Abstract
Blast-induced mild traumatic brain injury (mTBI) is of particular concern among military personnel due to exposure to blast energy during military training and combat. The impact of primary low-intensity blast mediated pathophysiology upon later neurobehavioral disorders has been controversial. Developing a military preclinical blast model to simulate the pathophysiology of human blast injury is an important first step. This article provides an overview of primary blast effects and perspectives of our recent studies demonstrating ultrastructural changes in the brain and behavioral disorders resulting from open-field blast exposures up to 46.6 kPa using a murine model. The model is scalable and permits exposure to varying magnitudes of primary blast injuries by placing animals at different distances from the blast center or by changing the amount of C4 charge. We here review the implications and future applications and directions of using this animal model to uncover the underlying mechanisms related to primary blast injury. Overall, these studies offer the prospect of enhanced understanding of the pathogenesis of primary low-intensity blast-induced TBI and insights for prevention, diagnosis and treatment of blast induced TBI, particularly mTBI/concussion related to current combat exposures.
Keywords: mild traumatic brain injury, open-field blast, primary blast wave, blast physics, animal model, ultrastructural abnormalities, behavior
Military personnel in theater of operations or during combat training are frequently exposed to blast waves produced by explosive weaponry, which can cause varying severity of traumatic brain injuries (TBIs). Blast-induced mild TBI (mTBI) is the most common form of TBI and is regarded as a ‘signature wound’ or ‘invisible injury’ of current combat activities. During 2000–2017, the Department of Defense reported that the vast majority (> 82%) of TBIs were classified as mTBI/concussion related to blast injury. Blast-induced mTBIs are usually not detectable using conventional imaging techniques including computerized tomographic scanning. They are characterized by scores of 13–15 on the Glasgow Coma Scale (GCS). Immediate signs and symptoms are usually transient (DeKosky et al., 2010), however some of these individuals are at a risk for developing post-traumatic stress disorder (PTSD), mental or physical abnormalities, and lifelong disabilities (DePalma et al., 2005; Griesbach et al., 2018). Later sequelae of these injuries impose immense burdens on affected patients, their families, and society.
While past blast injury experimental studies have helped to gain insights into TBI with a range of moderate- to high-intensity blast explosions (Cernak et al., 2011; Budde et al., 2013; Wang et al., 2016; Song et al., 2018a), our understanding of the pathogenesis of primary low-intensity blast (LIB) injury and its relation to later neurological outcomes remains poorly characterized. The pathogenesis of LIB injury from the primary blast shockwaves likely differs from that caused by impact/acceleration injuries. Therefore, an understanding of the neuropathological and cellular outcomes and associated behavioral consequences after blast exposures including primary LIB.
Experimental animal models have been widely used to address the fundamental questions related to the primary blast injury. Primary LIB injury models ideally should adhere to blast physics encountered in relevant combat scenarios. Detonation of high energy explosives entails almost instantaneous chemical decomposition with abrupt generation of kinetic, light, heat energy, sound, and generation of a supersonic shockwave front and overpressure followed by a blast wave (Goel et al., 2012). The primary blast wave is characterized by velocity in excess of the speed of sound in air, peak overpressure, duration, and impulse (integration of overpressure with respect to time). The Friedlander curve describes propagation of an ideal blast wave through time and space. However, this idealized waveform representation is altered by environmental interactions for an open-field blast exposure with a characteristic ‘ground bounce’ when the explosive and target are above ground (Goel et al., 2012). Our recent studies have provided a highly reproducible, open-field primary LIB injury mouse model which can be further scaled up for larger animals (Song et al., 2018a, b). The Missouri blast model employs an open-field detonation of a calibrated 350 g of high-energy C4 explosive to generate low-intensity primary blast waves (a static peak overpressure of 46.6 kPa and a maximal impulse of 8.7 pounds per square inch (PSI) × ms) manifesting a Friedlander waveform coupled with an enhanced impulse due to the ‘ground bounce’. Using this primary LIB injury mouse model, we have investigated potential links between blast physics and biological outcomes.
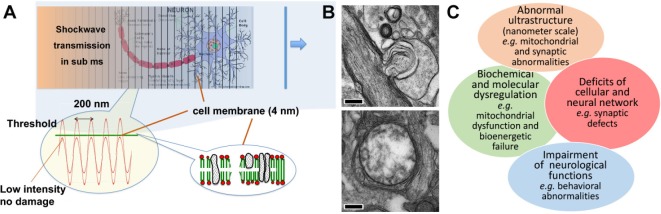
A prior physical model suggested that exposure(s) to primary blast waves may lead to nanoscale brain injury (Kucherov et al., 2012a). This injury paradigm suggested further study of the link between the biomedical outcomes and physical mechanisms causing mTBI. Our prior blast physics modeling predicted that the shockwave front traveling through liquid matters in the brain excites a phonon continuum decaying into specific acoustic waves with intensity exceeding the brain tissue’s strength (Figure 1A) (Kucherov et al., 2012b). This physical model predicted that energy deposited behind the primary blast wave causes nanoscale tissue damages occurring within microseconds (µs), well before the head acceleration. The model predicted periodic pressure waves at ~200 nm intervals with rupture peaks at ~4 nm, where peaks of the energy waves are the greatest (Figure 1A). Accordingly, primary LIB injury causes nanoscale subcellular damage, biochemical and molecular changes. The initial cellular effects predicted by these model calculations are much smaller and beyond the resolution offered by conventional imaging techniques and light microscopy.
Figure 1.
The predicted mechanism of cellular damage induced by primary shockwave in open-field blast.
(A) The acoustic wave modeling predicts ultrastructural damage to be rupturing of tissue in the primary blast direction at intervals of approximately 200 nm with rupture peaks at ~4 nm. (B) Representative nanoscale abnormalities, include myelin sheath ballooning (upper; scale bar: 0.5 μm) and swollen clear mitochondria (lower; scale bar: 0.2 μm). (C) Diagram of predicted biological changes, particular mitochondrial abnormalities, bioenergetics failure and synaptic defects that could lead to impairment of neurological functions.
In accordance with consideration of blast physics predicting ultrastructural injuries at nanoscale levels, we investigated fine structural neuropathological changes after primary LIB injury using transmission electron microscopy (TEM) and measured associated behavioral impairments. High speed videography confirmed that the open-field blast waves pass freely through the mice with no apparent head acceleration/rotation or bodily movement under such blast settings (Song et al., 2018a, b). As a result, the following biological consequences were attributable to the primary LIB wave effects. We observed the absence of macroscopic damage/necrosis and no apparent evident astrogliosis at blast exposure levels up to 46.6 kPa. Neuropathologically, myelinated axonal injury using silver staining was identified in blast exposed mice at 7 days post injury (DPI) (P < 0.0001), but not at 30 DPI (P > 0.05), suggesting some degrees of spontaneous recovery of myelinated axons. Importantly, as the blast wave passed through the brain from rostral to caudal, the level of silver staining intensities decreased, suggesting progressive reduction of the blast energy unloading occurred with greater damage to frontal brain areas. Using TEM, we observed and quantified myelin sheath defects at 7 DPI (P < 0.001), but not 30 DPI (P > 0.05), consistent with the silver staining findings. However, in contrast to the silver staining and myelination findings in axons, we observed and quantified persistent mitochondrial abnormalities both at 7 and 30 DPI (P < 0.0001) (Figure 1B). We observed extensive split layers, dense degeneration, ballooning, and disruption of the myelin in corpus callosum. We also noted swollen clear/dense and degenerated mitochondria in cortex, hippocampus, and striatum after LIB exposure. Our study further demonstrated transient neurobehavioral dysfunctions including: decreased locomotor activity, increased anxiety-like behaviors, compromised nesting behavior, and mild spatial learning and memory deficits after primary LIB injury. As about 15% of mTBI cases chronically associate with PTSD and other cognitive dysfunctions, it is often difficult to differentiate between the effects of blast mTBI and PTSD, which may also be the results of psychological stress/emotional trauma (Mac Donald et al., 2011, 2017; Wisco et al., 2014). In order to address this issue in our current animal model, future directions will include animal re-exposure to the testing ground to determine whether the observed behavioral changes might be re-introduced by the blast context in absence of actual blast exposures. Our current studies have uncovered unique ultrastructural brain abnormalities at nanoscale levels and associated neurobehavioral impairments due to primary blast injury. These findings address the critical gaps in knowledge about mTBI and provide insights into its pathogenesis of mTBI.
Our novel findings suggest the progression of additional molecular changes leading to secondary injuries, including mitochondrial dysfunction and disruption of axonal transport (Figure 1C). Recent evidence suggests that mitochondria play a critical role in TBI, as impairments of mitochondrial bioenergetics may be linked to neuronal excitotoxicity, disruption of Ca2+ homeostasis, production of reactive oxygen species (ROS), neuroinflammation, and ATP depletion (Hiebert et al., 2015). Further, the mitochondrial changes may lead to release of pro-apoptotic proteins causing potential neuronal damage. Defects in mitochondrial bioenergetics and associated metabolic function are also known to be linked with alterations of the maintenance of mitochondrial integrity, including mitochondrial clearance/dynamics. These effects can in turn, impact axonal transport and autophagy (Misgeld and Schwarz, 2017). Although mitochondrial dysfunction and bioenergetic failure have been implicated in TBI and in certain neurodegenerative diseases, it remains unclear whether such mitochondrial dysfunction is a cause or an effect of the underlying pathology. And whether or not mitochondrial dysfunction represents a viable therapeutic target particularly in primary blast injury to prevent or ameliorate its long term effects (Lezi and Swerdlow, 2012; Watts, 2016). Future investigations will require a focus upon direct links between primary LIB-induced changes in mitochondrial structures and functions and resulting cellular damages or neurochemical imbalance, including respiration, DNA/mRNA levels, and key proteins influencing energy production that may ultimately affect long-term brain functions.
In summary, preclinical animal models of TBI have provided substantial information about primary blast injury, but gaps remain in translating the pathophysiology of animal injury to human injury (Cernak et al., 2017). Ideally, autopsy studies of the human brain exposed to explosive devices are important and should be used to elucidate late injury effects. However, changing neurological and behavioral abnormalities observed in patients with TBI or blast injury may vary depending upon subtle of cellular, sub-cellular and molecular pathological processes. To study such changes, it remains important to use animal models that best simulate actual conditions of blast exposure, though differences in properties of soft tissues of the head or the major anatomical distinctions between human and animals remain as a limitation to be taken into account (Xiong et al., 2013; Jean et al., 2014); sensitive and respectful use of non-human primates suggests way of overcoming this limitation. Accurate and reproducible data are critical in translating and scaling animal findings into human injuries. Biological models using well defined blast conditions, detailed characterization of the physics of exposure, and model standardization offer better alignment between the actual blast exposures and biological consequences seen in real-life scenarios involving human subjects. As recently reported, we have implemented monitoring systems that ensure consistency and quality control of blast exposure in our open-field model. Our findings show that a primary LIB injury appears to contribute “invisible injuries”, solely detectable at ultrastructural levels and associated with well-defined neurobehavioral dysfunctions. The Missouri Blast model, provides a means to define underlying mechanisms of primary blast injury, the effects of repetitive blast exposures, sex differences, and body positions relative to blast source and intensity. Such quantitative observations will provide insights for preventing blast injuries and treatment blast-induced brain trauma. While further investigation is required, the resulting neuropathological and behavioral changes seen in this primary blast model appear to provide a link between experimental injury and human mTBI/concussion effects. Further, we are currently considering use of telemetric sensor probes to explore blast intensity and intracranial pressure effects.
The past decade of blast injury research yielded significant and novel findings regarding the blast physics and biological effects. Our recent observations revealed that primary LIB injury comprises an ‘invisible injury’ characterized by the absence of the macroscopic damage, necrosis, and related to nanoscale ultrastructural injuries and accompanied by neurobehavioral dysfunction up to 30 days after exposure. Using the Missouri blast model, we hope to further link blast physics to underlying injury mechanisms occurring as related to repetitive injury as well as in late non acute stages after blast injury.
Additional file: Open peer review report 1 (99.1KB, pdf) .
Footnotes
Conflicts of interest: None declared.
Financial support: This publication was made possible by funding from the DoD Congressionally Directed Medical Research Programs (CDMRP) for the Peer Reviewed Alzheimer’s Research Program Convergence Science Research Award (PRARP-CSRA; AZ140109) and the research funds of the University of Missouri (to ZG). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the United States government, the DoD, the United States Army or the Department of Veterans Affairs.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Ubaldo Armato, University of Verona Medical School, Italy.
Funding: This publication was made possible by funding from the DoD Congressionally Directed Medical Research Programs (CDMRP) for the Peer Reviewed Alzheimer’s Research Program Convergence Science Research Award (PRARP-CSRA; AZ140109) and the research funds of the University of Missouri (to ZG).
References
- Budde MD, Shah A, McCrea M, Cullinan WE, Pintar F, Stemper BD. Primary blast traumatic brain injury in the rat: relating diffusion tensor imaging and behavior. Front Neurol. 2013;4:154. doi: 10.3389/fneur.2013.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cernak I, Merkle AC, Koliatsos VE, Bilik JM, Luong QT, Mahota TM, Xu L, Slack N, Windle D, Ahmed FA. The pathobiology of blast injuries and blast-induced neurotrauma as identified using a new experimental model of injury in mice. Neurobiol Dis. 2011;41:538–551. doi: 10.1016/j.nbd.2010.10.025. [DOI] [PubMed] [Google Scholar]
- Cernak I, Stein DG, Elder GA, Ahlers S, Curley K, DePalma RG, Duda J, Ikonomovic M, Iverson GL, Kobeissy F. Preclinical modelling of militarily relevant traumatic brain injuries: Challenges and recommendations for future directions. Brain Inj. 2017;31:1168–1176. doi: 10.1080/02699052.2016.1274779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeKosky ST, Ikonomovic MD, Gandy S. Traumatic brain injury--football, warfare, and long-term effects. N Engl J Med. 2010;363:1293–1296. doi: 10.1056/NEJMp1007051. [DOI] [PubMed] [Google Scholar]
- DePalma RG, Burris DG, Champion HR, Hodgson MJ. Blast injuries. N Engl J Med. 2005;352:1335–1342. doi: 10.1056/NEJMra042083. [DOI] [PubMed] [Google Scholar]
- Goel MD, Matsagar VA, Gupta AK, Marburg S. An abridged review of blast wave parameters. Def Sci J. 2012;62:300–306. [Google Scholar]
- Griesbach GS, Masel BE, Helvie RE, Ashley MJ. The impact of traumatic brain injury on later life: effects on normal aging and neurodegenerative diseases. J Neurotrauma. 2018;35:17–24. doi: 10.1089/neu.2017.5103. [DOI] [PubMed] [Google Scholar]
- Hiebert JB, Shen Q, Thimmesch AR, Pierce JD. Traumatic brain injury and mitochondrial dysfunction. Am J Med Sci. 2015;350:132–138. doi: 10.1097/MAJ.0000000000000506. [DOI] [PubMed] [Google Scholar]
- Jean A, Nyein MK, Zheng JQ, Moore DF, Joannopoulos JD, Radovitzky R. An animal-to-human scaling law for blast-induced traumatic brain injury risk assessment. Proc Natl Acad Sci U S A. 2014;111:15310–15315. doi: 10.1073/pnas.1415743111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucherov Y, Hubler GK, DePalma RG. Blast induced mild traumatic brain injury/concussion: A physical analysis. J Appl Phys. 2012a;112:104701. [Google Scholar]
- Kucherov Y, Hubler G, Michopoulos J, Johnson B. Acoustic waves excited by phonon decay govern the fracture of brittle materials. J Appl Phys. 2012b;111:023514. [Google Scholar]
- Lezi E, Swerdlow RH. Mitochondria in neurodegeneration. Adv Exp Med Biol. 2012;942:269–286. doi: 10.1007/978-94-007-2869-1_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mac Donald CL, Barber J, Jordan M, Johnson AM, Dikmen S, Fann JR, Temkin N. Early clinical predictors of 5-year outcome after concussive blast traumatic brain injury. JAMA Neurol. 2017;74:821–829. doi: 10.1001/jamaneurol.2017.0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mac Donald CL, Johnson AM, Cooper D, Nelson EC, Werner NJ, Shimony JS, Snyder AZ, Raichle ME, Witherow JR, Fang R. Detection of blast-related traumatic brain injury in US military personnel. N Engl J Med. 2011;364:2091–2100. doi: 10.1056/NEJMoa1008069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misgeld T, Schwarz TL. Mitostasis in neurons: maintaining mitochondria in an extended cellular architecture. Neuron. 2017;96:651–666. doi: 10.1016/j.neuron.2017.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H, Cui J, Simonyi A, Johnson CE, Hubler GK, DePalma RG, Gu Z. Linking blast physics to biological outcomes in mild traumatic brain injury: Narrative review and preliminary report of an open-field blast model. Behav Brain Res. 2018a;340:147–158. doi: 10.1016/j.bbr.2016.08.037. [DOI] [PubMed] [Google Scholar]
- Song H, Konan LM, Cui J, Johnson CE, Langenderfer M, Grant D, Ndam T, Simonyi A, White T, Demirci U, Mott DR, Schwer D, Hubler GK, Cernak I, DePalma RG, Gu Z. Ultrastructural brain abnormalities and associated behavioral changes in mice after low-intensity blast exposure. Behav Brain Res. 2018b;347:148–157. doi: 10.1016/j.bbr.2018.03.007. [DOI] [PubMed] [Google Scholar]
- Wang H, Zhang YP, Cai J, Shields LB, Tuchek CA, Shi R, Li J, Shields CB, Xu XM. A compact blast-induced traumatic brain injury model in mice. J Neuropathol Exp Neurol. 2016;75:183–196. doi: 10.1093/jnen/nlv019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts LT. Stimulating mitochondria to protect the brain following traumatic brain injury. Neural Regen Res. 2016;11:1403–1404. doi: 10.4103/1673-5374.191205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisco BE, Marx BP, Holowka DW, Vasterling JJ, Han SC, Chen MS, Gradus JL, Nock MK, Rosen RC, Keane TM. Traumatic brain injury, PTSD, and current suicidal ideation among Iraq and Afghanistan US veterans. J Trauma Stress. 2014;27:244–248. doi: 10.1002/jts.21900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y, Mahmood A, Chopp M. Animal models of traumatic brain injury. Nat Rev Neurosci. 2013;14:128–142. doi: 10.1038/nrn3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.