Abstract
Approximately 15 million individuals suffer a stroke worldwide each year, and stroke results in death or permanent disability in two-thirds of these individuals. Due to increased knowledge and management of modifiable risk factors, stroke incidence in developed countries is declining, however remains high at just under 1 million patients per year in the United States alone. Further improving management of patients with cerebrovascular disease (CVD) ultimately will require development and clinical adoption of sensitive markers of hemodynamic and metabolic failure, as well as trials that evaluate how to interpret these markers to optimize therapies. Realizing this goal and reducing the complete burden of CVD is dependent on an improved understanding of the pathophysiological processes that underlie CVD in all stages, including sub-clinical disease processes, acute stroke, and post-stroke recovery mechanisms. This document serves as an introduction to the Journal of Cerebral Blood Flow and Metabolism special issue on cerebrovascular diseases, which is comprised of contributions from experts in each of the above stages of CVD, and outlines current standards for patient management and emerging directions that have potential for improving patient care over the next decade.
Keywords: Cerebrovascular disease, stroke, cerebral blood flow, metabolism, neuroimaging
Cerebrovascular disease remains a leading cause of death and the leading cause of adult disability in most developed countries.1 The etiology of overt stroke is well characterized and derives directly from disruption of the blood supply to the brain under either ischemic or hemorrhagic conditions. However, the manner in which brain parenchyma responds to either abrupt or chronic changes in oxygen delivery can be complex and encompasses a wide range of interactions between collateral flow, metabolism, and microvascular hemodynamics. The degree to which these secondary microvascular and tissue-level effects fully or partially compensate for flow disruption in large vessels is fundamental to functional outcomes and recurrent stroke incidence. Furthermore, stroke patients typically present as a result of large artery events but also often have pre-existing microvascular dysfunction secondary to other stroke risk factors and aging. As such, both macrovascular and microvascular status may have relevance for establishing recurrent stroke risk, portending how patients recover post-stroke, and even for neurodegenerative disease progression more generally. This special issue features both review and original research from leading researchers in multiple cerebrovascular disease domains, including ischemic physiology in chronic and acute disease, as well as emerging work focusing on post-stroke cerebral plasticity mechanisms.
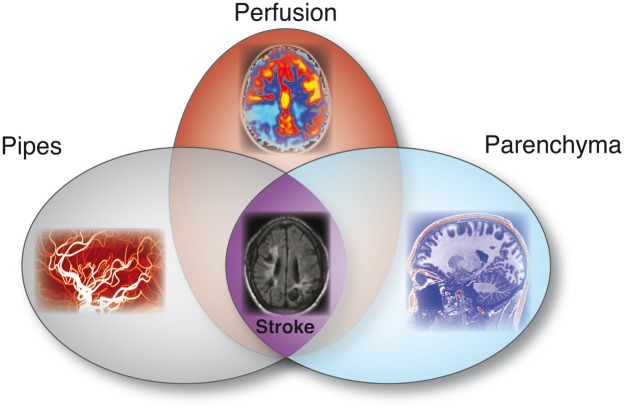
The overreaching theme for the issue is that cerebrovascular disease is complex and must be interpreted beyond the context of basic vascular steno-occlusion, and further, in the context of how such steno-occlusion impacts compensatory hemodynamic and metabolic behavior (Figure 1). Understanding these additional components will require improved imaging and analysis procedures aimed at more thoroughly characterizing the spectrum of brain signatures that predispose patients to good or poor outcomes. Emerging parameters that are discussed throughout the issue are cerebral blood flow (rate of blood delivery to tissue), cerebral blood volume (volume of blood/volume of brain), oxygen extraction fraction (oxygen consumed/oxygen delivered), cerebrovascular reserve (nearness of parenchyma to exhausting autoregulatory reserve capacity), and pH (e.g., tissue acidosis) in the context of genetic, angiographic, and modifiable risk factors. Furthermore, multiple aspects of cerebrovascular disease are considered, including adult atherosclerosis, pediatric stroke, non-atherosclerotic (e.g., moyamoya) steno-occlusive disease, sickle cell anemia, and post-stroke plasticity and rehabilitation. These topics were chosen due to both their fractional contribution to overall cerebrovascular disease burden as well as a need to refine diagnostic and treatment procedures in many of these conditions.
Figure 1.
A combined approach to fully characterize cerebrovascular disease likely involves accurate visualization of luminal steno-occlusion (e.g., pipes), how the brain tissue compensates for such steno-occlusion at the tissue level (e.g., perfusion and related hemodynamics), and whether such compensation mechanisms are adequate and lead to lesions or other abnormalities on anatomical imaging (e.g., parenchyma).
Multiple questions are also highlighted in light of recent clinical trial results. For instance, a major question in chronic disease stages may pertain to refining treatment options for patients with symptomatic atherosclerotic intracranial stenosis, in whom short-term recurrent stroke rates remain comparatively high at 14–20% even on standard-of-care medical management.2 Refining treatments in these patients or using more sensitive indicators of personalized risk profiles to triage patients at highest risk for recurrent stroke to aggressive interventions may be a topic of research over the next decade. For example, this could include (i) extracranial stenosis <70% and intracranial stenosis of the intracranial ICA, basilar, or first segment of the anterior cerebral artery, middle cerebral artery, or posterior cerebral artery >70%, (ii) evidence of white matter lesions or lacunar infarcts without cortical infarcts, and (iii) reduced cerebrovascular reserve in the flow territory of the stenotic vessel. Additionally, in non-atherosclerotic intracranial stenosis patients and specific patient groups in whom more aggressive treatment options such as direct and indirect surgical revascularizations are promising, hemo-metabolic neuroimaging may play a crucial role in the future for both triaging these patients for surgical revascularizations and also monitoring the parenchymal response to such interventions.3
In acute stroke, a recent significant finding has been the efficacy of thrombectomy4–8 and the utility of this approach well outside of a typical thrombolysis therapy window of 4.5 h. Work over the next decade will likely focus on identifying which patients will benefit from thrombectomy and personalizing the treatment window based on enduring tissue viability. One logical avenue worth investigating would focus on quantifying the presence or absence of collateral flow at the time treatment is considered, rather than simply the time since symptom onset. Addressing this question could require fast and quantitative neuroimaging and angiography to discern personalized risk/benefit profiles in terms of collateralization extent and ischemic tissue metabolism as a function of time after symptom onset.
In recent years, a series of guidelines, recommendations, and white papers have been published with the overall aim of standardized assessment of the brain vasculature, perfusion, and parenchyma.9–14 Still, this standardization is most often performed for clinical research or cohort studies where standardization is more easily performed relative to daily clinical practice. In the review articles presented, the authors describe steps that can be taken to bridge the gap between research tools and applying these tools in routine clinical practice. Both in research and clinical practice, the combined assessment of all aspects of cerebrovascular disease burden is a challenge that can be addressed. Most of the guidelines, recommendations, and white papers address a single aspect of cerebrovascular disease such as the measurements of small vessel disease,11 brain perfusion,14 or vessel wall pathology.15 The combined assessment of cerebrovascular disease burden should integrate the findings at the level of the brain vasculature, brain hemodynamics, and brain parenchyma. One could imagine that by building on the studies and reviews in this issue, the next step could be a practical severity score that combines all three aspects and could function as an Apgar score for the multi-level burden of cerebrovascular disease.
Biomarkers of cerebrovascular disease that facilitate personalized diagnostic and therapeutic decisions in patients with different causes, types and severity of cerebrovascular disease will be a main focus over the next decade. A combination of innovative efforts will be required where both new biomarkers of cerebrovascular disease are proposed together with the standardization and the translation to daily clinical practice of existing biomarkers. These biomarkers may be the basis for a better selection and stratification of patients in cohorts that benefit most from specific treatments. This will of course take time and will likely require large amounts of data and stroke researchers working synergistically between sites and trials. As a comparison, in acute stroke patients, it took three decades before computed tomography angiography became the standard of care to select stroke patients for thrombectomy based on the presence of an arterial occlusion. This example illustrates that both biomarkers and effective treatment options are needed to fulfill the promise of personalized medicine in chronic cerebrovascular disease.
Finally, few patients surviving overt stroke regain pre-stroke functional levels. Therefore, comprehensive networks of care are in place to help patients following the devastating functional consequences of stroke. Despite this, extremely limited information is available on how to select patients for post-stroke plasticity-inducing therapies. Furthermore, whether these therapies—either physical therapy, pharmacological, or neuro-stimulatory—can be applied to promote or accelerate cerebral plasticity remains controversial. Work related to this final often less-well investigated area of stroke research is also synopsized in the issue.
Stroke is often referred to as a cerebrovascular accident, but is associated with many well-defined lifestyle and vascular risk factors. As a result, the majority of strokes might be preventable with appropriate risk factor management and surveillance of cerebrovascular health. Neuroimaging is a critical component of theranostic care in most stages of cerebrovascular disease: from identification of stroke risk factors to stratifying acute and chronic stroke patients to the most effective treatments. In this issue, we outline current and emerging methods for understanding cerebrovascular disease in the settings of (i) chronic, (ii) acute, and (iii) post-stroke recovery stages and highlight major research directions in a consensus statement from contributing authors.16 The overall goal is to highlight advances in pathophysiological characterization of tissue in each of these stages of cerebrovascular disease and to identify unmet clinical needs and relevant future directions.
Acknowledgements
This article is intended as an introduction to the Journal of Cerebral Blood Flow and Metabolism (JCBFM) special issue on cerebrovascular disease.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Jeroen Hendrikse has received funding from the European Research Council under the European Union’s Horizon 2020 Programme (H2020) / ERC grant agreement n°637024 (HEARTOFSTROKE) and H2020 grant agreement No 666881, SVDs@target. Jeroen Hendrikse is supported by the Netherlands Organization for Scientific Research (NWO) under grant n°91712322.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Feigin VL. Stroke epidemiology in the developing world. Lancet 2005; 365: 2160–1. [DOI] [PubMed] [Google Scholar]
- 2.Derdeyn CP, Chimowitz MI, Lynn MJ, et al. Aggressive medical treatment with or without stenting in high-risk patients with intracranial artery stenosis (SAMMPRIS): the final results of a randomised trial. Lancet 2014; 383: 333–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scott RM, Smith ER. Moyamoya disease and moyamoya syndrome. N Engl J Med 2009; 360: 1226–1237. [DOI] [PubMed] [Google Scholar]
- 4.Berkhemer OA, Fransen PS, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 2015; 372: 11–20. [DOI] [PubMed] [Google Scholar]
- 5.Jovin TG, Chamorro A, Cobo E, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med 2015; 372: 2296–2306. [DOI] [PubMed] [Google Scholar]
- 6.Campbell BC, Mitchell PJ, Kleinig TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 2015; 372: 1009–1018. [DOI] [PubMed] [Google Scholar]
- 7.Saver JL, Goyal M, Bonafe A, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med 2015; 372: 2285–2295. [DOI] [PubMed] [Google Scholar]
- 8.Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 2015; 372: 1019–1030. [DOI] [PubMed] [Google Scholar]
- 9.Rosenberg GA, Wallin A, Wardlaw JM, et al. Consensus statement for diagnosis of subcortical small vessel disease. J Cereb Blood Flow Metab 2016; 36: 6–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Guio F, Jouvent E, Biessels GJ, et al. Reproducibility and variability of quantitative magnetic resonance imaging markers in cerebral small vessel disease. J Cereb Blood Flow Metab 2016; 36: 1319–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 2013; 12: 822–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wintermark M, Albers GW, Broderick JP, et al. Acute Stroke Imaging Research Roadmap II. Stroke 2013; 44: 2628–2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Warach SJ, Luby M, Albers GW, et al. Acute Stroke Imaging Research Roadmap III imaging selection and outcomes in acute stroke reperfusion clinical trials: consensus recommendations and further research priorities. Stroke 2016; 47: 1389–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alsop DC, Detre JA, Golay X, et al. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: a consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson Med 2015; 73: 102–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mandell DM, Mossa-Basha M, Qiao Y, et al. Intracranial vessel Wall MRI: principles and expert consensus recommendations of the American society of neuroradiology. AJNR Am J Neuroradiol 2017; 38: 218–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Donahue MJ, Achten E, Cogswell PM, et al. Consensus statement on current and emerging methods for the diagnosis and evaluation of cerebrovascular disease. J Cereb Blood Flow Metab. Epub ahead of print 1 January 2017. DOI: 10.1177/0271678X17721830. [DOI] [PMC free article] [PubMed]