex and are not completely understood. Unique features of SCA impact cerebral hemodynamics. First, a shift in the oxygen dissociation curve to the right alters the typical tight association between hemoglobin oxygen saturation measured with a pulse oximeter and measurement of the hemoglobin oxygen saturation with co-oximetry. Second, the red blood cells have abnormal rheological properties (non-deformability), and elevated hemoglobin S concentration increases blood viscosity. Both the presence of hemoglobin S and the abnormal rheological properties of the red blood cells influence cerebral blood flow. Most of the algorithms used to determine cerebral blood flow have been based on individuals in the general population who do not have profound anemia or abnormal hemoglobin as seen in SCA.
Multiple potentially overlapping mechanisms of cerebral infarction are possible in individuals with SCD. These include: (1) anemia, both chronic anemia with baseline hemoglobin in SCA of 6–8 g/dL and acute anemia (a drop in hemoglobin) in the setting of illness3; (2) inadequate oxygen delivery, including events that increase the need for oxygen, such as febrile illness or seizure, or reduce oxygen exchange such as acute chest syndrome3; (3) intracranial stenosis which is present in at least 10–15% of individuals with SCA22; (4) conventional stroke risk factors, such as hypertension, diabetes, and hyperlipidemia. Physiologically, risk for ischemic stroke occurs when cerebral blood flow (CBF; ml blood/100 g tissue/min) and cerebral blood volume (CBV; ml blood/ml parenchyma) are inadequate to maintain the cerebral metabolic rate of oxygen consumption (CMRO2; mmol O2/100 g tissue/min). CMRO2 is the product of CBF, the oxygen extraction fraction from blood (OEF; ratio of oxygen supplied to oxygen delivered) and blood oxygen content. Cerebral blood oxygen content is decreased in SCA due to reduced hemoglobin, the presence of hemoglobin S, and reduced hemoglobin-bound oxygen.
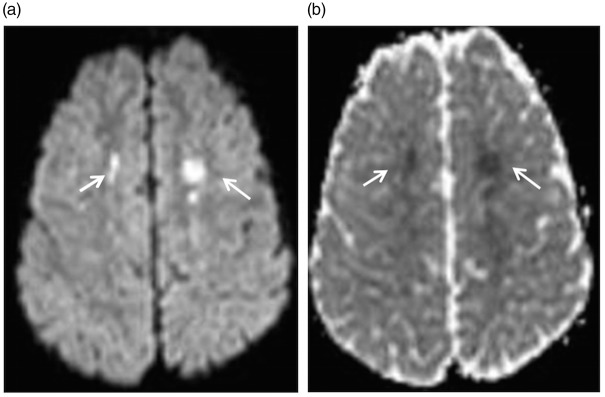
All untreated children and adults with SCA have chronic, hemolytic anemia. As in the general population, adapting to chronic anemia is based on passive physiologic mechanisms, including initial cerebral vasodilation (though capacity for vasodilation appears to be less in the setting of anemia)23–25 with increased cerebral blood flow and ultimately increased OEF if there is insufficient oxygen delivery based on cerebral demand. In children with SCA, even at baseline, there is a delicate relationship between oxygen demand and supply to the brain. In a cross-sectional study of 652 children with SCA, acute silent cerebral ischemic lesions (acute silent strokes) were detected with diffusion-weighted MRI of the brain (Figure 3).26 These lesions were identified in children that were asymptomatic at the time of the MRI of the brain and were not expected to have acute or subacute ischemia of the brain. Of the 10 asymptomatic children with SCA with acute silent cerebral ischemic events, two had follow-up imaging and one had a new silent stroke in the same region of the prior acute silent ischemic lesion detected by diffusion-weighted MRI of the brain. The incidence of acute silent cerebral ischemic events is approximately 10 times greater than the incidence of stroke or silent stroke recurrence in children with SCA. In the Silent Infarct Transfusion trial, the incidence of acute silent cerebral ischemic events was 47.3 per 100 patient years and the incidence of silent strokes was 4.8 events per 100 patient years.26 These data provide evidence that, in children with SCA, there are frequent discrete cerebral ischemic events occurring sporadically, with some events proceeding to permanent brain injury, strokes or silent strokes.
Figure 3.
Acute silent cerebral ischemic events. Children without focal neurological symptoms may have acute ischemic lesions seen on diffusion-weighted MRI sequences. Panel (a) shows bilateral restricted diffusion in the borderzones. Panel (b) shows corresponding changes on the apparent diffusion coefficient sequences to confirm that cerebral ischemia has occurred in the last seven days. Acute silent cerebral ischemic events can occur in any location in the brain. Ischemia in the borderzone region is most often seen when a child with SCA has had acute anemia, as was the case in this child, or significant hypotension.
Given the small margin of reserve between supply and demand of oxygen to the brain in SCA, acute drops in hemoglobin are a high-risk period for strokes. Dowling et al.27 tested the hypothesis that acute cerebral ischemic events would occur in children and would be temporally associated with a drop in hemoglobin levels to less than 5.5 g/d. In a cross-sectional study, the investigators screened children with acute, severe anemia and no clinical signs of stroke, both with and without SCA, with diffusion-weighted MRI of the brain within 10 days of the reduction in hemoglobin level. They demonstrated that 18.2% (4 of 22) of the children with SCA and 6.7% (2 of 30) the children without SCA had new ischemic lesions of the brain in the setting of profound anemia. Table 1 summarizes the incidence of silent strokes in children with SCA with and without prior infarcts. These studies provide strong evidence that children with profound anemia are at increased risk for cerebral ischemic injury, and children with SCA are higher risk because of their unique biological factors. Unfortunately, in adults with SCA, no similar risk stratification for initial and recurrent strokes and silent strokes has been published. Imaging methods to distinguish children and adults at highest risk for ischemic lesions are needed.
Table 1.
Silent ischemic events in children with sickle cell anemia: Identifying high-risk groups.
| Ischemic brain lesion | Study | Incidence of new ischemic events per 100 patient-years |
|---|---|---|
| Initial SCI | Pegelow et al.10 | 1.0 |
| Prior SCI with recurrent SCI while untreated | Pegelow et al.10 | 7.1 |
| Prior SCI with recurrent SCI while untreated | DeBaun48 | 4.8 |
| Prior SCI with recurrent SCI or stroke while treated with regular blood transfusion therapy | DeBaun et al.48 | 2.0 |
| Prior overt stroke with recurrent SCI while treated with regular blood transfusion therapy | Hulbert et al.28 | 5.0 |
| ASCIE | Quinn et al.26 | 47.3 |
| ASCIE during acute anemic event | Dowling et al.27 | 664 |
SCI: silent cerebral infarct; TCD: transcranial Doppler ultrasound; ASCIE: acute silent cerebral infarct event.
Methods to assess cerebral hemodynamics
Conventional neuroimaging methods such as MRI of the brain and CT of the head will show parenchymal brain injury that may be in a pattern suggestive of hemodynamic compromise (borderzone or watershed ischemia) but do not assess cerebral hemodynamics. Prior silent or overt stroke increases the risk of additional cerebral infarcts. MR and CT angiography of the brain assess cerebral blood vessels for intracranial stenosis, anatomic variants in the cerebral circulation and for aneurysms. Cerebral vasculopathy defined as stenosis > 50% is a known risk factor for recurrent cerebral ischemia.28 Given that prior cerebral ischemia and cerebral vasculopathy portend future stroke risk, in our practice, we assess clinically asymptomatic children with MRI and MRA of the brain at least once in childhood when these tests are able to be done without sedation and once in adulthood. If silent strokes or cerebral vasculopathy are present, we follow this group with surveillance MRI and MRA of the brain for progression and consider increasing the intensity of disease modifying therapy.
The standard method used to assess stroke risk in children with SCD is transcranial Doppler ultrasound (TCD). Adams et al.29 validated this technique in children with SCD and used TCD in their landmark study, the Stroke Prevention Trial in Sickle Cell Anemia (STOP). Children with elevated TCD velocity 200 cm/s or greater were randomized to monthly blood transfusion versus observation, resulting in a 92% risk reduction for stroke with blood transfusions. The results of this seminal trial led to the new standard of care for primary stroke prevention, namely, screening for elevated TCD measurements coupled with initial blood transfusion therapy for children with TCD velocities ≥ 200 cm/s.
Originally, elevations in TCD velocity were thought to be a sign of intracranial stenosis. In fact, the initial TCD validation study was designed to determine the sensitivity and specificity of TCD in detecting significant (>50% lumen diameter reduction) intracranial arterial stenosis in children with SCD.30 TCD detected intracranial stenosis that was >50% in 26 of 29 children with SCA (sensitivity 90%, specificity 100%). Despite this small validation study and the fact that elevated TCD velocity ≥200 cm/s clearly identifies children at risk for stroke,31 analysis of the STOP trial MRA of the brain data showed that in children with elevated TCD velocities, 79% had either no stenosis or less than 25% stenosis.32 In most children, TCD seems to reflect increased cerebral blood flow velocity. TCD velocity criteria used in children cannot be used to stratify risk of stroke in adults.33
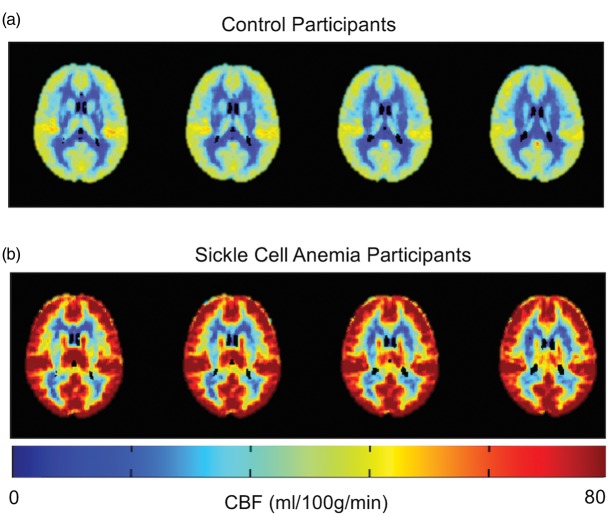
More recent methods to assess stroke risk include MR perfusion techniques such as arterial spin labelling (ASL). The two main types of ASL are continuous arterial spin labelling (CASL) and pseudocontinuous arterial spin labelling (pCASL). ASL MR perfusion allows for non-invasive quantification of global and regional CBF. ASL generates an image by magnetically “labelling” water molecules as an endogenous tracer. Selective radiofrequency irradiation inverts the magnetization of arterial blood water in the region or plane to which it is applied, usually in the neck for brain perfusion, and a downstream measurement is taken as labeled water molecules exchange into the brain. Labelled images are subtracted from control (unlabeled) images to estimate CBF. CBF has been shown to be globally increased in children34 and adults35,36 with SCD. Figure 4 shows this elevation in adults with SCA compared with healthy controls. One limitation of ASL in SCA is that with elevated CBF, labelling efficiency would be expected to be reduced. Cervical phase contrast angiography has been used to determine labelling efficiency in adults with SCA37 and confirmed elevated blood velocities. Labelling efficiency was found to be 0.60 compared with 0.85 in healthy age- and race-matched controls. Reduced labelling efficiency will result in an underestimation of CBF values without correction in SCA.
Figure 4.
Pseudo-continuous arterial spin labeling to assess cerebral blood flow in sickle cell disease. Mean cerebral blood flow (CBF) maps in control participants (a) (n = 43) and participants with SCA (b) (n = 22) are shown. Note that CBF is globally increased in SCA compared to controls.
A measure of the brain’s capacity to increase oxygen delivery is OEF, defined as the ratio of oxygen consumed over oxygen delivered to tissue. Elevation in cerebral OEF is postulated to be a compensatory mechanism when CBF is inadequate. OEF has classically been measured via PET. In a 1986 PET study in six adults with SCA, both regional cerebral blood flow and blood volume were found to be significantly elevated when compared to healthy controls.38 Oxygen extraction was not significantly different than controls. However, studies continued, primarily in other patient populations. The St. Louis Carotid Occlusion Study demonstrated convincingly that elevated OEF was a strong risk factor for stroke in adults without SCA, but with unilateral carotid occlusion. Specifically, ipsilateral-increased OEF was worrisome and a ratio of ipsilateral-to-contralateral mean regional carotid territory OEF greater than 1.13 was thought to be highly suggestive of stroke risk. In fact, the ischemic stroke rate over two years was 26.5% in 39 patients with increased OEF and 5.3% in 42 patients with normal OEF, P = 0.004).39 In the multicenter Carotid Occlusion Surgery Study (COSS), participants had (15)O PET and were randomized to observation versus extracranial to intracranial bypass surgery if the OEF ratio was >1.13 on the occluded side. The COSS study investigators did not find evidence that an OEF ratio >1.13 and surgery significantly decreased the rate of future strokes when compared to no surgery. Two-year rates of ipsilateral ischemic stroke were 21.0% for the surgical group and 22.7% for the nonsurgical group, a difference of 1.7% (95% CI, −10.4% to 13.8%). However, elevated OEF was independently associated with cognitive impairment in participants with carotid occlusion confirming that elevated OEF is a marker of cerebral impairment and injury.40
Research evaluating the utility of OEF in SCA is just beginning. Our group published data on 27 adults with SCA.35 We used T2-relaxation-under-spin-tagging (TRUST) MRI rather than PET to assess whole brain OEF. Global OEF measurement is logical in SCA, particularly in adults with SCA without significant extracranial or intracranial stenosis. Cerebral blood flow and OEF were both elevated. However, OEF, but not cerebral blood flow was increased in a linear fashion in participants with higher levels of clinical impairment defined as (1) moderate vasculopathy >50% of any major extracranial or intracranial vessel, (2) prior overt stroke or infarct on neuroimaging, and/or (3) chronic debilitating SCA-related pain that required treatment with chronic blood transfusion therapy. When a significant unilateral intracranial or extracranial stenosis is present, regional OEF measurements are logical. Regional OEF assessment has been completed using PET as described and recently using MRI in an animal model41 and non-SCD patient populations.42
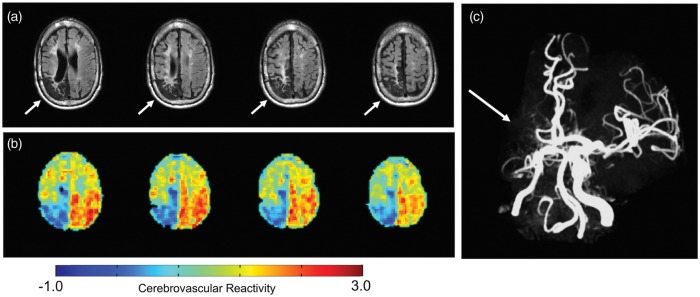
Cerebrovascular reactivity is the ability of cerebral blood vessels to change their diameter; hence, their capacity to regulate regional blood flow in the brain. Historically, adults with SCD have had assessment of global cerebral blood flow by the 133Xenon inhalation during normocapnia and hypercapnia.23 Currently, high resolution quantitative maps of cerebrovascular reactivity can be produced using blood-oxygen level-dependent (BOLD) MRI,43,44 and inhalation of carbon dioxide gases which assess for cerebral vasodilation. When an individual has little capacity to vasodilate to increase cerebral blood flow, this may be a worrisome sign. Prediction of cerebrovascular reserve is a key goal. Some combination of these techniques that assess OEF, CBF and perhaps cerebrovascular reactivity may provide a profile for individuals with SCA at the highest risk for stroke. Recent work has shown that cerebrovascular reactivity is significantly reduced in children with SCA, particularly in the setting of anemia.45 None of the children in this study had significant intracranial stenosis. Given that decreased cerebrovascular reactivity may occur before the presence of cerebral vasculopathy, in the future, cerebrovascular reactivity may be used to stratify individuals with SCA into high and low stroke risk groups. Studying cerebrovascular reactivity and other measures of cerebral hemodynamics in SCA, a homogeneous single gene disorder where individuals are predisposed to moyamoya provides a unique opportunity to understand the risk of initial and subsequent stroke in other high-risk populations, as typically moyamoya is a more heterogeneous group. To date, most of the work in this area has been in non-SCA populations with moyamoya.46,47 Figure 5 provides an example of using BOLD MRI to estimate cerebrovascular reactivity in SCD related moyamoya syndrome.
Figure 5.
Cerebrovascular reactivity in sickle cell anemia in the setting of cerebral vasculopathy. A 25-year-old male with SCA and history of overt stroke in the right posterior MCA territory (a) and unilateral moyamoya syndrome with right distal internal carotid and middle cerebral artery occlusion (b) had measurements of cerebral blood flow and cerebrovascular reactivity (CVR) (c). The patient was fitted with a nasal cannula (Salter Labs, Ref 400F) for end tidal CO2 monitoring and a non-rebreathing mask for administration of medical grade room air (21% O2, 79% N2) and hypercapnic hyperoxia (i.e. carbogen: 5% CO2, 95% O2). The CVR was assessed using a paradigm consisting of two blocks of 3-min carbogen administration interleaved with 3-min blocks of medical grade room air. Carbogen should produce cerebral vasodilation with good CVR. Gradient echo BOLD images were acquired. As expected, the patient had reduced CVR in the region of his remote stroke (blue). He also had relatively preserved reactivity in the left hemisphere, and posteriorly. Moyamoya preferentially affects the anterior circulation until very late stages.
Standard therapy fails to prevent stroke recurrence
Despite regular blood transfusion therapy, which is the standard of care for secondary stroke prevention in children with overt and silent stroke, over a course of five years, 45% of children with overt strokes will have a new infarct recurrence (overt or silent).28 For children with silent strokes, monthly blood transfusions significantly reduce their risk of new infarct; however, the number needed to treat to prevent 1 new infarct is 13 children.48 The most pressing clinical problem in this high-risk population, children and adults with SCA, is to clearly separate those likely to progress despite receiving regular blood transfusion therapy from the group not likely to have any recurrent cerebral infarcts.
Use of novel imaging strategies to better identify children that require more aggressive treatment is pivotal (e.g., revascularization surgery, stem cell transplantation or even gene therapy available through clinical trials). Clinical trials have answered many key questions regarding evaluation for stroke risk and treatment in children with SCA (Table 2). However, basic information regarding stroke risk in adults with SCA is lacking, and refinement of individual risk for stroke recurrence is necessary in children and adults.
Table 2.
Key clinical questions that were asked and answered from randomized clinical trials or meta-analyses in sickle cell anemia.
| Question | Answer | Adults |
|---|---|---|
| In individuals with SCA and overt strokes, are blood transfusions better than no transfusions for secondary stroke prevention? | Yes, in children with SCA (meta-analysis)49 | Unknown |
| In individuals with SCA and overt strokes, is hydroxyurea better than no treatment for secondary stroke prevention? | Yes, in children with SCA (meta-analysis)49 | Unknown |
| In individuals with SCA and prior infarct, is MRA-defined vasculopathy associated with future overt or silent strokes? | Yes, in children with SCA, Hulbert et al.28 | Unknown |
| Are individuals with SCA and abnormally high TCD measurements (>200 cm/s) at risk for strokes within a year? | Yes, for children between 2 and 16 years of age with SCA29,55 | No33 |
SCA: sickle cell anemia; MRA: magnetic resonance angiography.
How might modern neuroimaging methods help?
Clinicians providing medical care for children and adults with SCA have a keen interest in modern neuroimaging methods. In part, this attention is driven by the inability to identify a subgroup of children and adults that are likely to fail secondary stroke prevention with monthly blood transfusions.49 The burden of monthly blood transfusion is unacceptably high for individuals with SCA and their families in terms of time away from school and work, expense, excessive iron stores from transfusions, and other side effects.14,28,50 Currently, some families choose not to treat children identified as high-risk for stroke51 because the numbers needed to treat for secondary stroke prevention are so high. Improvements in both structural and hemodynamic imaging of the brain have the potential to increase the benefit-to-burden ratio by improving patient selection. If novel cerebral hemodynamic imaging could reduce the number needed to treat with blood transfusion to prevent recurrent infarcts, this would be a breakthrough.
Structural MRI identifies silent strokes and MR angiography demonstrates cerebral vasculopathy, and both of these findings are associated with increased stroke risk. However, improved structural imaging advances perhaps in combination with cerebral hemodynamic data may be helpful. For example, with increasing MRI magnet strengths, new lesions and potential pathologies will be identified that are not seen on lower resolution scans. Smaller areas of ischemic or hemorrhagic brain injury identified with ultra-high field magnets must be studied to assure that they are both predictive of future strokes and associated with expected location-specific cognitive profiles (frontal lobe lesions associated with executive function, working memory, attention). Simply identifying potentially new pathological lesions of the brain without evidence that they are associated with markers of abnormal cerebral hemodynamics such as increased OEF, decreased CVR and current or future neurological morbidity does not advance the field and may lead to over treatment.
Structural vascular imaging may also be helpful. For example, vessel wall imaging provides information regarding vessel wall thickening, luminal narrowing, and vessel wall enhancement.52 Improvements in spatial resolution, CSF suppression, and more quantitative thickness measurement procedures are expected over the next few years. Standard vascular imaging techniques only evaluate luminal narrowing or dilation. Cerebral vasculopathy is a strong risk factor for stroke and stroke recurrence in SCA. Intimal injury with proliferation is a consistent pathological finding in SCA.53,54 With vessel wall imaging, it may be possible to identify intimal proliferation at an earlier stage and implement new treatments to prevent progressive cerebral arteriopathy and stroke. Furthermore, a better understanding of the mechanism of arteriopathy in SCA could inform specific treatments. Vessel wall imaging is a new imaging modality worthy of further investigation in SCA.
Combining hemodynamic and structural neuroimaging may have the potential to refine estimation of future stroke risk. One major impact may come in relation to primary stroke prevention. Silent strokes are the most common neurological injury in children and adults with SCA; however, current methods to assess stroke risk do not identify children at risk for silent strokes. TCD has only been studied in relation to overt stroke risk and has not been shown to be useful to predict silent strokes. Modern and still investigational MRI methods may help elucidate cerebral hemodynamic profiles for individuals at high-risk for first silent strokes so that preventative strategies may be implemented before brain injury occurs.
New MRI methods are able to rapidly assess OEF, cerebral blood flow and cerebrovascular reactivity. These novel MRI methods as well as other currently undefined parameters may help identify thresholds for transfusion, particularly if such thresholds were associated with decreased cognitive performance as was demonstrated in the COSS trial. Cerebral hemodynamic parameters could be utilized in individuals with acute severe anemia to help guide thresholds for blood transfusion based on concerning cerebral hemodynamics (ongoing research will be necessary to define the proper hemodynamic thresholds). Similarly, MRI methods could be utilized with intracranial stenosis to assess effect of the stenosis on cerebral hemodynamics. Severely altered hemodynamics may suggest the need for neurosurgical revascularization in anemic patients or other therapies including maintaining hemoglobin at a higher level via transfusion.
Conclusion
Decades of research have defined several high-risk groups for initial and subsequent strokes in SCA (see Table 1); however, modern imaging methods may allow further refinement to avoid unnecessary and burdensome treatments and to identify groups where primary prevention for silent strokes is worthwhile. Novel neuroimaging strategies hold promise for identifying children and adults with SCA at highest risk for initial and recurrent cerebral infarcts (overt strokes and silent strokes). One potential strategy is using MRI assessments of regional or global OEF and cerebral blood flow to identify the subgroup of children and adults with altered cerebral hemodynamics that are most likely to have recurrent strokes. Such advancement in medical care would facilitate risk stratification, allowing the high-risk group to enroll in randomized controlled trials such as hematopoietic stem cell transplant or gene therapy, and consideration of cerebral re-vascularization procedures in setting of cerebral vasculopathy or some combination of these treatments. Another potential strategy is the combined use of MRA, CASL and OEF to identify whether select regions of the brain are at risk for cerebral ischemic injury and targeting interventions for those regions. The challenges of new imaging techniques in SCA are to validate novel imaging techniques such as MRI-measured elevated OEF and CBF and to determine whether these measures better predict stroke risk when compared to current known high-risk categories. Despite neurological injury being common in children and adults with SCA, in North America and Europe, SCA is considered a rare disease. Given the low prevalence of the disease in high income countries, where state of the art neuroimaging procedures are likely to occur, multi-center learning collaboratives are likely to advance the field faster than single center studies.
Acknowledgements
The findings and conclusions in this paper are those of the authors and do not necessarily represent the official position of the National Institutes of Health.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Time spent on this publication was supported by the National Institute of Neurological Disorders and Stroke of the National Institutes of Health Grant #1R01NS096127 and the American Heart Association Grant ##14CSA20380466.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
Jordan: drafting the manuscript, critical revision of the manuscript; DeBaun: drafting the manuscript, critical revision of the manuscript.
References
- 1.Hassell KL. Population estimates of sickle cell disease in the U.S. Am J Prev Med 2010; 38: S512–S521. [DOI] [PubMed] [Google Scholar]
- 2.Piel FB, Hay SI, Gupta S, et al. Global burden of sickle cell anaemia in children under five, 2010-2050: Modelling based on demographics, excess mortality, and interventions. PLoS Med 2013; 10: e1001484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ohene-Frempong K, Weiner SJ, Sleeper LA, et al. Cerebrovascular accidents in sickle cell disease: Rates and risk factors. Blood 1998; 91: 288–294. [PubMed] [Google Scholar]
- 4.Kassim AA, Pruthi S, Day M, et al. Silent cerebral infarcts and cerebral aneurysms are prevalent in adults with sickle cell anemia. Blood 2016; 127: 2038–2040. [DOI] [PubMed] [Google Scholar]
- 5.Strouse JJ, Jordan LC, Lanzkron S, et al. The excess burden of stroke in hospitalized adults with sickle cell disease. Am J Hematol 2009; 84: 548–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casella JF, King AA, Barton B, et al. Design of the silent cerebral infarct transfusion (SIT) trial. Pediatr Hematol Oncol 2010; 27: 69–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeBaun MR, Sarnaik SA, Rodeghier MJ, et al. Associated risk factors for silent cerebral infarcts in sickle cell anemia: Low baseline hemoglobin, sex, and relative high systolic blood pressure. Blood 2012; 119: 3684–3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bernaudin F, Verlhac S, Arnaud C, et al. Chronic and acute anemia and extracranial internal carotid stenosis are risk factors for silent cerebral infarcts in sickle cell anemia. Blood 2015; 125: 1653–1661. [DOI] [PubMed] [Google Scholar]
- 9.Miller ST, Macklin EA, Pegelow CH, et al. Silent infarction as a risk factor for overt stroke in children with sickle cell anemia: A report from the Cooperative Study of Sickle Cell Disease. J Pediatr 2001; 139: 385–390. [DOI] [PubMed] [Google Scholar]
- 10.Pegelow CH, Macklin EA, Moser FG, et al. Longitudinal changes in brain magnetic resonance imaging findings in children with sickle cell disease. Blood 2002; 99: 3014–3018. [DOI] [PubMed] [Google Scholar]
- 11.Nottage KA, Ware RE, Aygun B, et al. Hydroxycarbamide treatment and brain MRI/MRA findings in children with sickle cell anaemia. Brit J Haematol 2016; 175: 331–338. [DOI] [PubMed] [Google Scholar]
- 12.King AA, Strouse JJ, Rodeghier MJ, et al. Parent education and biologic factors influence on cognition in sickle cell anemia. Am J Hematol 2014; 89: 162–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.King AA, Rodeghier MJ, Panepinto JA, et al. Silent cerebral infarction, income, and grade retention among students with sickle cell anemia. Am J Hematol 2014; 89: E188–E192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wayne AS, Schoenike SE, Pegelow CH. Financial analysis of chronic transfusion for stroke prevention in sickle cell disease. Blood 2000; 96: 2369–2372. [PubMed] [Google Scholar]
- 15.Platt OS, Brambilla DJ, Rosse WF, et al. Mortality in sickle cell disease. Life expectancy and risk factors for early death. New Engl J Med 1994; 330: 1639–1644. [DOI] [PubMed] [Google Scholar]
- 16.Adams RJ, McKie VC, Brambilla D, et al. Stroke prevention trial in sickle cell anemia. Control Clin Trials 1998; 19: 110–129. [DOI] [PubMed] [Google Scholar]
- 17.Nabavizadeh SA, Vossough A, Ichord RN, et al. Intracranial aneurysms in sickle cell anemia: Clinical and imaging findings. J Neurointerv Surg 2015; 8: 434–440. [DOI] [PubMed] [Google Scholar]
- 18.Armstrong FD, Thompson RJ, Jr., Wang W, et al. Cognitive functioning and brain magnetic resonance imaging in children with sickle Cell disease. Neuropsychology Committee of the Cooperative Study of Sickle Cell Disease. Pediatrics 1996; 97: 864–870. [PubMed] [Google Scholar]
- 19.Schatz J, White DA, Moinuddin A, et al. Lesion burden and cognitive morbidity in children with sickle cell disease. J Child Neurol 2002; 17: 891–895. [PubMed] [Google Scholar]
- 20.Vichinsky EP, Neumayr LD, Gold JI, et al. Neuropsychological dysfunction and neuroimaging abnormalities in neurologically intact adults with sickle cell anemia. JAMA 2010; 303: 1823–1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanger M, Jordan L, Pruthi S, et al. Cognitive deficits are associated with unemployment in adults with sickle cell anemia. J Clin Exper Neuropsychol 2016; 38: 661–671. [DOI] [PubMed] [Google Scholar]
- 22.Silva GS, Vicari P, Figueiredo MS, et al. Brain magnetic resonance imaging abnormalities in adult patients with sickle cell disease: Correlation with transcranial Doppler findings. Stroke 2009; 40: 2408–2412. [DOI] [PubMed] [Google Scholar]
- 23.Prohovnik I, Hurlet-Jensen A, Adams R, et al. Hemodynamic etiology of elevated flow velocity and stroke in sickle-cell disease. J Cereb Blood Flow Metab 2009; 29: 803–810. [DOI] [PubMed] [Google Scholar]
- 24.Kuwabara Y, Sasaki M, Hirakata H, et al. Cerebral blood flow and vasodilatory capacity in anemia secondary to chronic renal failure. Kidney Int 2002; 61: 564–569. [DOI] [PubMed] [Google Scholar]
- 25.Nur E, Kim YS, Truijen J, et al. Cerebrovascular reserve capacity is impaired in patients with sickle cell disease. Blood 2009; 114: 3473–3478. [DOI] [PubMed] [Google Scholar]
- 26.Quinn CT, McKinstry RC, Dowling MM, et al. Acute silent cerebral ischemic events in children with sickle cell anemia. JAMA Neurol 2013; 70: 58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dowling MM, Quinn CT, Plumb P, et al. Acute silent cerebral ischemia and infarction during acute anemia in children with and without sickle cell disease. Blood 2012; 120: 3891–3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hulbert ML, McKinstry RC, Lacey JL, et al. Silent cerebral infarcts occur despite regular blood transfusion therapy after first strokes in children with sickle cell disease. Blood 2011; 117: 772–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adams RJ, McKie VC, Hsu L, et al. Prevention of a first stroke by transfusions in children with sickle cell anemia and abnormal results on transcranial Doppler ultrasonography. New Engl J Med 1998; 339: 5–11. [DOI] [PubMed] [Google Scholar]
- 30.Adams RJ, Nichols FT, Figueroa R, et al. Transcranial Doppler correlation with cerebral angiography in sickle cell disease. Stroke 1992; 23: 1073–1077. [DOI] [PubMed] [Google Scholar]
- 31.Adams R, McKie V, Nichols F, et al. The use of transcranial ultrasonography to predict stroke in sickle cell disease. New Engl J Med 1992; 326: 605–610. [DOI] [PubMed] [Google Scholar]
- 32.Abboud MR, Cure J, Granger S, et al. Magnetic resonance angiography in children with sickle cell disease and abnormal transcranial Doppler ultrasonography findings enrolled in the STOP study. Blood 2004; 103: 2822–2826. [DOI] [PubMed] [Google Scholar]
- 33.Valadi N, Silva GS, Bowman LS, et al. Transcranial Doppler ultrasonography in adults with sickle cell disease. Neurology 2006; 67: 572–574. [DOI] [PubMed] [Google Scholar]
- 34.Oguz KK, Golay X, Pizzini FB, et al. Sickle cell disease: Continuous arterial spin-labeling perfusion MR imaging in children. Radiology 2003; 227: 567–574. [DOI] [PubMed] [Google Scholar]
- 35.Jordan LC, Gindville MC, Scott AO, et al. Non-invasive imaging of oxygen extraction fraction in adults with sickle cell anaemia. Brain 2016; 139: 738–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prohovnik I, Pavlakis SG, Piomelli S, et al. Cerebral hyperemia, stroke, and transfusion in sickle cell disease. Neurology 1989; 39: 344–348. [DOI] [PubMed] [Google Scholar]
- 37.Juttukonda MR, Jordan LC, Gindville MC, et al. Quantification of arterial spin labeling efficiency in high cervical velocity conditions using phase contrast angiography. IEEE 2016; 1: 1350–1353. [Google Scholar]
- 38.Herold S, Brozovic M, Gibbs J, et al. Measurement of regional cerebral blood flow, blood volume and oxygen metabolism in patients with sickle cell disease using positron emission tomography. Stroke 1986; 17: 692–698. [DOI] [PubMed] [Google Scholar]
- 39.Grubb RL, Jr., Powers WJ, Derdeyn CP, et al. The carotid occlusion surgery study. Neurosurg Foc 2003; 14: e9. [DOI] [PubMed] [Google Scholar]
- 40.Marshall RS, Festa JR, Cheung YK, et al. Cerebral hemodynamics and cognitive impairment: Baseline data from the RECON trial. Neurology 2012; 78: 250–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chang FY, Xiao JX, Xie S, et al. Determination of oxygen extraction fraction using magnetic resonance imaging in canine models with internal carotid artery occlusion. Sci Rep 2016; 6: 303–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.An H, Ford AL, Chen Y, et al. Defining the ischemic penumbra using magnetic resonance oxygen metabolic index. Stroke 2015; 46: 982–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Donahue MJ, Dethrage LM, Faraco CC, et al. Routine clinical evaluation of cerebrovascular reserve capacity using carbogen in patients with intracranial stenosis. Stroke 2014; 45: 2335–2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smeeing DP, Hendrikse J, Petersen ET, et al. Arterial spin labeling and blood oxygen level-dependent MRI cerebrovascular reactivity in cerebrovascular disease: A systematic review and meta-analysis. Cerebrovasc Dis 2016; 42: 288–307. [DOI] [PubMed] [Google Scholar]
- 45.Kosinski PD, Croal PL, Leung J, et al. The severity of anaemia depletes cerebrovascular dilatory reserve in children with sickle cell disease: A quantitative magnetic resonance imaging study. BritJ Haematol 2017; 176: 280–287. [DOI] [PubMed] [Google Scholar]
- 46.Watchmaker JM, Juttukonda MR, Davis LT, et al. Hemodynamic mechanisms underlying elevated oxygen extraction fraction (OEF) in moyamoya and sickle cell anemia patients. J Cereb Blood Flow Metab Epub ahead of print 28 December 2016. DOI: 10.1177/0271678X16682509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Han JS, Mikulis DJ, Mardimae A, et al. Measurement of cerebrovascular reactivity in pediatric patients with cerebral vasculopathy using blood oxygen level-dependent MRI. Stroke 2011; 42: 1261–1269. [DOI] [PubMed] [Google Scholar]
- 48.DeBaun MR, Gordon M, McKinstry RC, et al. Controlled trial of transfusions for silent cerebral infarcts in sickle cell anemia. New Engl J Med 2014; 371: 699–6710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kassim AA, Galadanci NA, Pruthi S, et al. How I treat and manage strokes in sickle cell disease. Blood 2015; 125: 3401–3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brittenham GM. Iron-chelating therapy for transfusional iron overload. New Engl J Med 2011; 364: 146–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vichinsky EP, Ohene-Frempong K, Thein SL, et al. Transfusion and chelation practices in sickle cell disease: A regional perspective. Pediatr Hematol Oncol 2011; 28: 124–133. [DOI] [PubMed] [Google Scholar]
- 52.Obusez EC, Hui F, Hajj-Ali RA, et al. High-resolution MRI vessel wall imaging: Spatial and temporal patterns of reversible cerebral vasoconstriction syndrome and central nervous system vasculitis. Am J Neuroradiol 2014; 35: 1527–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rothman SM, Fulling KH, Nelson JS. Sickle cell anemia and central nervous system infarction: A neuropathological study. Ann Neurol 1986; 20: 684–690. [DOI] [PubMed] [Google Scholar]
- 54.Merkel KH, Ginsberg PL, Parker JC, Jr., et al. Cerebrovascular disease in sickle cell anemia: A clinical, pathological and radiological correlation. Stroke 1978; 9: 45–52. [DOI] [PubMed] [Google Scholar]
- 55.Adams RJ, Brambilla D. Optimizing Primary Stroke Prevention in Sickle Cell Anemia Trial I. Discontinuing prophylactic transfusions used to prevent stroke in sickle cell disease. New Engl J Med 2005; 353: 2769–2778. [DOI] [PubMed] [Google Scholar]