Abstract
Integrin signaling may modulate several different functions involved in cell migration, invasion, proliferation and motility, and is a potential candidate biomarker for oral cancer. In the present study, a total of four integrin genes were evaluated as potential biomarkers of oral squamous cell carcinoma (OSCC). Gene expression was determined using the reverse transcription-quantitative polymerase chain reaction in 55 OSCC and 55 matched normal oral tissues. The performance of individual and combined biomarkers was analyzed by receiver operating characteristic (ROC) analysis based on the relative mRNA expression (OSCC vs. matched oral tissue from the tumor-free margin), which was calculated using the ΔΔCq value (ΔCq of OSCC-ΔCq of oral tissue from the tumor-free margin of the same patient). In the individual ROC analysis, the areas under the ROC curve (AUCs) of relative mRNA expression (ΔΔCq) of integrin subunit α3 (ITGA3), integrin subunit α5 (ITGA5), integrin subunit β1 (ITGB1) and integrin subunit β6 (ITGB6) in all tumor locations were 0.724, 0.698, 0.640 and 0.657, respectively. For locations 2 (tongue/mouth part) and 3 (edentulous ridge), their individual AUC values were 0.840, 0.765, 0.725 and 0.763, respectively. In the cumulative ROC analysis, ITGA3, ITGA5 and ITGB1 genes exhibited the highest combined AUC values (0.809 and 0.871 for all locations and locations 2 and 3 combined, respectively) compared with other biomarker combinations. In conclusion, the results of the present study identified that higher mRNA expressions of ITGA3, ITGA5, ITGB1 and ITGB6 genes are suitable for OSCC diagnosis biomarkers. Cumulative ROC analysis indicated an improved overall performance compared with the best individual integrin biomarker of OSCC.
Keywords: oral cancer, integrin, tumor biomarker, receiver-operating characteristic curves
Introduction
Oral squamous cell carcinoma (OSCC) represents the sixth most common type of cancer worldwide (1). Several biomarkers for OSCC have been demonstrated previously (2–7) and may be useful for the diagnosis and prognosis of oral cancer. As carcinogenesis is a multistep process, a number of genes involved in the diagnosis of oral cancer have not been identified.
Integrins are a family of transmembrane-type receptor proteins on the cell surface, composed of heterodimeric complexes of 1α chain and 1β chain. The 18α and 8β subunits comprise ~24 different integrin receptors, each of which is capable of binding to a specific type of cell surface and extracellular matrix (ECM) protein ligand (8). They function in specific signal transduction and adhesive interactions between cells, and between cells and the ECM. Integrin signaling may modulate several different functions involved in cell motility, invasion, proliferation and migration (9,10). Furthermore, integrin signaling may also affect vascular neogenesis (11). Therefore, integrins serve an important role in tumor growth and metastasis (12–14).
Integrin subunit α3 (ITGA3), which combines with integrin subunit β1 (ITGB1) to form integrin α3β1, is the receptor for ECM molecules including fibronectin, laminin and collagen (8,12). Integrin α3β1 has been demonstrated to function in cell proliferation, migration and motility, and in the maintenance of basement membrane integrity (15–18). Several previous studies identified that integrin α3β1 was overexpressed and associated with tumor invasion and metastasis in the majority of types of cancer, including lung (19) and breast (20) cancer cell lines. Integrin subunit α5 (ITGA5) often combines with ITGB1 to form integrin α5β1, and serves as a receptor for fibronectin and fibrinogen to participate in cell differentiation, cell development and wound healing (8,12). It was identified that the emergence of integrin α5β1 expression is associated with tumor progression in lung cancer (21). ITGA5 may also promote tumor metastasis in oral cancer cell lines (22). Integrin subunit β6 (ITGB6) is the β subunit of integrin αvβ6, which is a receptor for fibronectin and cytotactin, and regulates cell invasion, inhibits apoptosis, modulates matrix metalloproteases and activates transforming growth factor β1 (8,23). Overexpression of integrin αvβ6 promotes epithelial-to-mesenchymal transition and is associated with cell invasion in oral cancer cell lines (24,25). However, the majority of these previous studies were focused on OSCC cell line models or non-quantitative immunohistochemical analyses of OSCC tissues. These studies did not use receiver-operating characteristic (ROC) curve analyses, which provide sensitivity and specificity data for the evaluation of OSCC biomarker performances.
In the present study, the reverse transcription-quantitative polymerase chain reaction (RT-qPCR) analysis was used to determine the mRNA expression levels of ITGA3, ITGA5, ITGB1 and ITGB6 genes in OSCC tissues from different tumor locations for ROC curve analyses, in order to identify suitable biomarker genes for the diagnosis of early-stage OSCC. The mRNA expression of ITGA3, ITGA5, ITGB1, and ITGB6 genes were identified as potential OSCC biomarkers. Furthermore, cumulative ROC analysis of integrin OSCC biomarkers had an improved diagnostic performance compared with individual ROC analysis.
Materials and methods
Tissue samples
The present study was approved by the Institutional Review Board at Kaohsiung Medical University (Kaohsiung, Taiwan) (approval no. KMUH-IRB-930104), and all patients provided written informed consent. A total of 55 oral tumors and 55 matched normal oral control tissues (at least 2.5 cm between tumor and control tissues) were collected (December 2004 to December 2009) from the Department of Oral and Maxillofacial Surgery, Kaohsiung Medical University Hospital. The age range of this patient cohort is 30-90 years and the median age is 50 years. All samples were blindly examined by at least three pathologists of the Department of pathology, Kaohsiung Medical University Hospital. All control tissues underwent pathological diagnosis for confirmation as non-tumor. All oral tumors underwent pathological diagnosis for OSCC and tumor stage classification used the Tumor-Node-Metastasis (TNM) system (26). The characteristics of the patients with OSCC are summarized in Table I, this basic patient information has been described previously (6).
Table I.
Basic characteristics of patients and tissue samples.
| (OSCC/matched control oral tissue) | ||
|---|---|---|
| Characteristics | n (total=55) | (%) |
| Age, years | ||
| ≤40 | 8 | 14.55 |
| 41-50 | 21 | 38.18 |
| 51-60 | 21 | 38.18 |
| ≥61 | 5 | 9.09 |
| Sex | ||
| Male | 50 | 90.91 |
| Female | 5 | 9.09 |
| TNM stage | ||
| I | 10 | 18.18 |
| II | 9 | 16.36 |
| III | 16 | 29.09 |
| IV | 20 | 36.37 |
| Lesion location | ||
| Buccal mucosa/retromolar area | 23 | 41.82 |
| Tongue/mouth floor | 17 | 30.91 |
| Edentulous ridge | 8 | 14.55 |
| Others | 7 | 12.72 |
| Carcinogenic factors | ||
| Alcohol | ||
| (+) | 46 | 83.64 |
| (−) | 9 | 16.36 |
| Betel quid | ||
| (+) | 50 | 90.91 |
| (−) | 5 | 9.09 |
| Cigarette smoking | ||
| (+) | 48 | 87.27 |
| (−) | 7 | 12.73 |
This basic patient information has been described previously (6). TNM, Tumor-Node-Metastasis; Others, lower lip/vestibule/soft palate; OSCC, oral squamous cell carcinoma; +, yes; -, no.
RT-qPCR
Tissues were sliced and placed into 1.5 ml microcentrifuge tubes containing TRIzol® reagent (Thermo Fisher Scientific, Inc., Waltham, MA, USA) for homogenization using a disposable tissue grinder pestle. Subsequently, the homogenized mixtures were used for total RNA extraction according to the manufacturer's protocol. RT was performed using an OmniScript RT kit (Qiagen GmbH, Hilden, Germany). qPCR was performed using an iQ SYBR Green Supermix (Bio-Rad Laboratories, Inc., Hercules, CA, USA) in an iCycler MyiQ real-time machine (Bio-Rad Laboratories, Inc.). The relative mRNA expressions of ITGA3, ITGA5, ITGB1 and ITGB6 genes in OSCCs and controls were examined. The forward and reverse primer sequences, and the lengths of PCR products, for these genes are provided in Table II. The thermocycling conditions are as follows: 94°C for 1 min; 4 cycles of 94°C for 15 sec, 64°C for 15 sec and 70°C for 15 sec; 4 cycles of 94°C for 15 sec, 61°C for 15 sec and 70°C for 15 sec; 4 cycles of 94°C for 15 sec, 58°C for 15 sec and 70°C for 15 sec; 60 cycles of 94°C for 15 sec, 55°C for 15 sec and 70°C for 15 sec; and finally 94°C for 1 min and 60°C for 5 min. The PCR assay was performed in duplicate. The relative mRNA expression levels (OSCC/oral tissue from the tumor-free margin ratio) for ITGA3, ITGA5, ITGB1 and ITGB6 genes were evaluated using the 2−ΔΔCq method (27). The threshold cycle (Cq) value of each integrin gene was subtracted from the Cq value for the GAPDH reference housekeeping gene. Melting curve analyses by qPCR analysis and gel electrophoresis by 1.5% agarose containing 0.5 µg/ml ethidium bromide for visualization under a UV box were used to validate the PCR products as described previously (28).
Table II.
Forward and reverse primer sequences and the lengths of PCR products for ITGA3, ITGA5, ITGB1, ITGB6, and GAPDH genes.
| Gene names | Primer sequences | Length of PCR products, bp |
|---|---|---|
| ITGA3 | Forward: 5′-TGCTGTGGAAGTGCGGCT-3′ | 206 |
| Reverse: 5′-GCGTGGTACTTGGGCATGAT-3′ | ||
| ITGA5 | Forward: 5′-TCATCTACATCCTCTACAAGCTTGG-3′ | 204 |
| Reverse: 5′-GCCGTCAGCACCTTCAAGA-3′ | ||
| ITGB1 | Forward: 5′-CGTATTCAGTGAATGGGAACAAC-3′ | 231 |
| Reverse: 5′-GATTTTCACCCGTGTCCCAT-3′ | ||
| ITGB6 | Forward: 5′-ACATGAAAGTGGGAGACACAGC-3′ | 215 |
| Reverse: 5′-ACACACCCCACACTGGAAAGA-3′ | ||
| GAPDH | Forward: 5′-GCATCCTGGGCTACACTGA-3′ | 162 |
| Reverse: 5′-CCACCACCCTGTTGCTGTA-3′ |
ITGA3, integrin subunit α3; ITGA5, integrin subunit α5; ITGB1, integrin subunit β1; ITGB6, integrin subunit β6.
Statistical analysis
Data were analyzed by the Mann-Whitney test and Kruskal-Wallis test using SPSS version 17.0 software (SPSS, Inc., Chicago, IL, USA). ROC curve analyses were commonly used to evaluate the performance of cancer diagnosis (29–34). Accordingly, ROC curves were used to examine the performance of ITGA3, ITGA5, ITGB1 and ITGB6 genes as predictive biomarkers for detecting OSCC. Cut-off values for individual ROC curves were calculated using the 60-ΔCq value, where 60 represents the total PCR cycle number, and no signal was assigned to the Cq value for 60, as described previously (5,6). For individual ROC analysis, the area under the ROC curve (AUC) of each biomarker was used to evaluate its performance as an OSCC biomarker.
To consider the combined effect of each biomarker, the cumulative ROC analysis of biomarkers was performed for OSCC prediction. At first, the critical (cut-off) point of the ROC curve for each biomarker was identified using JMP version 10 statistic software (SAS Institute Inc., Cary, NC, USA). Subsequently, biomarkers that were more relevant to the OSCC (those with values greater than the cut-off point in AUC of individual ROC results) were assigned a score of 1 (for example, values greater than the cut-off point from the AUC analysis of the individual ROC results), and the remaining biomarkers were assigned a score of 0. The combined scores for different combinations of biomarkers were calculated using the formula function in JMP 10. Finally, the combined scores were used for cumulative ROC analysis of biomarkers for comparison.
Results
Comparison of clinicopathological features and relative mRNA expressions of ITGA3, ITGA5, ITGB1 and ITGB6 genes in patients with OSCC
To evaluate the performance of the OSCC biomarkers, mRNA expression levels of ITGA3, ITGA5, ITGB1 and ITGB6 genes of OSCC samples were evaluated by comparing tumor samples with control samples (oral tissues from the tumor-free margin). In different locations (Table III), the differences in ITGA3 and ITGB6 gene expression levels were significant (P=0.05 and 0.005, respectively by Kruskal-Wallis test); however, the differences in the ITGA5 and ITGB1 gene expression levels were non-significant. In contrast, the differences in mRNA expression levels of ITGA3, ITGA5, ITGB1 and ITGB6 genes were non-significant at different stages (I–IV) (P=0.19, 0.58, 0.92, and 0.53, respectively by Kruskal-Wallis test) and for the T parameter (1–4) of the TNM staging system (a measure of tumor diameter/dimension) among patients with OSCC (P=0.36, 0.97, 0.75, and 0.83, respectively by Kruskal-Wallis test).
Table III.
Comparison of clinicopathological features and relative mRNA expression levels of ITGA3, ITGA5, ITGB1 and ITGB6 genes in oral squamous cell carcinoma tumor samples with control samples (oral tissue from the tumor-free margin).
| ITGA3 | ITGA5 | ITGB1 | ITGB6 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Clinical data | n (total=55) | Mean ± SD | P-valuea | Mean ± SD | P-valuea | Mean ± SD | P-valuea | Mean ± SD | P-valuea |
| Locationb | 0.01 | 0.33 | 0.16 | 0.05 | |||||
| 1 | 23 | 1.95±5.37 | 1.29±4.87 | 2.58±11.30 | 0.71±6.19 | ||||
| 2 | 17 | 4.32±4.74 | 4.04±4.99 | 2.41±3.23 | 0.91±11.08 | ||||
| 3 | 8 | 6.25±5.20 | 1.82±8.40 | 2.94±3.03 | 5.07±5.30 | ||||
| 4 | 7 | −6.87±14.91 | −1.74±12.27 | 2.15±3.58 | 2.66±5.95 | ||||
| TNM | 0.19 | 0.58 | 0.92 | 0.53 | |||||
| T1 | 14 | 1.52±12.93 | 2.88±5.64 | 1.20±4.29 | −2.12±13.83 | ||||
| T2 | 21 | 1.04±4.84 | 0.65±7.58 | 1.29±3.66 | 1.67±4.95 | ||||
| T3 and T4 | 20 | 3.86±5.48 | 2.34±6.82 | 4.74±11.37 | 2.66±3.69 | ||||
| Stage | 0.36 | 0.97 | 0.75 | 0.83 | |||||
| I | 10 | 8.26±15.43 | 2.53±5.56 | 0.87±5.03 | 0.16±9.95 | ||||
| II | 9 | 0.30±5.13 | 2.10±4.09 | 0.83±4.47 | 0.83±5.86 | ||||
| III and IV | 36 | 3.03±4.99 | 1.57±7.07 | 3.41±8.70 | 1.37±8.02 | ||||
Data are presented as mean ΔΔCq values (ΔCq of OSCC-ΔCq of its matched oral tissue from the tumor-free margin). TNM, Tumor-Node-Metastasis; T in TMN, tumor size and invasiveness in TMN classification.
Kruskal-Wallis test.
Location 1, buccal mucosa/retromolar area; 2, tongue/mouth floor; 3, edentulous ridge; 4, others (lower lip/vestibule/soft palate). SD, standard deviation; ITGA3, integrin subunit α3; ITGA5, integrin subunit α5; ITGB1, integrin subunit β1; ITGB6, integrin subunit β6.
Comparison of patient habits and relative mRNA expression of ITGA3, ITGA5, ITGB1, and ITGB6 genes in patients with OSCC
As indicated in Table IV, the differences in mRNA expression levels of ITGA3, ITGA5, ITGB1 and ITGB6 genes were not associated with cancer-causing habits, including alcohol consumption (P=0.83, 0.11, 0.68, and 0.18, respectively), betel nut chewing (P=0.65, 0.55, 0.81, and 0.75, respectively) and cigarette smoking (P=0.84, 0.45, 0.86, and 0.40, respectively by Mann-Whitney test).
Table IV.
Comparison of patient habits and relative mRNA expression levels of ITGA3, ITGA5, ITGB1 and ITGB6 genes in oral squamous cell carcinoma samples with control samples (oral tissue from the tumor-free margin).
| ITGA3 | ITGA5 | ITGB1 | ITGB6 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Habits | n (total=55) | Mean ± SD | P-valuea | Mean ± SD | P-valuea | Mean ± SD | P-valuea | Mean ± SD | P-valuea |
| Alcohol | 0.83 | 0.11 | 0.68 | 0.18 | |||||
| No | 9 | 3.02±3.60 | 4.66±3.38 | 1.91±2.26 | 4.53±5.73 | ||||
| Yes | 46 | 2.02±8.42 | 1.27±7.18 | 2.65±8.27 | 0.39±8.22 | ||||
| Betel quid chewing | 0.65 | 0.33 | 0.81 | 0.75 | |||||
| No | 5 | 3.59±5.84 | 4.25±5.21 | 0.73±3.33 | 3.74±7.03 | ||||
| Yes | 50 | 2.05±8.02 | 1.59±6.93 | 2.70±7.90 | 0.80±8.08 | ||||
| Cigarette smoking | 0.84 | 0.45 | 0.86 | 0.40 | |||||
| No | 5 | 3.51±4.90 | 3.53±6.61 | 1.13±2.31 | 4.56±6.98 | ||||
| Yes | 50 | 2.05±8.07 | 1.66±6.86 | 2.66±7.94 | 0.71±8.05 | ||||
Data are presented as mean ΔΔCq values (ΔCq of OSCC-ΔCq of its matched oral tissue from the tumor-free margin).
Mann-Whitney U test. SD, standard deviation; ITGA3, integrin subunit α3; ITGA5, integrin subunit α5; ITGB1, integrin subunit β1; ITGB6, integrin subunit β6.
AUC performances of individual OSCC biomarkers of ITGA3, ITGA5, ITGB1, and ITGB6 genes
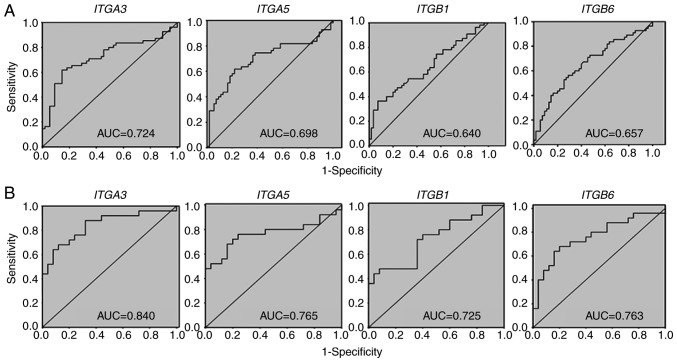
To test the predictive performance of each of the test biomarkers in OSCC diagnosis, individual ROC curves for ITGA3, ITGA5, ITGB1 and ITGB6 mRNA expression were constructed by comparing the mRNA expression level between OSCC tumor tissues and their controls (oral tissue from the tumor-free margin). The individual AUCs of the relative mRNA expression for ITGA3, ITGA5, ITGB1 and ITGB6 genes were 0.724 [95% confidence interval (CI), 0.630-0.819], 0.698 (95% CI, 0.600-0.796), 0.640 (95% CI, 0.536-0.743) and 0.657 (95% CI, 0.555-0.759), respectively (Fig. 1A). At a specificity of 50.91% for all locations (Table V, part A), sensitivities of relative mRNA expression for ITGA3, ITGA5, ITGB1 and ITGB6 were 90.91, 83.64, 70.91 and 74.55%, respectively. At a specificity of 61.82%, sensitivities of relative mRNA expression for ITGA3 and ITGA5 genes were decreased slightly to 85.45 and 78.18%, respectively. However, ITGB1 and ITGB6 genes were decreased to 50.91 and 61.82%, respectively. At a specificity of 67.27%, sensitivities of relative mRNA expression for ITGA3, ITGA5, ITGB1 and ITGB6 genes were markedly decreased, particularly for the ITGB1 gene (45.45%). Therefore, analysis of different sensitivities suggested that the ITGA3 and ITGA5 genes exhibited an improved specificity performance compared with ITGB1 and ITGB6 genes for all locations.
Figure 1.
AUC values of ITGA3, ITGA5, ITGB1 and ITGB6 genes as individual oral squamous cell carcinoma biomarkers. (A) All tumor locations (locations 1-4). (B) Only tumor locations 2/3. Location 1, buccal mucosa/retromolar area; location 2, tongue/mouth floor; location 3, edentulous ridge; location 4, others (lower lip/vestibule/soft palate); ITGA3, integrin subunit α3; ITGA5, integrin subunit α5; ITGB1, integrin subunit β1; ITGB6, integrin subunit β6; AUC, area under the curve.
Table V.
Different cut-offs and their relative sensitivity and specificity for ITGA3, ITGA5, ITGB1 and ITGB6 genes in patients with oral squamous cell carcinoma.
| A, All locations | ||||||||
| ITGA3 | ITGA5 | ITGB1 | ITGB6 | |||||
|---|---|---|---|---|---|---|---|---|
| Sensitivity, % | Specificity, % | Cut-off | Specificity, % | Cut-off | Specificity, % | Cut-off | Specificity, % | Cut-off |
| 50.91 | 90.91 | 63.20 | 83.64 | 56.26 | 70.91 | 61.93 | 74.55 | 57.90 |
| 61.82 | 85.45 | 62.87 | 78.18 | 55.00 | 50.91 | 61.17 | 61.82 | 57.21 |
| 67.27 | 67.27 | 61.94 | 63.64 | 54.35 | 45.45 | 60.96 | 58.18 | 57.06 |
| B, Locations 2/3 | ||||||||
| ITGA3 | ITGA5 | ITGB1 | ITGB6 | |||||
| Sensitivity, % | Specificity, % | Cut-off | Specificity, % | Cut-off | Specificity, % | Cut-off | Specificity, % | Cut-off |
| 64.00 | 92.00 | 62.97 | 84.00 | 55.00 | 64.00 | 61.07 | 84.00 | 57.90 |
| 68.00 | 88.00 | 62.67 | 84.00 | 55.00 | 64.00 | 60.99 | 80.00 | 57.65 |
| 76.00 | 76.00 | 61.73 | 76.00 | 53.94 | 60.00 | 60.78 | 60.00 | 56.61 |
All locations including the locations 1, 2, 3 and 4. Location 1, buccal mucosa/retromolar area; location 2, tongue/mouth floor; location 3, edentulous ridge; location 4, others (lower lip/vestibule/soft palate); ITGA3, integrin subunit α3; ITGA5, integrin subunit α5; ITGB1, integrin subunit β1; ITGB6, integrin subunit β6.
As the differences in ITGA3 and ITGB6 gene expression by location were significant (Table III), whether the different locations of OSCC may affect the mRNA expression levels of these four integrin genes was additionally investigated. In the example of locations 2 and 3 combined (locations 2/3), i.e., the tongue/mouth floor and edentulous ridge (Fig. 1B), it was demonstrated that the AUC values of relative mRNA expression for ITGA3, ITGA5, ITGB1 and ITGB6 genes in the tongue/mouth floor and edentulous ridge were 0.840 (95% CI, 0.728-0.952), 0.765 (95% CI, 0.632-0.898), 0.725 (95% CI, 0.584-0.866) and 0.762 (95% CI, 0.630-0.896), respectively. Notably, the AUC values of locations 2/3 combined (Fig. 1B) for these four integrin genes were increased compared with those of all locations together (Fig. 1A). Analysis of different sensitivities suggested that ITGA3 and ITGA5 genes exhibited an improved specificity performance compared with that of ITGB1 and ITGB6 genes for locations 2/3 (Table V, part B).
AUC performances of cumulative ROC analyses for different combinations of ITGA3, ITGA5, ITGB1, and ITGB6 biomarkers
To evaluate the diagnostic power of different combinations of these biomarkers, their cumulative ROC curves between OSCC and controls (oral tissue from the tumor-free margin) were calculated. The AUC values for different combinations (two, three and four) of the biomarkers are summarized in Table VI. The combination of ITGA3, ITGA5 and ITGB1 genes demonstrated the highest AUC values of 0.809 (CI, 0.728-0.890) and 0.871 (CI, 0.770-0.972), for the two types of locations (all locations and locations 2/3, respectively). The combination of ITGA3, ITGA5, ITGB1 and ITGB6 genes provided similar AUC values for the two locations.
Table VI.
Performances of cumulative ROC analyses for different combinations of ITGA3, ITGA5, ITGB1 and ITGB6 biomarkers in patients with OSCC.
| AUC of combined biomarkers for OSCCa | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AUC of combined biomarkers for OSCCa | ||||||||||
| Locations | ITGA3/ITGA5 | ITGA3/ITGB1 | ITGA3/ITGB6 | ITGA5/ITGB1 | ITGA5/ITGB6 | ITGB1/ITGB6 | ITGA3/ITGA5/ITGB1 | ITGA3/ITGA5/ITGB6 | ITGA5/ITGB1/ITGB6 | ITGA3/ITGA5/ITGB1/ITGB6 |
| Allb | 0.777 | 0.775 | 0.758 | 0.762 | 0.720 | 0.686 | 0.809c | 0.780 | 0.750 | 0.797 |
| 2/3 | 0.847 | 0.829 | 0.850 | 0.823 | 0.792 | 0.788 | 0.871c | 0.855 | 0.825 | 0.870 |
Biomarkers that were more relevant to the OSCC (those with values greater than the cut-off point in AUC of individual ROC results, as demonstrated in Fig. 1), were assigned a score of 1, and the others were assigned a score of 0. The combined scores for different combinations of biomarkers were used for cumulative ROC analysis.
All locations including the locations 1, 2, 3, and 4.
The best AUC performance among different combinations of biomarkers for OSCC. Location 1, buccal mucosa/retromolar area; location 2, tongue/mouth floor; location 3, edentulous ridge; location 4, others (lower lip/vestibule/soft palate); AUC, area under the curve; ROC, receiver operating characteristics; ITGA3, integrin subunit α3; ITGA5, integrin subunit α5; ITGB1, integrin subunit β1; ITGB6, integrin subunit β6; OSCC, oral squamous cell carcinoma.
Discussion
The overexpression of ITGA3, ITGA5, ITGB1 and ITGB6 genes has been demonstrated in several types of cancer (19–25); however, the suitability for ITGA3, ITGA5, ITGB1 and ITGB6 genes as OSCC biomarkers has rarely been considered.
The results of the present study identified that ITGA3 and ITGB1 mRNA were overexpressed in patients with OSCC. Similarly, it was demonstrated previously that ITGA3 and ITGB1 proteins were overexpressed in prostate tumor tissues, and knockdown of ITGA3 and ITGB1 genes by small interfering RNAs inhibited cell migration and invasion in prostate cancer cells (35). In the present study, ITGA5 mRNA was overexpressed in patients with OSCC. Similarly, ITGA5 and ITGB1 genes have been suggested to be potential biomarkers for non-small cell lung cancer (36). In addition, the ITGB6 gene may be a prognostic biomarker for invasive breast cancer (37). However, to the best of our knowledge, these integrin biomarkers have rarely been investigated in OSCC.
In the present study, individual ROC analyses for all locations identified ITGA3 and ITGA5 genes as good biomarkers for OSCC (AUC=0.724 and 0.698, respectively), but ITGB1 and ITGB6 genes performed poorly for OSCC prediction (AUC <0.66). Compared with all locations, improved AUC values for ITGA3, ITGA5, ITGB1 and ITGB6 genes were observed in combined locations 2 and 3 (the tongue/mouth part and edentulous ridge).
Previous studies have indicated that a combination of several variants generally provides an improved diagnostic power for ROC analysis compared with ROC analysis of individual markers (38–41). A combination of several biomarkers may therefore improve the diagnostic results for tumors, as has been demonstrated in thyroid cancer (42). Similarly, with the exception of the combination of ITGB1 and ITGB6 (AUC=0.686), the present study identified that the majority of the biomarker combinations, including a combination of two (ITGA3/ITGA5, ITGA3/ITGB1, ITGA3/ITGB6, ITGA5/ITGB1 and ITGA5/ITGB6), three (ITGA3/ITGA5/ITGB1, ITGA3/ITGA5/ITGB6 and ITGA5/IGB1/ITGB6) and four (ITGA3/ITGA5/ITGB1/ITGB6) genes, exhibited improved AUC values for all locations (ranging between 0.750 and 0.809) compared with the individual AUC values of individual integrin genes (ranging between 0.640 and 0.724). With the exception of the combinations of ITGA3/ITGB1, ITGA5/ITGB1, ITGA5/ITGB6, ITGB1/ITGB6 and ITGA5/ITGB1/ITGB6 genes (AUC values ranging between 0.788 and 0.829), it was identified that other combined biomarkers, including two, three and four genes, demonstrated improved AUC values for locations 2 and 3 (ranging between 0.847 and 0.871) compared with that of the individual AUC values of these integrin genes (ranging between 0.725 and 0.840). Among them, ITGA3, ITGA5 and ITGB1 genes exhibited the highest AUC values for all locations and locations 2 and 3 combined. Therefore, the cumulative ROC analysis, as a method of sensitive and specific evaluation, suggested a combination of multiple biomarkers for the diagnosis of OSCC.
mRNA has been repeatedly demonstrated to be a reliable material for the diagnosis of oral cancer (5,6,43,44). For example, tissue and salivary mRNA biomarkers are suggested to be associated with clinicopathological parameters for the diagnosis of OSCC (44). In clinical practice, the data from the present study of combined integrin biomarkers may be applied for non-invasive diagnosis of OSCC using saliva material for OSCC in the future.
An advantage of the proposed methods of the present study is a potential improvement of the AUC performance by using accumulative ROC analysis for the mRNA expression of a combination of integrin biomarkers. A disadvantage of this method is that it is based on tissue samples for mRNA evaluation, which are susceptible to degradation by RNase contamination (45). Additionally, expression at mRNA transcriptome levels will not always be consistent with those at protein levels in cases of posttranslational modification (46). Additionally, the present study did not provide information concerning depth of invasion, which is important for clinicians. Additional protein evaluation for ITGA3, ITGA5, ITGB1 and ITGB6 using immunohistochemistry is warranted. In addition, 5/55 the patients included in the present study were female, which may provide a gender bias for these biomarker predictions for oral cancer. Therefore, it is suggested that a larger cohort of female and male patients should be studied in the future to provide an unbiased prediction using these integrins. In the present study, the association between integrin expression and prognosis, was not analyzed which would have been beneficial to improve the reliability of the proposed integrin mRNA biomarker combination for oral cancer prediction.
Alcohol drinking, betel quid chewing and cigarette smoking are well-known risk factors for oral cancer (47), although non-smoking and betel quid non-chewing individuals may also suffer from oral cancer (48). It is possible that these habits may affect the initiation and progression of oral carcinogenesis, but do not directly affect the overexpression of certain genes in patients with OSCC. For example, no significant differences in the expression levels of the ITGA3, ITGA5, ITGB1 and ITGB6 biomarkers were observed between the OSCC and control tissues for each of these variables.
In conclusion, individual ROC analysis provides a sensitive and specific evaluation of biomarkers for OSCC diagnosis and prognosis. The results of the present study demonstrated that ITGA3 and ITGA5 genes were improved prognostic OSCC biomarkers for all locations compared with ITGB1 and ITGB6 genes, based on individual ROC analysis. However, ITGA3, ITGA5, ITGB1 and ITGB6 genes became more promising OSCC biomarkers when considering specific tumor locations (2 and 3; tongue/mouth part and edentulous ridge, respectively). Additionally, the cumulative ROC analyses indicated that the combination of ITGA3, ITGA5 and ITGB1 genes exhibited the highest AUC values for locations 2/3 and all locations. Therefore, ITGA3, ITGA5, ITGB1 and ITGB6 genes are suggested to be good OSCC biomarkers, and cumulative ROC analysis is hypothesized to provide an improved strategy for cancer biomarker identification.
Acknowledgements
The authors would like to thank Dr Hans-Uwe Dahms; Department of Biomedical Science and Environmental Biology, Kaohsiung Medical University, (Kaohsiung, Taiwan) for English editing.
Funding
The present study was partly supported by funds of the Ministry of Science and Technology, Taiwan (grant no. MOST 104-2320-B-037-013-MY3), the Health and Welfare Surcharge of Tobacco Products, the Ministry of Health and Welfare, Taiwan, Republic of China (grant no. MOHW107-TDU-B-212-114016), ChiMei-KMU Joint Project (grant no. 106CM-KMU-05) and the National Sun Yat-sen University-KMU Joint Research Project (grant no. NSYSU-KMU 107-p001).
Availability of data and materials
The datasets used and/or analyzed in the current study are available from the corresponding author on reasonable request.
Authors' contributions
HWC and CYY contributed significantly in writing the manuscript and data discussion/interpretation. CHC, JHT, and YHK collected the samples and analyzed pathological diagnosis of OSCC. HWC instructed JHT and YTC to extract RNA for qPCR analysis. JYT, YYW, and SSY assisted in clinical data collection and performed statistical analysis. SYL was responsible for study conception and overview. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The present study was approved by the Institutional Review Board at Kaohsiung Medical University (Kaohsiung, Taiwan) (KMUH-IRB-930104), and patients provided written informed consent.
Patient consent for publication
Patient provided written informed consent for the publication of associated data.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Shah JP, Gil Z. Current concepts in management of oral cancer-surgery. Oral Oncol. 2009;45:394–401. doi: 10.1016/j.oraloncology.2008.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dumache R, Rogobete AF, Andreescu N, Puiu M. Genetic and epigenetic biomarkers of molecular alterations in oral carcinogenesis. Clin Lab. 2015;61:1373–1381. doi: 10.7754/Clin.Lab.2015.150327. [DOI] [PubMed] [Google Scholar]
- 3.Nagata M, Noman AA, Suzuki K, Kurita H, Ohnishi M, Ohyama T, Kitamura N, Kobayashi T, Uematsu K, Takahashi K, et al. ITGA3 and ITGB4 expression biomarkers estimate the risks of locoregional and hematogenous dissemination of oral squamous cell carcinoma. BMC Cancer. 2013;13:410. doi: 10.1186/1471-2407-13-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elashoff D, Zhou H, Reiss J, Wang J, Xiao H, Henson B, Hu S, Arellano M, Sinha U, Le A, et al. Prevalidation of salivary biomarkers for oral cancer detection. Cancer Epidemiol Biomarkers Prev. 2012;21:664–672. doi: 10.1158/1055-9965.EPI-11-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yen CY, Chen CH, Chang CH, Tseng HF, Liu SY, Chuang LY, Wen CH, Chang HW. Matrix metalloproteinases (MMP) 1 and MMP10 but not MMP12 are potential oral cancer markers. Biomarkers. 2009;14:244–249. doi: 10.1080/13547500902829375. [DOI] [PubMed] [Google Scholar]
- 6.Yen CY, Huang CY, Hou MF, Yang YH, Chang CH, Huang HW, Chen CH, Chang HW. Evaluating the performance of fibronectin 1 (FN1), integrin α4β1 (ITGA4), syndecan-2 (SDC2) and glycoprotein CD44 as the potential biomarkers of oral squamous cell carcinoma (OSCC) Biomarkers. 2013;18:63–72. doi: 10.3109/1354750X.2012.737025. [DOI] [PubMed] [Google Scholar]
- 7.Wang S, Sun M, Gu C, Wang X, Chen D, Zhao E, Jiao X, Zheng J. Expression of CD163, interleukin-10, and interferon-gamma in oral squamous cell carcinoma: Mutual relationships and prognostic implications. Eur J Oral Sci. 2014;122:202–209. doi: 10.1111/eos.12131. [DOI] [PubMed] [Google Scholar]
- 8.Barczyk M, Carracedo S, Gullberg D. Integrins. Cell Tissue Res. 2010;339:269–280. doi: 10.1007/s00441-009-0834-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hood JD, Cheresh DA. Role of integrins in cell invasion and migration. Nat Rev Cancer. 2002;2:91–100. doi: 10.1038/nrc727. [DOI] [PubMed] [Google Scholar]
- 10.Desgrosellier JS, Cheresh DA. Integrins in cancer: Biological implications and therapeutic opportunities. Nat Rev Cancer. 2010;10:9–22. doi: 10.1038/nrc2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malinin NL, Pluskota E, Byzova TV. Integrin signaling in vascular function. Curr Opin Hematol. 2012;19:206–211. doi: 10.1097/MOH.0b013e3283523df0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mizejewski GJ. Role of integrins in cancer: Survey of expression patterns. Proc Soc Exp Biol Med. 1999;222:124–138. doi: 10.1046/j.1525-1373.1999.d01-122.x. [DOI] [PubMed] [Google Scholar]
- 13.Hwang R, Varner J. The role of integrins in tumor angiogenesis. Hematol Oncol Clin North Am. 2004;18:991–1006, vii. doi: 10.1016/j.hoc.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 14.Hynes RO. Integrins: Versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-S. [DOI] [PubMed] [Google Scholar]
- 15.Yamaguchi M, Ebihara N, Shima N, Kimoto M, Funaki T, Yokoo S, Murakami A, Yamagami S. Adhesion, migration, and proliferation of cultured human corneal endothelial cells by laminin-5. Invest Ophthalmol Vis Sci. 2011;52:679–684. doi: 10.1167/iovs.10-5555. [DOI] [PubMed] [Google Scholar]
- 16.DiPersio CM, Hodivala-Dilke KM, Jaenisch R, Kreidberg JA, Hynes RO. alpha3beta1 Integrin is required for normal development of the epidermal basement membrane. J Cell Biol. 1997;137:729–742. doi: 10.1083/jcb.137.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kreidberg JA. Functions of alpha3beta1 integrin. Curr Opin Cell Biol. 2000;12:548–553. doi: 10.1016/S0955-0674(00)00130-7. [DOI] [PubMed] [Google Scholar]
- 18.Tsuji T. Physiological and pathological roles of alpha3beta1 integrin. J Membr Biol. 2004;200:115–132. doi: 10.1007/s00232-004-0696-5. [DOI] [PubMed] [Google Scholar]
- 19.Takenaka K, Shibuya M, Takeda Y, Hibino S, Gemma A, Ono Y, Kudoh S. Altered expression and function of beta1 integrins in a highly metastatic human lung adenocarcinoma cell line. Int J Oncol. 2000;17:1187–1194. doi: 10.3892/ijo.17.6.1187. [DOI] [PubMed] [Google Scholar]
- 20.Coopman PJ, Thomas DM, Gehlsen KR, Mueller SC. Integrin alpha 3 beta 1 participates in the phagocytosis of extracellular matrix molecules by human breast cancer cells. Mol Biol Cell. 1996;7:1789–1804. doi: 10.1091/mbc.7.11.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adachi M, Taki T, Higashiyama M, Kohno N, Inufusa H, Miyake M. Significance of integrin alpha5 gene expression as a prognostic factor in node-negative non-small cell lung cancer. Clin Cancer Res. 2000;6:96–101. [PubMed] [Google Scholar]
- 22.Ryu MH, Park HM, Chung J, Lee CH, Park HR. Hypoxia-inducible factor-1alpha mediates oral squamous cell carcinoma invasion via upregulation of alpha5 integrin and fibronectin. Biochem Biophys Res Commun. 2010;393:11–15. doi: 10.1016/j.bbrc.2010.01.060. [DOI] [PubMed] [Google Scholar]
- 23.Bandyopadhyay A, Raghavan S. Defining the role of integrin alphavbeta6 in cancer. Curr Drug Targets. 2009;10:645–652. doi: 10.2174/138945009788680374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramos DM, Dang D, Sadler S. The role of the integrin alpha v beta6 in regulating the epithelial to mesenchymal transition in oral cancer. Anticancer Res. 2009;29:125–130. [PubMed] [Google Scholar]
- 25.Ramos DM, But M, Regezi J, Schmidt BL, Atakilit A, Dang D, Ellis D, Jordan R, Li X. Expression of integrin beta 6 enhances invasive behavior in oral squamous cell carcinoma. Matrix Biol. 2002;21:297–307. doi: 10.1016/S0945-053X(02)00002-1. [DOI] [PubMed] [Google Scholar]
- 26.Greene FL, Page DL, Fleming ID, Fritz AG, Balch CM, Haller DG, Morrow M, editors. AJCC cancer staging manual. Springer-Verlag; New York: 2002. [DOI] [Google Scholar]
- 27.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 28.Chang HW, Cheng CA, Gu DL, Chang CC, Su SH, Wen CH, Chou YC, Chou TC, Yao CT, Tsai CL, Cheng CC. High-throughput avian molecular sexing by SYBR green-based real-time PCR combined with melting curve analysis. BMC Biotechnol. 2008;8:12. doi: 10.1186/1472-6750-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shih IeM, Salani R, Fiegl M, Wang TL, Soosaipillai A, Marth C, Müller-Holzner E, Gastl G, Zhang Z, Diamandis EP. Ovarian cancer specific kallikrein profile in effusions. Gynecol Oncol. 2007;105:501–507. doi: 10.1016/j.ygyno.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 30.Wong HS, Chang WC. Correlation of clinical features and genetic profiles of stromal interaction molecule 1 (STIM1) in colorectal cancers. Oncotarget. 2015;6:42169–42182. doi: 10.18632/oncotarget.5888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Castilho LS, Cotta FV, Bueno AC, Moreira AN, Ferreira EF, Magalhães CS. Validation of DIAGNOdent laser fluorescence and the international caries detection and assessment system (ICDAS) in diagnosis of occlusal caries in permanent teeth: An in vivo study. Eur J Oral Sci. 2016;124:188–194. doi: 10.1111/eos.12257. [DOI] [PubMed] [Google Scholar]
- 32.Zhang B, Zhang Z, Zhang X, Gao X, Kernstine KH, Zhong L. Serological antibodies against LY6K as a diagnostic biomarker in esophageal squamous cell carcinoma. Biomarkers. 2012;17:372–378. doi: 10.3109/1354750X.2012.680609. [DOI] [PubMed] [Google Scholar]
- 33.Cai C, Shi R, Gao Y, Zeng J, Wei M, Wang H, Zheng W, Ma W. Reduced expression of sushi domain containing 2 is associated with progression of non-small cell lung cancer. Oncol Lett. 2015;10:3619–3624. doi: 10.3892/ol.2015.3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun L, Xu S, Liang L, Zhao L, Zhang L. Analysis of ROC: The value of HPV16 E6 protein in the diagnosis of early stage cervical carcinoma and precancerous lesions. Oncol Lett. 2016;12:1769–1772. doi: 10.3892/ol.2016.4844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kurozumi A, Goto Y, Matsushita R, Fukumoto I, Kato M, Nishikawa R, Sakamoto S, Enokida H, Nakagawa M, Ichikawa T, Seki N. Tumor-suppressive microRNA-223 inhibits cancer cell migration and invasion by targeting ITGA3/ITGB1 signaling in prostate cancer. Cancer Sci. 2016;107:84–94. doi: 10.1111/cas.12842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zheng W, Jiang C, Li R. Integrin and gene network analysis reveals that ITGA5 and ITGB1 are prognostic in non-small-cell lung cancer. Onco Targets Ther. 2016;9:2317–2327. doi: 10.2147/OTT.S91796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Desai K, Nair MG, Prabhu JS, Vinod A, Korlimarla A, Rajarajan S, Aiyappa R, Kaluve RS, Alexander A, Hari PS, et al. High expression of integrin β6 in association with the Rho-Rac pathway identifies a poor prognostic subgroup within HER2 amplified breast cancers. Cancer Med. 2016;5:2000–2011. doi: 10.1002/cam4.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.He M, Zhao Y, Yi H, Sun H, Liu X, Ma S. The combination of TP53INP1, TP53INP2 and AXIN2: Potential biomarkers in papillary thyroid carcinoma. Endocrine. 2015;48:712–717. doi: 10.1007/s12020-014-0341-8. [DOI] [PubMed] [Google Scholar]
- 39.Rozalski R, Gackowski D, Siomek-Gorecka A, Starczak M, Modrzejewska M, Banaszkiewicz Z, Olinski R. Urinary 5-hydroxymethyluracil and 8-oxo-7,8-dihydroguanine as potential biomarkers in patients with colorectal cancer. Biomarkers. 2015;20:287–291. doi: 10.3109/1354750X.2015.1068860. [DOI] [PubMed] [Google Scholar]
- 40.El-mezayen HA, Metwally FM, Darwish H. A novel discriminant score based on tumor-associated trypsin inhibitor for accurate diagnosis of metastasis in patients with breast cancer. Tumour Biol. 2014;35:2759–2767. doi: 10.1007/s13277-013-1366-y. [DOI] [PubMed] [Google Scholar]
- 41.Yin MZ, Tan S, Li X, Hou Y, Cao G, Li K, Kou J, Lou G. Identification of phosphatidylcholine and lysophosphatidylcholine as novel biomarkers for cervical cancers in a prospective cohort study. Tumour Biol. 2016;37:5485–5492. doi: 10.1007/s13277-015-4164-x. [DOI] [PubMed] [Google Scholar]
- 42.Arcolia V, Journe F, Renaud F, Leteurtre E, Gabius HJ, Remmelink M, Saussez S. Combination of galectin-3, CK19 and HBME-1 immunostaining improves the diagnosis of thyroid cancer. Oncol Lett. 2017;14:4183–4189. doi: 10.3892/ol.2017.6719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Michailidou E, Tzimagiorgis G, Chatzopoulou F, Vahtsevanos K, Antoniadis K, Kouidou S, Markopoulos A, Antoniades D. Salivary mRNA markers having the potential to detect oral squamous cell carcinoma segregated from oral leukoplakia with dysplasia. Cancer Epidemiol. 2016;43:112–118. doi: 10.1016/j.canep.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 44.Bu J, Bu X, Liu B, Chen F, Chen P. Increased expression of tissue/salivary transgelin mRNA predicts poor prognosis in patients with oral squamous cell carcinoma (OSCC) Med Sci Monit. 2015;21:2275–2281. doi: 10.12659/MSM.893925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grady LJ, Campbell WP, North AB. A comparison of several procedures for reducing RNase contamination in preparations of DNase and a particularly successful combination of methods. Anal Biochem. 1980;101:118–122. doi: 10.1016/0003-2697(80)90049-4. [DOI] [PubMed] [Google Scholar]
- 46.Febbraio F, Andolfo A, Tanfani F, Briante R, Gentile F, Formisano S, Vaccaro C, Scirè A, Bertoli E, Pucci P, Nucci R. Thermal stability and aggregation of sulfolobus solfataricus beta-glycosidase are dependent upon the N-epsilon-methylation of specific lysyl residues: Critical role of in vivo post-translational modifications. J Biol Chem. 2004;279:10185–10194. doi: 10.1074/jbc.M308520200. [DOI] [PubMed] [Google Scholar]
- 47.Ko YC, Huang YL, Lee CH, Chen MJ, Lin LM, Tsai CC. Betel quid chewing, cigarette smoking and alcohol consumption related to oral cancer in Taiwan. J Oral Pathol Med. 1995;24:450–453. doi: 10.1111/j.1600-0714.1995.tb01132.x. [DOI] [PubMed] [Google Scholar]
- 48.Petti S, Mohd M, Scully C. Revisiting the association between alcohol drinking and oral cancer in nonsmoking and betel quid non-chewing individuals. Cancer Epidemiol. 2012;36:e1–e6. doi: 10.1016/j.canep.2011.09.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed in the current study are available from the corresponding author on reasonable request.