Abstract
Nuclear factor κ-light-chain-enhancer of B cells (NF-κB) is one of the most important tumorigenic factors. Although it has been established that NF-κB is overly activated in human glioma cells, the molecular mechanisms that lead to the signal transduction to NF-κB and thereby the induction of resistance to apoptosis remain poorly understood. The present study demonstrated that mRNA and protein levels of E3 ubiquitin-protein ligase 2 (MIB2) were markedly upregulated in glioma cell lines and clinical samples. Immunohistochemical analysis also revealed high levels of MIB2 expression in glioma specimens. Ectopic overexpression of MIB2 was established in glioma cell lines to investigate its fundamental roles in the response of human glioma to apoptotic inducers. The results indicated that ultraviolet irradiation-induced cell apoptosis was inhibited with MIB2 overexpression in glioma cells. Notably, knockdown of MIB2 using RNA interference was able to increase the sensitivity of glioma cells to the pro-apoptotic agents. The present study identified that MIB2 induces NF-κB activation and facilitates the resistance of glioma cell to apoptosis. It was proposed that MIB2 may not only be an important hallmark to glioma disease progression, but that it may also offer novel clinical strategies to overcome resistance to cancer therapies.
Keywords: E3 ubiquitin-protein ligase 2, glioma, nuclear factor κ-light-chain-enhancer of B cells
Introduction
Gliomas are the most common and lethal type of primary intracranial tumor of the central nervous system and the median survival time for patients with glioma is ~15 months (1,2). Despite current advancements in diagnosis and therapeutic modalities, surgery remains the primary mode of treatment, as the majority of gliomas largely develop resistance to chemotherapy or radiotherapy (3). Due to the high levels of aggressiveness and inducible resistance to chemotherapy and radiotherapy associated with this disease, conventional treatments generally fail to produce positive outcomes (4). As a result, efforts have been redirected toward identifying a novel molecular target to glioma cells, for the future development of more effective therapies (5).
Apoptosis is a form of programmed cell death that functions to eliminate abnormal or transformed cells (6). Consequently, studies involving the utilization of the apoptotic pathway to target over-proliferating tumor cells have gained attention. It has been established that resistance to apoptotic signals and the inhibition of apoptosis are associated with glioma genesis (7). Dysregulation of apoptosis may not only promote the survival and proliferation of glioma cells, but also antagonize the effects of apoptotic inducers (e.g. Tumor necrosis factor-related apoptosis-inducing ligand) thereby developing resistance to chemotherapy and radiotherapy and increasing the risk of malignant phenotypes in cancer cells (8). However, much remains unknown regarding the underlying molecular mechanism of how glioma cells develop an apoptosis-resistant phenotype.
Nuclear factor κ-light-chain-enhancer of B cells (NF-κB) has been established as a pro-survival and anti-apoptotic oncogenic factor in the majority of the tumor types, and various oncogenes and growth factors may be additionally upregulated by NF-κB activation in tumor cells (8–10). Evidence has revealed that NF-κB activity is commonly elevated in glioma, and that tumor grading and prognosis are associated with its level of expression (11,12). Additional studies have also suggested the involvement of NF-κB activation in the anti-apoptotic responses of glioma cells (13,14). Therefore, it is important to identify novel molecular mechanisms that are associated with the aberrant activation of NF-κB in glioma that facilitate the anti-apoptotic responses.
In non-dividing cells, NF-κB is sequestered in the cytosol by the inhibitor of κB (IκB) family proteins, B-cell lymphoma (Bcl) 3 protein, p105 and p100 (15). However, the presence of certain antigens, cytokines and environmental stress signals may lead to the phosphorylation and degradation of IκBα through the 26S-proteosome-dependent system (16). Following the release of NF-κB and its nuclear translocation, NF-κB-targeted genes are activated (17). The phosphorylation of IκBα is accomplished by the IκB kinase (IKK) complex. Studies in breast cancer have previously identified IKK as being an oncogene and activator of the NF-κB pathway to facilitate cell transformation and to enable cancer cells to maintain a malignant phenotype (16). Elevated NF-κB expression has been detected in human ovarian and prostate cancer, which contributes to tumorigenesis, drug resistance and the promotion of cancer progression (18–20). However, a comprehensive understanding of what controls NF-κB expression and activity in cancer cells is lacking and, to the best of our knowledge, the upstream regulator of NF-κB signaling has yet to defined.
Ubiquitination of proteins is of key importance in post-translational modification, targeting proteins for degradation via the proteasome, membrane trafficking, DNA repair, protein kinase activation and chromatin modification (21). In an attempt to elucidate how glioma cells usurp the NF-κB signaling cascade to adopt an apoptosis-resistant phenotype, the present study aimed to investigate the involvement of E3 ubiquitin-protein ligase MIB2 (MIB2), an E3 ligase that catalyzes the addition of single or short chains of ubiquitin to lysine (22). The association between NF-κB activation and MIB2 expression is not well understood. The differential expression of MIB2 in glioma cells and its function in the NF-κB pathway may serve a role in the anti-apoptotic responses of glioma cells. The present study demonstrated a novel interaction between NF-κB and MIB2, and investigated the effects of these proteins on the induction of resistance to apoptosis. The presence of aberrant MIB2 expression in tumor specimens from patients with glioma also supports the hypothesis that the MIB2/NF-κB interaction serves an essential role in the development of drug resistance.
Materials and methods
Clinical specimens
Tissues used in the present study were obtained from 69 patients with glioma who had undergone craniotomy and tumor extirpation between March 2008 and December 2010 at the Shenzhen People's Hospital (Shenzhen, China). The present study was approved by the Ethics Committee of Shenzhen People's Hospital. The pathological diagnosis and grading of glioma were assessed according to World Health Organization (WHO) grade II–IV classification, which is based on the histological features of a heterogeneous population of tumors with varying prognoses and treatments (23). Clinical information of the samples is summarized in Table I. In addition, 4 normal brain tissues were obtained via donations from road traffic accident fatalities without any prior pathologically-detectable conditions. Written informed consent was obtained from all patients.
Table I.
Clinicopathological characteristics of patient samples and expression of MIB2 in glioma.
| Patient characteristic | n | % |
|---|---|---|
| Sex | ||
| Male | 39 | 56.5 |
| Female | 30 | 43.5 |
| Age, years | ||
| ≤50 | 53 | 76.8 |
| >50 | 16 | 23.2 |
| Grade | ||
| I | 8 | 11.6 |
| II | 24 | 34.8 |
| III | 21 | 30.4 |
| IV | 16 | 23.2 |
| MIB2 score | ||
| 0 | 3 | 4.3 |
| 1-4 | 15 | 21.7 |
| 5-8 | 23 | 33.3 |
| 9-12 | 28 | 40.7 |
MIB2, E3 ubiquitin-protein ligase.
Cell lines
Primary normal human astrocytes (NHA) were purchased from Lonza Group Ltd. (Basel, Switzerland) and maintained in Clonetics™ Astrocyte Growth Medium (Lonza Group Ltd.) supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA). Glioma T98G, LN-18 and A172 cell lines were purchased from the American Type Culture Collection (Manassas, VA, USA), and maintained in Dulbecco's modified Eagle's medium (Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.).
Plasmids and small interfering (si)RNA
The pNF-κB-Luc plasmids were purchased from the Clontech Laboratories, Inc. (Mountainview, CA, USA), and the Flag-MIB2 plasmids used for MIB2 overexpression were purchased from Addgene, Inc. (Cambridge, MA, USA). Human MIB2 siRNA was obtained from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). Glioma T98G cells were transfected with siRNAs targeting MIB2 (10 nM) or with control siRNA (10 nM) using Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific, Inc.). The transfection was performed at 37°C in a humidified incubator with 5% CO2. After 24 h, cells were harvested for subsequent experimentation.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
RNA was extracted from T98G, LN-18 and A172 cells or patient tissues using TRIzol® reagent (Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. A total of 5 µg RNA from each sample was reverse transcribed to complementary cDNA (at 42°C for 1 hr) using the SMART® MMLV Reverse Transcriptase kit (cat. no. 639523; Takara Bio, Inc., Otsu, Japan). qPCR was performed using QuantiTect SYBR Green PCR Master mix (Qiagen GmbH, Hilden, Germany) and was analyzed on an ABI Prism 7700 analyzer (Applied Biosystems; Thermo Fisher Scientific, Inc.). PCR was performed under the following conditions: Initial denaturation at 95°C for 5 min, followed by 35 cycles of 95°C for 30 sec, 55°C for 1 min and 72°C for 1 min, with a final extension step at 72°C for 5 min. GAPDH served as the internal control. The mRNA expression levels of MIB2 were normalized to those of GAPDH, and relative quantification was performed using the comparative cycle threshold method (2−ΔΔCq) (24). All PCR experiments were repeated ≥3 times. The sequences of the primers were as follows: MIB2 forward, 5′-CATCGGCGACCTTGACACA-3′ and reverse, 5′-CGACTGCTACACGCAGGTT-3′; and GAPDH forward, 5′-ACAACTTTGGTATCGTGGAAGG-3′ and reverse, 5′-GCCATCACGCCACAGTTTC-3′.
Western blot (WB) analysis
T98G, LN-18 and A172 cells were plated in tissue culture dishes overnight. Following harvest, adherent cells were washed with cold PBS twice and resuspended in lysis buffer (150 mM NaCl, 50 mM Tris-HCl, pH 7.4, 2 mM EDTA, 1% NP-40) containing protease inhibitor cocktail (Amresco, LLC, Solon, OH, USA). Tissues were dissociated using the Brain Tumor Dissociation kit (cat. no. 130-095-942; Miltenyi Biotec, Inc., Auburn, CA, USA). Protein levels in the extracts were quantified using a BCA assay (Pierce; Thermo Fisher Scientific, Inc.). Equal amounts of 100 µg total protein extracts were resolved by 10% standard sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred onto a polyvinylidene fluoride membrane (0.45 mm; EMD Millipore, Billerica, MA, USA). Membranes were blocked with 5% fat-free dry milk/Tris-buffered saline with 5% Tween-20 (cat. no. 9997; Cell Signaling Technology Inc., Danvers, MA, USA) at room temperature for 1 h, then incubated with the following anti-human primary antibodies overnight at 4°C: Anti-MIB2 (1:1,000; cat. no. sc-55149; Santa Cruz Biotechnology, Inc.), anti-α-tubulin (1:5,000; cat. no. T6074; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), anti-phosphorylated (p)-NF-κB p65 subunit (1:1,000; NF-κB p65; cat. no. sc-33020; Santa Cruz Biotechnology, Inc.), anti-caspase-3 (1:2,000; cat. no. sc-1225; Santa Cruz Biotechnology, Inc.) and anti-Bcl-2 (1:2,000; cat. no. sc-783 Santa Cruz Biotechnology, Inc.). Membranes were subsequently incubated with a horseradish peroxidase-conjugated goat anti-mouse immunoglobulin G (IgG) (cat no. sc-2005; 1:5,000; Santa Cruz Biotechnology, Inc.) or goat anti-rabbit IgG (cat no. sc-2004; 1:5,000; Santa Cruz Biotechnology, Inc.) secondary antibody for 1 h at room temperature and visualized using an enhanced chemiluminescence reagent (PerkinElmer, Inc., Waltham, MA, USA). ImageJ software (1.46p; National Institutes of Health, Bethesda, MD, USA) was used for densitometry analysis.
Immunohistochemistry (IHC)
Immunohistochemical staining of MIB2 protein was performed to examine changes in protein expression in 69 cases of human glioma samples. An anti-MIB2 antibody (anti-MIB2; 1:100; cat. no. sc-55149; Santa Cruz Biotechnology, Inc.) was used as the primary antibody to detect MIB2, and the procedure was performed as previously described. Immunohistochemistry was performed on 10% formalin-fixed (for 24-48 h at room temperature), paraffin-embedded tissue sections using the Envision peroxidase detection method (25). Sections (4-µm) were deparaffinized in xylene, dehydrated through a graded ethanol series at 10 min intervals (100, 70, 40 and 0%) and subsequently treated with 0.3% hydrogen peroxide in methanol for 30 min at room temperature to eliminate the endogenous peroxidase activity. For antigen retrieval, the sections were placed in 10 mM sodium citrate buffer (pH 6.0) and heated to near boiling (95-98°C) in a water bath for 20 min (or an oven) followed by cooling for 20 min at room temperature. The primary antibodies (anti-MIB2; 1:100; cat. no. sc-55149; Santa Cruz Biotechnology, Inc.) were incubated overnight at 4°C subsequent to blocking non-specific binding with 10% normal goat serum in PBS for 30 min at room temperature. For negative controls, PBS was used as a substitute for the primary antibodies. Sections were incubated with the secondary biotinylated antibody (Envision peroxidase complex; horseradish peroxidase conjugated; 1:200; cat. no. ZK-9600; OriGene Technologies, Inc., Rockville, MD, USA) for 20 min at room temperature. Sections were counterstained with Mayer's hematoxylin after immunostaining prior to mounting for 30 sec at room temperature (26). Immunohistochemical staining for protein expression in tumor lesions and normal tissues was assessed by 3 pathologists (1 chief pathologist and 2 associate chief pathologists; Pathology Department of Shenzhen People's Hospital, Shenzhen, China) blinded to the origin of the samples using a semi-quantitative method. An upright light microscope (Positive-ECLIPSE E200-LED; Nikon Corporation, Tokyo, Japan) was used at a magnification of ×200. H-scores were calculated for each sample according to the intensity of the nucleic and cytoplasmic staining. The expression levels of MIB2 were scored based on the staining intensity and distribution using the immunoreactive score (IRS), as follows: IRS = staining intensity (SI) × percentage of positive cells (PP). The SI was determined as follows: Absent, 0; weak, 1; moderate, 2; and strong, 3. The PP was scored as follows: 0%, 0; 0-25%, 1; 25-50%, 2; 50-75%, 3; and 75-100%, 4 (27,28).
Cell viability assay
Cells were plated in 6-well plates at a density of 5×105 cells per well and incubated for 24 h at 37°C, followed by irradiation with ultraviolet (UV; 20 or 40 J/m2) and an additional 12-h incubation at 37°C. The numbers of viable cells were determined by the trypan blue dye exclusion test. Briefly, the Glioma T98G cells were seeded in six-well plates (1×105 cells/ml). A 10 µl cell suspension was mixed with 10 µl 0.4% trypan blue for 3-5 min at room temperature (Nacalai Tesque, Inc., Kyoto, Japan), and live cells were counted manually using a hemacytometer (Erma, Inc., Tokyo, Japan). The results were expressed as the percentage of the values obtained when the cells were grown in the absence of reagents.
Annexin V binding assay
The ApopNexin Annexin V-fluorescein isothiocyanate apoptosis kit (cat. no. APT750; Merck KGaA) was used to quantitatively examine apoptotic cells using a flow cytometer using a BD FACSCanto™ II apparatus (BD Biosciences, Franklin Lakes, NJ, USA) with BD FACSChorus™ software (FCS 3.1) for analysis according to the manufacturer's protocol.
NF-κB-dependent reporter gene transcription assay
A pNF-κB-Luc plasmid for NF-κB luciferase reporter assay was obtained from Clontech Laboratories, Inc. NF-κB-dependent luciferase activity was measured using a Dual-Luciferase Reporter Assay system (Promega Corporation, Madison, WI, USA) according to the manufacturer's protocol. Briefly, Glioma T98G cells (1×105 cells/well) were seeded in a 96-well plate for 24 h. The cells were then transfected with plasmids using Lipofectamine® 2000 in each well and then incubated for a transfection period of 24 h at 37°C. Subsequently, the Dulbecco's modified Eagle's medium was removed and replaced with fresh medium. Luciferase activity was determined in a MicroLumat plus luminometer (Berthold Technologies GmbH Co., Bad Wildbad, Germany) by injecting 100 µl of assay buffer containing luciferin and measuring light emission for 10 sec. Co-transfection with pRL-CMV (Promega Corporation), which expresses Renilla luciferase, was performed to enable normalization of data for transfection efficiency.
Statistical analysis
All statistical analyses were performed using the SPSS 10.0 statistical software package (SPSS, Inc., Chicago, IL, USA) and data were expressed as the mean ± standard deviation. The differences between experimental conditions were compared individually using Student's t-tests. Comparisons within groups underwent P-values were calculated using one-way analysis of variance (ANOVA) followed by Tukey's post hoc test, two-way ANOVA followed by Tukey's post hoc test or Bonferroni's tests, or paired t-tests. P<0.05 was considered to indicate a statistically significant difference.
Results
Elevated MIB2 expression in glioma cell lines and human glioma specimens
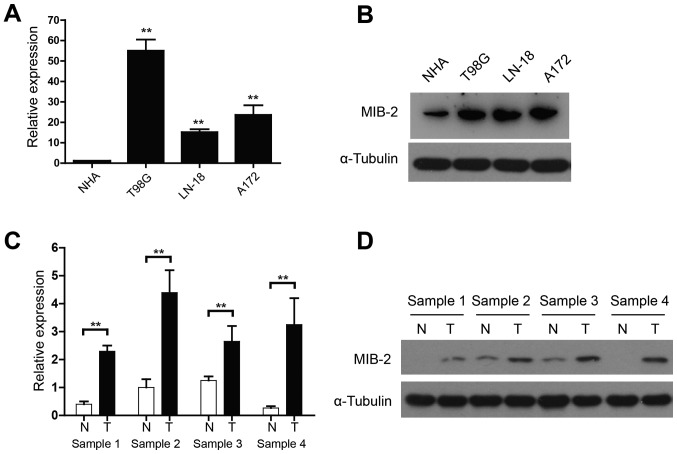
The functional and clinical relevance of MIB2 in human glioma remains to be investigated. The present study assessed the MIB2 expression in NHA and various human glioma cell lines using qPCR and WB analyses. When compared with NHA, mRNA expression of MIB2 was significantly increased in all glioma T98G, LN-18 and A172 cell lines (P<0.01; Fig. 1A). WB analysis also confirmed the upregulated MIB2 expression in all glioma cell lines, compared with NHA (Fig. 1B). In addition, four pairs of primary glioma samples and adjacent non-cancerous brain tissues were used to conduct a comparative analysis of MIB2 expression in human gliomas. While the adjacent non-cancerous brain tissue expressed a relatively low level of MIB2, the mRNA (Fig. 1C) and protein expression (Fig. 1D) levels of MIB2 indicated a significant elevation in all four sets of human primary glioma samples (P<0.01).
Figure 1.
Expression of MIB2 is elevated in human glioma cell lines and clinical glioma specimens. (A) RT-qPCR analysis of MIB2 mRNA in NHA and glioma T98G, LN-18 and A172 cell lines. Data are normalized to GAPDH and are presented as the mean ± standard deviation of 3 independent experiments. **P<0.01 by one-way ANOVA and Tukey's post hoc test. (B) Expression of MIB2 protein in NHA and indicated glioma cell lines. α-Tubulin was used as the loading control. (C) Reverse transcription-quantitative polymerase chain reaction analysis of MIB2 mRNA in four pairs of primary glioma tissues. Data are normalized to GAPDH and are presented as the mean ± SEM of three experiments, with statistical significance determined by one-way ANOVA and Tukey's post hoc test. **P<0.01. (D) Expression of MIB2 protein in paired T and N samples by western blot analysis. α-Tubulin was used as the loading control. NHA, normal human astrocyte; MIB2, E3 ubiquitin-protein ligase; ANOVA, analysis of variance; T, primary glioma samples; N, adjacent non-cancerous brain tissues.
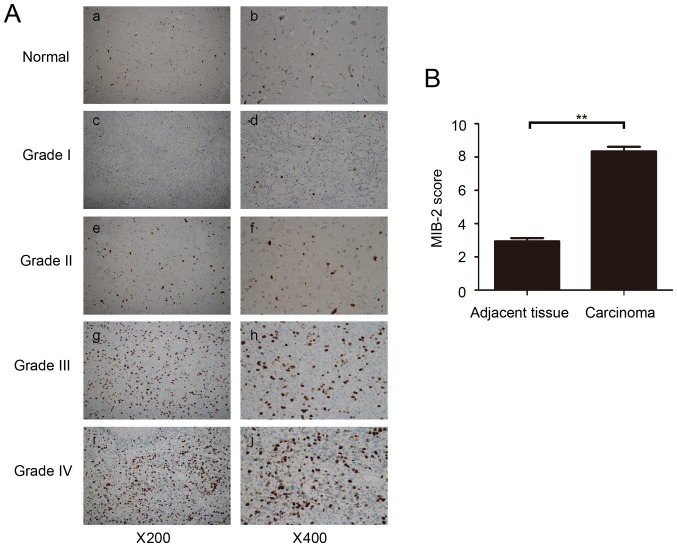
Additional confirmation of MIB2 expression in glioma was performed using immunohistochemical staining of tumor sections. Abundant MIB2 was detected and positively stained in all primary gliomas, while its expression in normal brain tissues was absent or only limited to a marginally measurable state (Fig. 2A). Additional analysis confirmed that the average scores of MIB2 staining in primary glioma clinical samples were significantly (P<0.01) increased compared with those of adjacent normal brain tissues (Fig. 2B). These data demonstrated that MIB2 was highly expressed in human glioma, suggesting the possibility of a specific role for MIB2 in glioma pathology.
Figure 2.
Immunohistochemical analysis of MIB2 expression in normal brain tissues and primary glioma tumors. (A) Representative images from IHC assays of normal brain tissues and specimens of 69 archived glioma cases. (a,b) Normal brain tissue; (c,d) WHO grade 1 pilocytic astrocytoma; (e,f) WHO grade 2 diffuse astrocytoma; (g,h) WHO grade 3 anaplastic astrocytoma; (i,j) WHO grade 4 glioblastoma multiforme. (a,c,e,g,i) Magnification, ×200. (b,d,f,h and j) Magnification, ×400. (B) Comparative statistical quantification of the mean values of MIB2 staining between normal brain tissues (n=3) and glioma specimens of different WHO grades. Student's t-test was used for statistical analysis. **P<0.01. IHC, immunohistochemistry; MIB2, E3 ubiquitin-protein ligase; WHO, World Health Organization.
UV-induced apoptosis was repressed in MIB2-overexpressing glioma cells
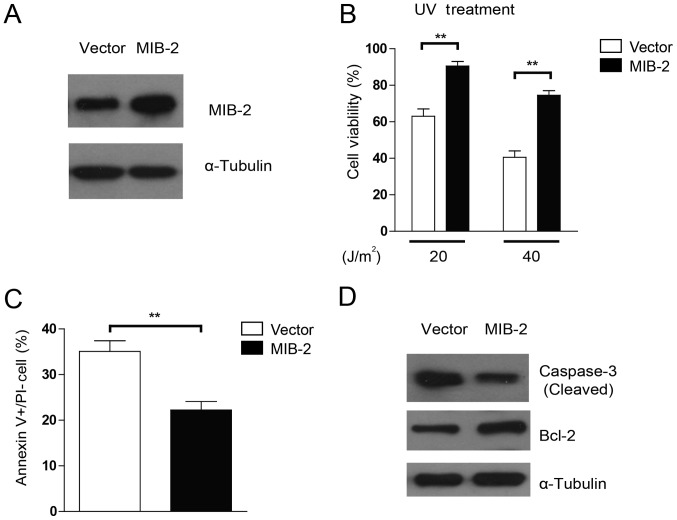
To examine whether the overexpression of MIB2 may affect the sensitivity of glioma cells to apoptosis inducers, stable transduction of MIB2 overexpression was achieved in T98G cells (Fig. 3A). As UV irradiation was administered to induce cell death, marked resistance toward different dosages of UV irradiation was observed in MIB2-overexpressing cell lines when compared with their vector-carrying control cells, in a dose-dependent manner (Fig. 3B). To investigate the specific effects of MIB2 overexpression in apoptosis, Annexin V binding assays were conducted. As indicated in Fig. 3C, T98G cells with MIB2 overexpression exhibited lower numbers of apoptotic cells post-UV irradiation compared with those of the vector control group, implying that MIB2 overexpression may confer protection in glioma cells against pro-apoptotic agents. Additionally, a pro-apoptotic protein, caspase-3, and an anti-apoptotic protein, Bcl-2, were assessed for their differential activities in cells with MIB2 overexpression. It was indicated that MIB2-overexpressing cells exhibited less activation and cleavage of caspase-3, but increased Bcl-2 expression, when compared with the vector control cells (Fig. 3D). Taken together, these results indicated that the functions of high MIB2 expression in glioma cells manifest in promoting cell survival and resistance to apoptosis.
Figure 3.
Overexpression of MIB2 enhances the anti-apoptotic activity of glioma cells. (A) Overexpression of MIB2 in the glioma T98G cell line. α-Tubulin was used as the loading control. (B) Overexpression of MIB2 inhibits cell death induced by UV irradiation. MIB2-overexpressing glioma cell lines were treated by UV irradiation (20 and 40 J/m2), and viable cells were counted using the trypan blue exclusion assay. Data are presented as the mean ± standard error of the mean of three experiments with statistical significance determined by one-way analysis of variance with Tukey's post hoc test. **P<0.01. (C) MIB2 reduces UV-induced apoptosis of glioma cells. MIB2-overexpressing glioma cell lines were treated with UV irradiation (40 J/m2), followed by Annexin V-fluorescein isothiocyanate and PI staining, and the number of Annexin V+/PI-cells was counted from 5 random fields. Results are expressed as percentages of total cells. Student's t-test was used for statistical analysis. **P<0.01. (D) MIB2-overexpressing glioma cell lines were treated with UV irradiation (40 J/m2) and harvested for cell lysate preparation. Western blotting was performed to assess the levels of cleaved caspase-3 and Bcl-2 protein. α-Tubulin was used as the loading control. UV, ultraviolet; MIB2, E3 ubiquitin-protein ligase; PI, propidium iodide; Bcl-2, B-cell lymphoma 2.
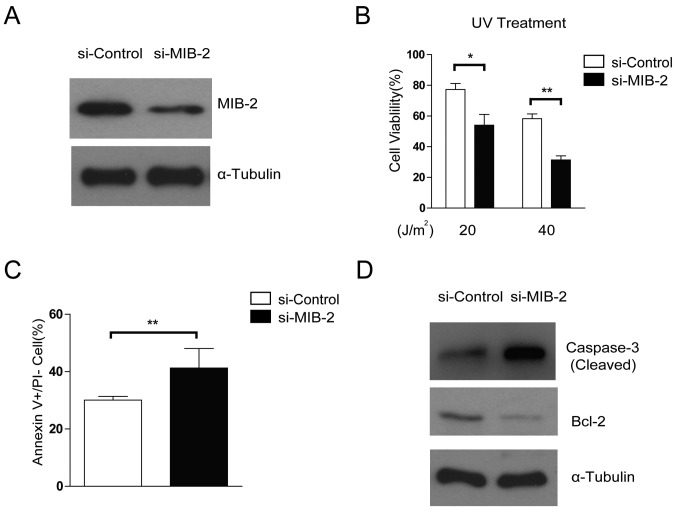
Depletion of MIB2 increases the susceptibility of glioma cells toward apoptosis
The effects of specific MIB2 knockdown on glioma cell survival and apoptosis were then compared using treatment with RNA interference. The glioma T98G cell line with MIB2 depletion exhibited increased rates of apoptosis following UV irradiation (Fig. 4A and B), compared with the control. Knockdown of MIB2 increased the rates of cellular apoptosis in glioma cells compared with the control cells, as confirmed by Annexin V binding assays (Fig. 4C). In addition, promoted activation and cleavage of caspase-3 and a decreased level of Bcl-2 was observed when MIB2 was depleted in T98G cell lines (Fig. 4D). These data suggested that MIB2 may be a key controller in the adaptation of the anti-apoptotic phenotype in glioma cells.
Figure 4.
Knockdown of endogenous MIB2 attenuates the resistance of glioma cells to apoptosis. (A) Knockdown of MIB2 in glioma cell lines was analyzed by WB. α-tubulin was used as the loading control. (B) Knockdown of MIB2 increases cell death. The indicated cells were treated by UV irradiation (20 and 40 J/m2) and counted for viable cells using the trypan blue exclusion assay. Data are presented as the mean ± standard error of the mean of three experiments with statistical significance determined by one-way analysis of variance with Tukey's post hoc test. **P<0.01. (C) Knockdown of MIB2 enhances the sensitivity of glioma cells to UV-induced apoptosis. MIB2-knockdown glioma cell lines were treated with UV irradiation (40 J/m2), followed by Annexin V-fluorescein isothiocyanate and PI staining, and the number of Annexin V+/PI-cells was counted from 5 random fields. Results are expressed as percentages of total cells. Columns represent the mean of three experiments. A Student's t-test was used for statistical analysis. **P<0.01. (D) T98G cells were treated with UV irradiation (40 J/m2) and were harvested for cell lysate preparation. WB was performed to assess the levels of cleaved caspase-3 and Bcl-2 protein. α-Tubulin was used as the loading control. WB, western blotting; MIB2, E3 ubiquitin-protein ligase; PI, propidium iodide; UV, ultraviolet; Bcl-2, B-cell lymphoma 2.
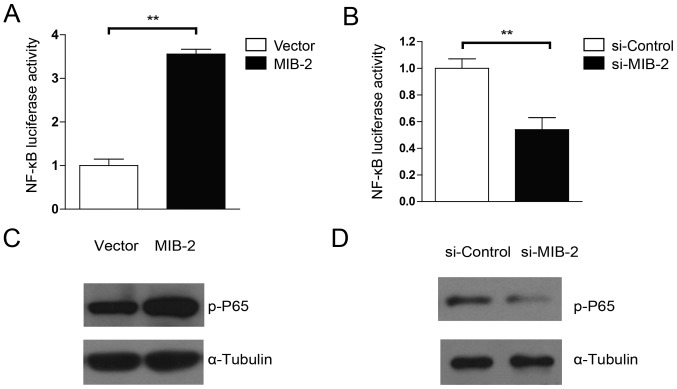
MIB2 is capable of activating the NF-κB pathway
Evidence has suggested that Bcl-2 expression is controlled by NF-κB activity, and that activation of NF-κB promotes drug resistance in glioma cells (9,29). Therefore, the present study examined whether the anti-apoptotic activity of MIB2 in human glioma cells was associated with NF-κB activation. MIB2-overexpressing T98G cells exhibited significantly (P<0.01) more marked luciferase activity driven by the NF-κB inducible promoter, compared with the vector control cells (Fig. 5A). By contrast, cells with MIB2 knockdown exhibited significantly decreased NF-κB activity (Fig. 5B). The WB analysis substantiated these results. NF-κB p65 phosphorylation was upregulated in glioma T98G cells with MIB2 overexpression (Fig. 5C), whereas a significant decrease in NF-κB p65 phosphorylation levels was observed in MIB2 knockdown glioma cells (Fig. 5D). Taken together, these results supported our hypothesis that MIB2 mediates an increase in NF-κB signaling.
Figure 5.
MIB2 activates NF-κB signaling in glioma cells. (A) Activation of NF-κB-dependent reporter gene by overexpression of MIB2. Cells co-transfected with 1 µg pNF-κB-luciferase and 10 ng pRL-TK Renilla plasmids for 24 h in 12-well plates were lysed and the luciferase activity was measured. Data are presented as the mean of three independent experiments. Student's t-test was used for statistical analysis. **P<0.01. (B) Downregulation of NF-κB-dependent reporter gene by knockdown of MIB2. Cells co-transfected with 1 µg pNF-κB-luciferase and 10 ng pRL-TK Renilla plasmids for 24 h in 12-well plates were lysed and the luciferase activity was measured. Data are presented as the mean of three independent experiments. Student's t-test was used for statistical analysis. **P<0.01. (C) Western blot analysis of NF-κB p65 phosphorylation in MIB2-overexpressing glioma cell lines. α-Tubulin was used as the loading control. (D) Western blot analysis of NF-κB p65 phosphorylation in MIB2-knockdown glioma cell lines. α-Tubulin was used as the loading control. MIB2, E3 ubiquitin-protein ligase; p, phosphorylated; NF-κB, nuclear factor κ-light-chain-enhancer of B cells; p65, NF-κB p65 subunit.
Discussion
In the present study, MIB2, an E3 ligase, was identified as a key contributor that enabled glioma cells to acquire anti-apoptotic properties through the activation of the NF-κB pathway. Previous data have implicated NF-κB activation in malignant transformation and resistance to apoptosis in human glioma cells (15). However, the underlying molecular mechanism of how NF-κB activity is regulated remains unknown. The present study demonstrated that MIB2 expression was highly upregulated in glioma cells compared with control cells. As a result, the cellular function of MIB2 was suggested to be the activation of NF-κB signaling and the induction of an anti-apoptotic phenotype. Furthermore, the overexpression of MIB2 was significantly associated with the development of resistance to UV irradiation in the primary human glioma samples examined, while the downregulation of MIB2 restored the pro-apoptotic responses toward apoptotic inducers. These data indicated that MIB2 is an activator of NF-κB, and that activation of the NF-κB pathway by MIB2 is a key mechanism for promoting glioma cell survival.
Protein degradation via ubiquitination is a catalytic mechanism that targets various cellular proteins, and is therefore pivotal in regulating a wide variety of cellular signaling pathways and cell survival (30). As neurons are particularly vulnerable to changes in cellular proteins and alterations in signaling processes (31), the ubiquitination system is a key regulator of neuronal physiology, pathology and tumorigenesis. At present, MIB2 has been elucidated to be an important modulator of the Notch and glutamate receptor signaling pathway, and to have an essential role in mammalian development (32,33). Although suggestions that MIB2 may be potent in inducing NF-κB targeted gene activation have been made, the primary question remaining is how MIB2 expression in glioma is associated with NF-κB activity, and how these interactions affect cancer cell survival (34). MIB2 was first characterized as an actin-binding, cytoskeleton-associated protein in melanoma (35). In Drosophila, MIB2 is responsible for proper muscle development and maintenance of muscle integrity by preventing their apoptotic degradation (36,37). The roles of MIB2 in immunity have been studied extensively: Matsuda et al (31) presented the first study on the involvement of MIB2 in cytokine signaling as being an activator of NF-κB and a mitogen-activated protein kinase promoter (38). Additionally, Stempin et al (32) revealed that the complex of Bcl-10 and MIB2 promoted auto-ubiquitination and ubiquitination of IKKα, and led to the activation of NF-κB. The results of these studies supported the data indicating that MIB2 is specifically involved in NF-κB activation.
In addition to its roles in causing cancer through inflammation, NF-κB is known to regulate a range of downstream anti-apoptotic genes, including cellular inhibitor of apoptosis 2 and Bcl-extra large (38,39). Owing to this anti-apoptotic function, NF-κB has been demonstrated to actively participate in the development of resistance to chemotherapy and radiotherapy in numerous neoplasms (40–42). Notably, it has been established that NF-κB is activated in response to treatment with chemotherapeutic drugs or irradiation (43,44). More specifically, inhibiting NF-κB activation enhances the cytotoxic effects of apoptotic inducers (26). This association has been described in various cancer cell types, including glioma (45). Therefore, a number of preclinical studies have been conducted on agents that inhibit the activation of the NF-κB signaling pathway. At present, the only NF-κB inhibitors used clinically are proteasome inhibitors that function to prevent the degradation of IκB, but the uses of these strategies are limited due to their lack of efficacy and high cytotoxicity (46). Additional analysis of the mechanisms behind the induction of NF-κB may identify more targets for NF-κB inhibition strategies. In light of this, the present study aimed to clarify the roles of MIB2 in the activation of NF-κB in response to UV irradiation, a DNA-damage agent known to induce NF-κB activity (47,48). By first examining the expression of MIB2 in glioma cell lines and clinical specimens, it was demonstrated that MIB2 was expressed in a high abundance in tumors. In addition, MIB2 was identified to mediate anti-apoptotic responses via activating NF-κB. Depleting MIB2 resulted in enhanced susceptibility of glioma cells to UV-induced cell death, suggesting that the key downstream role for MIB2 is NF-κB-driven mediators of cell survival and anti-apoptosis.
In conclusion, the results of the present study propose a novel and important role for MIB2 in NF-κB-mediated resistance to apoptosis in glioma cells. This suggests the potential for developing a therapeutic strategy of inhibiting MIB2 to overcome anti-apoptotic responses.
Acknowledgements
Not applicable.
Funding
This research is supported by the 309th Hospital of Chinese People's Liberation Army (grant no. 2015ZD-002) and Liaoning Natural Funding (grant no. 2015020714).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
JB, LX, YH, WF, XK, XM, YG, LB, WC and BS planned the experiment, collected the data and wrote the paper. ZY and BC analyzed the data. XL collected the samples.
Ethics approval and consent to participate
The present study was approved by the Ethics Committee of Shenzhen People's Hospital (Shenzhen, China). Written informed consent was obtained from all patients.
Consent for publication
Consent for publication was obtained from all patients.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359:492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 2.Furnari FB, Fenton T, Bachoo RM, Mukasa A, Stommel JM, Stegh A, Hahn WC, Ligon KL, Louis DN, Brennan C, et al. Malignant astrocytic glioma: Genetics, biology, and paths to treatment. Genes Dev. 2007;21:2683–2710. doi: 10.1101/gad.1596707. [DOI] [PubMed] [Google Scholar]
- 3.Nieder C, Mehta MP, Jalali R. Combined radio- and chemotherapy of brain tumours in adult patients. Clin Oncol (R Coll Radiol) 2009;21:515–524. doi: 10.1016/j.clon.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 4.Fidoamore A, Cristiano L, Antonosante A, d'Angelo M, Di Giacomo E, Astarita C, Giordano A, Ippoliti R, Benedetti E, Cimini A. Glioblastoma stem cells microenvironment: The paracrine roles of the niche in drug and radioresistance. Stem Cells Int. 2016;2016:6809105. doi: 10.1155/2016/6809105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Argyriou AA, Kalofonos HP. Molecularly targeted therapies for malignant gliomas. Mol Med. 2009;15:115–122. doi: 10.2119/molmed.2008.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kasibhatla S, Tseng B. Why target apoptosis in cancer treatment? Mol Cancer Therap. 2003;2:573–580. [PubMed] [Google Scholar]
- 7.Wong ML, Kaye AH, Hovens CM. Targeting malignant glioma survival signalling to improve clinical outcomes. J Clin Neurosci. 2007;14:301–308. doi: 10.1016/j.jocn.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 8.Sun SY, Hail N, Jr, Lotan R. Apoptosis as a novel target for cancer chemoprevention. J Natl Cancer Inst. 2004;96:662–672. doi: 10.1093/jnci/djh123. [DOI] [PubMed] [Google Scholar]
- 9.Biswas DK, Shi Q, Baily S, Strickland I, Ghosh S, Pardee AB, Iglehart JD. NF-kappa B activation in human breast cancer specimens and its role in cell proliferation and apoptosis. Proc Natl Acad Sci USA. 2004;101:10137–10142. doi: 10.1073/pnas.0403621101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shen HM, Tergaonkar V. NFkappaB signaling in carcinogenesis and as a potential molecular target for cancer therapy. Apoptosis. 2009;14:348–363. doi: 10.1007/s10495-009-0315-0. [DOI] [PubMed] [Google Scholar]
- 11.Guan H, Zhang H, Cai J, Wu J, Yuan J, Li J, Huang Z, Li M. IKBKE is over-expressed in glioma and contributes to resistance of glioma cells to apoptosis via activating NF-κB. J Pathol. 2011;223:436–445. doi: 10.1002/path.2815. [DOI] [PubMed] [Google Scholar]
- 12.Kumar DM, Patil V, Ramachandran B, Nila MV, Dharmalingam K, Somasundaram K. Temozolomide-modulated glioma proteome: Role of interleukin-1 receptor-associated kinase-4 (IRAK4) in chemosensitivity. Proteomics. 2013;13:2113–2124. doi: 10.1002/pmic.201200261. [DOI] [PubMed] [Google Scholar]
- 13.Yamagishi N, Miyakoshi J, Takebe H. Enhanced radiosensitivity by inhibition of nuclear factor kappa B activation in human malignant glioma cells. Int J Radiat Biol. 1997;72:157–162. doi: 10.1080/095530097143374. [DOI] [PubMed] [Google Scholar]
- 14.Angileri FF, Aguennouz M, Conti A, La Torre D, Cardali S, Crupi R, Tomasello C, Germanò A, Vita G, Tomasello F. Nuclear factor-kappaB activation and differential expression of survivin and Bcl-2 in human grade 2-4 astrocytomas. Cancer. 2008;112:2258–2266. doi: 10.1002/cncr.23407. [DOI] [PubMed] [Google Scholar]
- 15.Wong ET, Tergaonkar V. Roles of NF-kappaB in health and disease: Mechanisms and therapeutic potential. Clin Sci (Lond) 2009;116:451–465. doi: 10.1042/CS20080502. [DOI] [PubMed] [Google Scholar]
- 16.Sizemore N, Lerner N, Dombrowski N, Sakurai H, Stark GR. Distinct roles of the Ikappa B kinase alpha and beta subunits in liberating nuclear factor kappa B (NF-kappa B) from Ikappa B and in phosphorylating the p65 subunit of NF-kappa B. J Biol Chem. 2002;277:3863–3869. doi: 10.1074/jbc.M110572200. [DOI] [PubMed] [Google Scholar]
- 17.Mankan AK, Lawless MW, Gray SG, Kelleher D, McManus R. NF-kappaB regulation: The nuclear response. J Cell Mol Med. 2009;13:631–643. doi: 10.1111/j.1582-4934.2009.00632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Antoon JW, White MD, Slaughter EM, Driver JL, Khalili HS, Elliott S, Smith CD, Burow ME, Beckman BS. Targeting NFκB mediated breast cancer chemoresistance through selective inhibition of sphingosine kinase-2. Cancer Biol Ther. 2011;11:678–689. doi: 10.4161/cbt.11.7.14903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Santini D, Schiavon G, Vincenzi B, Gaeta L, Pantano F, Russo A, Ortega C, Porta C, Galluzzo S, Armento G, et al. Receptor activator of NF-kB (RANK) expression in primary tumors associates with bone metastasis occurrence in breast cancer patients. PLoS One. 2011;6:e19234. doi: 10.1371/journal.pone.0019234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoshiya Y, Gupta V, Segev DL, Hoshiya M, Carey JL, Sasur LM, Tran TT, Ha TU, Maheswaran S. Mullerian inhibiting substance induces NFkB signaling in breast and prostate cancer cells. Mol Cell Endocrinol. 2003;211:43–49. doi: 10.1016/j.mce.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 21.Mani A, Gelmann EP. The ubiquitin-proteasome pathway and its role in cancer. J Clin Oncol. 2005;23:4776–4789. doi: 10.1200/JCO.2005.05.081. [DOI] [PubMed] [Google Scholar]
- 22.Rajan N, Elliott RJ, Smith A, Sinclair N, Swift S, Lord CJ, Ashworth A. The cylindromatosis gene product, CYLD, interacts with MIB2 to regulate notch signalling. Oncotarget. 2014;5:12126–12140. doi: 10.18632/oncotarget.2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vigneswaran K, Neill S, Hadjipanayis CG. Beyond the world health organization grading of infiltrating gliomas: Advances in the molecular genetics of glioma classification. Ann Transl Med. 2015;3:95. doi: 10.3978/j.issn.2305-5839.2015.03.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nature Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 25.Kämmerer U, Kapp M, Gassel AM, Richter T, Tank C, Dietl J, Ruck P. A new rapid immunohistochemical staining technique using the EnVision antibody complex. J Histochem Cytochem. 2001;49:623–630. doi: 10.1177/002215540104900509. [DOI] [PubMed] [Google Scholar]
- 26.Bednarski BK, Ding X, Coombe K, Baldwin AS, Kim HJ. Active roles for inhibitory kappaB kinases alpha and beta in nuclear factor-kappaB-mediated chemoresistance to doxorubicin. Mol Cancer Therap. 2008;7:1827–1835. doi: 10.1158/1535-7163.MCT-08-0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McClelland RA, Finlay P, Walker KJ, Nicholson D, Robertson JF, Blamey RW, Nicholson RI. Automated quantitation of immunocytochemically localized estrogen receptors in human breast cancer. Cancer Res. 1990;50:3545–3550. [PubMed] [Google Scholar]
- 28.Jung Y, Joo KM, Seong DH, Choi YL, Kong DS, Kim Y, Kim MH, Jin J, Suh YL, Seol HJ, et al. Identification of prognostic biomarkers for glioblastomas using protein expression profiling. Int J Oncol. 2012;40:1122–1132. doi: 10.3892/ijo.2011.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pitlick FA. Binding of bovine brain tissue factor to concanavalin A-Sepharose. Partial purification of coagulant, arylamidase, and alkaline phosphatase activities. Biochim Biophys Acta. 1976;428:27–34. doi: 10.1016/0304-4165(76)90105-7. [DOI] [PubMed] [Google Scholar]
- 30.Tu Y, Chen C, Pan J, Xu J, Zhou ZG, Wang CY. The ubiquitin proteasome pathway (UPP) in the regulation of cell cycle control and DNA damage repair and its implication in tumorigenesis. Int J Clin Exp Pathol. 2012;5:726–738. [PMC free article] [PubMed] [Google Scholar]
- 31.Wang X, Michaelis EK. Selective neuronal vulnerability to oxidative stress in the brain. Front Aging Neurosci. 2010;2:12. doi: 10.3389/fnagi.2010.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koo BK, Yoon KJ, Yoo KW, Lim HS, Song R, So JH, Kim CH, Kong YY. Mind bomb-2 is an E3 ligase for Notch ligand. J Biol Chem. 2005;280:22335–22342. doi: 10.1074/jbc.M501631200. [DOI] [PubMed] [Google Scholar]
- 33.Koo BK, Yoon MJ, Yoon KJ, Im SK, Kim YY, Kim CH, Suh PG, Jan YN, Kong YY. An obligatory role of mind bomb-1 in notch signaling of mammalian development. PLoS One. 2007;2:e1221. doi: 10.1371/journal.pone.0001221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ye JS, Kim N, Lee KJ, Nam YR, Lee U, Joo CH. Lysine 63-linked TANK-binding kinase 1 ubiquitination by mindbomb E3 ubiquitin protein ligase 2 is mediated by the mitochondrial antiviral signaling protein. J Virol. 2014;88:12765–12776. doi: 10.1128/JVI.02037-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takeuchi T, Heng HH, Ye CJ, Liang SB, Iwata J, Sonobe H, Ohtsuki Y. Down-regulation of a novel actin-binding molecule, skeletrophin, in malignant melanoma. Am J Pathol. 2003;163:1395–1404. doi: 10.1016/S0002-9440(10)63497-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nguyen HT, Voza F, Ezzeddine N, Frasch M. Drosophila mind bomb2 is required for maintaining muscle integrity and survival. J Cell Biol. 2007;179:219–227. doi: 10.1083/jcb.200708135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barsi JC, Rajendra R, Wu JI, Artzt K. Mind bomb1 is a ubiquitin ligase essential for mouse embryonic development and Notch signaling. Mech Dev. 2005;122:1106–1117. doi: 10.1016/j.mod.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 38.Wang Q, Wang X, Evers BM. Induction of cIAP-2 in human colon cancer cells through PKC delta/NF-kappa B. J Biol Chem. 2003;278:51091–51099. doi: 10.1074/jbc.M306541200. [DOI] [PubMed] [Google Scholar]
- 39.Hinz M, Loser P, Mathas S, Krappmann D, Dörken B, Scheidereit C. Constitutive NF-kappaB maintains high expression of a characteristic gene network, including CD40, CD86, and a set of antiapoptotic genes in Hodgkin/Reed-Sternberg cells. Blood. 2001;97:2798–2807. doi: 10.1182/blood.V97.9.2798. [DOI] [PubMed] [Google Scholar]
- 40.Baldwin AS. Control of oncogenesis and cancer therapy resistance by the transcription factor NF-kappaB. J Clin Invest. 2001;107:241–246. doi: 10.1172/JCI11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakshatri H, Bhat-Nakshatri P, Martin DA, Goulet RJ, Jr, Sledge GW., Jr Constitutive activation of NF-kappaB during progression of breast cancer to hormone-independent growth. Mol Cell Biol. 1997;17:3629–3639. doi: 10.1128/MCB.17.7.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cusack JC, Liu R, Baldwin AS. NF- kappa B and chemoresistance: Potentiation of cancer drugs via inhibition of NF-kappa B. Drug Resist Updat. 1999;2:271–273. doi: 10.1054/drup.1999.0094. [DOI] [PubMed] [Google Scholar]
- 43.Cusack JC, Jr, Liu R, Houston M, Abendroth K, Elliott PJ, Adams J, Baldwin AS., Jr Enhanced chemosensitivity to CPT-11 with proteasome inhibitor PS-341: Implications for systemic nuclear factor-kappaB inhibition. Cancer Res. 2001;61:3535–3540. [PubMed] [Google Scholar]
- 44.Lu TP, Lai LC, Lin BI, Chen LH, Hsiao TH, Liber HL, Cook JA, Mitchell JB, Tsai MH, Chuang EY. Distinct signaling pathways after higher or lower doses of radiation in three closely related human lymphoblast cell lines. Int J Radiat Oncol Biol Phys. 2010;76:212–219. doi: 10.1016/j.ijrobp.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roos WP, Batista LF, Naumann SC, Wick W, Weller M, Menck CF, Kaina B. Apoptosis in malignant glioma cells triggered by the temozolomide-induced DNA lesion O6-methylguanine. Oncogene. 2007;26:186–197. doi: 10.1038/sj.onc.1209785. [DOI] [PubMed] [Google Scholar]
- 46.Alberts SR, Foster NR, Morton RF, Kugler J, Schaefer P, Wiesenfeld M, Fitch TR, Steen P, Kim GP, Gill S. PS-341 and gemcitabine in patients with metastatic pancreatic adenocarcinoma: A north central cancer treatment group (NCCTG) randomized phase II study. Ann Oncol. 2005;16:1654–1661. doi: 10.1093/annonc/mdi324. [DOI] [PubMed] [Google Scholar]
- 47.Ali F, Sultana S. Repeated short-term stress synergizes the ROS signalling through up regulation of NFkB and iNOS expression induced due to combined exposure of trichloroethylene and UVB rays. Mol Cell Biochem. 2012;360:133–145. doi: 10.1007/s11010-011-1051-7. [DOI] [PubMed] [Google Scholar]
- 48.Triscott J, Rose Pambid M, Dunn SE. Concise review: Bullseye: Targeting cancer stem cells to improve the treatment of gliomas by repurposing disulfiram. Stem cells. 2015;33:1042–1046. doi: 10.1002/stem.1956. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.