Abstract
Atrial myxoma is the most common type of primary cardiac tumor and it is closely associated with stroke in adults. Early diagnosis and treatment of atrial myxomas is essential for the prevention of embolic events. The aim of the present study was to assess neurological complications associated with atrial myxoma. The neurological signs of atrial myxoma were retrospectively assessed in individuals who underwent treatment at West China Hospital (Chengdu, China) and The Affiliated Hospital of Hainan Medical University (Haikou, China), between March 2003 and February 2015. A total of 130 patients with atrial myxoma were included and 22 (17%) exhibited neurologic signs. These patients were aged 39.9±12.6 years (range, 13–78 years) and there were 13 female and 9 male patients. Ischemic cerebral infarct constituted the dominant clinical symptom (68.2%) and 3 patients exhibited concomitant cardiac manifestations. Atrial myxoma was diagnosed by echocardiography in all patients. Irregular surface of atrial myxomas was associated with a high risk of embolic events. The patients with myxoma successfully underwent surgery with no mortality recorded. In conclusion, atrial myxomas frequently manifest as cerebral infarction in individuals without cardiovascular risk factors. These tumors more commonly affect the middle cerebral artery. Irregular surface of myxomas appears to be associated with embolic events. Echocardiography may improve the diagnosis and early treatment of atrial myxomas.
Keywords: atrial myxomas, neurological complications, echocardiography, acute ischemic stroke, aneurysm
Introduction
Atrial myxomas are the most common primary cardiac tumors, comprising up to 80% of all primary tumors of the heart (1). It is widely admitted that these tumors originate from subendocardial multipotential mesenchymal cells (2). Anatomically, atrial myxomas are mostly pedunculated and soft, and which are in consistency with a smooth, villous, or irregular surface. Atrial myxomas predominantly occur between the third and the sixth decades of life and show a 2.05:1 female predominance (3). Over 75% of myxomas originate from the left atrium, 18% of them from the right atrium, while less than 5% are found in bilateral atrium (4). Clinical manifestations of atrial myxoma vary greatly and patients usually present one of the symptoms in the following tetrad: Arrhythmias, intracardiac flow obstruction, embolic phenomena and constitutional symptoms (5). Atrial myxomas are asymptomatic in ~10% of the affected individuals (6). In clinic, neurologic signs occur in 25–45% of myxoma patients, and may appear as the initial symptom in 12% individuals (7). Surgical resection is the most effective treatment and preventive intervention in atrial myxomas.
The neurological signs of atrial myxoma commonly result from ischemic cerebral infarction complications, which are found in 30–50% of patients with myxoma (8). Manifestation as haemorrhagic stroke is uncommon, and this seems to be related to the formation of cerebral aneurysms (9). The tumor type, size, location and mobility appear to be closely associated with embolism in patients with atrial myxomas (10). Cerebral infarction might recur for non-diagnosed and treated myxomas. The delayed neurological complications of myxomatous disease include oncotic aneurysms and myxomatous metastasis. They usually present with manifestations similar to cerebral vasculitis and infective endocarditis (3). Accordingly, echocardiography, computed tomography, and magnetic resonance imaging of the heart should be performed in patients with suspected stroke.
Studies of atrial myxoma and secondary brain infarction are generally small series and case reports. In this study, clinical cases of atrial myxoma-associated neurologic complications were assessed for morphology and clinical data, also summarizing their imaging characteristics.
Patients and methods
Patients
Patient records of West China Hospital (Chengdu, China) and Affiliated Hospital of Hainan Medical University (Haikou, China) were queried for myxoma detection between March 10, 2003 and February 5, 2015, and a total of 130 patients definitely diagnosed with atrial myxoma were enrolled. After approval by the institutional review boards, 22 consecutive patients were identified with neurologic complications resulting from cardiac myxoma. The medical history of each patient was reviewed in detail, recording neurological conditions related to atrial myxoma. The patients were classified into two groups (embolic vs. non-embolic), and sex, age, cardiovascular risk factors, myxoma location and size, initial manifestation, neuroimaging assays, and clinical outcome were recorded for each of them.
Immunohistochemistry
For immunohistochemical analysis of atrium myxoma, paraffin-embedded samples were sliced and mounted on microscopic slides. Rabbit monoclonal anti-CD34 and anti-CD68 antibodies (Santa Cruz Biotechnology Inc., Dallas, TX, USA) were the first antibodies and were diluted 200 times. Antigens retrieved using citric acid buffer (10 mmol/l, pH 7.2) and by microwaving slides. The samples were treated with primary antibody at 4°C overnight followed by incubation with biotinylated secondary antibody (1:1,000 dilution; Zhongshan Biology Company, Beijing, China). Finally, freshly prepared DAB coloring solution was added, and CD34 or CD68 would stain brown under the microscope.
Statistical analysis
Statistical analysis was performed with SPSS 19.0 (IBM Corp., Armonk, NY, USA). Data were presented as mean ± standard deviation (SD). Categorical variables were assessed by the chi-square test or Fisher's exact test. P<0.05 was considered to indicate a statistically significant difference.
Results
Patient population
Neurologic complications associated with cardiac myxoma were found in 22 patients (17%), comprising 9 male and 13 female individuals (Table I). Ages were 39.9±12.6 (range 13–78) years. Seven patients (32%) had no remarkable medical history before diagnosis. Meanwhile, 15 subjects (68%) showed cerebrovascular risk factors such as hyperlipidemia (n=10), hypertension (n=6), and smoking (n=5). Myxomas were limited to the left atrium in 20 cases (90.9%). A total of 19 patients (86.4%) had neurologic signs as initial presentations, while three patients initially showed cardiac signs, with neurologic symptoms occurring 2 years after surgical treatment. All patients showing neurologic complications associated with cardiac myxoma underwent neuroimaging. Brain computed tomography (CT) was performed in 19 patients (86.4%). Twenty-one individuals (95.5%) had brain magnetic resonance (MRI) examinations. Twelve patients (54.5%) underwent cerebrovascular arterial imaging with magnetic resonance angiography (MRA) and computed tomography angiography (CTA). Five patients (22.7%) were submitted to digital subtraction angiography (DSA) examinations.
Table I.
Baseline clinical characteristics of patients with atrial myxoma.
| Patient characteristic | N (%) |
|---|---|
| Sex | |
| Male | 9 (40.9) |
| Female | 13 (59.1) |
| Age, mean ± SD | 39.9±12.6 |
| Age range, years | 13–78 |
| Medical conditions | |
| Hyperlipidemia | 10 (45.5) |
| Hypertension | 6 (27.3) |
| Smoking | 5 (22.7) |
| Myxoma location | |
| Left atrium | 20 (90.9) |
| Right atrium | 1 (4.5) |
| Multichamber | 1 (4.5) |
| Initial symptoms | |
| Dyskinesia | 12 (54.5) |
| Speech dysfunction | 3 (13.6) |
| Disturbance of consciousness | 2 (9.1) |
| Headache | 1 (4.5) |
| Vision changes | 1 (4.5) |
| Palpitations | 2 (9.1) |
| Dyspnoea | 1 (4.5) |
| Neuroimaging | |
| Non-contrast CT | 19 (86.4) |
| CTA | 2 (9.1) |
| MRI | 21 (95.5) |
| MRA | 12 (54.5) |
| DSA | 5 (22.7) |
SD, standard deviation; CT, computed tomography; CTA, computed tomography angiography; MRI, magnetic resonance; MRA, magnetic resonance angiography; DSA, digital subtraction angiography.
Characteristics of subjects with myxoma associated cerebral infarction
The characteristics of 15 individuals developing cerebral infarction secondary to myxomatous emboli are listed in Table II. Sudden-onset unilateral motor deficit was most frequent, occurring in 13 subjects (87%). Among the 15 cases with cerebral infarctions, 8 (53.3%) and 7 (46.7%), respectively, had infarcts in multiple and single vascular territories. Cardiac myxomas were surgically resected with success in all patients. The time period between the onset of neurological symptoms and resection averaged 20 days, ranging from 1 to 27 days, excluding patient 5 who suffered a transient ischemic attack (TIA) 2 years before myxoma diagnosis. Three patients (20.0%) showed persistent neurologic deficits at final follow-up.
Table II.
Characteristics of patients presenting with cerebral infarction complications of myxoma.
| Age/sex | Neurological syndrome | Infarction location on imaging | Time elapsed between cerebral infarction and myxoma diagnosis | Neurological disability on last follow-up |
|---|---|---|---|---|
| 44/F | Right-sided weakness | Left frontoparietal infarct | Simultaneous | None |
| 58/F | Aphasia and right-sided hemiparesis | Left temporoparietal infarct | Simultaneous | Mild persistent right-sided weakness |
| 22/M | Left-sided hemiparesis | Right frontal lobe infarct | Simultaneous | None |
| 71/F | Left-sided hemiparesis, left arm paraesthesia | Right parietal infarct | Simultaneous | None |
| 41/M | Left-sided hemiparesis, facial droop | Right basal ganglia and corona radiata infarcts | 2 years | None |
| 67/M | Visual changes, bilateral Frontal headache | Left occipital ischaemic infarct | Simultaneous | Persistent left sided weakness |
| 30/F | Left-sided arm and leg weakness | Right parietal infarct, posterior putamen and right centrum semiovale | Simultaneous | None |
| 50/F | Right arm and face weakness | Left thalamus, midbrain and temporal lobe infarct | Simultaneous | None |
| 69/F | Left-sided arm weakness | Right parietal infarct | Simultaneous | None |
| 55/M | Dysarthria, left hemiparesis | Multiple infarctions | Simultaneous | Persistent right sided weakness |
| 23/F | Right-sided weakness | Left frontoparietal infarct | Simultaneous | None |
| 49/M | Right facial palsy, right hemiparesis | Multiple infarctions | Simultaneous | None |
| 61/F | Left arm weakness | Left frontal and left occipital lobe infarcts | Simultaneous | None |
| 62/F | Right-sided hemiparesis and mixed aphasia | Left temporoparietal infarct | Simultaneous | None |
| 38/M | Visual changes | Left occipital ischaemic infarct | Simultaneous | None |
M, male; F, female.
Neuroimaging findings
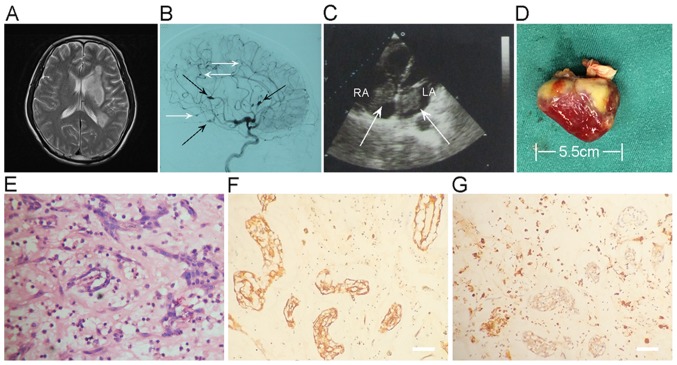
An example of combined cerebral infarction and intracranial aneurysm in a myxoma case is shown in Fig. 1. An emergency MRI of the brain demonstrated multifocal punctate acute infarction throughout the left temporal, basal ganglia and centrum semiovale (Fig. 1A). Digital subtraction angiography showed multiple typical distal fusiform and saccular aneurysms in left internal carotid arterial territories (Fig. 1B). Cardiac ultrasound revealed masses in the left and right atria that were resected, and identified as double atrial myxomas. The left atrium tumor and right atrium tumor measure 60×19 and 43×16 mm, respectively (Fig. 1C). The excised left atrium myxoma presents as an irregular, and measures 55×19×15 mm (Fig. 1D). Hematoxylin and eosin (H&E) stain disclosed typical atrial myxoma cells, embedded in a loose myxoid matrix (Fig. 1E). Immunohistochemical analysis of resected atrial myxoma showed positivity for CD34 (Fig. 1F) and CD68 (Fig. 1G).
Figure 1.
A 64-year-old male had multiple infarctions. (A) T2WI showing multiple infarctions throughout the left temporal, basal ganglia, and centrum semiovale. (B) DSA imaging showing multiple fusiform (black arrow) and saccular aneurysms (white arrow) or irregular aneurismal dilatation of the branches of ICA. Left-sided ICA, lateral view. (C) Cardiac ultrasound showing double atrial myxomas (arrows) attached to the interatrial septal wall. The LA tumor and RA tumor measure 60×19 and 43×16 mm, respectively. (D) Gross features of the excised left atrium myxoma. The tumor presents as an irregular, and measures 55×19×15 mm. (E) Histologic and morphological observations of myxomas. (F) Immunohistochemistry assay. CD34 expression is positive in resected myxoma. (G) Expression of CD68 in resected myxoma was assessed by immunohistochemistry assay. Scale bar, 50 µm. LA, left atrium; RA, right atrium.
Myxoma characteristics
No significant differences were found in the diameter of myxomas between the embolic and non-embolic groups (4.26±1.6 vs. 3.18±1.1 cm; P=0.3720). However, significantly more individuals in the embolic group had large myxomas (>5 cm) compared with the non-embolic group (47.06 vs. 20.35%, P=0.0230). Microscopy revealed 10 myxomas with intratumoral hemorrhage (58.82%) in the embolic group, while 61 myxomas showed intratumoral hemorrhage (53.98%) in the non-embolic group (P=0.4510). Myxoma attachment was similar in both groups (1.0±0.2 vs. 1.1±0.4 cm; P=0.1335; Table III). However, irregular surface of myxomas was markedly more frequent in the embolic group compared with patients with no embolism (76.47 vs. 36.28%, P=0.0273).
Table III.
Atrial myxoma characteristics: Embolic vs. non-embolic groups.
| Myxoma characteristics | Embolic group, n=17 (%) | Non-embolic group, n=113 (%) | P-value |
|---|---|---|---|
| Tumor diameter (cm), mean ± SD | 4.26±1.6 | 3.18±1.1 | 0.3720 |
| >5 cm, n | 8 (47.06) | 23 (20.35) | 0.0230 |
| Intratumoral haemorrhage | 10 (58.82) | 61 (53.98) | 0.4510 |
| Attachment diameter (cm), mean ± SD | 1.0±0.2 | 1.1±0.4 | 0.1335 |
| >1 cm, n | 7 (41.18) | 51 (45.13) | 0.4750 |
| Irregular surface, n | 13 (76.47) | 41 (36.28) | 0.0273 |
n, number of cases; SD, standard deviation.
Discussion
Atrial myxoma is found in the left atrium in 75% of patients in the solid or papillary form (2). In this study, ages (age at onset of 30–60 years) and sex distribution (female predominance) of atrial myxoma cases corroborated previous reports (6,7). Neurological symptoms of atrial myxoma might appear before or at the time of primary tumor diagnosis. They were found in 16.9% of patients, a rate lower than previously reported (4,8).
In this study, ischemic cerebral infarction was the most commonly encountered neurologic complication of atrial myxoma. Although embolism is a relatively common precursor of cerebral infarction in the youth, the majority of existing reports merely describe isolated cases. The present series assessed 22 patients with neurological manifestations associated with myxoma, including 15 with ischemic cerebral infarction. These data differ from previous series, which showed TIA as the most frequent neurological presentation of atrial myxoma (9). Nevertheless, multiple studies found percentages of ischemic cerebral infarction similar to the present values (4,5,10). This discrepancy may be associated with different pathological characteristics of atrial myxomas. This study showed that myxomas with irregular surface were more prone to embolism. Fracturing of small myxoma fragments is considered to be responsible for embolic events. Indeed, the irregular surface contributes to myxoma fragmentation and increases interactive areas, resulting in embolism (5,11,12).
Additionally, oncotic aneurysms are late signs of atrial myxoma. In patients with cardiac myxoma, oncotic aneurysms are caused by metastatic emboli, which invade and disrupt the vessel wall (13,14). In the current study, 1 patient presented with recurrent cerebral infarction caused by a partially thrombosed 1.7 cm myxomatous oncotic aneurysm. Previous studies have examined endovascular embolization, chemotherapy, and radiation in the treatment of such aneurysms, and the findings are equivocal (15–20). Therefore, the optimal treatment of myxomatous oncotic aneurysms needs to be established.
Atrial myxoma causes cerebral infarction, but can be treated. In 22 cases in this series, neurological manifestations were found as initial sign of atrial myxoma. In 14/15 patients with cerebral infarction, tumor diagnosis occurred during the hospital stay, with surgical removal within 1 month. All patients were diagnosed by echocardiography, which was used routinely after a stroke of uncertain cause. There was no recurrence of atrial myxoma in the 22 subjects that underwent surgical treatment during follow-up (6 months to 12 years). Our findings advocate for early diagnosis and surgical resection of atrial myxoma before further embolic or cardiac complications occur.
In summary, atrial myxomas frequently present with cerebral infarction in individuals without known cardiovascular risk factors. The middle cerebral artery is more commonly concerned. Irregular surface of myxomas appears to be related to embolic events. Echocardiography could improve diagnosis and early treatment of atrial myxomas.
Acknowledgments
Help and advice from all members of the Clinical Laboratory Center of the Affiliated Hospital of Hainan Medical University is acknowledged.
Funding
The present study was funded by the National Natural Science Foundation of China (grant no. 81460184), the Health and Family Planning Commission of Hainan Province, China, Scientific Research Project (2014–07) and the Social Development Project of Science and Technology in Hainan Province (grant no. 2015SF13).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
XYW, YMC, XPL and QFL conceived and designed the study. XYW, YMC, LLY and HBZ performed the experiments. XYW, ZBC and YMC analyzed the data. LM and SRW assisted in the study design and discussed and interpreted the data. QFL and XYW wrote the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The present study was approved by the First Affiliated Hospital of Hainan Medical University's Institutional Review Board. Individual consent was waived by the committee because of the retrospective nature of the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Gošev I, Paić F, Durić Z, Gošev M, Ivčević S, Jakuš FB, Biočina B. Cardiac myxoma the great imitators: Comprehensive histopathological and molecular approach. Int J Cardiol. 2013;164:7–20. doi: 10.1016/j.ijcard.2011.12.052. [DOI] [PubMed] [Google Scholar]
- 2.Liao WH, Ramkalawan D, Liu JL, Shi W, Zee CS, Yang XS, Li GL, Li J, Wang XY. The imaging features of neurologic complications of left atrial myxomas. Eur J Radiol. 2015;84:933–939. doi: 10.1016/j.ejrad.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 3.Thyagarajan B, Kumar MP, Patel S, Agrawal A. Extracardiac manifestations of atrial myxomas. J Saudi Heart Assoc. 2017;29:37–43. doi: 10.1016/j.jsha.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aiello VD, de Campos FP. Cardiac Myxoma. Autopsy Case Rep. 2016;6:5–7. doi: 10.4322/acr.2016.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yin L, Wang J, Li W, Ling X, Xue Q, Zhang Y, Wang Z. Usefulness of CHA2DS2-VASc scoring systems for predicting risk of perioperative embolism in patients of cardiac myxomas underwent surgical treatment. Sci Rep. 2016;6:39323. doi: 10.1038/srep39323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rios RE, Burmeister DB, Bean EW. Complications of atrial myxoma. Am J Emerg Med. 2016;34:2465.e1–2465.e2. doi: 10.1016/j.ajem.2016.05.079. [DOI] [PubMed] [Google Scholar]
- 7.Andreu Perez J, Parrilla G, Arribas JM, García-Villalba B, Lucas JJ, Navarro Garcia M, Marín F, Gutierrez F, Moreno A. Neurological manifestations of cardiac myxoma: Experience in a referral hospital. Neurologia. 2013;28:529–534. doi: 10.1016/j.nrl.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 8.He DK, Zhang YF, Liang Y, Ye X, Wang C, Kang B, Wang ZN. Risk factors for embolism in cardiac myxoma: A retrospective analysis. Med Sci Monit. 2015;21:1146–1154. doi: 10.12659/MSM.893855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Z, Chen S, Zhu M, Zhang W, Zhang H, Li H, Yuan G, Zou C. Risk prediction for emboli and recurrence of primary cardiac myxomas after resection. J Cardiothorac Surg. 2016;11:22. doi: 10.1186/s13019-016-0420-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spartalis M, Tzatzaki E, Spartalis E, Moris D, Athanasiou A, Kyrzopoulos S, Tsiapras D, Kalogris P, Voudris V. Atrial myxoma mimicking mitral stenosis. Cardiol Res. 2017;8:128–130. doi: 10.14740/cr558w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh S, Tripathy MP, Mohanty BB, Biswas S. Sporadic multicentric right atrial and right ventricular myxoma presenting as acute pulmonary thromboembolism. Heart Views. 2016;17:19–22. doi: 10.4103/1995-705X.182642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sabzi F, Faraji R. Preoperative emboli in a pregnant woman with myxoma. Iran J Med Sci. 2016;41:345–349. [PMC free article] [PubMed] [Google Scholar]
- 13.Ekinci EI, Donnan GA. Neurological manifestations of cardiac myxoma: A review of the literature and report of cases. Int Med J. 2004;34:243–249. doi: 10.1111/j.1444-0903.2004.00563.x. [DOI] [PubMed] [Google Scholar]
- 14.Brinjikji W, Morris JM, Brown RD, Thielen KR, Wald JT, Giannini C, Cloft HJ, Wood CP. Neuroimaging findings in cardiac myxoma patients: A single-center case series of 47 patients. Cerebrovasc Dis. 2015;40:35–44. doi: 10.1159/000381833. [DOI] [PubMed] [Google Scholar]
- 15.Lee SJ, Kim JH, Na CY, Oh SS. Eleven years' experience with Korean cardiac myxoma patients: Focus on embolic complications. Cerebrovasc Dis. 2012;33:471–479. doi: 10.1159/000335830. [DOI] [PubMed] [Google Scholar]
- 16.Alvarez-Sabin J, Lozano M, Sastre-Garriga J, Montoyo J, Murtra M, Abilleira S, Codina A. Transient ischaemic attack: A common initial manifestation of cardiac myxomas. Eur Neurol. 2001;45:165–170. doi: 10.1159/000052116. [DOI] [PubMed] [Google Scholar]
- 17.Isogai T, Yasunaga H, Matsui H, Tanaka H, Hisagi M, Fushimi K. Factors affecting in-hospital mortality and likelihood of undergoing surgical resection in patients with primary cardiac tumors. J Cardiol. 2017;69:287–292. doi: 10.1016/j.jjcc.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 18.Lee TH, Huang SC, Su TM, Yang KY, Rau CS. Multiple cerebral aneurysms and brain metastasis from primary cardiac myxosarcoma: A case report and literature review. Chang Gung Med J. 2011;34:315–319. [PubMed] [Google Scholar]
- 19.Saffie P, Riquelme F, Mura J, Urra A, Passig C, Castro Á, Illanes S. Multiple myxomatous aneurysms with bypass and clipping in a 37-year-old man. J Stroke Cerebrovasc Dis. 2015;24:e69–e71. doi: 10.1016/j.jstrokecerebrovasdis.2014.10.017. [DOI] [PubMed] [Google Scholar]
- 20.Al-Said Y, Al-Rached H, Baeesa S, Kurdi K, Zabani I, Hassan A. Emergency excision of cardiac myxoma and endovascular coiling of intracranial aneurysm after cerebral infarction. Case Rep Neurol Med. 2013;2013:839270. doi: 10.1155/2013/839270. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.