Keywords: nerve regeneration, aminooxyacetic acid, chronic alcoholism rat model, hydrogen sulfide, Ca2+ overload, brain injury, learning and memory abilities, Morris water maze, myelin basic protein, mitochondria, ATP enzyme activity, neural regeneration
Abstract
Chronic alcoholism seriously damages the central nervous system and leads to impaired learning and memory. Cell damage in chronic alcoholism is strongly associated with elevated levels of hydrogen sulfide (H2S) and calcium ion overload. Aminooxyacetic acid is a cystathionine-β-synthase activity inhibitor that can reduce H2S formation in the brain. This study sought to observe the effect of aminooxyacetic acid on learning and memory in a chronic alcoholism rat model. Rats were randomly divided into three groups. Rats in the control group were given pure water for 28 days. Rats in the model group were given 6% alcohol for 28 days to establish an alcoholism rat model. Rats in the aminooxyacetic acid remedy group were also given 6% alcohol for 28 days and were also intraperitoneally injected daily with aminooxyacetic acid (5 mg/kg) from day 15 to day 28. Learning and memory was tested using the Morris water maze test. The ultrastructure of mitochondria in the hippocampus was observed by electron microscopy. H2S levels in the hippocampus were measured indirectly by spectrophotometry, and ATPase activity was measured using a commercial kit. The expression of myelin basic protein was determined by immunohistochemistry and western blotting. Compared with the control group, latency and swimming distance were prolonged in the navigation test on days 2, 3, and 4 in the model group. In the spatial probe test on day 5, the number of platform crosses was reduced in the model group. Cristae cracks, swelling or deformation of mitochondria appeared in the hippocampus, the hippocampal H2S level was increased, the mitochondrial ATPase activity was decreased, and the expression of myelin basic protein in the hippocampus was down-regulated in the model group compared with the control group. All the above indexes were ameliorated in the aminooxyacetic acid remedy group compared with the model group. These findings indicate that aminooxyacetic acid can improve learning and memory in a chronic alcoholism rat model, which may be associated with reduction of hippocampal H2S level and mitochondrial ATPase activity, and up-regulation of myelin basic protein levels in the hippocampus.
Introduction
Chronic alcoholism caused by prolonged overdrinking results in central nervous system damage. Chronic alcoholism is a progressive, potentially fatal disease, characterized by a strong desire for alcohol, an increase in tolerance, increased dependence, and non-control (Nam et al., 2012; Du et al., 2014). Long-term heavy drinking causes damage to the central nervous system and peripheral nerves (Li et al., 2005). The neurotoxicity of ethanol has been extensively studied, but its mechanism of action remains unclear. Decreased activity of cytochrome oxidase causes increased neuronal adenosine triphosphate (ATP) metabolism and calcium overload (Yang et al., 2011). Ethanol produces oxygen free radicals by the action of alcohol dehydrogenase and aldehyde dehydrogenase that damage neuronal DNA. N-methyl-D-aspartic acid (NMDA) and/or γ-aminobutyric acid (GAGB) mediate alcohol-induced neuronal apoptosis (Sandra et al., 2001). Aminooxyacetic acid (AOAA) can inhibit the expression of cystathionine-beta-synthase, thereby reducing the formation of H2S, inhibiting Ca2+ influx, and alleviating intracellular Ca2+ overload (Li et al., 2009). However, there is currently no study addressing AOAA treatment for chronic alcoholism. We speculate that AOAA can relieve nerve cell damage caused by chronic alcoholism and can improve central nervous system function by reducing the formation of H2S and alleviating intracellular Ca2+ overload.
Myelin sheath is a single layer fat-protein tissue that envelops the axons of neurons. Myelination injury or shedding has an adverse effect on neuronal function (Sandell and Peters, 2003; Marina et al., 2008). Previous studies have shown cognitive and memory function defects related to changes in the myelination of nerve fibers (Luo et al., 2001; Long et al., 2005). In the central nervous system, myelin basic protein (MBP), accounts for 30–40% of myelin in the myelin sheath. MBP is an extrinsic protein localized exclusively at the cytoplasmic surface in the major dense line of the myelin sheath (Xie al., 2013). It is synthesized and secreted by oligodendrocytes (Li, 2007). MBP can promote axonal regeneration (Xu et al., 2005) and can also combine with calcium to inhibit calcium overload caused by free radicals during membrane lipid degradation (Li et al., 2006). A decrease in the level of MBP can inhibit myelin formation and affect the normal conduction function of the myelin sheath (Zhang et al., 2017). The hippocampus is strongly associated with advanced neural activities, such as learning and memory and emotions. There is a clear synaptic pathway within the hippocampus and the integrity of the hippocampal formation is important for learning and memory (Zao et al., 2015). In this study, we investigated a rat model of chronic alcoholism and observed the effect of AOAA treatment on changes in H2S levels, mitochondrial structure and MBP levels in the hippocampus. We also tested the learning and memory capabilities of the model rats.
Materials and Methods
Animals
Sixty 2-month-old, male, Sprague-Dawley rats weighing 120–160 g were provided by the Animal Management Center of Zhengzhou University of China [SCXK (Yu) 2015-0004]. The experiment was approved by the Animal Ethics Committee of Xinxiang Medical College of China (No. 4107000081551).
The rats were housed at 22–25°C for 5–7 days to acclimatize to the laboratory environment. The rats were randomly divided into a control group, an AOAA remedy group, and a model group (chronic alcoholism model) (n = 20 per group).
Model establishment and drug administration
The control group was given free access to pure water every day, the water being changed every morning at 9:00 a.m. To establish a model of chronic alcoholism (Rong et al., 2008), the model and AOAA remedy groups were given free access to 6% (v/v) alcohol solution for 28 days, with the alcohol solution being changed at 9:00 a.m. every day. From day 15 to day 28, rats in the AOAA remedy group were intraperitoneally injected once a day with 5 mg/kg AOAA (Geel, Antwerp, Belgium) dissolved in 1 mL of saline (Tang et al., 2015). The control and model groups were intraperitoneally injected with 1 mL saline once a day from day 15 to day 28. After establishment of the model, the water maze test was performed to verify whether the model was successful. A significant difference in learning and memory abilities between the model and control groups indicates the model has been successfully established.
Evaluation of learning and memory abilities
On the 28th day after the start of model establishment, each group was tested in the Morris water maze test. This labyrinth system is widely used with rats in basic and applied neurobiology (Hu et al., 2000). The Morris water maze apparatus (Noldus, Wageningen, Gelderland, Netherlands) consisted of a cylindrical pool, a movable platform and a drainage system (Yu et al., 2015). The pool was circular with a black wall and bottom. The pool was surrounded by blue curtains to block visual references in the room. References of various shapes and colors can be fixed to the inner wall of the pool or other visible high points (Yan et al., 2004). During training, a platform was submerged 0.5 cm below the surface in a fixed position within a quadrant. The pool was divided into four quadrants: southwest, southeast, northwest and northeast. The submerged platform was placed in the center of the southeast quadrant. On the first day of the test, the platform was removed as a pre-experiment for the rats to become acclimatized to the environment and to identify and remove rats with poor swimming skills. Eight rats from each group were randomly selected for the navigation test and the spatial probe test. The positioning navigation test was performed on four consecutive days. The starting quadrant was changed each day in an anti-clockwise manner. Each rat was trained four times (in a different quadrant each time) every day. The swimming trajectory of rats was recorded with a camera and analyzed using behavioral record software, EthoVision XT 8 (Noldus). Each time the rat found the submerged platform, the rat was allowed to stay on the platform for 30 seconds to consolidate its memory. The maximum duration for each training was 60 seconds. If the submerged platform was not found within 60 seconds, the rat was directed to the platform and allowed to stay on the platform for 30 seconds. In the navigation test, the latency period to find the underwater platform and swimming distance of each rat were recorded. A shorter delay time and trajectory length indicated stronger learning ability. The spatial probe test was performed following the navigation test. The spatial probe test was conducted only once to measure the ability of rats to retain the memory of the platform position. The difference between this experiment and the previous one was that the platform was removed. The rats were then released from the center of the northwest quadrant, which was opposite the target quadrant, and the swimming time was 180 seconds. In the spatial probe test, the more times the rat crossed the platform position and the longer the swimming distance within the platform quadrant, the stronger the learning ability. After the end of the Morris water maze test, the rats were euthanized. Four rats in each group were subjected to immunohistochemical staining and a total of eight hippocampi were analyzed. The left and right hippocampi of the remaining four rats were cryopreserved. H2S levels, ATPase activity, and MBP protein levels in the eight hippocampi were measured, and the mitochondria were observed.
H2S levels in the hippocampus measured by spectrophotometry
Twelve hours after euthanasia, H2S absorbance in hippocampal tissue was measured using an ELx800 automatic microplate reader (Bio-Tek, Venuschi, VT, USA) at the wavelength of 670 nm. H2S levels in the hippocampus were measured according to an H2S standard curve and expressed in terms of H2S level per unit weight of tissue (nmol/g) (Ou et al., 2007). The levels of H2S in the eight hippocampi were measured.
Morphology of mitochondria observed by electron microscopy
Twelve hours after euthanasia, 1 cubic millimeter pieces of hippocampus were prepared. The pieces were fixed with 4% glutaraldehyde for 1 minute, and then stored at 4°C for 3 hours. The samples were then washed with PBS and immersed in 1% citric acid for approximately 1 hour. After washes with PBS, these samples were dehydrated with acetone and embedded in epoxy resin. The samples were then sliced into ultrathin sections. The sections were stained with citrate and uranyl acetate and observed by transmission electron microscopy (Hitachi, Tokyo, Japan). The morphology of mitochondria in neurons of the hippocampus was compared among groups (with assistance from the Electron Microscopy Department of Xinxiang Medical College of China).
Determination of mitochondrial ATPase activity
Twelve hours after euthanasia, according to the instructions of the Ultramicro Total ATP Enzyme Assay Kit (Nanjing Institute of Bioengineering, Nanjing, China), samples were pretreated, then tissues were accurately weighed, and physiological saline added to give a weight (g):volume (mL) ratio of 1:9. Samples were mechanically homogenized under ice-water bath conditions and centrifuged at 2500 × g for 10 minutes. The supernatant was taken to measure mitochondrial ATPase activity. The supernatant was taken for enzymatic reaction, and centrifuged at 3500 × g for 10 minutes, and then treated with the fixed phosphorus reagent. The optical density (OD) values were measured using a spectrophotometer at 636 nm. The ATPase activity was calculated by (measured OD value − control OD value)/(standard OD value − blank OD value) × standard concentration × 6 × 7.8 ÷ sample protein concentration.
MBP immunohistochemistry
The brains of four rats from each group were fixed with 4% paraformaldehyde for 12 hours, paraffin embedded and sectioned. Using an SP Kit for immunohistochemistry (Bioss, Beijing, China), sections were dewaxed and rehydrated, and 0.01 mol/L citrate solution was used for antigen retrieval. The slices were washed in PBS for 5 minutes three times, incubated in 5% H2O2 solution at 37°C for 20 minutes (SP Kit; Bioss Inc.), washed in PBS for 5 minutes three times, and blocked with normal goat serum working fluid at 37°C for 20 minutes. A rabbit anti-MBP polyclonal antibody (Bioss Inc.) was added, incubated at 37°C for 20 minutes and then rinsed with PBS for 5 minutes three times. Biotin-labeled secondary antibody working fluid was added and incubated at 37°C for 60 minutes. Sections were then washed with PBS for 5 minutes three times. Horseradish peroxidase-labeled streptavidin working fluid was added and incubated at 37°C for 50 minutes. Staining was developed with diaminobenzidine at 37°C for 5 minutes and observed and photographed under a light microscope. Image-pro-plus 6.0 image processing software was used to analyze the positive expression of MBP. The value of the integrated OD (mean density) of the effective target protein divided by the effective target distribution area is the mean density value.
MBP western blot assay
Three days after euthanasia, the hippocampus was added in tissue lysate (1 mg: 10 μL, Beyotime Biotechnology Co., Ltd., Shanghai, China) containing protease inhibitor (50:1; Beyotime Biotechnology Co., Ltd.) and incubated for 30 minutes and then centrifuged to extract whole protein. Protein concentration was measured using a bicinchoninic acid protein quantification kit (Beyotime Biotechnology Co., Shanghai, China). The extracted protein was diluted to give the same concentration in all samples and incubated at 100°C in a water bath for 10 minutes, followed by storage at −80°C. Sodium dodecyl sulfate polyacrylamide gel electrophoresis was performed on 40 μg of protein at 120 V for 90 minutes (BioRad, Hercules, CA, USA). Proteins were then transferred onto polyvinylidene fluoride membrane at 100 V for 90 minutes (BioRad). The membrane was then incubated with a rabbit anti-MBP polyclonal antibody (1:500; Bioss) at 4°C overnight. The blot was then rinsed with Tris-buffered saline containing Tween 20 (1:1000), incubated with goat anti-rabbit IgG (1:1000; Beyotime Biotechnology Co.) and then rinsed again in Tris-buffered saline with Tween 20. Strip analysis was then performed using an imaging system (UVP, Upland, CA, USA). The value of the integrated optical density of the target protein divided by the integrated optical density of the internal reference protein gave the relative value of the target protein.
Statistical analysis
Data are expressed as the mean ± SEM and were analyzed using SPSS 13.0 software (SPSS, Chicago, IL, USA). The comparison between groups was performed by one-way analysis of variance followed by the least significant difference post hoc test. A value of P < 0.05 was considered statistically significant.
Results
Learning and memory in each group
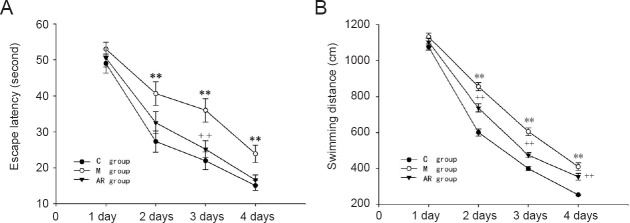
As shown in Figure 1, the learning and memory of the the model group was significantly worse than that of the control group, showing longer latency periods and swimming distances. There was a significant difference between the groups on day 2 and day 4 (P < 0.01). The latency periods and swimming distances in the AOAA remedy group were significantly decreased compared with the model group, especially on day 2 and day 4 (P < 0.01).
Figure 1.
Morris water maze navigation test.
(A) Latency to the platform for the three groups; (B) swimming distance to the platform for three groups. The data are presented as the mean ± SEM. Comparisons between groups were conducted by one-way analysis of variance followed by the least significant difference post hoc test. **P < 0.01, vs. C group; ++P < 0.01, vs. M group. C group: Control group; M group: model group; AR group: AOAA remedy group (chronic alcoholism model + aminooxyacetic acid remedy). AOAA: Aminooxyacetic acid.
Figure 2 shows the representative exploratory trajectories of the rats on day 5. Compared with the control group, the trajectories of the rats in the model group were remarkably more evenly distributed in each quadrant with a decreased number of platform crossings. The trajectories of the rats in the AOAA remedy group were significantly concentrated in the quadrant of the platform with an increased number of platform crossings compared with the model group.
Figure 2.
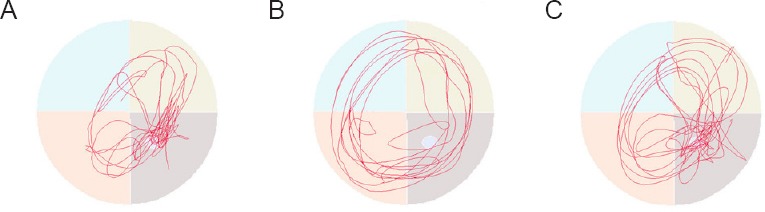
Representative trajectories of rats in the Morris water maze spatial probe test on day 5.
(A–C) Representative trajectories of rats in the control (A), model (B)and AOAA remedy groups (C) (chronic alcoholism model + AOAA remedy), respectively. The more trajectories in the target quadrant (lower left quadrant), the better the memory of the rat; the more platform crossings (circle in the lower left quadrant), the better the memory of the rat. The trajectories of rats in the model group were significantly more evenly distributed in each quadrant compared with the control group, and the number of platform crossings was reduced. The trajectories of the rats in the control and the AOAA remedy groups were significantly concentrated in the platform quadrant and many crossed the platform. AOAA: Aminooxyacetic acid.
H2S levels in the hippocampus
As shown in Figure 3, compared with the control group, H2S levels in the hippocampus of rats of the model group were significantly increased (P < 0.01). H2S levels were significantly decreased in the hippocampus in the AOAA remedy group compared with the model group (P < 0.01).
Figure 3.
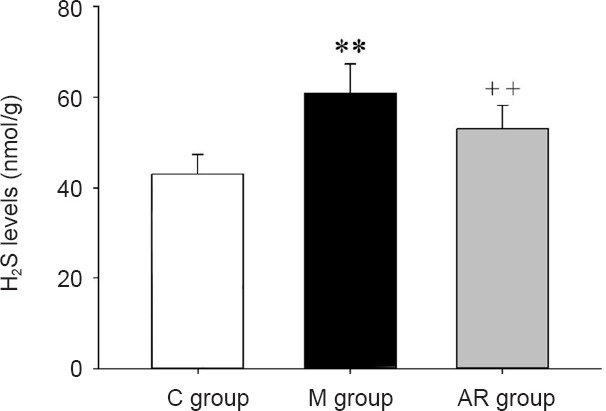
H2S levels in the hippocampus.
The data are presented as the mean ± SD (n = 8). Comparisons between groups were conducted by one-way analysis of variance followed by the least significant difference post hoc test. **P < 0.01, vs. C group; ++P < 0.01, vs. M group. C group: Control group; AR group: AOAA remedy group (chronic alcoholism model + aminooxyacetic acid remedy); M group: model group. AOAA: Aminooxyacetic acid.
Morphology of mitochondria in the hippocampus observed by electron microscopy
The morphology of mitochondria in the hippocampus of each group was observed by electron microscopy. As shown in Figure 4, mitochondria indicated by black arrows were intact. There was no cavitation, cristae breakage, or swelling. The mitochondria indicated with white arrows show vacuolization, and broken and deformed cristae. The mitochondria in the control group were intact and there were no vacuoles, cristae breaks, or swelling. The mitochondria in the model group showed cristae breakage, swelling and deformation. Mitochondrial morphology was improved after AOAA treatment.
Figure 4.
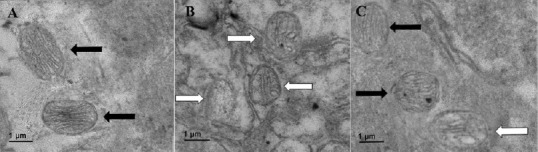
Ultrastructure of hippocampal mitochondria.
(A–C) Ultrastructure of mitochondria in the control (A), model (B) and AOAA remedy groups (C), respectively (scanning electron microscopy, original magnification, 10,000 ×). Black arrows indicate intact mitochondria without vacuoles, cristae cracks, swelling or deformation. White arrows indicate damaged mitochondria. They contain vacuoles and the cristae are broken and deformed. The mitochondria in the control group were intact without vacuoles, cristae cracks or swelling. The mitochondria in the model group showed vacuoles, cristae cracks and swelling. Mitochondrial ultrastructure was improved after AOAA treatment. AOAA: Aminooxyacetic acid.
Mitochondrial ATPase activity
As shown in Figure 5, mitochondrial ATPase activity in the hippocampus of the model group was significantly decreased compared with that of the control group (P < 0.01). The activity of mitochondrial ATPase in the AOAA remedy group was significantly higher compared with that in the model group (P < 0.01).
Figure 5.
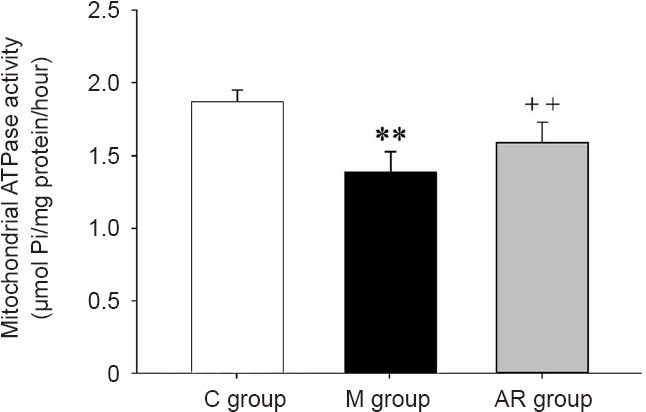
Mitochondrial ATPase activity in the hippocampus.
The data are presented as the mean ± SD (n = 8). Comparisons between groups were conducted by a one-way analysis of variance followed by the least significant difference post hoc test. **P < 0.01, vs. C group; ++P < 0.01, vs. M group. C group: Control group; M group: model group; AR group: AOAA remedy group (chronic alcoholism model + aminooxyacetic acid remedy). AOAA: Aminooxyacetic acid.
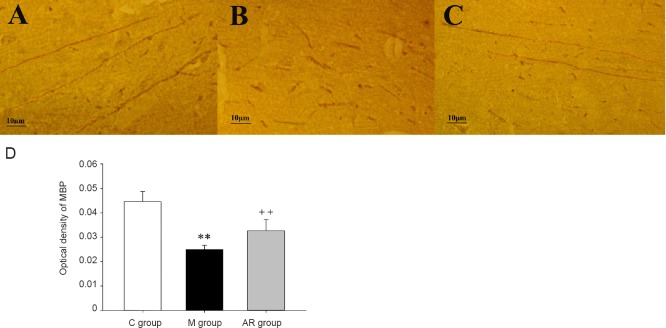
MBP expression in the hippocampus
MBP expression was observed by immunohistochemistry in the hippocampal CA1 region in each group (Figure 6A–C). The average OD shows the level of MBP in the hippocampal CA1 region. Compared with the control group, the MBP level was significantly decreased in the model group (P < 0.01). The MBP level was significantly increased in the AOAA remedy group compared with the model group (P < 0.01) (Figure 6D).
Figure 6.
Mean optical density of MBP in the hippocampus.
(A–C) Light microscopy images of MBP immunohistochemistry in the hippocampus of the control (A), model (B) and AOAA remedy groups (C), respectively. (D) Mean optical density of MBP in the hippocampus of each groups (C). The data are presented as the mean ± SD (n = 8). Comparisons between groups were conducted by a one-way analysis of variance followed by the least significant difference post hoc test. **P < 0.01, vs. C group; ++P < 0.01, vs. M group. C group: Control group; M group: model group; AR group: AOAA remedy group (chronic alcoholism model + aminooxyacetic acid remedy); MBP: myelin basic protein. AOAA: Aminooxyacetic acid.
Western blot assay of MBP is shown in Figure 7. MBP levels were significantly decreased in the hippocampus of the model group compared with the control group (P < 0.01). MBP levels were significantly increased in the AOAA remedy group compared with the model group (P < 0.01).
Figure 7.
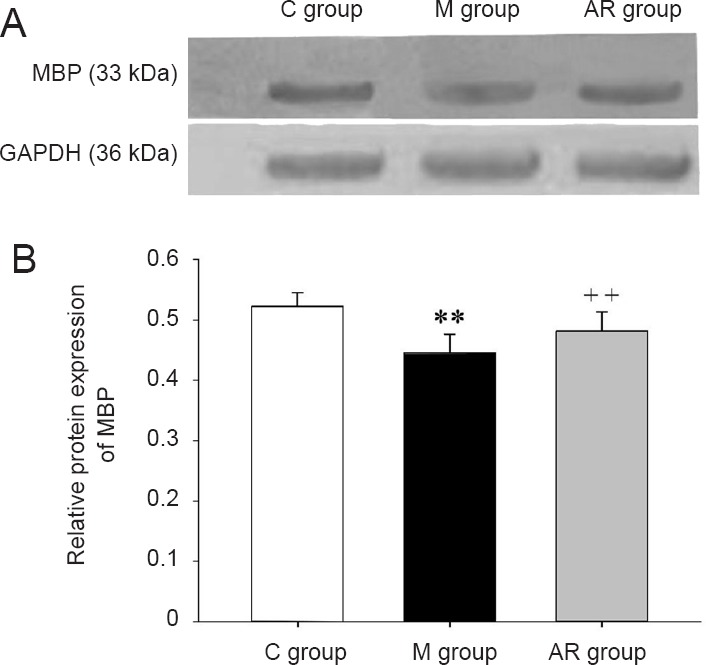
MBP levels in the hippocampus (western blot assay).
(A) Protein bands of MBP and GAPDH in the hippocampus of each group. (B) The levels of MBP in the hippocampus of each group. Data are presented as the mean ± SD (n = 8). Comparisons between groups were conducted by a one-way analysis of variance followed by the least significant difference post hoc test. **P < 0.01, vs. C group; ++P < 0.01, vs. M group. C group: Control group; M group: model group; AR group: AOAA remedy group (chronic alcoholism model + aminooxyacetic acid remedy); MBP: myelin basic protein. AOAA: Aminooxyacetic acid.
Discussion
Chronic alcoholism seriously damages the central nervous system and leads to impaired learning, memory, and cognitive judgment (Li et al., 2016; Le et al., 2017). The mechanism of nerve damage by alcohol has been investigated by different studies. Decreased activity of cytochrome oxidase causes reduced neuronal ATP metabolism and calcium overload. Ethanol produces oxygen free radicals under the action of alcohol dehydrogenase and aldehyde dehydrogenase, which injure neuronal DNA. Ethanol-induced neuronal apoptosis is mediated by NMDA and/or GAGB, and serotonin receptors (Sandra et al., 2001). AOAA can inhibit the expression of cystathionine-beta-synthase, thereby reducing the formation of H2S, inhibiting Ca2+ influx and alleviating intracellular Ca2+ overload, which relieves cell damage (Li et al., 2009). However, no study has addressed AOAA treatment for chronic alcoholism. The results of this study indicate that AOAA can alleviate the impaired learning and memory caused by chronic alcoholism.
Along with CO and NO, H2S is a gaseous signaling molecule (Li et al., 2016; Le et al., 2017). In the brain, cystathionine-beta-synthase catalyzes the formation of H2S (Zhao et al., 2001; Cui et al., 2016) from cysteine. Physiological concentrations of H2S can help blood vessel dilation and the anti-oxidative response (Jung et al., 2014; Huang et al., 2017) to maintain learning and memory abilities (Li et al., 2015; Tian et al., 2016). High concentrations of H2S can, however, impair organs (Jiang et al., 2016). It inhibits central nervous system function, and specifically inhibits the excitatory postsynaptic membrane potential and blocks synaptic transmission. It also enhances NMDA receptor-mediated calcium overload, which leads to increased intracellular calcium concentration and damage to mitochondrial morphology and energy metabolism, eventually resulting in cell necrosis. Chronic alcoholism can activate cystathionine-beta-synthase, and catalyze the formation of H2S (Zao et al., 2001). AOAA is a cystathionine-beta-synthase inhibitor (Sandell and Peters, 2003; Niu et al., 2018), and can reduce H2S formation in the brain by inhibiting cystathionine-beta-synthase activity (Donovan et al., 2017).
ATPase can catalyze the oxidation of ADP to ATP, which plays an important role in material transport and information transfer (King et al., 2016). The activity of ATPase can change with the onset of diseases (Li et al., 2000). By detecting the activity of mitochondrial ATPase in the hippocampus of each group, we found that compared with the control group, ATPase activity was remarkably decreased in the model group. Compared with the model group, ATPase activity was dramatically increased in the AOAA remedy group.
MBP is the main protein of central nervous system myelin and can promote axonal regeneration (Luo et al., 2001; Zhou et al., 2017). In addition, MBP can also inhibit the calcium-induced degradation effect of free radicals on membrane lipids (Xu et al., 2005). Sandell and Peters (2003) found that cognitive and memory function defects are associated with structural changes of myelinated myelin sheath in the central nervous system. High levels of H2S can have effects on the central nervous system. Accumulation of ethanol can cause oligodendrocyte damage (Hu et al., 2016) and reduce MBP levels, causing the myelin sheath to come off axons, leading to impairment of information transmission among synapses (Bai et al., 2017).
In summary, AOAA can ameliorate the effects of chronic alcoholism on brain tissue, which might be achieved by reducing H2S levels. Thus, AOAA protects mitochondrial function, reduces alcohol-induced MBP damage, and protects the structure and function of neurons, thereby improving the learning and memory of chronic alcoholism model rats. However, in this study, we only focused on the learning and memory capabilities of rats, and H2S levels, mitochondrial ATPase activity and MBP levels in the hippocampus. Therefore, further investigations are needed to support AOAA treatment for chronic alcoholism.
Additional file:Open peer review report 1 (122.1KB, pdf) .
Footnotes
Conflicts of interest: The authors declare that there are no conflicts of interest associated with this manuscript.
Financial support: This study was supported by the National Natural Science Foundation of China (to YMX), No. 81530037, 81471158; a grant from the Department of Education of Henan Province of China (to ALD), No. 15A310006. The funding sources had no role in study conception and design, data analysis or interpretation, paper writing or deciding to submit this paper for publication.
Institutional review board statement: All experimental procedures and protocols were approved by the Animal Ethics Committee of Xinxiang Medical College of China (approval No. 4107000081551). All experimental procedures described here were in accordance with the National Institutes of Health (NIH) guidelines for the Care and Use of Laboratory Animals.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Rong Xie, Fudan University Huashan Hospital, China.
Funding: This study was supported by the National Natural Science Foundation of China (to YMX), No. 81530037, 81471158; a grant from the Department of Education of Henan Province of China (to ALD), No. 15A310006.
(Copyedited by Wang J, Li CH, Qiu Y, Song LP, Zhao M)
References
- Bai RX, Hou XJ, Bi XY. Clinical and imaging analysis of chronic alcoholic demyelinating encephalopathy. Linchuang Neike Zazhi. 2017;34:191–194. [Google Scholar]
- Cui Y, Duan X, Li H, Dang B, Yin J, Wang Y, Gao A, Yu Z, Chen G. Hydrogen sulfide ameliorates early brain injury following subarachnoid hemorrhage in rats. Mol Neurobiol. 2016;53:3646–3657. doi: 10.1007/s12035-015-9304-1. [DOI] [PubMed] [Google Scholar]
- Donovan J, Wong PS, Roberts RE, Garle MJ, Alexander SPH, Dunn WR, Ralevic V. A critical role for cystathionine-β-synthase in hydrogen sulfide-mediated hypoxic relaxation of the coronary artery. Vasc Pharmacol. 2017;93-95:20–32. doi: 10.1016/j.vph.2017.05.004. [DOI] [PubMed] [Google Scholar]
- Du A, Jiang H, Xu L, An N, Liu H, Li Y, Zhang R. Damage of hippocampal neurons in rats with chronic alcoholism. Neural Rege Res. 2014;9:1610. doi: 10.4103/1673-5374.141787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu F, Dai YJ, Hou DR. Establishment of brain injury model in mouse by intracranial injection of anhydrous ethanol. Shiyan Dongwu yu Bijiao Yixue. 2016;36:428–432. [Google Scholar]
- Huang YQ, Tang CS, Du JB, Jin HF. Cardiovascular regulation by sulfur-containing gaseous signaling molecules and the underlying mechanisms: updated research evidence. Prog Physiol Sci. 2017;48:4–11. [PubMed] [Google Scholar]
- Jiang L, Zhang P, Luo Y, Li WT, Bi L, Zou W. Protective effects of hydrogen sulfide on chronic stress-induced hippocampal neural injury in rats and its mechanisms. Zhongguo Xingwei Kexue yu Naokexue Zazhi. 2016;25:19–23. [Google Scholar]
- Jing Q, H U, Wen Z, Lai S. A preliminary study on the memory attribution and methodology in Morris water maze test. Guangzhou Zhongyiyao Daxue Xuebao. 2000;2:117–119. [Google Scholar]
- Jung J, Jeong NY. Hydrogen sulfide controls peripheral nerve degeneration and regeneration: a novel therapeutic strategy for peripheral demyelinating disorders or nerve degenerative diseases. Neural Rege Res. 2014;9:2119–2121. doi: 10.4103/1673-5374.147940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura H. Hydrogen polysulfide (h2sn) signaling along with hydrogen sulfide (H2S) and nitric oxide (No) J Neural Transm (Vienna) 2016;123:1235. doi: 10.1007/s00702-016-1600-z. [DOI] [PubMed] [Google Scholar]
- King MS, Kerr M, Crichton PG, Springett R, Kunji ERS. Formation of a cytoplasmic salt bridge network in the matrix state is a fundamental step in the transport mechanism of the mitochondrial adp/atp carrier. Biochim Biophys Acta. 2016;1857:14–22. doi: 10.1016/j.bbabio.2015.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le BA, Fama R, Sullivan EV. Executive functions, memory, and social cognitive deficits and recovery in chronic alcoholism: a critical review to inform future research. Alcohol Clin Exp Res. 2017:1432–1443. doi: 10.1111/acer.13431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Lang SY, Zhang Y, Neurology DO. Brain physiological function in patients with chronic alcoholism peripheral neuropathy. Zhongguo Jiankang Xinlixue Zazhi. 2016;24:1287–1291. [Google Scholar]
- Li C, Yang S, Zhang W, Lu W, Nyengaard JR, Morrison JH, Tang Y. Demyelination induces the decline of the myelinated fiber length in aged white matter. Anat Rec. 2009;292:528–535. doi: 10.1002/ar.20884. [DOI] [PubMed] [Google Scholar]
- Li J, Yuan XR, LI YH, Cui SZ, Zhou R. Establishment of rat alcohol dependent model. Zhongguo Yaowu Yilaixing Zazhi. 2006;15:433–436. [Google Scholar]
- Li XJ, Li CK, Wei LY, Lu N, Wang GH, Zhao HG, Li DL. Hydrogen sulfide intervention in focal cerebral ischemia/reperfusion injury in rats. Neural Regen Res. 2015;10:932–937. doi: 10.4103/1673-5374.158353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XM, Deng Y. Alcohol abuse in the injury of central nervous system. Zhongguo Linchuang Kangfu. 2005;9:181–183. [Google Scholar]
- Li Y, Xu F, Xu X. Effect of nitric oxide donor on activity of pancreatic ATPase after hepatic blood flow blocking. Linchang Mazuixue Zazhi. 2000;16:877–880. [Google Scholar]
- Li YJ. Detection of Chronic Alcoholics’s-100β Protein in Serumand Density of Their Myelin Basic Protein. Yanji, China: Yanbian University; 2007. [Google Scholar]
- Liu H. The Effect of Chronic Alcoholism on H2S/CBS Systems and Myelin in Hippocampus. Urumqi, China: Xinxiang Medical University; 2014. [Google Scholar]
- Long C, Li J. Brief review on degenerative disease of central nervous system. Jichu Yixue yu Linchuang. 2005;25:673–678. [Google Scholar]
- Luo WL, Lin DC, Li YM. Preliminary study of facial nerve regeneration in the chamber of myelin basic protein. Zhonghua Erbiyanhouke Zazhi. 2001;36:14–18. [PubMed] [Google Scholar]
- Nam HW, Mclver SR, Hinton DJ. Adenosine and glutamate signaling in neuron–glial interactions: implications in alcoholism and sleep disorders. Alcohol Clin Exp Res. 2012;36:1117–1125. doi: 10.1111/j.1530-0277.2011.01722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu W, Wang J, Qian J, Wang M, Wu P, Chen F, Yan S. Allosteric control of human cystathionine beta synthase activity by a redox active disulfide bond. J Biol Chem. 2018;293:2523–2533. doi: 10.1074/jbc.RA117.000103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu WD, Wu DS, Wei DP. The effect on proliferation of oligodendrocytes of rats induced by alcohol and metabolite acetaldehyde. Weisheng Dulixue Zazhi. 2000;14:35–37. [Google Scholar]
- Ren CL, Li DL, Zhao HG, Zhen WL, Wang SQ, Hou ZH, Yin XF. Dynamic changes of endogenous hydrogen sulfide during global cerebral ischemia-reperfusion in rats. Zhongxiyi Jiehe Xinnao xueguan Bing Zazhi. 2008;5:181. [Google Scholar]
- Rong M, Sheng SL, Zhao ZW. Effect of APP17 Peptide on Aβ related protein expression in APP transgenic mice. Zhongguo Yaolixue Tongbao. 2008;28:1045–1048. [Google Scholar]
- Sandell JH, Peters A. Disrupted myelin and axon loss in the anterior commissure of the aged rhesus monkey. Comp Neurol. 2003;466:14–30. doi: 10.1002/cne.10859. [DOI] [PubMed] [Google Scholar]
- Sandra MM, Michael WM. Effects of prenatal exposure to ethanol on the expression of bcl-2, bax and caspase-3 in the developing rat cerebral cortex and thalamus. Brain Res. 2001;911:71–81. doi: 10.1016/s0006-8993(01)02718-4. [DOI] [PubMed] [Google Scholar]
- Saresella M, Marventano, Guerini FR, Zanzottera M, Delbue S, Marchioni E, Maserati R, Longhi R, Ferrante P, Clerici M. Myelin basic protein-specific T lymphocytes proliferation and programmed cell death in demyelination diseases. Clin Immunol. 2008;129:509–517. doi: 10.1016/j.clim.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang WH. Suzhou, China: Soochow University; 2015. Hydrogen sulfide mediates chronic visceral pain induced by mediate chronic visceral pain induced by prenatal maternal stress in rat offspring (Dissertation) [Google Scholar]
- Tian M, Huang Y, Dong YJ, Xiao Y, Guan Z. Effect of exogenous H2S on H2S concentration and cystathionine β-synthasw expreaaion inhippocampus in a rat model of vascular dementia. Guoji Naoxueguanbing Zazhi. 2016;24:1091–1095. [Google Scholar]
- Wang Y, Li YD, Peng C, Cheng GH, Chen L, Lu W, Kong JM, Xiao L, Tang Y. Demyelination in the hippocampus and its correlation with altered behaviors of the cuprizone-induced mouse model of schizophrenia. Chongqing Yike Daxue Xuebao. 2014;39:129–136. [Google Scholar]
- Xie F, Zhang JC, Fu H, Chen J. Age-related decline of myelin proteins is highly correlated with activation of astrocytes and microglia in the rat cns. Int J Mol Med. 2013;32:1021–1028. doi: 10.3892/ijmm.2013.1486. [DOI] [PubMed] [Google Scholar]
- Xu XW. Research progress on alcoholic encephalopathy. Jinri Jiankang. 2017;5:31–32. [Google Scholar]
- Yan H, DU JB. Changes and significance of hydrogen sulfide/cystathionine γ-lyase system in hypertension: an experimental study with rats. Zhonghua Yixue Zazhi. 2004;84:1114–1117. [PubMed] [Google Scholar]
- Yang Q, Li XX. Advances in mitochondrial injury induced by complete cerebral ischemia-reperfusion. Yixue Zongshu. 2011;17:1619–1621. [Google Scholar]
- Yu QX. Study of learning and memory method based on moeeis water maze (Dissertation) Harbin, China: Harbin University of Commerce; 2015. [Google Scholar]
- Zhang XF, Jin H, Lin BB, Long LI, Song CM, LI ZF, Liang SX, Mao JJ, Liu WL, Tao J Chen LD. Learning and memory deficit and demyelination of corpus callosum in app/ps1 transgenic mice. Zhongguo Kangfu Lilun Yu Shijian. 2017;23:1027–1031. [Google Scholar]
- Zhao LL. MiR-101 in rat hippocampus regulates cognition via Nrf2-ARE pathway after traumatic brain injury. Wuhan, China: Huazhong University of Science and Technology; 2015. [Google Scholar]
- Zhao W, Zhang J, Lu Y, Wang R. The vasorelaxant effect of H(2)S as a novel endogenous gaseous K (ATP) channel opener. EMBO Journal. EMBO J. 2001;20:6008–6016. doi: 10.1093/emboj/20.21.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou XH, Feng SQ, Liu S, Hao Y, Wei ZJ, Lin W, Fan BY, Ren YM, Shi GD, Chen JT. Activated Schwann cells combined with umbilical cord blood mesenchymal stem cell transplantation for repair of spihal cord injury. Zhonghua Shiyan Waike Zazhi. 2017;34:1734–1737. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.