Abstract
Increased reactive oxygen species by the activation of NADPH oxidase (NOX) contributes to the development of diabetic complications. Apocynin, a NOX inhibitor, increases sciatic nerve conductance and blood flow in diabetic rats. We investigated potential protective effect of apocynin in rat diabetic neuropathy and its precise mechanism of action at molecular level. Rat models of streptozotocin-induced diabetes were treated with apocynin (30 and 100 mg/kg per day, intragastrically) for 4 weeks. Mechanical hyperalgesia and allodynia were determined weekly using analgesimeter and dynamic plantar aesthesiometer. Western blot analysis and histochemistry/immunohistochemistry were performed in the lumbar spinal cord and sciatic nerve respectively. Streptozotocin injection reduced pain threshold in analgesimeter, but not in aesthesiometer. Apocynin treatment increased pain threshold dose-dependently. Western blot analysis showed an increase in catalase and NOX-p47phox protein expression in the spinal cord. However, protein expressions of neuronal and inducible nitric oxide synthase (nNOS, iNOS), superoxide dismutase, glutathion peroxidase, nitrotyrosine, tumor necrosis factor-α, interleukin-6, interleukin-1β, aldose reductase, cyclooxygenase-2 or MAC-1 (marker for increased microgliosis) in the spinal cord remained unchanged. Western blot analysis results also demonstrated that apocynin decreased NOX-p47phox expression at both doses and catalase expression at 100 mg/kg per day. Histochemistry of diabetic sciatic nerve revealed marked degeneration. nNOS and iNOS immunoreactivities were increased, while S-100 immunoreactivity (Schwann cell marker) was decreased in sciatic nerve. Apocynin treatment reversed these changes dose-dependently. In conclusion, decreased pain threshold of diabetic rats was accompanied by increased NOX and catalase expression in the spinal cord and increased degeneration in the sciatic nerve characterized by increased NOS expression and Schwann cell loss. Apocynin treatment attenuates neuropathic pain by decelerating the increased oxidative stress-mediated pathogenesis in diabetic rats.
Keywords: apocynin, diabetic complications, experimental diabetes mellitus, neuropathic pain, NADPH oxidase, sciatic nerve, spinal cord, Western blotting, peripheral nerve injury, neural regeneration
Introduction
Diabetic neuropathy (DN) is one of the major complications of diabetes mellitus that is characterized by spontaneous pain, hyperalgesia, and allodynia (Russell and Zilliox, 2014). So far, two theories (i.e., vascular and neurochemical) have been proposed to explain the complex pathogenesis of peripheral neuropathy. According to the “vascular” theory, diabetes-induced endothelial dysfunction results in vascular reactivity, subsequent decrease in nerve blood flow and endoneurial hypoxia (Cameron et al., 2001). “Neurochemical” theory suggests the involvement of Schwann cells, dorsal root ganglion neurons, and spinal cord oligodendrocytes in the pathogenesis (Zenker et al., 2013).
Several pathways are involved to trigger neuroinflammation probably ascending from peripheral nerve to spinal cord, namely, sorbitol pathway through the activation of aldose reductase; non-enzymatic glycation/glycoxidation and formation of advanced glycation end products, oxidative/nitrosative stress, pro-inflammatory response resulting from the activation of protein kinase C, poly(ADP-ribose)polymerase (PARP), transcription factor NF-κB, mitogen-activated protein kinase (MAPK), cyclooxygenase-2 (COX-2) and 12/15-lipoxygenase (12/15-LO) and finally abnormal Ca2+ homeostasis/signaling (Obrosova, 2009). Among them, oxidative stress induced by reactive oxygen species (ROS) is the key factor in the development and progression of diabetic complications and chronic neuropathic pain (Guedes et al., 2008; Babizhayev et al., 2015). ROS are produced intracellularly through multiple mechanisms, one of which is NADPH oxidase enzymes (NOXs) (de M Bandeira et al., 2013). NOXs are expressed primarily in the phagocytic cells, however, all of the major cell types in the brain (neurons, astrocytes, microglia, and vascular endothelial cells) constitutively express several NOXs (Drummond et al., 2011). NOXs mediate neuropathic pain processing (Kallenborn-Gerhardt et al., 2013).
Options for the treatment of painful DN are limited. New therapeutic interventions targeting primary mechanisms contributing to nerve damage are crucial for the promising future treatment of this complication. Recent research focuses on molecules targeting the inhibition of NOX signalling (Kallenborn-Gerhardt et al., 2013). Apocynin, a naturally occurring compound extracted from Picrorhiza kurroa plant, demonstrates NOX inhibitory properties (‘t Hart et al., 1990). Apocynin provides protection against some complications of diabetes, i.e., endothelial dysfunction (Olukman et al., 2010), nephropathy (Nam et al., 2009), and retinopathy (Al-Shabrawey et al., 2008). Current evidence regarding prevention of DN is limited. Available data from rat models show that apocynin partially corrects diabetes-induced reduction in sciatic nerve motor conductance in a dose-independent manner (Cotter and Cameron, 2003) and reverses diabetes-induced decrease in pain withdrawal threshold when given after the clinical features of diabetic neuropathy settle down (Zhao et al., 2014). Apocynin suppresses oxidative stress in the spinal cord of diabetic rats by preventing the increase in NOX expression and decrease in superoxide dismutase (SOD) activity, and reducing hydrogen peroxide and malonyldialdehyde concentration (Zhao et al., 2014). However, it has been noticed that some effects of apocynin in in vitro and in vivo experimental models could not depend on NOX inhibition per se (Aldieri et al., 2008). Therefore, we aimed primarily to investigate the potential protective effect of apocynin in DN rats in the early developmental phase of the disease, and secondarily to clarify its mechanisms of action at the molecular level in diabetic spinal cord with special interest in some of the mechanisms triggering neuroinflammation mentioned above.
Materials and Methods
Induction of diabetes and drug administration
Male Wistar rats (Ege University Experimental Animal Research Centre, 200–250 g, 10–12 weeks old, n = 8–12) were used in the study. The experimental protocol was approved by the Local Ethics Committee for Animal Experiments Ege University (Approval No. 2010-06). The instructions and policies of this committee conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No: 85-23, revised 1996) and the experiments were performed in accordance with these guidelines. The animals were housed in groups of 5 in plastic cages covered with sawdust under a standard 12-hour light/dark cycle in a room maintained at 22 ± 3°C and were given food and water ad libitum.
Diabetes was induced by a single intraperitoneal injection of streptozotocin (55 mg/kg, pH 4.0, Sigma-Aldrich, Interlab A.Ş, İstanbul, Turkey). Hyperglycemia was confirmed by measuring tail vein blood glucose levels with glucometer (Accu-Chek GO, Roche Diagnostics, Turkey) 72 hours after diabetes induction, and expressed as mM. Rats with blood glucose levels ≥ 13.9 mM were considered to be diabetic.
Diabetic rats were randomly divided into three experimental groups with 8–12 rats per group; group 1 received 0.5 mL/d saline (DM), groups 2 and 3 received apocynin (Sigma, St. Louis, MO, USA) 30 mg/kg per day (DM + Apo30) and 100 mg/kg per day (DM + Apo100) in 0.5 mL of saline respectively. Another group of age-matched normal rats (n = 8) receiving 0.5 mL/d saline served as the normal control group (NC). Apocynin or saline was intragastrically administered once a day for 4 weeks via an orogastric catheter (INSTECH, Plymouth Meeting, PA, USA).
Behavioral pain tests
Mechanical hyperalgesia and allodynia were evaluated in both paws of diabetic rats. Pain tests were recorded before the administration of streptozotocin and on post-injection weeks 1, 2, 3 and 4 respectively. Analgesimeter and dynamic plantar aestesiometer (Ugo Basile, Milan, Italy) were used to measure mechanical hyperalgesia and mechanical allodynia respectively. Pain threshold tests were carried out before and weekly after injection of streptozotocin. The tests were performed by an experimenter blinded to the treatments. All rats were habituated to the test environment an hour before the experiments; meanwhile food and water were withdrawn. Stimulus was applied three times to each paw with 5-min intervals and both the mechanical and the allodynia test results were recorded. The mean of three measurements was calculated and accepted as the final test result. Nociceptive thresholds were expressed in grams as the force required to elicit a withdrawal response. Response to the noxious stimulus was expressed as the percentage change in threshold, which was calculated using the following formula: %change in threshold = (post-surgery paw withdrawal latency/pre-surgery paw withdrawal latency) × 100%.
Western blot analysis in the spinal cord
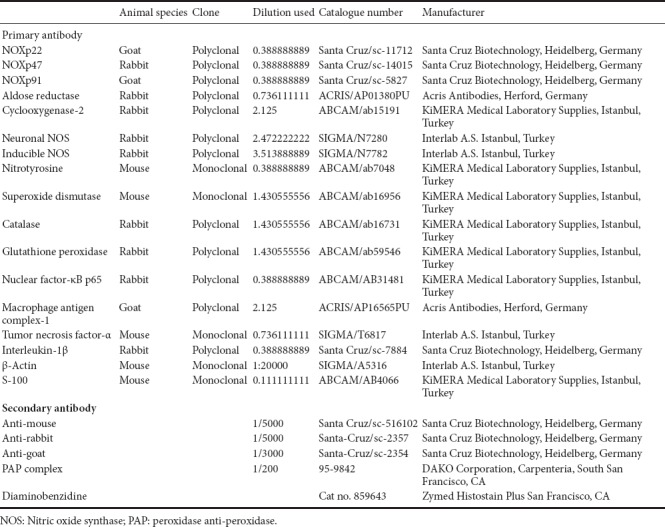
Lumbar segment (L3–4) of spinal cord from rats in all experimental groups was dissected under anesthesia (85/15 mg/kg of ketamine/xylasine, intraperitoneal, Ketalar Pfizer, Istanbul, Turkey, Alfazyne 2% Ege-Vet, Istanbul, Turkey) at the end of 4-week treatment period. Approximate 100 mg samples were homogenized on ice in lysis buffer containing protease inhibitors (1:10, w/v) and centrifuged. Protein concentration in the supernatant was measured by a spectrophotometer. Extracted proteins (80–100 µg) were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto nitrocellulose membrane. Blots were blocked for an hour at room temperature in blocking solutions containing 5% non-fat dried milk to saturate nonspecific protein binding and then incubated overnight at 4°C with antibodies against NOXp47, aldose reductase, COX-2, iNOS, nitrotyrosine, SOD, catalase, glutathione peroxidase, p65NF-κB, macrophage antigen complex (MAC)-1, tumor necrosis factor (TNF)-α, interleukin (IL)-1β and β-actin as reference. After extensive washing, blots were incubated in horseradish peroxidase-linked anti-mouse or anti-rabbit secondary antibodies as appropriate for an hour at room temperature. The names, species and clones of the primary and secondary antibodies, dilutions, catalogue numbers and the names of the suppliers are presented in Table 1. Chemiluminescence detection was developed using an ECL Plus detection kit (Amersham Pharmacia, Pittsburgh, PA, USA) and exposed to X-ray film (Amersham Hyperfilm ECL, Buckinghamshire, UK). The autoradiographs were analyzed by scanning densitometry with subtraction of the background counts measured outside loaded lanes and the intensity of the signal on the blots was measured with ImageJ analysis software of NIH (Bethesda, Maryland, USA). The data were presented as the ratio of protein band density over β-actin band density.
Table 1.
Detailed information on antibodies used for western blotting and immunohistochemistry
Histochemistry and immunohistochemistry in the sciatic nerve
Sciatic nerves from rats in all experimental groups were dissected under anesthesia (85/15 mg/kg of ketamine/xylasine, intraperitoneal, Ketalar Pfizer, Istanbul, Turkey, Alfazyne 2% Ege-Vet, Istanbul, Turkey) at the end of 4-week treatment period and fixed with 2.5% glutaraldehyde in 0.1 M Sørensen’s phosphate buffer (pH 7.2). Postfixation was made with 1% osmium tetraoxide in the same buffered solution. After dehydration through a graded series of ethanol solution, tissue was embedded in epoxy resin (Fluka; Sigma). The specimens were cross-sectioned (20 cross-sections for each specimen) at 0.5-μm using an ultramicrotome (Leica Microsystems) and stained with toluidine blue (Sigma-Aldrich). Sections were examined and photographed by a light microscope (Olympus BX51, Olympus C-5050 digital camera, Olympus, Tokyo, Japan). After epon removal process, sections were subjected to immunohistochemical staining. For immunohistochemistry, paraffin sections were immersed in xylene overnight and immunohistochemical staining was performed using nNOS, iNOS and S-100 and PAP (peroxidase antiperoxidase) complex. Briefly, paraffin sections were immersed in xylene overnight and incubated in methanol containing 1% H2O2 (hydrogen peroxide) to reduce endogenous peroxidase activity. Sections were kept in sodium citrate solution in the microwave oven at 90 W for 5 minutes and at 360 W for 15 minutes. After washing in 0.2 M Tris-HCl, including 0.5% Triton X, the sections were incubated with mouse S-100, nNOS and nNOS primary antibodies overnight at 4°C. Sections were then incubated with anti-iNOS and anti-nNOS and mouse monoclonal PAP complex and reacted with 0.05% diaminobenzidine and 0.01% H2O2 at room temperature.
S-100-immunoreactive cells were counted using imaging analysis software Image-Pro Express 4.5 (Media-Cybernetics Inc., Silver Springs, MD, USA). To ensure the objectivity of S-100 method, the measurement was performed by two independent researchers blind to the treatment groups. An average was taken from the results of these two researchers and accepted as the final S-100-positive cell counts. Cell density (cells/mm2) was calculated for each animal using ten areas randomly selected from ten sciatic nerve sections, and the average S-100-immunoreactive cell density was calculated for each animal. S-100 positive cell number represented the mean S-100-immunoreactive cell density value of each experimental group.
Semiquantitative analysis of nNOS and iNOS immunoreactivities in the sciatic nerve was performed by two independent researchers who examined the slides immunostained for each specific isoform and assigned a score of relative intensity. Relative intensity scores were given solely through visual inspection. Score 0 represented the absence of any immunolocalization, score 1 represented light staining, score 2 represented moderate staining, score 3 represented strong staining, and finally score 4 indicated intense staining. An average of the scoring results of the two independent researchers was taken to confirm the identification and intensity of cellular staining for each isoform.
Statistical analysis
Data were presented as the mean ± standard error of mean (SEM). GraphPad Prism version 6.01 (GraphPad software Inc., La Jolla, CA, USA) was used to analyze the data. The statistical significance of the differences among groups was evaluated by repeated measures of analysis of variance (ANOVA) followed by post-hoc Bonferroni test in the pain threshold tests. Statistical analysis of data obtained from Western blotting experiments and immunohistochemistry was performed using one-way ANOVA followed by post-hoc Bonferroni test. A P-value of < 0.05 was accepted as statistically significant.
Results
Apocynin effect on body weight, blood glucose levels, and pain tests
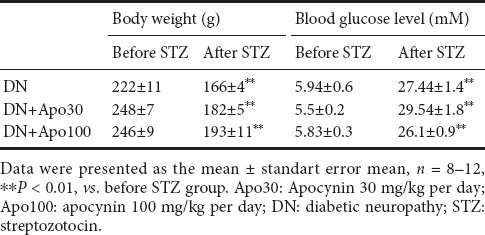
Body weight and fasting blood glucose levels of control rats were 11.9 ± 0.5 and 4.65 ± 0.2 mM, respectively. Rats developing hyperglycaemia (blood glucose levels ≥ 13.9 mM) after streptozotocin injection significantly lost weight (P < 0.01, Table 2). Treatment with apocynin for 4 weeks neither changed the decrease in body weight, nor affected blood glucose levels (Table 2).
Table 2.
Effect of 4-week apocynin treatment on body weight and blood glucose levels of streptozotocin-induced diabetic rats and control rats
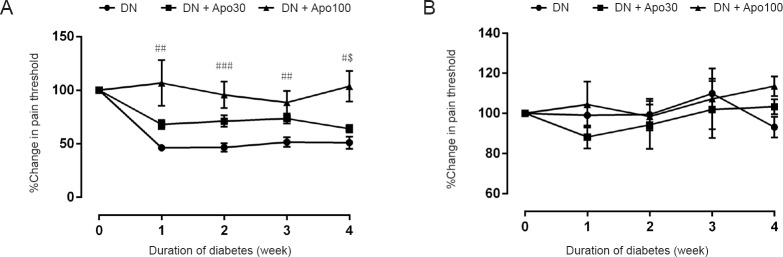
Streptozotocin administration reduced the pain threshold in rats significantly in analgesimeter (P < 0.05 and P < 0.01, Figure 1A), but not in aesthesiometer (Figure 1B). Almost a 50% decrease was observed starting from the first week of injection and persisting throughout the course of experiments. Apocynin treatment slightly increased the pain threshold in analgesimeter at 30 mg/kg per day in DN rats; however, this increase was statistically insignificant (Figure 1A). Meanwhile, pain threshold was markedly increased by apocynin at 100 mg/kg per day, almost returning to normal values recorded before the onset of diabetes.
Figure 1.
Change in pain threshold in diabetic neuropathic (DN) rats at analgesimeter (A) and dynamic plantar aesthesiometer (B) and the effect of apocynin (Apo) treatment on the decreased pain threshold.
Closed circles represent the threshold of DN rats (DN group). Closed squares and closed triangles represent DN rats treated with Apo 30 mg/kg per day (DN + Apo30) and 100 mg/kg per day (DN + Apo100), respectively. Data are presented as the mean ± standard error mean, n = 8–12 rats. The statistical significance of the differences among groups was evaluated by repeated measures of analysis of variance, followed by post-hoc Bonferroni test in the pain threshold tests. #P < 0.05, ##P < 0.01, ###P < 0.01, vs. DN group; $P < 0.05, vs. DN + Apo30 group.
Apocynin effect on protein expression of catalase and NOXp47 in the spinal cord
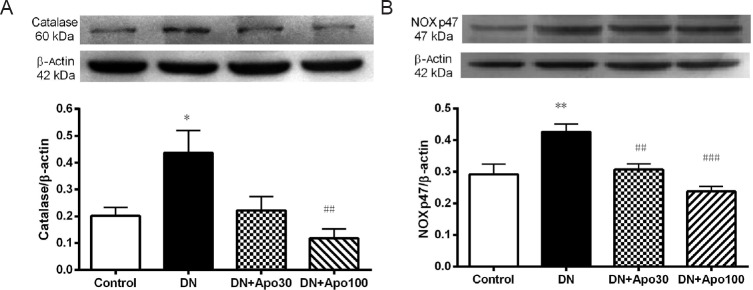
Western blot experiments in spinal cord of DN rats did not show significant changes from control in the expression of nNOS, iNOS, aldose reductase, COX-2, SOD, glutathione peroxidase, nitrotyrosine, TNF-α, IL-6, IL-1β, MAC-1, NF-κB p65 or NOX subunits p22 and gp91 (data not shown). Apocynin treatment did not significantly affect the expression of these proteins. Only the expression of catalase (P < 0.05) and NOXp47 (P < 0.01), the active subunit of NOX, in the spinal cord of DN rats increased significantly (Figure 2). Apocynin treatment significantly prevented the increase in catalase expression at 100 mg/kg/day (P < 0.01; Figure 2A) and NOXp47 expression at either doses (P < 0.05 and P < 0.001 respectively; Figure 2B).
Figure 2.
Effect of apocynin treatment on the protein expression of catalase (A) and NOXp47 (B) in the spinal cord of DN rats.
Control group was composed of healthy rats receiving 1 mL/kg saline for 4 weeks. Data are presented as the mean ± standard error mean. Statistical analysis of data obtained from Western blotting experiments was performed using one-way analysis of variance, followed by post-hoc Bonferroni test. n = 6 rats. *P < 0.05, **P < 0.01, vs. control; #P < 0.05, ##P < 0.01, vs. DN group. DN: Diabetic neuropathy; DN + Apo30; DN rats treated with apocynin 30 mg/kg per day; DN + Apo100: DN rats treated with apocynin 100 mg/kg per day.
Apocynin effect of sciatic nerve histochemistry/immunohistochemistry
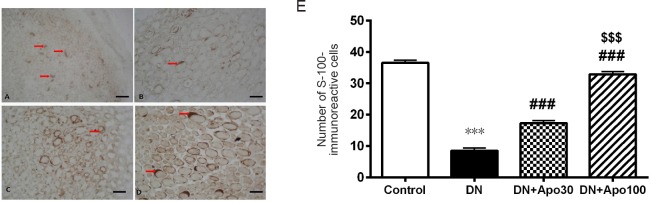
Toluidine staining of sciatic nerve from DN rats revealed marked vasoconstriction, increased endothelial cell activation and proliferation, thickening in basal laminae in the capillary vessels (Figure 3B). There was an increase in extracellular matrix, atrophy in axonal structures, myelin degeneration with loss of Schwann cells and myelin and impairment in configuration (Figure 3B). Schwann cell loss was also confirmed by S-100 immunostaining, a good marker for the cells derived from neural crest (Figure 4). The number of S-100 positive cells significantly decreased in DN rats (P < 0.001; Figure 4). Apocynin treatment at both doses reversed these histopathological changes dramatically (Figure 3C and D) and prevented Schwann cell degeneration and loss dose-dependently (P < 0.001; Figure 4).
Figure 3.
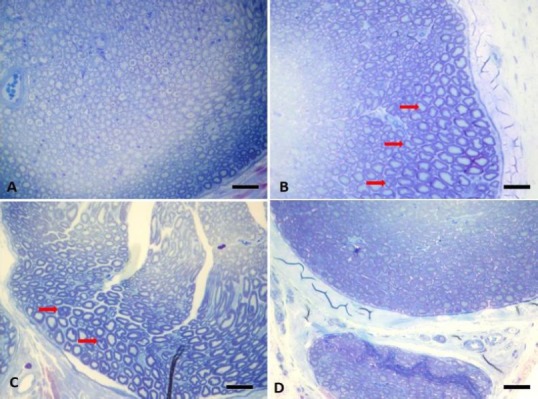
Light microscopic images of the sciatic nerve from normal control rats (A) and DN rats untreated (B) or treated daily either with 30 mg/kg (C) or 100 mg/kg apocynin (D) for 4 weeks.
Toluidine blue staining was performed. Original magnification 40×, scale bars: 125 µm (A). Red arrows represent degenerated axonal structures (B). Toluidine staining of sciatic nerve from DN rats revealed marked vasoconstruction, increased endothelial cell activation and proliferation, thickening in basal lamina in the capillary vessels (B). There was an increase in extracellular matrix, atrophy in axonal structures, myelin degeneration with loss of Schwann cells and myelin and impairment in configuration (B). Apocynin treatment at both doses reversed these histopathological changes dramatically and dose-dependently prevented Schwann cell degeneration (C and D). DN: Diabetic neuropathy.
Figure 4.
Effect of apocynin (Apo) treatment for 4 weeks on the immunoreactivity of S-100 in sciatic nerve of DN rats.
Representative immunohistochemical images of S-100 staining in the sciatic nerve sections: (A) control group, (B) DN group, (C) DN + Apo30 group, (D) DN + Apo100 group. Original magnification, 100×, black bars correspond to 50 µm scale (A). The number of S-100-immunoreactive cells significantly decreased in DN rats (B). Apocynin treatment at both doses reversed these histopathological changes dramatically and dose-dependently prevented Schwann cell degeneration and loss (C and D). Red arrows represent S-100 immunostained axoplasmic structures. (E) Bar graphics show the number of S-100 immunoreactive cells. ***P < 0.001, vs. control group; ###P < 0.001, vs. DN group; $$$P < 0.001, vs. DN + Apo30 group. n = 3 rats. DN: Diabetic neuropathy; Apo30: DN rats treated with apocynin 30 mg/kg per day; Apo100: DN rats treated with Apo 100 mg/kg per day.
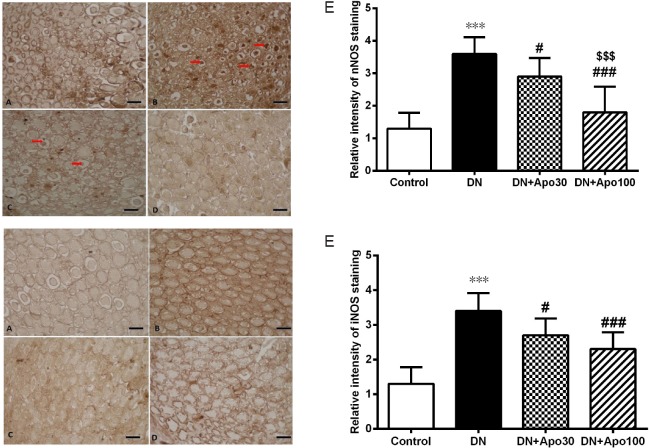
Immunostaining showed marked increases in the expression of both nNOS and iNOS in the sciatic nerve of DN rats (Figure 5). Both doses of apocynin reversed these changes significantly (P < 0.05 for Apo30 group, P < 0.001 for Apo100 group; Figure 5). The names, species, and clones of the primary and secondary antibodies, dilutions, catalogue numbers and the names of the suppliers are presented in Table 2.
Figure 5.
Effect of apocynin (Apo) treatment for 4 weeks on the protein expression of nNOS and iNOS in sciatic nerve of DN rats.
Representative immunohistochemical images of nNOS (upper panel) and iNOS (lower panel) staining in the sciatic nerve sections: control rats (A), DN rats untreated (B) or treated daily with 30 mg/kg Apo (C) or 100 mg/kg Apo (D) for 4 weeks. Original magnification, 100×, black bars correspond to 50 µm scale (A). Red arrows represent nNOS immunostained axoplasmic structures. Immunostaining showed marked increases in the expression of both nNOS and iNOS in sciatic nerve of DN rats (B). Both doses of Apo reversed these changes significantly (C and D). Relative intensity of nNOS (upper panel) and iNOS (lower panel) immunostaining in the sciatic nerve sections is shown in E. ***P < 0.001, vs. control; #P < 0.05, ###P < 0.001, vs. DN group; $$$P < 0.001, vs. DN + Apo30 group. n = 3 rats. Apo30: DN rats treated with Apo 30 mg/kg per day; Apo100: DN rats treated with Apo 100 mg/kg per day. nNOS: Neuronal nitric oxide synthase; iNOS: inducible nitric oxide synthase; DN: diabetic neuropathy.
Discussıon
Data of the present study revealed that a single intraperitoneal injection of streptozotocin (55 mg/kg) to adult rats caused stable hyperglycaemia and a gradual decrease in body weight over 4 weeks. There was a marked decrease in pain threshold (nearly 50%) starting from the first week of streptozotocin injection and persisting throughout the experimental period for 4 weeks. These findings in diabetic rats were accompanied by a marked decrease in Schwann cell count, loss of myelin and impairment in configuration and an increase in the expression of nNOS and iNOS protein in sciatic nerve, indicating a significant breakdown of sciatic nerve health. These pathological findings in DN rat model mimic human disease, indeed, it has been well documented that axonal degeneration and segmental demyelination are the main pathological characteristics of neuropathic damage induced by hyperglycaemia in humans (Román-Pintos et al., 2016). Experiments at the spinal level revealed that sustainable hyperglycaemia increased the expression of NOXp47, the active subunit of NOX, and catalase in the spinal cord of DN rats. Four-week treatment with apocynin had no effect on hyperglycaemia or weight loss, however, it ameliorated hyperglycaemia-induced hyperalgesia and prevented sciatic nerve damage as well as the increase in spinal NOXp47 and catalase expressions. These findings suggest that apocynin does not improve hyperalgesia by enhancing diabetes control, but through direct effects on oxidation and/or nerve cells.
We used two tests to evaluate pain threshold in DN rats. Analgesimeter performs mechanical pressure mostly on the dorsal surface of paws according to the Randall-Selitto method (Santos-Nogueira et al., 2012). It evaluates mechanical nociceptive withdrawal threshold even at early stages of rat neuropathic pain, assesses “hyperalgesia” (augmented pain response to normally painful stimuli) and it is used as an indicator of peripheral sensitization. Dynamic plantar aesthesiometer assesses “touch sensitivity” on the plantar surface of rodents and it is used to evaluate “allodynia” (exaggerated pain sensations to normally nonpainful stimuli) as an indicator of central sensitization (Sandkühler, 2009). Decreased pain threshold in analgesimeter, but not in dynamic plantar aestesiometer, in our DN rats suggests that hyperglycaemia, as a chemical stimulus, is capable of activating nociceptors at this stage of diabetes inducing peripheral, but not central, sensitization. Longer exposure to hyperglycaemia may be required to impair touch sensitivity. Indeed, both mechanical hyperalgesia and tactile allodynia has been reported in streptozotocin-diabetic animals, allodynia developing at later stages of diabetes probably after the first 4 weeks of streptozotocin injection (Morrow, 2004).
Treatment with apocynin at 100 mg/kg/day completely prevented the development of hyperalgesia and the beneficial effect of apocynin was observed from the first week of hyperglycaemic state, suggesting the contribution of NOX activity in the whole developmental process of DN. In parallel, spinal expression of the active form of NOX, i.e., p47 protein of NOX2, was higher in DN rats which was completely blocked by apocynin treatment. This finding suggests that although signs of central sensitization is not available after 4-week exposure to hyperglycaemia, molecular signs of central sensitization exists at spinal cord level. Indeed, it has been reported that streptozotocin injection results in elevated NOX expression, increased H2O2 and malondialdehyde levels and decreased SOD activity in the spinal cord of DN rats (Zhao et al., 2014). Spinal cord NOXp47 expression increased in our model without any change in SOD or glutathione peroxidase expression. Such controversy in the levels of antioxidant enzymes in diabetes exists in other tissues. Among the parameters evaluating oxidative-nitrosative stress in spinal cord in our study (i.e., expression of iNOS, nitrotyrosine, SOD, catalase and glutathione peroxidase), only catalase expression was higher in the diabetic group. Papers evaluating protein or mRNA expression or the activity of oxidative stress parameters in diabetic neuropathy are controversial. Increase in mRNA of catalase has similarly been reported in the superior cervical ganglion of diabetic rats without any change in SOD or glutathione peroxidase expression, however, the duration of diabetic state is longer, i.e., 12 months after induction of diabetes (Kishi et al., 2000). There is probably time-dependency of oxidative stress in diabetes. Increased catalase activity may be due to induction by superoxide anion and hydrogen peroxide per se which has already been shown in diabetic kidney, liver and aorta (Majithiya and Balaraman, 2005) and may be suggested as a compensatory response due to increased oxidative stress at earlier stages of diabetes. This possible compensatory response was prevented by apocynin treatment in our study, re-confirming that NOX is at least one of the first inducers of oxidative stress in diabetic spinal tissue. Although neuropathy is a late complication of diabetes, it can develop at the earliest stages of glucose dysregulation in humans as well (Russell and Zilliox, 2014).
Among NOXs, NOX2 isoform is extensively expressed in the spinal cord and active NOX2 produces superoxide radical, which can be converted into other superoxide-derived oxidants such as H2O2, hydroxyl radicals, and peroxynitrite (Patel et al., 2005). Reactive oxygen species (ROS) mediate activation of pro-inflammatory pathways through MAPK and NFκB signaling which, then, regulate the expression of downstream factors such as TNF-α, iNOS, and monocyte chemotractant protein (MCP)-1 (Aldskogius and Kozlova, 1998). This oxidative process and spinal microglial activation are well proven in traumatic neuropathic and inflammatory pain models, however, evidence on the contribution of oxidative stress in the pathogenesis of DN at spinal level is considerably limited. It is noteworthy in our study to mention that NOX contributes to diabetic neuropathy at spinal level even at the early stages of hyperglycaemic insult. However, the expression of downstream factors (i.e., TNF-α, IL-6, IL-1β, MAC-1, NF-κB p65) and other mediators that may involve in DN pathogenesis (i.e., aldose reductase, COX or NOS pathways) remained unchanged at spinal level. Looking at peripheral nerve level, both nNOS and iNOS expression increased at sciatic nerve and sciatic nerve histology showed marked impairment, indicating that hyperglycaemia primarily affects sciatic nerve and spinal changes occur later. This finding suggests that metabolic alterations can lead to structural changes over time from peripheral to central nervous system and the earlier the disease is treated, the better the neuronal damage and neuropathic pain is prevented. NOX-mediated oxidative stress both in sciatic nerve and spinal cord contributes significantly in the pathophysiology of DN, therefore, it might be a novel target for the development of adjuvant therapy in DN.
In conclusion, streptozotocin-induced diabetes in rats results in hyperalgesia, but not allodynia, at early stages of the experimental model. Decreased pain threshold of hyperglycaemic rats is accompanied by increased expression of NOX and catalase protein in spinal cord and increased axonal degeneration in sciatic nerve with Schwann cell loss and increased NOS expression. Apocynin treatment attenuates neuropathic pain by decelerating the increased oxidative stress-mediated pathogenesis in diabetic rats. While NOX activation alone cannot completely account for oxidative stress-related dysfunction, it may provide a novel potential therapeutic target for DN.
Footnotes
Conflicts of interest: There is no conflict of interest to declare in this work.
Financial support: This study was supported by the Research Fund of Ege University (Project No. 2010-TIP-076). The funding body played no role in the study design, in the collection, analysis and interpretation of data, in the writing of the paper, and in the decision to submit the paper for publication.
Institutional review board statement: The experimental protocol was approved by the Ethics Committee for Animal Experiments Ege University (Approval No. 2010-06) and performed in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996).
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Funding: This study was supported by the Research Fund of Ege University (Project No. 2010-TIP-076).
References
- ‘t Hart BA, Simons JM, Knaan-Shanzer S, Bakker NP, Labadie RP. Antiarthritic activity of the newly developed neutrophil oxidative burst antagonist apocynin. Free Radic Biol Med. 1990;9:127–131. doi: 10.1016/0891-5849(90)90115-y. [DOI] [PubMed] [Google Scholar]
- Al-Shabrawey M, Rojas M, Sanders T, Behzadian A, El-Remessy A, Bartoli M, Parpia AK, Liou G, Caldwell RB. Role of NADPH oxidase in retinal vascular inflammation. Invest Ophthalmol Vis Sci. 2008;49:3239–3244. doi: 10.1167/iovs.08-1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldieri E, Riganti C, Polimeni M, Gazzano E, Lussiana C, Campia I, Ghigo D. Classical inhibitors of NOX NAD(P)H oxidases are not specific. Curr Drug Metab. 2008;9:686–696. doi: 10.2174/138920008786049285. [DOI] [PubMed] [Google Scholar]
- Aldskogius H, Kozlova EN. Central neuron-glial and glial-glial interactions following axon injury. Prog Neurobiol. 1998;55:1–26. doi: 10.1016/s0301-0082(97)00093-2. [DOI] [PubMed] [Google Scholar]
- Babizhayev MA, Strokov IA, Nosikov VV, Savel’yeva EL, Sitnikov VF, Yegorov YE, Lankin VZ. The role of oxidative stress in diabetic neuropathy: generation of free radical species in the glycation reaction and gene polymorphisms encoding antioxidant enzymes to genetic susceptibility to diabetic neuropathy in population of type I diabetic patients. Cell Biochem Biophys. 2015;71:1425–1443. doi: 10.1007/s12013-014-0365-y. [DOI] [PubMed] [Google Scholar]
- Cameron NE, Eaton SE, Cotter MA, Tesfaye S. Vascular factors and metabolic interactions in the pathogenesis of diabetic neuropathy. Diabetologia. 2001;44:1973–1988. doi: 10.1007/s001250100001. [DOI] [PubMed] [Google Scholar]
- Cotter MA, Cameron NE. Effect of the NAD(P)H oxidase inhibitor, apocynin, on peripheral nerve perfusion and function in diabetic rats. Life Sci. 2003;73:1813–1824. doi: 10.1016/s0024-3205(03)00508-3. [DOI] [PubMed] [Google Scholar]
- de M Bandeira S, da Fonseca LJ, da S Guedes G, Rabelo LA, Goulart MO, Vasconcelos SM. Oxidative stress as an underlying contributor in the development of chronic complications in diabetes mellitus. Int J Mol Sci. 2013;14:3265–3284. doi: 10.3390/ijms14023265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond GR, Selemidis S, Griendling KK, Sobey CG. Combating oxidative stress in vascular disease: NADPH oxidases as therapeutic targets. Nat Rev Drug Discov. 2011;10:453–471. doi: 10.1038/nrd3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guedes RP, Araújo AS, Janner D, Belló-Klein A, Ribeiro MF, Partata WA. Increase in reactive oxygen species and activation of Akt signaling pathway in neuropathic pain. Cell Mol Neurobiol. 2008;28:1049–1056. doi: 10.1007/s10571-008-9279-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallenborn-Gerhardt W, Schröder K, Geisslinger G, Schmidtko A. NOXious signaling in pain processing. Pharmacol Ther. 2013;137:309–317. doi: 10.1016/j.pharmthera.2012.11.001. [DOI] [PubMed] [Google Scholar]
- Kishi Y, Nickander KK, Schmelzer JD, Low PA. Gene expression of antioxidant enzymes in experimental diabetic neuropathy. J Peripher Nerv Syst. 2000;5:11–18. doi: 10.1046/j.1529-8027.2000.00144.x. [DOI] [PubMed] [Google Scholar]
- Majithiya JB, Balaraman R. Time-dependent changes in antioxidant enzymes and vascular reactivity of aorta in streptozotocin-induced diabetic rats treated with curcumin. J Cardiovasc Pharmacol. 2005;46:697–705. doi: 10.1097/01.fjc.0000183720.85014.24. [DOI] [PubMed] [Google Scholar]
- Morrow TJ. Animal models of painful diabetic neuropathy: the STZ rat model. Curr Protoc Neurosci. 2004;(Chapter 9: Unit 9.18) doi: 10.1002/0471142301.ns0918s29. [DOI] [PubMed] [Google Scholar]
- Nam SM, Lee MY, Koh JH, Park JH, Shin JY, Shin YG, Koh SB, Lee EY, Chung CH. Effects of NADPH oxidase inhibitor on diabetic nephropathy in OLETF rats: the role of reducing oxidative stress in its protective property. Diabetes Res Clin Pract. 2009;83:176–182. doi: 10.1016/j.diabres.2008.10.007. [DOI] [PubMed] [Google Scholar]
- Obrosova IG. Diabetes and the peripheral nerve. Biochim Biophys Acta. 2009;1792:931–940. doi: 10.1016/j.bbadis.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Olukman M, Orhan CE, Celenk FG, Ulker S. Apocynin restores endothelial dysfunction in streptozotocin diabetic rats through regulation of nitric oxide synthase and NADPH oxidase expressions. J Diabetes Complications. 2010;24:415–423. doi: 10.1016/j.jdiacomp.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Patel M, Li QY, Chang LY, Crapo J, Liang LP. Activation of NADPH oxidase and extracellular superoxide production in seizure-induced hippocampal damage. J Neurochem. 2005;92:123–131. doi: 10.1111/j.1471-4159.2004.02838.x. [DOI] [PubMed] [Google Scholar]
- Román-Pintos LM, Villegas-Rivera G, Rodríguez-Carrizalez AD, Miranda-Díaz AG, Cardona-Muñoz EG. Diabetic polyneuropathy in type 2 diabetes mellitus: inflammation, oxidative stress, and mitochondrial function. J Diabetes Res. 2016;2016:3425617. doi: 10.1155/2016/3425617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell JW, Zilliox LA. Diabetic neuropathies. Continuum (Minneap Minn) 2014;20:1226–1240. doi: 10.1212/01.CON.0000455884.29545.d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandkühler J. Models and mechanisms of hyperalgesia and allodynia. Physiol Rev. 2009;89:707–758. doi: 10.1152/physrev.00025.2008. [DOI] [PubMed] [Google Scholar]
- Santos-Nogueira E, Redondo Castro E, Mancuso R, Navarro X. Randall-Selitto test: a new approach for the detection of neuropathic pain after spinal cord injury. J Neurotrauma. 2012;29:898–904. doi: 10.1089/neu.2010.1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenker J, Ziegler D, Chrast R. Novel pathogenic pathways in diabetic neuropathy. Trends Neurosci. 2013;36:439–449. doi: 10.1016/j.tins.2013.04.008. [DOI] [PubMed] [Google Scholar]
- Zhao WC, Zhang B, Liao MJ, Zhang WX, He WY, Wang HB, Yang CX. Curcumin ameliorated diabetic neuropathy partially by inhibition of NADPH oxidase mediating oxidative stress in the spinal cord. Neurosci Lett. 2014;560:81–85. doi: 10.1016/j.neulet.2013.12.019. [DOI] [PubMed] [Google Scholar]