Keywords: nerve regeneration, sevoflurane, neural stem cells, apoptosis, vascular endothelial growth factor, PI3K, p-AKT, anesthesia, learning, memory, developmental neurobiology, neural regeneration
Abstract
Sevoflurane is the most commonly used volatile anesthetic during pregnancy. The viability of neural stem cells directly affects the development of the brain. However, it is unknown whether the use of sevoflurane during the second trimester affects the survival of fetal neural stem cells. Therefore, in this study, we investigated whether exposure to sevoflurane in mid-gestation induces apoptosis of neural stem cells and behavioral abnormalities. On gestational day 14, pregnant rats were anesthetized with 2% or 3.5% sevoflurane for 2 hours. The offspring were weaned at 28 days and subjected to the Morris water maze test. The brains were harvested to examine neural stem cell apoptosis by immunofluorescence and to measure Nestin and SOX-2 levels by western blot assay at 6, 24 and 48 hours after anesthesia as well as on postnatal day (P) 0, 14 and 28. Vascular endothelial growth factor (VEGF) and phosphoinositide 3-kinase (PI3K)/AKT pathway protein levels in fetal brain at 6 hours after anesthesia were assessed by western blot assay. Exposure to high-concentration (3.5%) sevoflurane during mid-gestation increased escape latency and path length to the platform, and it reduced the average duration spent in the target quadrant and platform crossing times. At 6, 24 and 48 hours after anesthesia and at P0, P14 and P28, the percentage of Nestin/terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)-positive cells was increased, but Nestin and SOX-2 protein levels were decreased in the hippocampus of the offspring. At 6 hours after anesthesia, VEGF, PI3K and phospho-AKT (p-AKT) levels were decreased in the fetal brain. These changes were not observed in animals given low-concentration (2%) sevoflurane exposure. Together, our findings indicate that exposure to a high concentration of sevoflurane (3.5%) in mid-gestation decreases VEGF, PI3K and p-AKT protein levels and induces neural stem cell apoptosis, thereby causing learning and memory dysfunction in the offspring.
Introduction
As intrauterine fetal therapy has evolved, there has been an increasing role for fetal surgery in the second trimester. Relatively lengthy general anesthesia is required for most intrauterine procedures (Van et al., 2007; Hoagland et al., 2017). Usually, 2% sevoflurane is the lowest inhalation concentration that can meet the needs of the operation and maintain uterine relaxation; however, once the concentration exceeds 3.5%, sevoflurane will have a serious effect on the circulation of the mother, causing circulatory changes that can directly affect the blood supply and health of the fetus. Therefore, in clinical practice, sevoflurane is used at a concentration of 2% to 3.5%.
Anesthesiologists must not only provide an adequate depth of anesthesia, but must also consider the short- and long-term effects of fetal exposure to anesthetic drugs. The potentially detrimental effect of anesthetic drugs on the development of the neonatal central nervous system is of particular concern.
General anesthesia can cause neurodevelopmental damage and cognitive dysfunction in infants (Sun, 2010). Recent studies have shown that the use of anesthetic drugs during the critical rapid growth phase of early brain development in neonatal animals can cause neurodegenerative changes and impaired learning (Jevtovic et al., 2003; Fredriksson et al., 2007; Satomoto et al., 2009). As pregnant women are less likely to undergo anesthesia, most studies on the effect of maternal anesthetic exposure on the fetus have focused on the teratogenic effects of anesthetic drugs in early pregnancy, and its influence on the neonatal Apgar score and acid–base balance (Littleford, 2004; Cheek and Baird, 2009). To date, there are few studies on the long-term neurobehavioral effects of maternal exposure to general anesthetics during pregnancy on the offspring. Exposure of pregnant rats to a single episode of anesthesia with 1.4% isoflurane (1.3 minimum alveolar concentration) during mid-gestation reportedly induces long-term spatial memory impairment in the offspring (Kong et al., 2011; Palanisamy et al., 2011). Sevoflurane is the most commonly used inhalational anesthetic in clinical practice, and is the first choice for maintenance of anesthesia during pregnancy (Dong et al., 2009). However, the long-term effects of sevoflurane exposure in the second trimester are unclear. Therefore, in the present study, we sought to clarify the acute and long-term effects of sevoflurane exposure in mid-gestation, using both low (2%) and high (3.5%) concentrations of the anesthetic.
Neural development in rats is similar to that in humans, and 14 days of gestation in rats is equivalent to mid-gestation in humans. Although the neurodevelopmental timeframe differs between rats and humans, neural stem cell (NSC) proliferation and apoptosis in the developing hippocampus are similar (Sanes et al., 2005). Therefore, we used 14-day-pregnant rats for the experiments.
From a neuro-embryologic perspective, the second trimester is a critically important period in neural development. During this period, NSCs are present throughout the brain. Neurodevelopment is a complex and dynamic process of morphological and functional changes, which are achieved through the proliferation, differentiation, apoptosis and migration of NSCs (Urbán et al., 2014). Therefore, the number and functional status of NSCs directly affect the neurodevelopmental process. Furthermore, brain development can easily be altered by environmental and drug effects in the second trimester. For example, in rodents, even a relatively non-invasive intervention such as abdominal ultrasonography can prevent neurons from migrating to the cortex (Ang et al., 2006). Furthermore, maternal intake of ethanol in the first or second trimester can impair neuronal proliferation, migration and cortical formation in the brain during embryonic development, resulting in behavioral abnormalities (Carneiro et al., 2005; Hausknecht et al., 2005; Manent et al., 2007; Cuzon et al., 2008; Hellemans et al., 2008; Guerri et al., 2009; Kuwagata et al., 2009). Therefore, the use of anesthetics in the second trimester is likely to affect NSC number and functional status, and cause behavioral impairments.
Studies have shown that vascular endothelial growth factor (VEGF)-mediated angiogenesis is very important for embryonic development, and the loss of VEGF can trigger neuronal apoptosis (Ryu et al., 2009; Lee et al., 2011). VEGF, as reported in many studies, is strongly associated with neonatal hypoxic-ischemic encephalopathy and long-term learning and memory deficits in Alzheimer’s disease patients (Garcia et al., 2014; Echeverria et al., 2017; Durán-Carabali et al., 2018). Zhang et al. (2016) suggested that activating the phosphatidylinositol 3-kinase (PI3K)/AKT pathway reduces neuronal apoptosis induced by anesthesia and enhances learning and memory abilities. Pretreatment with minocycline activates the PI3K/AKT pathway to reduce neurological damage caused by exposure to ketamine and improve behavioral performance in adulthood (Lu et al., 2017). In addition, dextromethorphan reduces repeated propofol exposure-induced apoptosis and cognitive impairment by activating the PI3K/AKT signaling pathway (Wang et al., 2016). However, it is unknown whether VEGF and the PI3K/AKT pathway are affected by gestational sevoflurane exposure.
In the present study, we investigate the effects of sevoflurane exposure in mid-gestation on learning and memory abilities and NSC apoptosis in the offspring. Furthermore, we examine the underlying molecular mechanisms.
Materials and Methods
Animals and anesthetic procedures
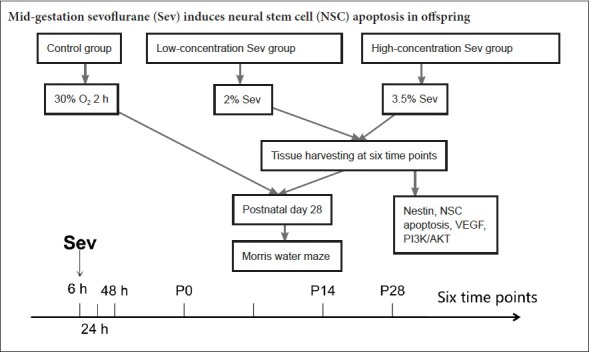
For this study, 168 healthy female Sprague-Dawley rats and an additional 30 healthy male Sprague-Dawley rats weighing 380–420 g were provided by the Animal Center, Shengjing Hospital of China Medical University in China (license No. SCXK (Jing) 2014-0004). All rats, aged 8–10 weeks old, were given free access to water and food, and housed at 20–25°C with a relative humidity of 40–60% under natural light. The experiment was approved by the Animal Ethics Committee of Shengjing Hospital (No. 2018PS07K). Female and male rats were paired, and a positive vaginal smear was noted as pregnancy day 0.
To assess the effect of different anesthetic concentrations, we randomly allocated rats at 14 days of gestation into control, and low- and high-concentration sevoflurane groups (n = 56 each). All pregnant rats were intraperitoneally anesthetized with 300 mg/kg 10% chloral hydrate (R 00635, Shanghai Xingke Biotechnology Co., Ltd., Shanghai, China). An internal carotid artery catheter was placed to monitor heart rate and blood pressure with the Biological Function Experiment System (ChengDu TaiMeng Software Co., Ltd., Chengdu, China). The catheter was fixed on the back of the neck subcutaneously to prevent grasping. The next day, on the 14th day of gestation, pregnant rats were put in an anesthesia box. The anesthesia box was a self-made transparent glass box with a flip design at the top. Each side of the box has a small hole, one of which is connected to an anesthesia machine to provide oxygen and anesthesia gas. Another hole is connected with an anesthesia gas monitor to measure the concentration of the anesthesia gas in the box. Rats in the low- and high-concentration sevoflurane groups were respectively administered 2% and 3.5% sevoflurane in 30% oxygen for two hours. In the control group, pregnant rats were administered 30% oxygen for 2 hours. A previous study showed that at least 2 hours of anesthetic exposure is required to observe neuronal apoptosis or injury, and approximates the duration of anesthesia in fetal surgery (Wang, 2009). Rats breathed spontaneously throughout the procedure. The minimum alveolar concentration value of the exhaled gas, heart rate and blood pressure were monitored throughout anesthesia (Biological Function Experiment System, ChengDu TaiMeng Software Co., Ltd.; Anesthesia Gas Monitor, Detex-Ohmeda, Louisville, CO, USA). Intermittent arterial blood gas analysis (pH and PaCO2) was performed, and the rectal temperature was maintained at 37 ± 0.5°C. In the event of hypotension during anesthesia, phenylephrine 0.3 mg/kg was administered through the tail vein. After recovery from anesthesia, the pregnant rats were caged for rearing. The brain tissues of offspring were obtained at six time points for analysis: 6, 24 and 48 hours after anesthesia, and on postnatal days 0, 14 and 28. The number of NSCs (Nestin-positive cells), apoptotic cells [terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)-positive cells] and apoptotic NSCs (Nestin and TUNEL double-positive cells) were detected by immunofluorescence staining. In addition, western blot assays were used to detect the expression of Nestin in the hippocampus. To investigate the mechanisms leading to apoptosis, VEGF, PI3K, AKT and phospho-AKT (p-AKT) levels were detected by western blot assay in the fetal brain 6 hours after anesthesia.
Morris water maze test
Eight litters (with three offspring per litter as replicates) were randomly selected to undergo the Morris water maze test 28 days after birth. Dim lighting was used in the laboratory and a quiet environment was maintained. Food and drink were forbidden in the laboratory to avoid odor interference. The experiment began at 8:00 a.m. every day. After each experiment, the rats were dried and caged to keep warm. The spatial navigation test was conducted within 5 days, and each rat was subjected to four rounds of testing, each for 90 seconds with an interval of ≥ 30 minutes to ensure that the rats had adequate rest. Rats were placed in the water, and escape latency (the time taken to find the platform to escape the water for 5 seconds), path length to finding the platform, swimming speed and swimming distance were recorded. If the rat was unable to find the platform within 90 seconds, the computer automatically stopped recording, and the rat was gently guided to the platform to ensure that it would stay on the platform for 30 seconds. By the end of the navigation task, the underwater platform was removed, and the midpoint of the quadrant corresponding to the original platform location was used as the water entry point. Then, the rat was placed into the tank facing the wall. Average duration spent in the target quadrant and platform crossings were recorded. An image acquisition and analysis system (Shenyang Furui Infrared Technology, Shenyang, China; Shanghai Science and Technology Co., Ltd., Shanghai, China) was used to record the frequency that the rat passed through the area of the original platform location within 90 seconds.
Western blot assay for Nestin and SOX-2 in fetal and postnatal hippocampus and for VEGF, PI3K, AKT and p-AKT levels in fetal brain
Fetal brains from each group (n = 8 gravid females, with three fetuses per female as replicates) were harvested 6, 24 and 48 hours after anesthesia, and the hippocampi were harvested on postnatal days 0, 14 and 28 from each group as well (n = 8 litters, with three offspring per litter as replicates) to assess Nestin and SOX-2 expression. Furthermore, 6 hours after anesthesia, the fetal brains in each group (n = 8 gravid females, with three fetuses per female as replicates) were harvested to detect VEGF, PI3K, p-AKT and AKT protein expression. Western blot assay was performed as previously reported (Zhang, 2012). Anti-Nestin polyclonal antibody (MAB353, Millipore, Bedford, MA, USA) was diluted at 1:1000 and incubated overnight at 4°C. Anti-SOX-2 antibody was diluted at 1:1000. Anti-VEGF, anti-PI3K, anti-p-AKT and anti-AKT antibodies were diluted at 1:200, 1:200, 1:200 and 1:100, respectively, and incubated overnight at 4°C (sc-365964, sc-81670, sc-376641, sc-5298, sc-293125, CA, USA). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was detected simultaneously as an internal reference. The Molecular Image VersaDoc MP 5000 system was used to image the gel. Signal intensities were determined using an imaging system (Quantity One, Bio-Rad, Beijing, China) and imaging software (NIH Image Version 1.37, National Institutes of Health, Bethesda, MD, USA). For the western blot assay, a relative ratio of the gray value of each protein to the corresponding internal reference (GAPDH) was calculated.
Nestin/TUNEL immunofluorescence staining
At 6, 24 and 48 hours after anesthesia, and on postnatal days 0, 14 and 28, brain tissues were harvested from fetuses or postnatal animals. Offspring from eight mothers (three rats per mother as replicates) were tested for immunostaining from each group. Brain tissues were fixed in 4% paraformaldehyde, and cut into 8-mm coronal brain sections. Three brain sections were used for each rat, and incubated with 10% bovine serum albumin in an incubator at 37°C for 30 minutes, blocked, and incubated with mouse anti-Nestin monoclonal antibody (1:100) at 4°C overnight. The next day, a rabbit anti-mouse IgG secondary antibody was incubated with TUNEL reagent (1:10) and tetramethyl rhodamine isothiocyanate (TRITC; 1:50) at 37°C for 2 hours, followed by three washes with PBS. Immunofluorescence controls were routinely performed in which primary antibody was not included. Slides were covered with mounting medium (Vector Laboratories, Burlingame, CA, USA). Cells expressing an NSC-specific marker (Nestin, red) and stained with 4′,6-diamidino-2-phenylindole (DAPI: blue) were identified as NSCs. TUNEL-positive (green) cells represented apoptotic cells. Cells positive for both Nestin and TUNEL (Nestin+/TUNEL+) were considered apoptotic NSCs.
Cell counting
Immunofluorescence images were captured on a fluorescence microscope (Nikon, Tokyo, Japan), and were analyzed using NIH Image J software. The numbers of apoptotic cells, NSCs and apoptotic NSCs in fetal and postnatal rat hippocampus were counted and expressed as a percentage of the control group. Mean numbers were used for analysis. Quantification of positive cells was performed in five sections per rat, and eight rats per mother were analyzed.
Statistical analysis
All data are expressed as the mean ± SEM. All analyses were performed with SPSS 19.0 software (IBM, Corp., Armonk, NY, USA). The mean significant difference among the experimental groups was determined by one-way analysis of variance followed by the Bonferroni post hoc test. A value of P < 0.05 was considered statistically significant.
Results
Effect of sevoflurane on hemodynamic parameters in pregnant rats
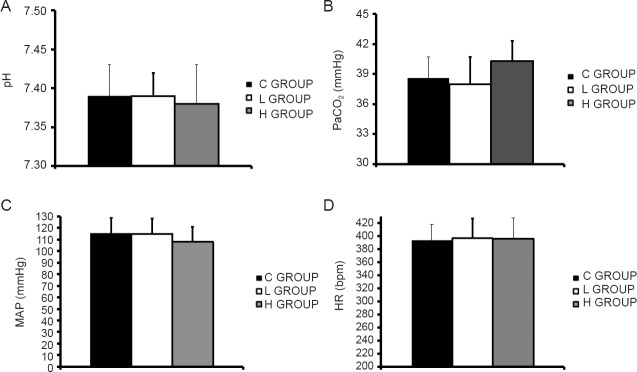
Compared with the control group, there was no significant difference in hemodynamic or acid-base parameters in either sevoflurane group (P > 0.05; Figure 1).
Figure 1.
Comparison of hemodynamic and acid-base parameters in anesthetized pregnant rats in the various groups.
(A–C) pH, PaCO2 and MAP in the maternal internal carotid artery at the end of anesthesia; 1 mmHg = 0.133 kPa. (D) Maternal HR at the end of anesthesia. C GROUP: Control group; L GROUP: low-concentration sevoflurane (2%) group; H GROUP: high-concentration sevoflurane (3.5%) group; PaCO2: arterial partial pressure of carbon dioxide; MAP: mean arterial pressure; HR: heart rate.
Effect of sevoflurane exposure during mid-gestation on behavior of offspring at 28 postnatal days
One-way analysis of variance showed a significantly prolonged escape latency and swimming distance to the platform, reduced frequency of crossing the platform, and reduced time of target quadrant activity in the high-concentration sevoflurane group compared with the control group (P < 0.01; Figure 2). There were no significant differences between the low-concentration sevoflurane group and the control group. The control group had a locus of motion in the fourth quadrant that was similar to that of rats in the low-concentration sevoflurane group. In the high-concentration sevoflurane group, however, trajectories were evenly distributed throughout the region with no defined target area.
Figure 2.
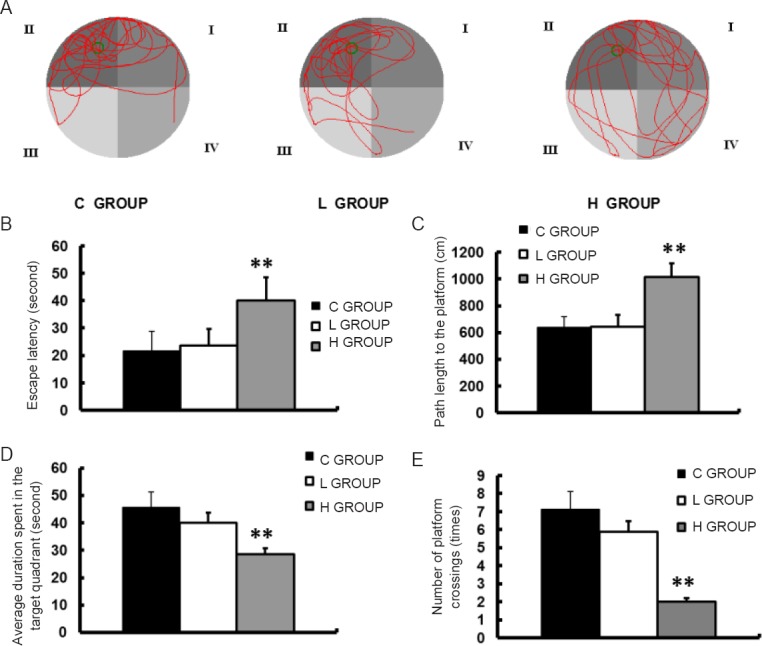
Neurobehavioral characteristics of progeny in the three groups on postnatal day 28.
Morris water maze test was performed on postnatal day 28. (A) Trace of movements in the Morris water maze. (B) Escape latency. (C) Path length to the platform. (D) Average duration spent in the target quadrant in the probe test. (E) Number of platform crossings in a 90-second period in the probe test. Data are expressed as the mean ± SD (n = 8 litters, with three rats per litter as replicates). **P < 0.01, vs. C GROUP (one-way analysis of variance followed by the Bonferroni post hoc test). C GROUP: Control grousp; L GROUP: low-concentration sevoflurane (2%) group; H GROUP: high-concentration sevoflurane (3.5%) group.
Effect of sevoflurane exposure in mid-gestation on apoptosis in the offspring
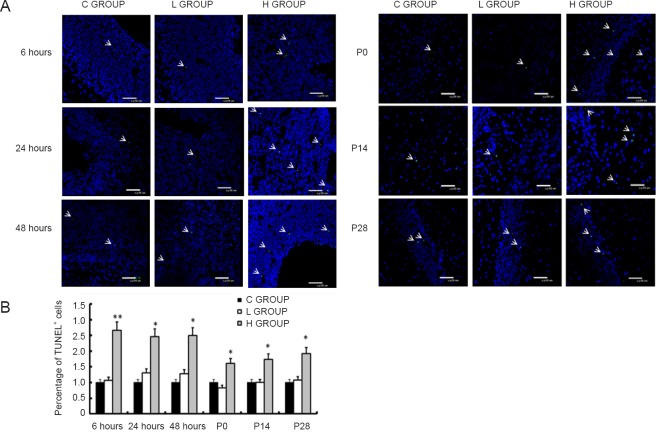
TUNEL staining was performed to examine apoptosis in the brain. Compared with the control group, there was no significant difference in the number of apoptotic cells in the low-concentration sevoflurane group at any of the six time points (P > 0.05). In contrast, compared with the control group, apoptotic cells were more numerous in the high-concentration sevoflurane group at all six time points (P < 0.05 or P < 0.01; Figure 3). These findings indicate that high-concentration sevoflurane increases apoptosis in the brain in offspring.
Figure 3.
Effect of sevoflurane exposure in mid-gestation on apoptosis in the fetal brain (6, 24 and 48 hours after anesthesia) and the postnatal hippocampus (P0, P14 and P28).
(A) Representative images show that the H GROUP has increased numbers of TUNEL+ cells (green, indicated by white arrows; original magnification, 400×) compared with the C GROUP at the different time points. There was no difference between the L GROUP and the C GROUP. Blue: nuclei; green: TUNEL+ apoptotic cells. (B) Quantitative analysis of the effect of sevoflurane on TUNEL+ cells at the different time points. Three sections were selected from each rat to analyze the ratio of apoptotic cells in each section. Data are expressed as the mean ± SD (n = 8 mothers, with three offspring per mother as replicates). *P < 0.05, **P < 0.01, vs. C GROUP (one-way analysis of variance followed by the Bonferroni post hoc test). C GROUP: Control group; L GROUP: low-concentration sevoflurane (2%) group; H GROUP: high-concentration sevoflurane (3.5%) group.
Effect of sevoflurane exposure in mid-gestation on Nestin and SOX-2 expression in fetal brain and postnatal hippocampus
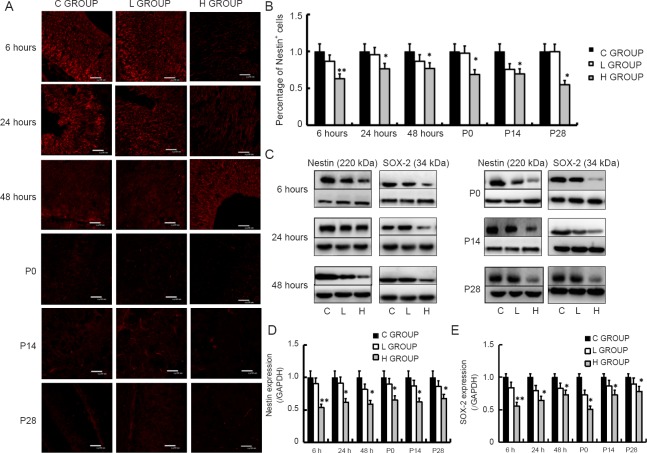
Nestin immunostaining was performed to detect NSCs. Compared with the control group, there was no significant difference in the area of red staining (Nestin-positive cells) in the low-concentration sevoflurane group at any time point (P > 0.05). In contrast, the number of Nestin-positive cells was significantly less in the high-concentration sevoflurane group at all six time points (P < 0.05 or P < 0.01; Figure 4A, B). The western blot results confirmed that Nestin and SOX-2 protein levels were significantly lower in the high-concentration sevoflurane group than in the control group at all time points (P < 0.05 or P < 0.01; Figure 4C–E). These results indicate that high-concentration sevoflurane exposure during mid-gestation reduces the number of NSCs in the offspring. There was no significant difference between the low-concentration sevoflurane group and the control group.
Figure 4.
Effect of sevoflurane exposure in mid-gestation on Nestin and SOX-2 expression in the fetal brain (6, 24 and 48 hours after anesthesia) and the postnatal hippocampus (P0, P14 and P28).
(A) Representative images showing that the H GROUP exhibits decreased Nestin+ (red) neural stem cells in the fetal brain and postnatal hippocampus compared with the C GROUP at the different time points. There was no difference between the L GROUP and the C GROUP (original magnification, 400×). (B) Effect of sevoflurane on Nestin+ cells at the various time points. (C) Western blot assay for Nestin and SOX-2 in the fetal brain and postnatal hippocampus, with GAPDH as the internal reference. (D, E) Quantitation of Nestin and SOX-2 protein in the fetal brain and postnatal hippocampus in the three groups. Three sections were selected from each rat to analyze Nestin expression. Data are expressed as the mean ± SD (n = 8 mothers, with three rats per mother as replicates). *P < 0.05, **P < 0.01, vs. C GROUP (one-way analysis of variance followed by the Bonferroni post hoc test). C GROUP: Control group; L GROUP: low-concentration sevoflurane (2%) group; H GROUP: high-concentration sevoflurane (3.5%) group.
Effect of sevoflurane exposure in mid-gestation on neural stem cell apoptosis in offspring
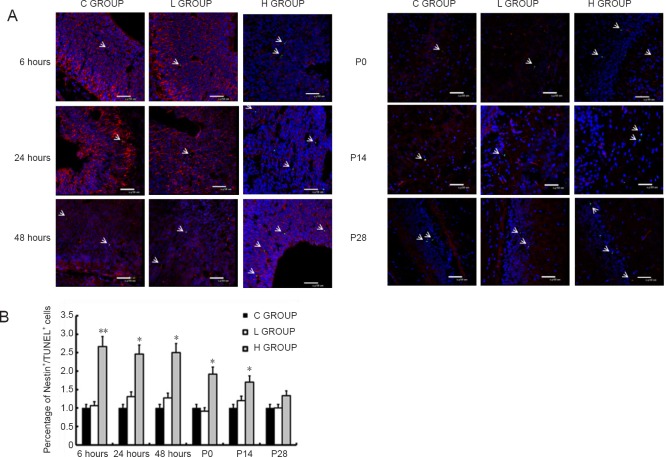
Nestin+/TUNEL+ cells were considered apoptotic NSCs. There was no significant difference in the number of apoptotic NSCs between the low-concentration sevoflurane group and the control group at any time point (Figure 5). Compared with the control group, the number of apoptotic NSCs in the high-concentration sevoflurane group was significantly higher at 6, 24 and 48 hours after anesthesia, and on postnatal days 0 and 14 (P < 0.05). These findings indicate that high-concentration sevoflurane promotes the apoptosis of NSCs in the brain of offspring. There was no significant difference between the groups on postnatal day 28 (P > 0.05).
Figure 5.
Effect of sevoflurane exposure in mid-gestation on neural stem cell apoptosis in the fetal brain (6, 24 and 48 hours after anesthesia) and postnatal hippocampus (P0, P14 and P28).
(A) Presence of TUNEL+/Nestin+ cells in the fetal brain or postnatal hippocampus in the three groups (original magnification, 400×). Blue: nuclei; green: TUNEL+ (apoptotic) cells; red: Nestin+ (neural stem) cells. TUNEL+/Nestin+ cells are apoptotic neural stem cells. The arrows indicate neural stem cell apoptosis. (B) Effect of sevoflurane on TUNEL+/Nestin+ cells at the different time points. Three sections were used from each rat to analyze the ratio of apoptotic neural stem cells in each section. Data are expressed as the mean ± SD (n = 8 mothers in each group, with 3 rats per mother as replicates). *P < 0.05, **P < 0.01, vs. C GROUP (one-way analysis of variance followed by the Bonferroni post hoc test). C GROUP: Control group; L GROUP: low-concentration sevoflurane (2%) group; H GROUP: high-concentration sevoflurane (3.5%) group. TUNEL: terminal deoxynucleotidyl transferase dUTP nick end labeling.
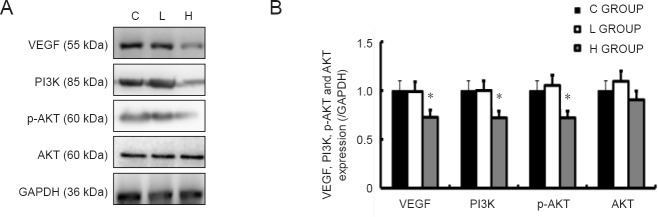
Effect of sevoflurane exposure in mid-gestation on VEGF, PI3K, AKT and p-AKT levels in the fetal brain
Western blot assay showed that VEGF, PI3K and p-AKT protein levels were significantly lower in the high-concentration sevoflurane group than in the control group. However, there was no difference in AKT expression between the high-concentration sevoflurane group and the control group. There were no significant differences between the low-concentration sevoflurane group and the control group (Figure 6).
Figure 6.
Effect of sevoflurane exposure in mid-gestation on VEGF, PI3K, AKT and p-AKT levels in the fetal brain 6 hours after anesthesia.
(A) Western blots for VEGF, PI3K, AKT and p-AKT in the fetal brain. (B) Quantitation of VEGF, PI3K, AKT and p-AKT levels in the fetal brain in the three groups. Data are expressed as the mean ± SD (n = 8 mothers, with 3 rats per mother as replicates). *P < 0.05, vs. C GROUP (one-way analysis of variance followed by the Bonferroni post hoc test). C GROUP: Control group; L GROUP: low-concentration sevoflurane (2%) group; H GROUP: high-concentration sevoflurane (3.5%) group. VEGF: Vascular endothelial growth factor; PI3K: phosphatidylinositol 3-kinase; p-AKT: phospho-AKT.
Discussion
Our findings are consistent with most previous studies on gestational exposure models. For example, a single episode of exposure to 1.4% isoflurane (1.3 mininum alveolar concentration) for 4 hours in mid-gestation was reported to result in long-term spatial memory impairment in rat offspring (Kong et al., 2011; Palanisamy et al., 2011). It has also been reported that gestational exposure to 2.5% sevoflurane for 2 hours causes learning and memory dysfunction in the offspring in mice (Zheng et al., 2013). In addition, neuronal death has been reported in the embryos of macaques administered ketamine anesthesia in the second or third trimester (Slikker, 2007). However, the mechanisms underlying the neurobehavioral toxicity of anesthetic use during pregnancy remained unclear.
Recent studies on hippocampal neuronal death mechanisms have implicated the N-methyl-D-aspartic acid receptor and gamma-aminobutyric acid A receptor pathways, mitochondrial damage, intracellular calcium imbalance, neuroinflammatory pathways, and the brain-derived neurotrophic factor pathway (Lei et al., 2012). However, a study showed that hippocampal neuronal death after exposure to isoflurane in young rats does not cause cognitive impairment (Stratmann et al., 2009). In another study, cell death in the dentate gyrus of young rats was not increased after repeated exposure to isoflurane, but a reduction in NSC pools and neurogenesis, and impaired spatial memory were observed (Zhu et al., 2010). In addition, there is a close correlation between NSCs in the hippocampal dentate gyrus and learning and memory functions in the adult (Lie et al., 2004). These findings suggest that a decrease in hippocampal NSC pools underlies cognitive dysfunction in experimental animals.
The second trimester is an important period for embryonic neurodevelopment. During this period, the hippocampus gradually forms through the proliferation, apoptosis, migration and differentiation of neural precursor cells from hippocampal and dentate neuroepithelium (Sanes et al., 2005). Therefore, exposure to narcotic drugs during this period is likely to impact the number and function of NSCs, thereby resulting in functional impairments.
Here, we found that the number of NSCs in the whole fetal brain as well in the hippocampus of postnatal animals is reduced by exposure to high-concentration sevoflurane during mid-gestation. This reduction in the number of NSCs is likely to impact hippocampal development and cause learning and memory dysfunctions (Snyder et al., 2001; van et al., 2002; Laplagne et al., 2006; Toni et al., 2008; Zhao et al., 2008). Co-staining for TUNEL and Nestin showed that high-concentration sevoflurane provoked apoptosis of NSCs, but only until the 14th day after birth. This suggests that a protective or compensatory mechanism might reduce the number of apoptotic NSCs on postnatal day 28. However, this protective mechanism is not able to reverse the neurobehavioral effect of high-concentration sevoflurane. Another possibility is that the number of NSCs had decreased on postnatal day 28 compared with the previous time points, resulting in a commensurate reduction in the number of detectable apoptotic NSCs.
Here, we observed that in the high-concentration sevoflurane group, apoptosis was significantly lower at P0 (newborn) compared with 6, 24 or 48 hours (fetal) after sevoflurane exposure. Possibly, over time, the fetus initiates some type of compensatory mechanism. Perinatal fetal-maternal interactions might also inhibit apoptosis. Nevertheless, because mid-gestation is critical for hippocampal development, the damage caused by high-concentration sevoflurane persists.
VEGF is essential for the development of the nervous system (Kershner and Welshhans, 2017). Studies have shown that VEGF-mediated angiogenesis is very important for embryonic development, and the loss of VEGF triggers neuronal apoptosis (Ryu, 2009; Lee, 2011). VEGF, as reported in many studies, is strongly associated with neonatal hypoxic-ischemic encephalopathy and long-term learning and memory in Alzheimer’s disease patients (Garcia et al., 2014; Durán-Carabali et al., 2017; Echeverria et al., 2017). Our results, for the first time, show that exposure to high-concentration sevoflurane downregulates PI3K and p-AKT in the fetal brain. Zhang et al. (2016) suggested that activating the PI3K/AKT pathway reduces neuronal apoptosis induced by anesthesia and enhances learning and memory abilities. Pretreatment with minocycline activates the PI3K/AKT pathway to reduce neurological damage caused by exposure to ketamine and improve behavioral performance in adulthood (Lu et al., 2017). Dextromethorphan reduces propofol-induced apoptosis and cognitive impairment by activating the PI3K/AKT signaling pathway (Wang et al., 2016). These results suggest that high-concentration sevoflurane can regulate NSC apoptosis by inhibiting the VEGF/PI3K/AKT pathway.
This study has some limitations. First, we were not able to fully elucidate the mechanisms responsible for the sevoflurane-induced apoptosis of NSCs. Second, we found that sevoflurane induced NSC apoptosis starting 6 hours after anesthesia until postnatal day 14; however, we were not able to identify potential neuroprotective mechanisms. Third, it has been reported that the duration of anesthetic exposure also affects the neurotoxicity of anesthetic drugs, in addition to concentration (Li et al., 2007). Further studies are needed to identify the critical concentration and exposure time needed to elicit neurotoxicity in the offspring. In addition, we found that the number of NSCs, the levels of NSC marker proteins and apoptosis in NSCs were impacted at 6 hours after the use of high-concentration sevoflurane. Therefore, we only evaluated VEGF, PI3K, AKT and p-AKT levels 6 hours after anesthesia, without examining other time points. Therefore, additional time points should be examined in future studies. Furthermore, a number of studies (Yon et al., 2005; Zhang et al., 2010; Boscolo et al., 2011; Sanchez et al., 2011) suggest that anesthetics impair mitochondrial morphogenesis, integrity or function, leading to neurodevelopmental changes and altered synaptic function. Moreover, some studies (Chen et al., 2012; Luo, 2014; McGee et al., 2015) have shown that ethanol can damage mitochondria in neural cells of neonatal rats and increase the number of reactive oxygen species, which in turn regulate apoptosis through the PI3K/AKT/mTOR pathway. Whether sevoflurane exposure is associated with mitochondrial damage in our model is unknown and needs further investigation. Recent studies have shown that sevoflurane affects the Wnt/β-catenin pathway in neural stem cells and also perturbs prefrontal cortical development. Wnt/β-catenin pathway is a classic pathway that participates in the proliferation of neural stem cells. Normal proliferation can compensate for excessive apoptosis of neural stem cells. Excessive apoptosis of neural stem cells observed in this study may be caused by the reduction in neural stem cell proliferation, which needs to be confirmed by further investigations.
In summary, low-concentration sevoflurane, commonly used in clinical practice, has no significant effect on NSC apoptosis or number, suggesting that it can be used safely in pregnancy. In comparison, high-concentration sevoflurane may cause apoptosis of NSCs and reduce their number, thereby upsetting the balance between cell proliferation and cell death. This in turn perturbs the normal development of the central nervous system, resulting in learning and memory dysfunction in the offspring. Therefore, high concentrations of sevoflurane should be avoided in pregnancy if possible.
Additional file:Open peer review report 1 (29.4KB, pdf) .
Footnotes
Conflicts of interest: The authors declare no competing financial interests.
Financial support: This work was supported by the National Natural Science Foundation of China, No. 81671311 (to PZ), No. 81503273 (to NZ); the Science and Technology Foundation of Liaoning Province of China, No. 2015020467 (to PZ); the Outstanding Scientific Fund of Shengjing Hospital of China Medical University, No. 201708. The funders did not participant in the study design, in the collection, analysis and interpretation of data, in the writing of the paper, and in the decision to submit the paper for publication.
Institutional review board statement: The procedures were in accordance with Animal Ethics Committee of Shengjing Hospital of China Medical University (approval No. 2018PS07K). The experimental procedure followed the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1985).
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Cyrus David Mintz, Johns Hopkins School of Medicine, USA.
Funding: This work was supported by the National Natural Science Foundation of China, No. 81671311 (to PZ), No. 81503273 (to NZ); the Science and Technology Foundation of Liaoning Province of China, No. 2015020467 (to PZ); the Outstanding Scientific Fund of Shengjing Hospital of China Medical University, No. 201708.
(Copyedited by Patel B, Wysong S, Wang J, Li CH, Qiu Y, Song LP, Zhao M)
References
- Ang ES Jr, Gluncic V, Duque A, Schafer ME, Rakic P. Prenatal exposure to ultrasound waves impacts neuronal migration in mice. Proc Natl Acad Sci U S A. 2006;103:12903–12910. doi: 10.1073/pnas.0605294103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boscolo A, Starr J, Sanchez V, Lunardi N, DiGruccio M, Ori C, Erisir A, Trimmer P, Bennett J, Jevtovic-Todorovic V. The abolishment of anesthesia-induced cognitive impairment by timely protection of mitochondria in the developing rat brain: The importance of free oxygen radicals and mitochondrial integrity. Neurobiol Dis. 2011;45:1031, 1041. doi: 10.1016/j.nbd.2011.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carneiro LM, Diógenes JP, Vasconcelos SM. Behavioral and neurochemical effects on rat offspring after prenatal exposure to ethanol. Neurotoxicol Teratol. 2005;27:585–592. doi: 10.1016/j.ntt.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Cheek TG, Baird E. Anesthesia for nonobstetric surgery: maternal and fetal considerations. Clin Obstet Gynecol. 2009;52:535–545. doi: 10.1097/GRF.0b013e3181c11f60. [DOI] [PubMed] [Google Scholar]
- Chen G, Ke Z, Xu M, Liao M, Wang X, Qi Y, Zhang T, Frank JA, Bower KA, Shi X, Luo J. Autophagy is a protective response to ethanol neurotoxicity. Autophagy. 2012;8:1577–1589. doi: 10.4161/auto.21376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuzon VC, Yeh PW, Yanagawa Y, Obata K, Yeh HH. Ethanol consumption during early pregnancy alters the disposition of tangentially migrating GABAergic interneurons in the fetal cortex. J Neurosci. 2008;28:1854–1864. doi: 10.1523/JNEUROSCI.5110-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Zhang G, Zhang B. The common inhalational anesthetic sevoflurane induces apoptosis and increases beta-amyloid protein levels. Arch Neurol. 2009;66:620–31. doi: 10.1001/archneurol.2009.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durán-Carabali LE, Arcego DM, Odorcyk FK. Prenatal and early postnatal environmental enrichment reduce acute cell death and prevent neurodevelopment and memory impairments in rats submitted to neonatal hypoxia ischemia. Mol Neurobiol. 2018;55:3627–3641. doi: 10.1007/s12035-017-0604-5. [DOI] [PubMed] [Google Scholar]
- Echeverria V, Barreto GE, Ávila-Rodriguez M, Tarasov VV, Aliev G. Is VEGF a key target of cotinine and other potential therapies against Alzheimer disease? Curr Alzheimer Res. 2017;14:1155–1163. doi: 10.2174/1567205014666170329113007. [DOI] [PubMed] [Google Scholar]
- Fredriksson A, Pontén E, Gordh T, Eriksson P. Neonatal exposure to a combination of N-methyl-D-aspartate and gamma-aminobutyric acid type A receptor anesthetic agents potentiates apoptotic neurodegeneration and persistent behavioral deficits. Anesthesiology. 2007;107:427–436. doi: 10.1097/01.anes.0000278892.62305.9c. [DOI] [PubMed] [Google Scholar]
- Garcia KO, Ornellas FL, Martin PK. Therapeutic effects of the transplantation of VEGF overexpressing bone marrow mesenchymal stem cells in the hippocampus of murine model of Alzheimer’s disease. Front Aging Neurosci. 2014;6:30. doi: 10.3389/fnagi.2014.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerri C, Bazinet A, Riley EP. Foetal alcohol spectrum disorders and alterations in brain and behaviour. Alcohol Alcohol. 2009;44:108–114. doi: 10.1093/alcalc/agn105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausknecht KA, Acheson A, Farrar AM. Prenatal alcohol exposure causes attention deficits in male rats. Behav Neurosci. 2005;119:302–310. doi: 10.1037/0735-7044.119.1.302. [DOI] [PubMed] [Google Scholar]
- Hellemans KG, Verma P, Yoon E, Yu W, Weinberg J. Prenatal alcohol exposure increases vulnerability to stress and anxiety-like disorders in adulthood. Ann N Y Acad Sci. 2008;1144:154–175. doi: 10.1196/annals.1418.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoagland MA, Chatterjee D. Anesthesia for fetal surgery. Paediatr Anaesth. 2017;27:873. doi: 10.1111/pan.13185. [DOI] [PubMed] [Google Scholar]
- Jevtovic-Todorovic V, Hartman RE, Izumi Y. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci. 2003;23:876–882. doi: 10.1523/JNEUROSCI.23-03-00876.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kershner L, Welshhans K. RACK1 regulates neural development. Neural Regen Res. 2017;12:1036–1039. doi: 10.4103/1673-5374.211175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong F, Xu L, He D, Zhang X, Lu H. Effects of gestational isoflurane exposure on postnatal memory and learning in rats. Eur J Pharmacol. 2011;670:168–174. doi: 10.1016/j.ejphar.2011.08.050. [DOI] [PubMed] [Google Scholar]
- Kuwagata M, Ogawa T, Shioda S, Nagata T. Observation of fetal brain in a rat valproate-induced autism model: a developmental neurotoxicity study. Int J Dev Neurosci. 2009;27:399–405. doi: 10.1016/j.ijdevneu.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Laplagne DA, Espósito MS, Piatti VC. Functional convergence of neurons generated in the developing and adult hippocampus. PLoS Biol. 2006;4:e409. doi: 10.1371/journal.pbio.0040409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HK, Chauhan SK, Kay E, Dana R. Flt-1 regulates vascular endothelial cell migration via a protein tyrosine kinase-7-dependent pathway. Blood. 2011;117:5762–5771. doi: 10.1182/blood-2010-09-306928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei X, Guo Q, Zhang J. Mechanistic insights into neurotoxicity induced by anesthetics in the developing brain. Int J Mol Sci. 2012;13:6772–6799. doi: 10.3390/ijms13066772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Liang G, Wang S, Meng Q, Wang Q, Wei H. Effects of fetal exposure to isoflurane on postnatal memory and learning in rats. Neuropharmacology. 2007;53:942–950. doi: 10.1016/j.neuropharm.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie DC, Song H, Colamarino SA, Ming GL, Gage FH. Neurogenesis in the adult brain: new strategies for central nervous system diseases. Annu Rev Pharmacol Toxicol. 2004;44:399–421. doi: 10.1146/annurev.pharmtox.44.101802.121631. [DOI] [PubMed] [Google Scholar]
- Littleford J. Effects on the fetus and newborn of maternal analgesia and anesthesia: a review. Can J Anaesth. 2004;51:586–609. doi: 10.1007/BF03018403. [DOI] [PubMed] [Google Scholar]
- Lu Y, Giri PK, Lei S. Pretreatment with minocycline restores neurogenesis in the subventricular zone and subgranular zone of the hippocampus after ketamine exposure in neonatal rats. Neuroscience. 2017;352:144–154. doi: 10.1016/j.neuroscience.2017.03.057. [DOI] [PubMed] [Google Scholar]
- Luo J. Autophagy and ethanol neurotoxicity. Autophagy. 2014;10:2099–2108. doi: 10.4161/15548627.2014.981916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manent JB, Jorquera I, Mazzucchelli I. Fetal exposure to GABA-acting antiepileptic drugs generates hippocampal and cortical dysplasias. Epilepsia. 2007;48:684–693. doi: 10.1111/j.1528-1167.2007.01056.x. [DOI] [PubMed] [Google Scholar]
- McGee MA, Abdel-Rahman AA. Ethanol attenuates peripheral NMDAR-mediated vascular oxidative stress and pressor response. Alcohol. 2015;49:499–506. doi: 10.1016/j.alcohol.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palanisamy A, Baxter MG, Keel PK, Xie Z, Crosby G, Culley DJ. Rats exposed to isoflurane in utero during early gestation are behaviorally abnormal as adults. Anesthesiology. 2011;114:521–528. doi: 10.1097/ALN.0b013e318209aa71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu JK, Cho T, Choi HB, Wang YT, McLarnon JG. Microglial VEGF receptor response is an integral chemotactic component in Alzheimer’s disease pathology. J Neurosci. 2009;29:3–13. doi: 10.1523/JNEUROSCI.2888-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez V, Feinstein SD, Lunardi N, Joksovic PM, Boscolo A, Todorovic SM, Jevtovic-Todorovic V. General anesthesia causes long-term impairment of mitochondrial morphogenesis and synaptic transmission in developing rat brain. Anesthesiology. 2011;115:992–1002. doi: 10.1097/ALN.0b013e3182303a63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes DH, Reh TA, Harris WA. Development of the nervous system. Oxford: Academic Press, UK; 2005. [Google Scholar]
- Satomoto M, Satoh Y, Terui K. Neonatal exposure to sevoflurane induces abnormal social behaviors and deficits in fear conditioning in mice. Anesthesiology. 2009;110:628–637. doi: 10.1097/ALN.0b013e3181974fa2. [DOI] [PubMed] [Google Scholar]
- Slikker W Jr, Zou X, Hotchkiss CE. Ketamine-induced neuronal cell death in the perinatal rhesus monkey. Toxicol Sci. 2007;98:145–158. doi: 10.1093/toxsci/kfm084. [DOI] [PubMed] [Google Scholar]
- Snyder JS, Kee N, Wojtowicz JM. Effects of adult neurogenesis on synaptic plasticity in the rat dentate gyrus. Neurophysiol. 2001;85:2423–2431. doi: 10.1152/jn.2001.85.6.2423. [DOI] [PubMed] [Google Scholar]
- Stratmann G, Sall JW, May LD. Isoflurane differentially affects neurogenesis and long-term neurocognitive function in 60-day-old and 7-day-old rats. Anesthesiology. 2009;110:834–848. doi: 10.1097/ALN.0b013e31819c463d. [DOI] [PubMed] [Google Scholar]
- Sun L. Early childhood general anaesthesia exposure and neurocognitive development. Br J Anaesth. 2010;105(Suppl 1):61–68. doi: 10.1093/bja/aeq302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toni N, Laplagne DA, Zhao C. Neurons born in the adult dentate gyrus form functional synapses with target cells. Nat Neurosci. 2008;11:901–907. doi: 10.1038/nn.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbán N, Guillemot F. Neurogenesis in the embryonic and adult brain: same regulators, different roles. Front Cell Neurosci. 2014;8:396. doi: 10.3389/fncel.2014.00396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van De Velde M, De Buck F. Anesthesia for non-obstetric surgery in the pregnant patient. Minerva Anestesiol. 2007;73:235–240. [PubMed] [Google Scholar]
- van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Peretich K, Zhao Y, Liang G, Meng Q, Wei H. Anesthesia-induced neurodegeneration in fetal rat brains. Pediatr Res. 2009;66:435–440. doi: 10.1203/PDR.0b013e3181b3381b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wu C, Han B. Dexmedetomidine attenuates repeated propofol exposure-induced hippocampal apoptosis, PI3K/AKT/Gsk-3beita signal disruption, and juvenile cognitive deficits in neonatal rats. Mol Med Rep. 2016;14:769–775. doi: 10.3892/mmr.2016.5321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yon JH, Daniel-Johnson J, Carter LB, Jevtovic-Todorovic V. Anesthesia induces neuronal cell death in the developing rat brain via the intrinsic and extrinsic apoptotic pathways. Neuroscience. 2005;135:815–827. doi: 10.1016/j.neuroscience.2005.03.064. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Dong Y, Wu X, Lu Y, Xu Z, Knapp A, Yue Y, Xu T, Xie Z. The mitochondrial pathway of anesthetic isoflurane-induced apoptosis. Bio Chem. 2010;285:4025–4037. doi: 10.1074/jbc.M109.065664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Xu Z, Wang H. Anesthetics isoflurane and desflurane differently affect mitochondrial function, learning, and memory. Ann Neurol. 2012;71:687–698. doi: 10.1002/ana.23536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YH, Zhang J, Song JN. The PI3K-AKT-mTOR pathway activates recovery from general anesthesia. Oncotarget. 2016;7:40939–40952. doi: 10.18632/oncotarget.10172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- Zheng H, Dong Y, Xu Z. Sevoflurane anesthesia in pregnant mice induces neurotoxicity in fetal and offspring mice. Anesthesiology. 2013;118:516–526. doi: 10.1097/ALN.0b013e3182834d5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C, Gao J, Karlsson N. Isoflurane anesthesia induced persistent, progressive memory impairment, caused a loss of neural stem cells, and reduced neurogenesis in young, but not adult, rodents. J Cereb Blood Flow Metab. 2010;30:1017–1030. doi: 10.1038/jcbfm.2009.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.